Abstract
Aims
Low-energy distal radius fractures (DRFs) are the most common upper arm fractures correlated with bone fragility. Vitamin D deficiency is an important risk factor associated with DRFs. However, the relationship between DRF severity and vitamin D deficiency is not elucidated. Therefore, this study aimed to identify the correlation between DRF severity and serum 25-hydroxyvitamin-D level, which is an indicator of vitamin D deficiency.
Methods
This multicentre retrospective observational study enrolled 122 female patients aged over 45 years with DRFs with extension deformity. DRF severity was assessed by three independent examiners using 3D CT. Moreover, it was categorized based on the AO classification, and the degree of articular and volar cortex comminution was evaluated. Articular comminution was defined as an articular fragment involving three or more fragments, and volar cortex comminution as a fracture in the volar cortex of the distal fragment. Serum 25-hydroxyvitamin-D level, bone metabolic markers, and bone mineral density (BMD) at the lumbar spine, hip, and wrist were evaluated six months after injury. According to DRF severity, serum 25-hydroxyvitamin-D level, parameters correlated with bone metabolism, and BMD was compared.
Results
The articular comminuted group (n = 28) had a significantly lower median serum 25-hydroxyvitamin-D level than the non-comminuted group (n = 94; 13.4 ng/ml (interquartile range (IQR) 9.8 to 17.3) vs 16.2 ng/ml (IQR 12.5 to 20.4); p = 0.005). The AO classification and volar cortex comminution were not correlated with the serum 25-hydroxyvitamin-D level. Bone metabolic markers and BMD did not significantly differ in terms of DRF severities.
Conclusion
Articular comminuted DRF, referred to as AO C3 fracture, is significantly associated with low serum 25-hydroxyvitamin-D levels. Therefore, vitamin D3 supplementation for vitamin D deficiency might prevent articular comminuted DRFs. Nevertheless, further studies must be conducted to validate the results of the current study.
Cite this article: Bone Jt Open 2022;3(3):261–267.
Keywords: Distal radius fracture, 25-hydroxyvitamin-D, Vitamin D, Fracture severity, Osteoporosis, distal radius fractures, Serum, Vitamin D deficiency, bone mineral density (BMD), Bone fragility, arm fractures, bone metabolism, 3D CT scan, deformity
Introduction
Low-energy distal radius fractures (DRFs) correlated with bone fragility are commonly caused by falling from standing position. 1 Moreover, DRFs are the most frequent upper arm fractures associated with osteoporosis and are believed to be the first fractures among other osteoporotic fractures. 2 A previous study revealed that patients with DRF are 3.22 times at higher risk for future femoral hip fracture than those with non-DRF. 3 DRF severity is based on fracture pattern, and severe DRF is correlated with poor clinical and radiological outcomes. 4,5 Some studies have revealed an association between DRF severity and injury intensity 6 and low bone mineral density (BMD). 7,8 However, other factors affecting DRF severity are not fully elucidated.
Vitamin D is a hormone that regulates calcium ion concentration in the body, and vitamin D deficiency is associated with osteoporosis. 9 Recently, vitamin D was found to have multiple effects on the human body, which include preventing diabetes and enhancing the immune system. 10 Most importantly, it affects bone metabolism. Vitamin D deficiency decreases bone mass. Thus, it is considered a risk factor for osteoporotic fractures including DRFs. 11 Vitamin D sufficiency is reflected by serum 25-hydroxyvitamin-D levels. Approximately 76% of patients with DRFs had vitamin D deficiency (serum 25-hydroxyvitamin-D levels: < 20 ng/ml). 12 Previous studies have compared the serum 25-hydroxyvitamin-D levels between patients with DRFs and those with non-DRFs, and the results varied. 13-15 However, the relationship between DRF severity and serum 25-hydroxyvitamin-D level was not fully elucidated. We hypothesized that 25-hydroxyvitamin-D level is correlated with DRF severity. This multicentre retrospective observational study aimed to identify the relationship between DRF severity and serum 25-hydroxyvitamin-D level among perimenopausal and postmenopausal woman by assessing serum 25-hydroxyvitamin-D levels according to DRF severity. Moreover, bone metabolic markers and BMD were evaluated according to DRF severity.
Methods
Ethical approval
This multicentre, retrospective observational study was approved by the institutional review board of Toyonaka Municipal Hospital, Kansai Rosai Hospital, and JCHO Hoshigaoka Medical Center. Moreover, it was performed in accordance with the Declaration of Helsinki. 16 Not all patients were required to provide informed consent because the clinical data were anonymized after each patient agreed to treatment. We applied the opt-out method, which enabled patients to refuse to be included in this study. The consent in this study was obtained from the website of Toyonaka Municipal Hospital.
