Abstract
Aims
To evaluate the impact of negative pressure wound therapy (NPWT) on the odds of having deep infections and health-related quality of life (HRQoL) following open fractures.
Methods
Patients from the Fluid Lavage in Open Fracture Wounds (FLOW) trial with Gustilo-Anderson grade II or III open fractures within the lower limb were included in this secondary analysis. Using mixed effects logistic regression, we assessed the impact of NPWT on deep wound infection requiring surgical intervention within 12 months post-injury. Using multilevel model analyses, we evaluated the impact of NPWT on the Physical Component Summary (PCS) of the 12-Item Short-Form Health Survey (SF-12) at 12 months post-injury.
Results
After applying inverse probability treatment weighting to adjust for the influence of injury characteristics on type of dressing used, 1,322 participants were assessed. The odds of developing a deep infection requiring operative management within 12 months of initial surgery was 4.52-times higher in patients who received NPWT compared to those who received a standard wound dressing (95% confidence interval (CI) 1.84 to 11.12; p = 0.001). Overall, 1,040 participants were included in our HRQoL analysis, and those treated with NPWT had statistically significantly lower mean SF-12 PCS post-fracture (p < 0.001). These differences did not reach the minimally important difference for the SF-12 PCS.
Conclusion
Our analysis found that patients treated with NPWT had higher odds of developing a deep infection requiring operative management within 12 months post-fracture. Due to possible residual confounding with the worst cases being treated with NPWT, we are unable to determine if NPWT has a negative effect or is simply a marker of worse injuries or poor access to early soft-tissue coverage. Regardless, our results suggest that the use of this treatment requires further evaluation.
Cite this article: Bone Jt Open 2022;3(3):189–195.
Keywords: Negative Pressure Wound Therapy, Fractures, Infection, negative pressure wound therapy, open fractures, infection, deep infections, wounds, SF-12 scores, logistic regression analysis, physical component summary (PCS), Short Form Health Survey, soft-tissue
Introduction
Open fractures continue to be devastating presentations in orthopaedic trauma, as they are associated with superficial and deep infections and various bone healing problems. 1-3 These complications are associated with increased costs to the health care system. 4 Furthermore, patients with open fractures have been found to have decreased health-related quality of life (HRQoL). 5
Once initial standard management of open fractures is completed, including thorough irrigation and debridement of the wound, the surface of the wound is then dressed. Dressings can take the form of a non-adhesive sealed dressing layer, with or without antibiotic beads, which is applied to protect the wound from further contamination. 6 Another form of dressing commonly used for open fracture management is negative pressure wound therapy (NPWT). This technology has been previously thought to aid wound healing through removal of fluid, promotion of wound healing through angiogenesis, and cell division activation, as well as optimization of the wound environment. 7,8 This, however, comes at a considerable cost increase compared to conventional dressings.
International expert panels, as well as clinical guidelines, 9 have supported the use of NPWT for wounds in the setting of open fractures. More recently, the Wound management of Open Lower Limb Fractures (WOLLF) randomized controlled trial (RCT) compared the effect of NPWT to standard wound management on 12-month disability and deep infection rates among patients with severe open fractures of the lower limb. 10 In their study of 460 patients, no statistically significant differences were found between the two groups. This secondary analysis of the Fluid Lavage in Open Fracture Wounds (FLOW) trial patient cohort aims to evaluate the effect of NPWT compared to standard dressings on the rate of deep infections and HRQoL within this large cohort of open fracture patients.
Methods
The FLOW trial was an RCT, which enrolled patients with open fractures from 41 clinical sites from Australia, Canada, India, Norway and the USA. 11 This study was approved by the ethics committees at each participating site, as well as the two co-ordinating centres at McMaster University (Research Ethics Board no. 08-268) and Prisma Health (formerly Greenville Health System) (Institutional Review Committee no. 03-08-06). Furthermore, the study was prospectively registered at www.clincaltrials.gov with identifier NCT00788398. The two-by-three factorial designed study randomized 2,551 patients who had open fractures to undergo one of three irrigation pressures and one of two irrigation solutions. This study found that the reoperation rate was higher with the soap group compared to the saline group. Meanwhile, the reoperation rate was similar regardless of irrigation pressure.
