Abstract
Purpose of Review
Despite an overall reduction in lung cancer incidence and mortality rates worldwide, Blacks still have higher mortality rates compared to Whites. There are many factors that contribute to this difference. This review seeks to highlight racial disparities in treatment and the possible reasons for these disparities.
Recent Findings
Factors attributing to racial disparities in lung cancer treatment include social determinants of health, differences in the administration of guideline-concordant therapy as well as molecular testing that is essential for most NSCLC patients.
Summary
One way to circumvent disparities in lung cancer survivorship is to ensure equal representation of race in research at all levels that will provide insight on interventions that will address social determinants of health, differences in treatment patterns, molecular testing, and clinical trial involvement.
Keywords: Racial disparities, Non-small cell lung cancer, Guideline-concordant treatment, Molecular biomarker testing utilization, Social determinants of health, Next-generation sequencing, Clinical trials, Chemotherapy, Radiation and surgery, Black-White disparity
Introduction
Racial Disparities in Lung Cancer
Lung cancer is the second most commonly diagnosed cancer worldwide, after breast cancer [1], and its incidence continues to be of concern globally [2]. In 2020, an estimated 2.2 million new cases of lung cancer were diagnosed globally, accounting for approximately 11.4% of the global cancer burden [1]. In the USA, lung cancer continues to be a concern even though there has been a decrease in incidence and mortality in recent decades [3]. From 1999–2017, the age-adjusted incidence rate for lung cancer was 59.2 per 100,000 for all racial groups and a higher incidence was reported for Blacks (71.4 per 100,000) compared to Whites (37.8 per 100,000) [1]. An analysis of the multi-ethnic cohort study suggests that there may be inherent biological differences driving the disparity in lung cancer incidence. In this analysis, Blacks had a significantly greater risk of developing lung cancer than Whites and other racial groups. This disparity was most evident among light smokers, i.e. individuals who smoked less than 10 cigarettes per day and those who smoked 11–20 cigarettes per day. However, the racial/ethnic differences are less pronounced for persons who smoked 35 cigarettes per day compared to persons who smoked 10 cigarettes per day with the p value for interaction 0.04 and < 0.001, respectively [4]. These findings may be driven by different susceptibility to carcinogens found in cigarette smoke or ancestry-related biological differences which may increase the risk of lung cancer development independent of tobacco use, but more research is needed. A recent study by Blackman et al. revealed that a majority of Black cigarette smokers are poor metabolizers of tobacco smoke carcinogens [5]. This suggests that Blacks may indeed be more susceptible to the damaging effects of cigarette smoke, thus having an increased risk of lung cancer.
Another hypothesis is that Black patients exhibit higher risk behaviors which contribute to the disparity in lung cancer incidence. When comparing tobacco use patterns and lung cancer incidence among Black and White lung cancer patients, the disparity is quite different when men and women are observed separately. Lung cancer incidence is 13% higher among Black men compared to White men but 14% lower among Black women compared to White women. These differences are thought to be related to differences in tobacco exposure as data shows that Black men smoke cigarettes at a higher rate than White men, while Black women smoke cigarettes at a lower rate than White women [6]. Black individuals also have lower rates of smoking cessation compared to Whites [7]. Blacks referred to a lung cancer screening program had significantly lower odds of receiving low-dose computed tomography compared to Whites (odds ratio of 0.537, 95% CI = 0.384–0.750), even after adjusting for individual lung cancer risk factors and neighborhood-level factors (adjusted odds ratio of 0.483, 95% CI = 0.331–0.707). Blacks also had a trend toward delayed follow up, nonadherence and loss to follow up. However, adherence was poor among all races and this difference was not statistically significant [8].