Patients
Female patients aged 45 years or over with low-energy DRF who were treated at our institutions from January 2018 to January 2021 were included in this study. Low-energy injury was defined as falling from standing position. The exclusion criteria were as follows: high-energy injury; age under 45 years; flexion deformity DRF; hyperparathyroidism due to chronic kidney disease; vitamin D3 supplementation at the time of injury; lost to follow-up within six months of surgery or non-agreement with osteoporosis examination; and male sex. All patients underwent CT scans of the distal forearm before treatment.
Assessment of DRF severity
Because DRF severity was correlated with treatment strategy and clinical outcome, we focused on comminution of the articular surface and the volar cortex of the distal radius, which are also important factors for validating the AO classification. 5,17,18 Although metaphyseal comminution was commonly observed in the dorsal site, 19 the volar cortex was thicker than the dorsal side, and it is correlated with the clinical outcome of DRF. 4,17 Therefore, this study focused on volar cortex comminution. DRF severity was eventually defined based on the following three classifications: AO classification, comminution of the articular surface, and comminution of the volar cortex of the distal radius. 4 Although AO classification was defined as A1–C3 based on the initial classification, to prevent small group categorization, we allocated patients with extra-articular fracture in group A, those with partial articular fracture in group B, and those with complete articular fracture in group C. 20 Regarding comminution of the articular surface, comminution fracture was defined as articular fragment involving three or more fragments, and this is referred to as C3 fractures based on the AO classification. Comminution of the volar cortex was defined as a fracture line existing in the volar cortex of the distal fragment (Figure 1). Categorization into these classifications was independently performed by three examiners (SA, TS, KK) using preoperative 3D CT scan. Three examiners were two hand surgeons and one was an orthopaedic resident who was trained for hand surgery. If the classification decision was not reached among the three examiners, the classification was confirmed via a discussion.
Fig. 1.
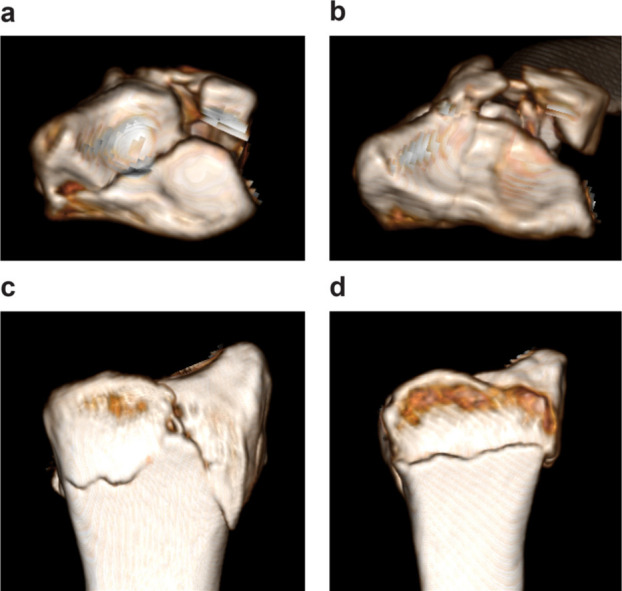
Articular comminution was defined as a fragment involving three or more fragments. a) Articular comminution and b) non-comminution. Volar cortex comminution was defined as a fracture in the volar cortex of the distal fragment. c) Volar cortex comminution and d) non-comminution.
Demographic characteristics and osteoporosis
Age, sex, BMI, estimated glomerular filtration ratio (eGFR; an indicator of renal function), current smoking status, steroid intake, and comorbidities (diabetes mellitus (DM)), and osteoporosis treatment at the time of DRF injury were assessed.
Measurement of bone metabolic markers
To prevent the effect of fracture healing on bone metabolism and to standardize the measurement period, we evaluated bone metabolic marker levels at six months after the injury. 21 Total type 1 procollagen N-terminal propeptide (P1NP) was a marker of bone formation. Tartrate-resistant acid phosphatase 5b (TRACP-5b) was used to measure bone resorption. Undercarboxylated osteocalcin (ucOC) was adopted for vitamin K sufficiency and bone quality. Intact parathyroid hormone (PTH) levels were evaluated to exclude primary or secondary hyperparathyroidism due to advanced-stage chronic kidney disease.