Deep infection analysis
Patients within the FLOW trial who suffered Gustilo-Anderson 12 grade II or III lower limb open fractures were included in the study. Patients with a deep infection diagnosed before the date of NPWT application or on the same day were excluded from the analysis. To adjust for the influence of injury characteristics among the NPWT and standard wound dressing groups, an inverse probability treatment weighting model using the covariate balancing propensity score method was performed to calculate the propensity score and generate a weighted cohort. Inverse probability treatment weighting creates groups that are otherwise similar when assessing the impact of a treatment or exposure. 13 Opposed to matching treated and untreated individuals on a particular selection of confounders, the inverse probability treatment weighting approach uses the entire cohort and can include numerous confounding variables. 13 Every individual in the cohort is assigned a weight, dependent on the probability of exposure to the treatment effect being explored, and applying this weight to regression models lessens or eliminates the influence of confounders. 13 Since most observations are kept in the analysis, this method offers increased precision in estimating treatment effects. 14 The following variables were controlled for: Gustilo-Anderson grade (II vs IIIA vs IIIB), irrigation solution (soap vs saline), fracture location (tibia vs other), mechanism of injury (low vs high energy), degree of contamination (low vs high), skin loss (yes vs no), and muscle loss (yes vs no).
To ensure covariate balance of the weighted cohort, we checked the standardized mean differences (SMDs) of each covariate. An SMD no greater than 0.1 implied a negligible correlation and good balance between the group and each covariate. A mixed effects logistic regression analysis was then completed, with centre included as a random effect, NPWT versus standard wound dressing included as a fixed effect, and the propensity score weights included as an adjustment variable. Results were reported as odds ratios (ORs), 95% confidence intervals (CIs), and associated p-values. All tests were two-tailed with α = 0.05. Deep infection was defined as an infection requiring surgical intervention in the form of irrigation and debridement within 12 months post-injury.
Health-related quality of life analysis
The FLOW trial included HRQoL as a secondary outcome within the study. This was assessed by using the 12-Item Short-Form Health Survey (SF-12) which measures self-reported HRQoL via an eight domain profile of functional health and wellbeing, as well as physical and mental health measures. 15 SF-12 scores were collected at baseline, as well as six weeks, and three, six, nine, and 12 months post-open fracture. Norm-based scoring methods were used to calculate the physical component summary (PCS) scores with a range of 0 to 100. We performed multilevel model analyses with two levels (patient and time). SF-12 PCS was the dependent variable with patient entered as a random effect. NPWT versus standard wound dressing was entered as a fixed effect as well as the propensity score weights and pre-injury SF-12 scores. A threshold for minimally important difference (MID) was set at five points. 15
Statistical analysis
Results were reported as adjusted mean differences (AMDs) with 95% CIs and associated p-values. All tests were two-tailed with α = 0.05. All analyses were performed using R software version 4.0.2 (R Project for Statistical Computing, Austria).
Results
Patient characteristics
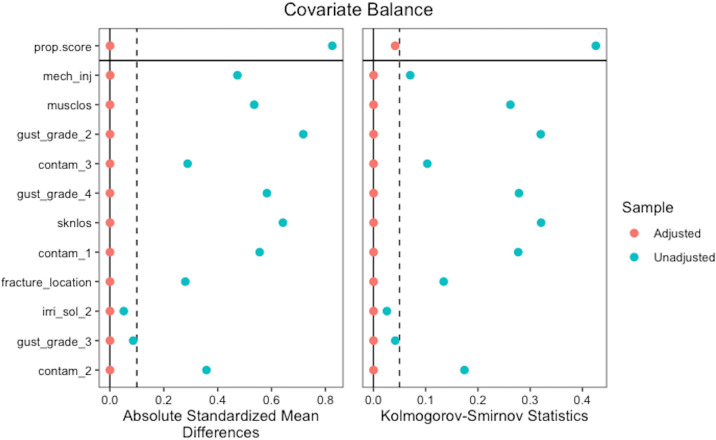
Overall, 1,322 patients met the inclusion criteria of Gustilo-Anderson grade II or III open fractures within the lower limb from the FLOW trial. Of the 1,322 included patients, 266 received NPWT and 1,056 received a standard wound dressing. The mean duration of NPWT was 11.5 days (standard deviation (SD) 21). Table I demonstrates the patient demographics prior to propensity score weighting. After propensity score weighting, adequate balance between the NPWT and standard wound dressing groups was obtained as shown by standardized mean differences less than 0.1 for each of the covariates (Table II and Figure 1).
Table I.
Baseline patient characteristics.
| Variable | NPWT group (n = 266) | Standard wound dressing group (n = 1,056) |
|---|---|---|
| Mean age, yrs (SD) | 43.0 (15.6) | 43.7 (16.9) |
| Sex, n (%) | ||
| Female | 52 (19.5) | 328 (31.1) |
| Male | 214 (80.5) | 728 (68.9) |
| Gustilo grade, n (%) | ||
| II | 73 (27.4) | 628 (59.5) |
| IIIA | 99 (37.2) | 349 (33.0) |
| IIIB | 94 (35.3) | 79 (7.5) |
| Mechanism of Injury, n (%) | ||
| Low energy | 6 (2.3) | 98 (9.3) |
| High energy | 260 (97.7) | 958 (90.7) |
| Fracture location, n (%) | ||
| Other | 96 (36.1) | 523 (49.5) |
| Tibia | 170 (63.9) | 533 (50.5) |
| Contamination, n (%) | ||
| Mild | 124 (46.6) | 785 (74.3) |
| Moderate | 102 (38.3) | 221 (20.9) |
| Severe | 40 (15.0) | 50 (4.7) |
| Irrigation solution, n (%) | ||
| Soap | 130 (48.9) | 920 (87.1) |
| Saline | 136 (51.1) | 567 (53.7) |
| Amount of fascial tissue debrided, n (%) | ||
| None | 47 (17.7) | 383 (36.3) |
| Small (< 1 cm2) | 135 (50.8) | 560 (53.0) |
| Moderate (1 to 5 cm2) | 72 (27.1) | 102 (9.7) |
| Large (> 5 cm2) | 12 (4.5) | 11 (1.0) |
| Amount of bone debrided (n = 265), n (%) | ||
| None | 98 (36.8) | 547 (51.8) |
| Small (< 1 cm2) | 100 (37.6) | 364 (34.5) |
| Moderate (1 to 5 cm2) | 53 (19.9) | 124 (11.7) |
| Large (> 5 cm2) | 14 (5.3) | 21 (2.0) |
| Amount of skin debrided, n (%) | ||
| None | 38 (14.3) | 303 (28.7) |
| Small (< 1 cm2) | 124 (46.6) | 619 (58.6) |
| Moderate (1 to 5 cm2) | 86 (32.3) | 111 (10.5) |
| Large (> 5 cm2) | 18 (6.8) | 23 (2.2) |
| Amount of muscle debrided (n = 1,055), n (%) | ||
| None | 61 (22.9) | 502 (47.5) |
| Small (< 1 cm2) | 117 (44.0) | 434 (41.1) |
| Moderate (1 to 5 cm2) | 64 (24.1) | 105 (9.9) |
| Large (> 5 cm2) | 24 (9.0) | 14 (1.3) |
| Mean wound width, cm (SD) | 6.4 (6.3) | 3.1 (3.3) |
| Mean wound length, cm (SD) | 8.6 (8.9) | 5.6 (5.6) |
| Wound degloving injury, n (%) | 124 (46.6) | 193 (18.3) |
| Skin loss, n (%) | 132 (49.6) | 185 (17.5) |
| Muscle loss, n (%) | 106 (39.8) | 144 (13.6) |
NPWT, negative pressure wound therapy; SD, standard deviation.
Table II.
Covariate balance across comparison groups before and after propensity score weighting.