Assessment of BMD
The BMD of lumber spine, femoral neck, and distal forearm of the contralateral normal side were assessed via dual X-ray absorptiometry (Horizon W, Hologic, USA; PRODIGY, GE Healthcare Japan, Japan). T score was calculated at all measured sites.
Measurement of serum 25-hydroxyvitamin-D
The serum 25-hydroxyvitamin-D level (standard range: 3.0–99.9 ng/mL) was investigated using a commercially available electrochemiluminescence immunoassay (ECLIA) (Roche Diagnostics KK, Japan) and was quantified with intra-assay and inter-assay coefficients of variation of 1.47% and 2.64%, respectively. We evaluated serum 25-hydroxyvitamin-D level at six months after the injury, which was the same timing of measurement of abovementioned bone metabolic marker levels. We compared bone markers including serum 25-hydroxyvitamin-D and BMD according to DRF severity. To evaluate the effect of the seasons on serum 25-hydroxyvitamin-D level, we evaluated seasons during which the serum 25-hydroxyvitamin-D level was to be measured. Because serum 25-hydroxyvitamin-D level is affected by age, 22 the serum 25-hydroxyvitamin-D level between the DRF severity groups was compared after adjusting for age.
Statistical analysis
To assess the reliability of DRF severity classification, the Cohen’s kappa coefficient was calculated. We defined kappa value as follows: 0.0 to 0.2, slight agreement; 0.2 to 0.4, fair agreement; 0.4 to 0.6, moderate agreement; 0.6 to 0.8, substantial agreement; and 0.8 to 1.0, almost perfect agreement. 23 The Mann-Whitney U test and the chi-squared test were used to compare parameters between the two groups. Data were presented as median value (interquartile range (IQR)). The Kruskal-Wallis test was used to compare serum 25-hydroxyvitamin-D levels in terms of seasons. After adjusting for age, we performed analysis of covariance (ANCOVA) to compare serum 25-hydroxyvitamin-D levels between the two groups. A p value of < 0.05 was considered statistically significant.
To conduct a power analysis, we assessed effect size in advance. The effect size was calculated based on a previous study that compared serum 25-hydroxyvitamin-D levels between the DRF and control group. 13 Power analysis revealed that a minimum sample size of 114 was required to achieve a significance level of 0.05, with a power of 80% power and with an effect size of 0.564, for two-group comparison.
Statistical tests were performed using JMP Pro 15.0 (SAS Institute, USA), SPSS v. 22.0 (IBM, USA), and G‐power 3.1 (University of Kiel, Germany).
Results
In total, 319 consecutive patients were treated at our institutions. However, male patients (n = 85); those aged 45 years or below (n = 3); those with high-energy injury (n = 25), flexion deformity (n = 28), and hyperparathyroidism (n = 1); those receiving vitamin D3 supplementation (n = 13); and those who were lost to follow-up or who refused to join the BMD survey (n = 42) were excluded. In total, 122 female patients were enrolled in this study. All injuries were caused by falling from standing position, and all cases of DRFs involved extension deformity.
Regarding fracture severity based on the AO classification, 35 patients presented with type A fracture, and 87 with type C. None of the patients had type B fracture. In total, 28 and 20 patients presented with articular comminuted and volar cortex comminuted fracture, respectively. Table I shows the kappa coefficient for the reliability of the classifications. Articular comminution classification had the highest reliability among the three classifications, with substantial to almost perfect agreement in both inter- and intraobserver reliability.
Table I.
Reliability of distal radius fracture classification.