| Before propensity score weighting | After propensity score weighting | |||||
|---|---|---|---|---|---|---|
| Variable | NPWT group (n = 266) | Standard wound dressing group(n = 1,056) | SMD | NPWT group (n = 266) | Standard wound dressing group (n = 1,056, weighted as 318) |
SMD |
| Gustilo grade, % | ||||||
| II | 27.4 | 59.5 | -0.72 | 27.4 | 27.5 | -0.0002 |
| IIIA | 37.2 | 33.1 | 0.09 | 37.2 | 37.2 | 0 |
| IIIB | 35.3 | 7.5 | 0.58 | 35.3 | 35.3 | 0.0002 |
| Mechanism of injury, % | ||||||
| High energy | 97.7 | 90.7 | 0.47 | 97.7 | 97.7 | 0.0008 |
| Fracture location, % | ||||||
| Tibia | 63.9 | 50.5 | 0.28 | 63.9 | 63.9 | 0.0001 |
| Contamination, % | ||||||
| Mild | 46.6 | 74.3 | -0.56 | 46.6 | 46.6 | -0.0001 |
| Moderate | 38.4 | 20.9 | 0.36 | 38.4 | 38.4 | 0 |
| Severe | 15 | 4.7 | 0.29 | 15 | 15 | 0.0002 |
| Irrigation solution, % | ||||||
| Saline | 51.1 | 53.7 | -0.05 | 51.1 | 51.1 | 0.0001 |
| Skin loss | 49.6 | 17.5 | 0.64 | 49.6 | 49.6 | 0.0002 |
| Muscle loss | 39.9 | 13.6 | 0.54 | 39.9 | 39.8 | 0.0002 |
| Propensity score, probability | 0.35 | 0.16 | 0.83 | 0.35 | 0.35 | -0.0005 |
NPWT, negative pressure wound therapy; SMD, standardized mean difference.
Fig. 1.
Covariate balance across comparison groups before and after propensity score weighting.
Rate of infection
We found that the odds of developing a deep infection requiring operative management within 12 months of initial injury was 4.52-times higher (95% CI 1.84 to 11.12; p = 0.001, mixed effects logistic regression analysis) in patients who received NPWT compared to those who did not (Table III). A sensitivity analysis was completed by excluding Gustilo IIIB injuries, leaving 1,149 patients with either Gustilo II or IIIA injuries available. Within this analysis, the odds of developing a deep infection requiring operative management within 12 months of initial injury was 4.16-times higher (95% CI 0.86 to 20.25; p < 0.001, mixed effects logistic regression analysis) in patients who received NPWT compared to those who did not (Table III). A second sensitivity analysis was completed by excluding participants with more than four irrigation and debridement procedures done during reoperation. Within this analysis of 1,308 participants, the odds of developing a deep infection requiring operative management within 12 months of initial injury was 3.81-times higher (95% CI 1.53 to 9.47; p = 0.004, mixed effects logistic regression analysis) in patients who received NPWT compared to those who did not (Table III).
Table III.
Rates of deep infection rates at 12 months.
| Deep infection complication | NPWT group, n (%) | Standard wound dressing group, n (%) |
Odds ratio (95% CI) | p-value* |
|---|---|---|---|---|
| Analysis 1: Participants with Gustilo-Anderson II or IIIA or IIIB open fractures (n = 1,322 patients; 116 events) | n = 266 patients 50 (18.8) |
n = 1,056 patients 66 (6.3) |
4.52 (1.84 to 11.12) | 0.001 |
| Analysis 2: Participants with Gustilo-Anderson II or IIIA open fractures (IIIB fractures excluded) (n = 1,149 patients; 91 events) | n = 172 patients 31 (18.0) |
n = 977 patients 60 (6.1) |
4.16 (0.86 to 20.25) | < 0.001 |
| Analysis 3: Exclusion of participants with more than four irrigation and debridement procedures done during reoperation (n = 1,308 patients; 102 events) | n = 253 patients 42 (16.6) |
n = 1,055 patients 60 (5.7) |
3.81 (1.53 to 9.47) | 0.004 |
Mixed effects logistic regression analysis.
CI, confidence interval; NWPT, negative pressure wound therapy.
Health-related quality of life
A total of 1,040 patients met the inclusion criteria for the analysis (Table IV). NPWT was associated with a statistically significantly lower mean post-fracture SF-12 PCS, indicating worse physical health (AMD -4.23; 95% CI -5.73 to -2.73; p < 0.001). This difference did not reach the MID for the SF-12 PCS.
Table IV.
Comparison of 12-Item Short Form Health Survey Physical Component Summary (SF-12 PCS) Score.
| Endpoint | Patients with data | Adjusted mean difference in score, NPWT vs standard wound dressing (95% CI)* | p-value† |
|---|---|---|---|
| SF-12 PCS‡ | 5,168 observations among 1,040 participants | -4.23 (-5.73 to -2.73) | < 0.001 |
The mean difference was obtained from the multi-level model.
Asymptotic Wald test.
Minimally important difference was set at five points.
CI, confidence interval; NWPT, negative pressure wound therapy.
Discussion
Our study assessed patients with severe lower limb fractures within the FLOW trial, and found statistically significantly increased odds of developing wound infections that require surgical intervention in patients treated with NPWT compared to those who received standard wound dressings. There was also a significant decrease in PCS scores of the SF-12 quality of life measure at various time points for those treated with NPWT compared to standard treatment.
In contrast, the recent WOLLF trial of patients with severe open fractures of the lower limb found no significant difference in infection between those treated with NPWT compared to standard wound care. 10 Their study included 460 patients across 24 trauma hospitals within the UK. It should be noted that this study only included patients with wounds that surgeons deemed not able to close primarily. Additionally, differences in our results and the WOLLF trial may be attributed to the possibility that varying standard dressing interventions were used in the WOLLF and FLO trials. Another RCT had demonstrated a reduction in the rate of deep wound infections in those treated with NPWT compared to those with standard wound dressing. 16 However, this was a small, single-centre study including only 59 patients.
Similar to the WOLLF trial, our study found lower SF-12 PCS scores over 12 months in the patients treated with NPWT versus standard treatment. Despite the statistically significant difference of -4.23 points for the SF-12 PCS scores post-fracture, these were still less than the minimally important SF-12 difference of five points. 15 Nonetheless, our study suggests that those treated with NPWT approach the minimally clinically important difference when compared to those who received standard treatment. Further analysis should be completed to assess possible confounding variables, such as the severity of injury on patients’ HRQoL.
Upon observing that NPWT in Gustilo grade IIIB open tibial fractures sometimes led to delays in definitive soft-tissue coverage due to a reduced sense of urgency of returning to the operating room, Hou et al 17 examined the impact of prolonged NPWT in 32 patients with Gustilo type IIIB open tibia fractures and compared them with a similar cohort of type IIIB tibial fractures treated by primary NPWT in the literature. Hou et al 17 discovered that the rate of infection was significantly higher in patients who had a NPWT usage interval of more than seven days from the time of injury to flap coverage (45% rate), as compared to those whose usage interval was seven days or less (10% rate) (p = 0.001). It may be possible that our finding of there being a higher odds of deep infection when receiving NPWT was influenced by prolonged use of NPWT (mean duration 11.5 days (SD 21)), leading to a delay in soft-tissue coverage. However, we were unable to determine if longer duration of NPWT was a confounder or the cause of the worse outcome in our analysis. The optimal time periods and methods for NPWT are currently unknown and should be better defined.
The greatest strength of our analysis was the use of inverse probability treatment weighting to address the possible influence of injury characteristics on our results and create balance for covariates among the comparison groups without needing to drop any eligible cases. To further minimize any residual confounding, we performed a sensitivity analysis by excluding the more severe Gustilo IIIB injuries, and we found that the increased odds of infection remained both clinically and statistically significant. Despite the use of inverse probability treatment weighting to minimize possible confounding from variables we could control for, accounting for unknown confounders is often a challenge and a potential limitation. We are suspicious that residual confounding exists with the worst cases being treated with NPWT. Given the observational nature of the design, we are unable to determine if the NPWT has a negative effect or is simply a marker of worse injuries or poor access to soft-tissue coverage. However, with multiple sensitivity analyses to control for as many potential confounders as possible, our results still identified a significant increased odds of infection with the use of NPWT suggesting the need for further high-quality studies to investigate the use of NPWT for early wound management after open fractures.
In conclusion, the current study suggests that the use of NPWT in the setting of severe lower limb open fractures may be less beneficial than previously believed, may be associated with an increased risk of infection under some circumstances, and that further research is needed to confirm these findings.
Take home message
- The odds of developing a deep infection requiring operative management within 12 months of initial surgery was 4.52-times higher in patients who received negative pressure wound therapy (NPWT) compared to those who received a standard wound dressing.
- Those treated with NPWT had statistically significantly lower mean 12-Item Short-Form Health Survey Physical Component Summary (SF-12 PCS) post-fracture.