| Variable | AO classification | Articular comminution | Volar cortex comminution |
|---|---|---|---|
| Interobservers 1 and 2 | 0.49 | 0.68 | 0.42 |
| Interobservers 1 and 3 | 0.64 | 0.70 | 0.41 |
| Interobservers 2 and 3 | 0.48 | 0.69 | 0.38 |
| Intraobserver 1 | 0.74 | 0.73 | 0.56 |
| Intraobserver 2 | 0.94 | 0.91 | 0.86 |
| Intraobserver 3 | 0.91 | 0.96 | 0.73 |
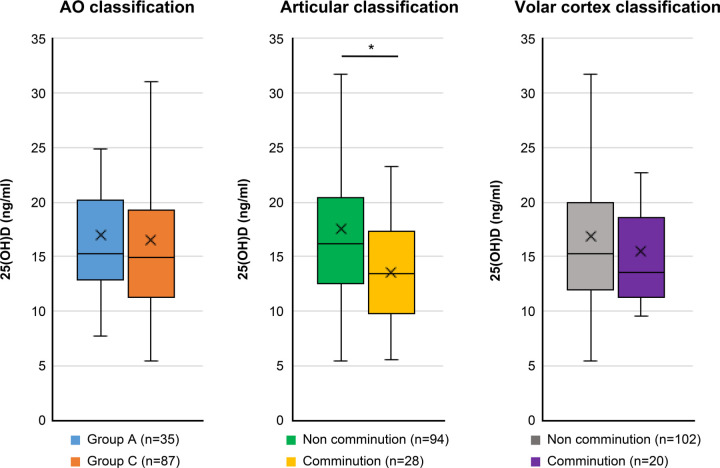
The articular comminuted group had significantly lower median serum 25-hydroxyvitamin-D levels than the non-articular comminuted group (13.4 ng/ml (IQR 9.8 to 17.3) vs 16.2 ng/ml (IQR 12.5 to 20.4); p = 0.005, Mann-Whitney U test). Age-adjusted ANCOVA indicated that the articular comminuted group had a significantly lower serum 25-hydroxyvitamin-D level than the non-comminuted group (13.5 ng/ml (95% confidence interval (CI) 10.8 to 16.3) vs 17.6 ng/ml (95% CI 16.1 to 19.0); p = 0.012, ANCOVA). Renal function was quite similar between the articular comminuted and non-comminuted groups. However, the articular comminuted group had a relatively higher median intact PTH level than the non-articular comminuted group (44.2 ng/ml (IQR 36.9 to 56.0) vs 39.0 ng/ml (IQR 31.0 to 50.0); p = 0.072, Mann-Whitney U test). This result indicated secondary PTH elevation caused by vitamin D deficiency in the articular comminuted group. There were no significant differences in terms of serum 25-hydroxyvitamin-D levels based on the AO classification and volar cortex comminution classification (Figure 2). The serum 25-hydroxyvitamin-D levels did not significantly differ in terms of seasons (p = 0.322. Kruskal-Wallis test) (Figure 3). Furthermore, there were no significant differences in terms of demographic characteristics, bone metabolic markers, and BMD between the articular comminuted and non-comminuted groups (Table II).
Fig. 2.
Serum 25-hydroxyvitamin-D level (25(OH)D) was compared according to fracture classifications. The box plot indicated interquartile range and median as well as mean value (cross mark). *p < 0.05.
Fig. 3.
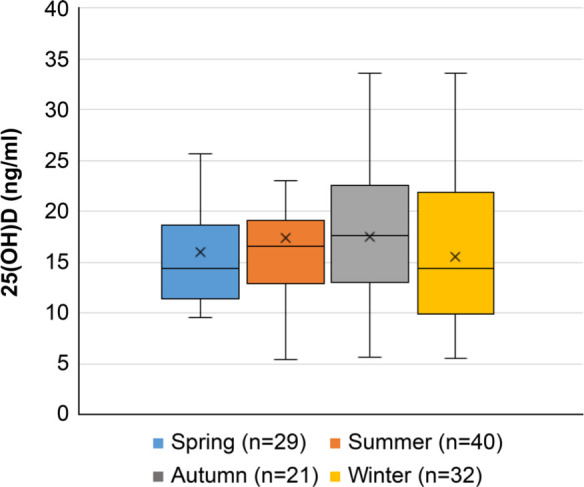
The serum 25-hydroxyvitamin-D levels (25(OH)D) according to each season are shown in box plots. Spring, March to May; summer, June to August; autumn, September to November; and winter, December to February.
Table II.
Comparison of demographic characteristics between patients with articular non-comminuted fracture and those with comminuted fracture.
| Characteristic | Non-comminuted fracture (n = 94) | Comminuted fracture (n = 28) | p-value |
|---|---|---|---|
| Median age, yrs (IQR) | 72 (61 to 78) | 72.5 (67 to 79) | 0.439* |
| Median BMI, kg/m2 (IQR) | 22.3 (19.6 to 24.8) | 21.9 (20.2 to 23.8) | 0.801* |
| Current smoking status, n (%) | 2 (2) | 0 | 0.421† |
| Steroid intake, n | 0 | 0 | |
| Diabetes mellitus, n (%) | 9 (9.5) | 2 (7.1) | 0.693† |
| Median eGFR, ml/min (IQR) | 68.5 (59.2 to 79) | 71.2 (58.2 to 78.9) | 0.801* |
| Osteoporosis treatment, n (%) | 10 (10.6%) | 5 (17.8%) | 0.786† |
| Bisphosphonate | 9 | 3 | |
| Denosumab | 1 | 1 | |
| Others | 0 | 1 | |
| Median BMD T-score (IQR) | |||
| Lumbar spine | -2.0 (-2.9 to -0.8) | -2.0 (-2.7 to -1.2) | 0.859* |
| Femoral neck | -2.2 (-3.1 to -1.6) | -2.6 (-3.0 to -1.7) | 0.491* |
| Forearm | -2.7 (-3.8 to -1.0) | -2.2 (-3.4 to -1.5) | 0.911* |
| Median P1NP, μg/l (IQR) | 58.0 (43.6 to 74.5) | 56.8 (42.6 to 68.7) | 0.712* |
| Median TRACP-5b, mU/dl (IQR) | 413 (319 to 522) | 416 (319 to 496) | 0.801* |
| Median intact-PTH level, pg/m (IQR) | 39.0 (31.0 to 50.0) | 44.2 (36.9 to 56.0) | 0.072* |
| Median ucOC level, ng/ml (IQR) | 4.9 (3.4 to 7.5) | 6.0 (3.8 to 9.1) | 0.244* |
Mann-Whitney U test.
Chi-squared test.
BMD, bone mineral density; eGFR, estimated glomerular filtration ratio; IQR, interquartile range; P1NP, total type 1 procollagen N-terminal propeptide; PTH, parathyroid hormone; TRACP-5b, tartrate-resistant acid phosphatase 5b; ucOC, undercarboxylated osteocalcin.
Discussion
This study showed the relationship between DRF severity and serum 25-hydroxyvitamin-D level among women with low-energy DRFs with extension deformity. Compared with articular non-comminuted DRFs, articular comminuted DRFs are associated with lower serum 25-hydroxyvitamin-D levels.
Vitamin D is a hormone that regulates serum calcium concentration, and vitamin D deficiency is considered a risk factor for osteoporosis. 9,24 Moreover, vitamin D deficiency increases body sway and the risk for falls and possible fall-related fracture. 25 Therefore, DRF is commonly associated with vitamin D deficiency, and it is the first fracture that was discovered among the osteoporotic fractures. 2,26 The serum 25-hydroxyvitamin-D levels between the DRF and control groups were compared. However, the results were not consistent. Jang et al 13 showed that the 25-hydroxyvitamin-D level of the DRF group was significantly lower than that of the control group. By contrast, Rozental et al 14 and Ting et al 15 revealed that there were no significant differences in terms of 25-hydroxyvitamin-D levels between the DRF and control groups.
In terms of DRF severity, vitamin D affects bone microstructure, and vitamin D deficiency increases the risk of fractures. 27,28 Therefore, vitamin D deficiency may affect both the cartilage and subchondral bone. An in vitro study revealed that vitamin D deficiency has deleterious effects on the cartilage based on a rat model, 29 thereby supporting our suggestion of the mechanism for articular comminution. Vitamin D deficiency has an impact on the subchondral bone, 30 which might be correlated with articular surface comminution. In this study, the articular comminuted group had a higher intact PTH level than the non-comminuted group. Vitamin D deficiency causes intact PTH elevation. A substantial elevation in serum intact PTH levels cause not only osteoporosis but also mineralization disorder. 31 Subchondral bone fragility caused by severe osteoporosis and mineralization disorder may be correlated with articular comminuted DRFs.
According to our results, the 25-hydroxyvitamin-D level did not differ according to fracture type based on the AO classification and volar cortex comminution classification. Articular comminution specifically reflects cancellous bone fragility, and volar cortex comminution may indicate cortical bone fragility. Moreover, the AO classification showed mixing cancellous and cortical bone fragility. Hence, vitamin D deficiency is strongly correlated with articular comminution compared with cortical comminution. This result is supported by a previous study showing that the cortical bone is maintained under vitamin D deficiency. 32
The serum 25-hydroxyvitamin-D level was affected by several factors including race, ageing, and duration of sunshine exposure. 12,33 In our cohort, all patients were Asian, and serum 25-hydroxyvitamin-D levels did not significantly differ according to seasons. ANCOVA revealed that the serum 25-hydroxyvitamin-D level of patients with articular comminuted DRF was significantly lower than that of patients with articular non-comminuted DRF regardless of age.