- These differences did not reach the minimally important difference for the SF-12 PCS.
Footnotes
Author contributions: Y. Atwan: Conceptualization, Writing – original draft.
S. Sprague: Conceptualization, Methodology, Writing – original draft.
G. Slobogean: Methodology, Writing – review and editing.
S. Bzovsky: Data curation, Formal analysis, Writing – original draft.
K. J. Jeray: Methodology, Writing – review and editing.
B. Petrisor: Methodology, Writing – review and editing
M. Bhandari: Conceptualization, Funding acquisition, Methodology, Writing – review and editing.
E. Schemitsch: Conceptualization, Methodology, Writing – original draft.
Funding statement: The authors declare that the FLOW Study was supported by research grants from the Canadian Institutes of Health Research No. MCT-93173, United States Army Institute of Surgical Research, Orthopaedic Trauma Research Program (OTRP) and Peer Reviewed Orthopaedic Research Program (PRORP), and Association Internationale pour l’Ostéosynthèse Dynamique (AIOD). Stryker provided Surgilav irrigators for the trial for clinical sites in Asia. Zimmer provided the Pulsavac irrigator at discounted rates to selected clinical sites in North America. Triad Medical donated the initial supply of castile soap for the study. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Orthopaedic Trauma Research Program under award no. W81XWH-08-1-0473 and the Peer Reviewed Orthopaedic Research Program, under award no. W81XWH-12-1-0530. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
ICMJE COI statement: S. Sprague reports board or committee member for Orthopaedic Trauma Association, employment from Global Research Solutions, and consultant fees from the University of Sherbrooke and Platform Life Sciences, all outside the submitted work. G. P. Slobogean reports editorial or governing board for the Journal of Orthopaedic Trauma, board or committee member for the Orthopaedic Trauma Association, paid consultant for Smith & Nephew, and paid consultant for Zimmer, all outside the submitted work. K. J. Jeray reports board or committee member for the American Board of Orthopaedic Surgery, board or committee member for the American Orthopaedic Association, editorial or governing board for the International Journal of Orthopedic Trauma, editorial or governing board for the Journal of Bone and Joint Surgery – American, editorial or governing board for the Journal of Orthopaedic Trauma, editorial or governing board for the Journal of the American Academy of Orthopaedic Surgeons, board or committee member for the Orthopaedic Trauma Association, paid presenter or speaker for Radius, board or committee member for the Southeastern Fracture Consortium, and paid consultant for Zimmer, all outside the submitted work. B. Petrisor reports paid consultant, paid presenter or speaker, and research support from Stryker and other financial or material support from Pfizer, all outside the submitted work. Dr. Bhandari reports paid consultant from AgNovos Healthcare, research support from the Canadian Institutes of Health Research (CIHR), board or committee member for the International Society of Orthopaedic Surgery and Traumatology (SICOT), research support from the National Institutes of Health (NIAMS & NICHD), research support from Physicians' Services Incorporated, paid consultant for Sanofi-Aventis, paid consultant for Smith & Nephew, and research support from the U.S. Department of Defense, all outside the submitted work. E. Schemitsch reports paid consultant for Acumed, paid consultant for Amgen, research support for Biocomposites, board or committee member for the Canadian Orthopaedic Association, other financial or material support for DePuy, paid consultant for Heron Therapeutics, IP royalties and paid consultant for ITS, editorial or governing board for the Journal of Orthopaedic Trauma, board or committee member for the Orthopaedic Trauma Association, editorial or governing board for Orthopaedic Trauma Association International, paid consultant for Pentopharm, paid consultant for Sanofi-Aventis, publishing royalties and financial or material support for Saunders/Mosby-Elsevier, other financial or material support, paid consultant, and research support for Smith & Nephew, publishing royalties and financial or material support for Springer, IP royalties, other financial or material support, and paid consultant for Stryker, paid consultant for Swemac, paid consultant for Synthes, and other financial or material support for Zimmer, all outside the submitted work. All other authors have nothing to report.
Ethical review statement: The FLOW trial was approved by the ethics committees at each participating site, as well as the two coordinating centerscentres at McMaster University (Research Ethics Board no. 08-268) and Greenville Health System (Institutional Review Committee no. 03-08-06). Furthermore, the study was prospectively registered at www.clincaltrials.gov with identifier: NCT00788398.