Bone fragility is caused by low bone mass and low bone quality. 34 Previous studies revealed that low BMD is correlated with severe DRF. 7,8 However, the definition of DRF severity in a previous study was based on the risk of early and late displacement, which was different from our method. 8 Moreover, in another research, BMD was calculated based on the peripheral quantitative CT scan of the distal radius and was analyzed using absolute values, which was also differed from our method. 7 Previous studies revealed that the osteoporosis treatment ratio before and after surgery among patients with DRF ranged from 7.8% to 21% and from 13% to 27%, respectively. 35,36 In the current study, 15 patients (12%) received treatments for osteoporosis other than vitamin D3 supplementation before sustaining fractures, which is similar to previous reports. Vitamin D sufficiency is correlated with DRF severity. Thus, we should focus on vitamin D supplementation, which might prevent severe DRF.
The current study had several limitations. First, DRF severity was affected by bone fragility and trauma degree. This analysis focused on low-energy DRF. Thus, we wanted to standardize the degree of trauma. The manner of falling or body weight affects the degree of trauma. However, they could not be completely standardized. Furthermore, other bony metabolism markers that might affect DRF severity were not assessed. These include pentosidine and homocysteine levels, which are markers of bone quality. 37 Second, BMD measurement was not performed using a single, standardized set of equipment because the study was conducted at multiple centres. Therefore, we could compare T score, but not absolute bone density, via a relative evaluation. Third, because bone metabolic markers and BMD were assessed six months after the injury and not at the time of the initial injury, our data do not exactly indicate the markers present at the time of injury. Finally, each group included patients who received osteoporotic treatment, which might have affected bone structure. This factor might be correlated with articular or volar cortex comminuted DRF. However, the radius could hardly benefit from osteoporotic treatment, and there was no significant difference in the number of patients treated between the groups.
In summary, vitamin D deficiency was correlated with articular comminuted DRF. Vitamin D supplementation could decrease DRF severity. However, further studies must be conducted to validate whether vitamin D supplementation might prevent articular comminuted DRFs.
Take home message
- Articular comminuted distal radius fractures (DRFs), are associated with a significantly lower serum 25-hydroxyvitamin-D level compared to non-comminuted DRFs.
- Vitamin D deficiency is related to DRF severity.
- Vitamin D3 supplementation for vitamin D deficiency might prevent articular comminuted DRFs.
Acknowledgements
We would like to thank Yohei Tomiyama, Hiroki Kondo, Masayuki Bun, Akihiko Ezaki, Tomoki Asano, and Yoshiki Yamada, and all of the medical staff in the Toyonaka Municipal Hospital for their contribution to the fracture treatment and osteoporotic fracture study.
Footnotes
Author contributions: S. Abe: Conceptualization, Methodology, Project administration, Supervision, Resources, Software, Investigation, Formal analysis, Data curation, Validation, Visualization, Writing – original draft, Writing – review & editing.
M. Kashii: Methodology, Project administration, Supervision, Investigation, Writing – original draft, Writing – review & editing.
T. Shimada: Conceptualization, Methodology, Resources, Investigation, Data curation, Formal analysis.
K. Suzuki: Conceptualization, Methodology, Resources, Investigation, Data curation.
S. Nishimoto: Conceptualization, Methodology, Resources, Investigation, Data curation.
R. Nakagawa: Conceptualization, Methodology, Resources, Investigation, Data curation.
M. Horiki: Conceptualization, Methodology, Resources, Investigation, Data curation.
Y. Yasui: Conceptualization, Methodology, Resources, Investigation, Data curation.
J. Namba: Conceptualization, Methodology, Resources, Investigation, Data curation.
K. Kuriyama: Conceptualization, Methodology, Project administration, Resources, Supervision, Data curation, Investigation, Validation, Writing – original draft.
Funding statement: The author(s) disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: This work is supported by The Grant-in-aid for Community Health and Medical Care from the Osaka University Medical School Alumni.
Ethical review statement: This multicenter, retrospective observational study was approved by the institutional review board of Toyonaka Municipal Hospital, Kansai Rousai Hospital, Japan Community Health Care Organization of Hoshigaoka Medical Center.
Open access funding: The open access funding was supported by Toyonaka Municipal Hospital.
Contributor Information
Shingo Abe, Email: a.shingo13@gmail.com.
Masafumi Kashii, Email: mkashii0323@gmail.com.
Toshiki Shimada, Email: to.shi.ki.808@gmail.com.
Koji Suzuki, Email: kouji_szk@hotmail.co.jp.
Shunsuke Nishimoto, Email: tennishun37@gmail.com.
Reiko Nakagawa, Email: reiko_tanigake@yahoo.co.jp.
Mitsuru Horiki, Email: mhoriki2003@yahoo.co.jp.
Yukihiko Yasui, Email: hikobosy@yahoo.co.jp.
Jiro Namba, Email: dtwvk@mx5.canvas.ne.jp.
Kohji Kuriyama, Email: kuriyama@kkf.biglobe.ne.jp.
References
- 1. Cooper C, Melton L. Magnitude and impact of osteoporosis and fractures. San Diego, California, USA: Academic Press, 1996: 419–434. [Google Scholar]
- 2. Sontag A, Krege JH. First fractures among postmenopausal women with osteoporosis. J Bone Miner Metab. 2010;28(4):485–488. 10.1007/s00774-009-0144-9 [DOI] [PubMed] [Google Scholar]
- 3. Robinson CM, Royds M, Abraham A, McQueen MM, Court-Brown CM, Christie J. Refractures in patients at least forty-five years old. A prospective analysis of twenty-two thousand and sixty patients. J Bone Joint Surg Am. 2002;84-A(9):1528–1533. 10.2106/00004623-200209000-00004 [DOI] [PubMed] [Google Scholar]
- 4. Wadsten MÅ, Buttazzoni GG, Sjödén GO, Kadum B, Sayed-Noor AS. Influence of cortical comminution and intra-articular involvement in distal radius fractures on clinical outcome: a prospective multicenter study. J Wrist Surg. 2017;6(4):285–293. 10.1055/s-0037-1601577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mignemi ME, Byram IR, Wolfe CC, et al. Radiographic outcomes of volar locked plating for distal radius fractures. J Hand Surg Am. 2013;38(1):40–48. 10.1016/j.jhsa.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sander AL, Leiblein M, Sommer K, Marzi I, Schneidmüller D, Frank J. Epidemiology and treatment of distal radius fractures: current concept based on fracture severity and not on age. Eur J Trauma Emerg Surg. 2020;46(3):585–590. 10.1007/s00068-018-1023-7 [DOI] [PubMed] [Google Scholar]
- 7. Lill CA, Goldhahn J, Albrecht A, Eckstein F, Gatzka C, Schneider E. Impact of bone density on distal radius fracture patterns and comparison between five different fracture classifications. J Orthop Trauma. 2003;17(4):271–278. 10.1097/00005131-200304000-00005 [DOI] [PubMed] [Google Scholar]
- 8. Clayton RAE, Gaston MS, Ralston SH, Court-Brown CM, McQueen MM. Association between decreased bone mineral density and severity of distal radial fractures. J Bone Joint Surg Am. 2009;91-A(3):613–619. 10.2106/JBJS.H.00486 [DOI] [PubMed] [Google Scholar]
- 9. Fischer V, Haffner-Luntzer M, Amling M, Ignatius A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur Cell Mater. 2018;35:365–385. 10.22203/eCM.v035a25 [DOI] [PubMed] [Google Scholar]
- 10. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):E988. 10.3390/nu12040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25(4):585–591. 10.1016/j.beem.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 12. Yamanaka Y, Menuki K, Zenke Y, et al. Serum 25-hydroxyvitamin D concentrations in Japanese postmenopausal women with osteoporotic fractures. Osteoporos Sarcopenia. 2019;5(4):116–121. 10.1016/j.afos.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jang WY, Chung MS, Baek GH, Song CH, Cho HE, Gong HS. Vitamin D levels in post-menopausal Korean women with a distal radius fracture. Injury. 2012;43(2):237–241. 10.1016/j.injury.2011.10.020 [DOI] [PubMed] [Google Scholar]
- 14. Rozental TD, Herder LM, Walley KC, et al. 25-hydroxyvitamin-D and bone turnover marker levels in patients with distal radial fracture. J Bone Joint Surg Am. 2015;97-A(20):1685–1693. 10.2106/JBJS.O.00313 [DOI] [PubMed] [Google Scholar]
- 15. Ting BL, Walley KC, Travison TG, Rozental TD. Elevated bone turnover markers are associated with distal radius fractures in premenopausal women. J Hand Surg Am. 2017;42(2):71–77. 10.1016/j.jhsa.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 16. World Medical Association . World Medical association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17. Bain GI, MacLean SBM, McNaughton T, Williams R. Microstructure of the distal radius and its relevance to distal radius fractures. J Wrist Surg. 2017;6(4):307–315. 10.1055/s-0037-1602849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walenkamp MMJ, Aydin S, Mulders MAM, Goslings JC, Schep NWL. Predictors of unstable distal radius fractures: a systematic review and meta-analysis. J Hand Surg Eur Vol. 2016;41(5):501–515. 10.1177/1753193415604795 [DOI] [PubMed] [Google Scholar]