Group Investigators
The FLOW Investigators group are S. Sprague, K. J. Jeray, B. Petrisor, M. Bhandari, and E. Schemitsch.
Open access funding: The open access funding for FLOW Study was supported by research grants from the Canadian Institutes of Health Research No. MCT-93173, United States Army Institute of Surgical Research, Orthopaedic Trauma Research Program, and Peer Reviewed Orthopaedic Research Program, and Association Internationale pour l’Ostéosynthèse Dynamique.
Contributor Information
Yousif Atwan, Email: yatwan@uwo.ca.
Sheila Sprague, Email: sprags@mcmaster.ca.
Gerard P. Slobogean, Email: gslobogean@som.umaryland.edu.
Sofia Bzovsky, Email: bzovskys@mcmaster.ca.
Kyle J. Jeray, Email: Kyle.Jeray@prismahealth.org.
Brad Petrisor, Email: petrisor@hhsc.ca.
Mohit Bhandari, Email: bhandam@mcmaster.ca.
Emil Schemitsch, Email: Emil.Schemitsch@lhsc.on.ca.
References
- 1. Fernandes M de C, Peres LR, de Queiroz AC, Lima JQ, Turíbio FM, Matsumoto MH. Open fractures and the incidence of infection in the surgical debridement 6 hours after trauma. Acta Ortop Bras. 2015;23(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hull PD, Johnson SC, Stephen DJG, Kreder HJ, Jenkinson RJ. Delayed debridement of severe open fractures is associated with a higher rate of deep infection. Bone Joint J. 2014;96-B(3):379–384. [DOI] [PubMed] [Google Scholar]
- 3. Westgeest J, Weber D, Dulai SK, Bergman JW, Buckley R, Beaupre LA. Factors associated with development of nonunion or delayed healing after an open long bone fracture: a prospective cohort study of 736 subjects. J Orthop Trauma. 2016;30(3):149–155. [DOI] [PubMed] [Google Scholar]
- 4. MacKenzie EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am. 2007;89-A(8):1685–1692. [DOI] [PubMed] [Google Scholar]
- 5. Sprague S, Bhandari M, Heetveld MJ, et al. Factors associated with health-related quality of life, hip function, and health utility after operative management of femoral neck fractures. Bone Joint J. 2018;100-B(3):361–369. [DOI] [PubMed] [Google Scholar]
- 6. Dhivya S, Padma VV, Santhini E. Wound dressings - a review. Biomedicine. 2015;5(4):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51(7):301–331. [DOI] [PubMed] [Google Scholar]
- 8. Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg. 2008;122(3):786–797. [DOI] [PubMed] [Google Scholar]
- 9. Cross WW, Swiontkowski MF. Treatment principles in the management of open fractures. Indian J Orthop. 2008;42(4):377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costa ML, Achten J, Bruce J, et al. Effect of negative pressure wound therapy vs standard wound management on 12-month disability among adults with severe open fracture of the lower limb: The wollf randomized clinical trial. JAMA. 2018;319(22):2280–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FLOW Investigators, Bhandari M, Jeray KJ, et al. A trial of wound irrigation in the initial management of open fracture wounds. N Engl J Med. 2015;373(27):2629–2641. [DOI] [PubMed] [Google Scholar]
- 12. Gustilo RB, Merkow RL, Templeman D. The management of open fractures. J Bone Joint Surg Am. 1990;72:299–304. [PubMed] [Google Scholar]
- 13. Ruth C, Brownell M, Isbister J, et al. Manitoba Centre for Health Policy, ; 2015. http://mchp-appserv.cpe.umanitoba.ca/reference/insight_report_web.pdf (date last accessed 22 February 2022). [Google Scholar]
- 14. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657. [DOI] [PubMed] [Google Scholar]
- 15. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 16. Stannard JP, Volgas DA, Stewart R, McGwin G, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma. 2009;23(8):552–557. [DOI] [PubMed] [Google Scholar]
- 17. Hou Z, Irgit K, Strohecker KA, et al. Delayed flap reconstruction with vacuum-assisted closure management of the open IIIB tibial fracture. J Trauma. 2011;71(6):1705–1708. [DOI] [PubMed] [Google Scholar]