- 19. Rhee SH, Kim J. Distal radius fracture metaphyseal comminution: a new radiographic parameter for quantifying, the metaphyseal collapse ratio (MCR). Orthop Traumatol Surg Res. 2013;99(6):713–718. 10.1016/j.otsr.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 20. Meinberg EG, Agel J, Roberts CS, Karam MD, Kellam JF. Fracture and dislocation classification compendium-2018. J Orthop Trauma. 2018;32 Suppl 1:S1–S170. 10.1097/BOT.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 21. Ichimura S, Hasegawa M. Biochemical markers of bone turnover. New aspect. Changes in bone turnover markers during fracture healing. Clin Calcium. 2009;19(8):1102–1108. CliCa090811021108 [PubMed] [Google Scholar]
- 22. Schöttker B, Hagen L, Zhang Y, et al. Serum 25-Hydroxyvitamin D levels as an aging marker: strong associations with age and all-cause mortality independent from telomere length, epigenetic age acceleration, and 8-isoprostane levels. J Gerontol A Biol Sci Med Sci. 2019;74(1):121–128. 10.1093/gerona/gly253 [DOI] [PubMed] [Google Scholar]
- 23. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 24. Nakamura K, Saito T, Oyama M, et al. Vitamin D sufficiency is associated with low incidence of limb and vertebral fractures in community-dwelling elderly Japanese women: the Muramatsu Study. Osteoporos Int. 2011;22(1):97–103. 10.1007/s00198-010-1213-6 [DOI] [PubMed] [Google Scholar]
- 25. Pfeifer M, Begerow B, Minne HW, et al. Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp Clin Endocrinol Diabetes. 2001;109(2):87–92. 10.1055/s-2001-14831 [DOI] [PubMed] [Google Scholar]
- 26. Oyen J, Apalset EM, Gjesdal CG, Brudvik C, Lie SA, Hove LM. Vitamin D inadequacy is associated with low-energy distal radius fractures: a case-control study. Bone. 2011;48(5):1140–1145. 10.1016/j.bone.2011.01.021 [DOI] [PubMed] [Google Scholar]
- 27. Wintermeyer E, Ihle C, Ehnert S, et al. Crucial role of vitamin D in the musculoskeletal system. Nutrients. 2016;8(6):E319. 10.3390/nu8060319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goltzman D. Functions of vitamin D in bone. Histochem Cell Biol. 2018;149(4):305–312. 10.1007/s00418-018-1648-y [DOI] [PubMed] [Google Scholar]
- 29. Pascual-Garrido C, Angeline ME, Ma R, et al. Low levels of vitamin D have a deleterious effect on the articular cartilage in a rat model. HSS J. 2016;12(2):150–157. 10.1007/s11420-016-9492-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nawabi DH, Chin KF, Keen RW, Haddad FS. Vitamin D deficiency in patients with osteoarthritis undergoing total hip replacement: a cause for concern? J Bone Joint Surg Br. 2010;92-B(4):496–499. 10.1302/0301-620X.92B3.23535 [DOI] [PubMed] [Google Scholar]
- 31. Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am. 2010;39(2):321–331. 10.1016/j.ecl.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 32. Lee AMC, Anderson PH, Sawyer RK, et al. Discordant effects of vitamin D deficiency in trabecular and cortical bone architecture and strength in growing rodents. J Steroid Biochem Mol Biol. 2010;121(1–2):284–287. 10.1016/j.jsbmb.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 33. van der Wielen RP, Löwik MR, van den Berg H, et al. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346(8969):207–210. 10.1016/s0140-6736(95)91266-5 [DOI] [PubMed] [Google Scholar]
- 34. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy . Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–795. 10.1001/jama.285.6.785 [DOI] [PubMed] [Google Scholar]
- 35. Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90-A(5):953–961. 10.2106/JBJS.G.01121 [DOI] [PubMed] [Google Scholar]
- 36. Baba T, Hagino H, Nonomiya H, et al. Inadequate management for secondary fracture prevention in patients with distal radius fracture by trauma surgeons. Osteoporos Int. 2015;26(7):1959–1963. 10.1007/s00198-015-3103-4 [DOI] [PubMed] [Google Scholar]
- 37. Shiraki M, Kuroda T, Shiraki Y, Tanaka S, Higuchi T, Saito M. Urinary pentosidine and plasma homocysteine levels at baseline predict future fractures in osteoporosis patients under bisphosphonate treatment. J Bone Miner Metab. 2011;29(1):62–70. 10.1007/s00774-010-0191-2 [DOI] [PubMed] [Google Scholar]