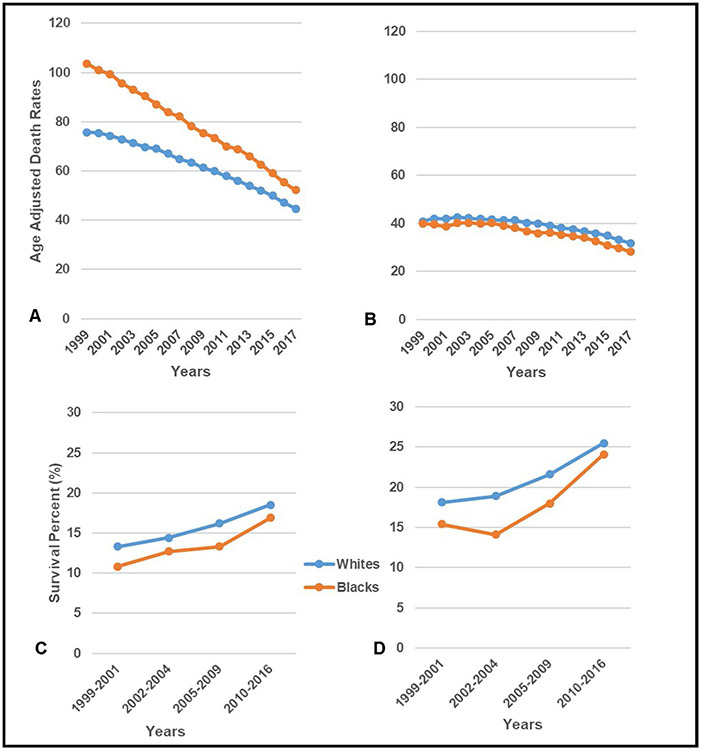
Similar to lung cancer incidence, there is also a disparity in lung cancer death rates. For the same period 1999–2017, the age-adjusted death rate was 47.8 per 100,000 and a higher age-adjusted death rate was reported for Blacks (51.9 per 100,000) compared to Whites (48.5 per 100,000) [9]. Lung cancer death rates steadily declined from 55.4 per 100,000 in 1999 to 36.7 per 100,000 in 2017 but disparities by race and sex persisted [1]. Similar declines in death rates were observed for Blacks (1999: 65 per 100,000/2017:37.9 per 100,000) and Whites (1999: 55.3 per 100,000/2017: 37.5 per 100,000), with the disparity gap narrowing between Whites and Blacks in the latter years [1] However, a more detailed look at the age-adjusted rates by sex and race over time reveals a slight difference in disparity patterns. The racial disparity was larger among males than females [1, 3]. Black males had higher death rates compared to White males and the disparity gap steadily decreased over time [1]. In contrast, White females had higher death rates compared to Black females. While the disparity gap was much smaller compared to males, despite the declining death rates, the racial disparity gap remained unchanged over time (Fig. 1A-B).
Fig. 1.
Age-adjusted US death rates for lung cancer among males (A) and females (B) by race (1999–2017) [9], and 5-year relative survival among males (C) and females (D) by race (1999–2016) [13]
As it relates to 5-year survival for Black men relative to White men and Black women relative to White women, as is expected, there have been improvements in all groups over the 5-year periods from 1999–2016. [4] This is likely due to the implementation of more targeted, advanced immunotherapies and treatments for lung cancer [10, 11, 12•]. Data from the Surveillance, Epidemiology, and End Results Program from 1999–2016 [13] show that for both males and females, Black patients continue to have lower survival rates than their White counterparts (Fig. 1C-D). More recently, Siegel et al. [14] reported in 2021 that the 5-year survival rate for lung cancer is lower in Blacks (18%) compared to Whites (21%) patients [14] and more importantly, the disparity is most prominent in early-stage patients. The rate of survival for Black patients with localized lung cancer was 55% compared to 59% for White patients [14]. There is conflicting prior data as to whether the lung cancer survival disparity between Black and White lung cancer patients is solely driven by socioeconomic status (SES), higher risk behaviors, healthcare access and quality of care or also by inherent differences in tumor biology and treatment response [15••, 16••, 17•]. While the survival gap has narrowed between racial groups, further investigation into the cause of the persistent disparity is needed.
There are many factors that contribute to this persistent disparity in lung cancer survival and among them is racial disparities in treatment. Numerous studies have suggested reasons for racial disparities in lung cancer treatment; these include social determinants of health such as economics and factors that influence access and quality of care, education, neighborhood/environment and social and community support, as well as molecular testing that is essential for administration of guideline-concordant therapy for most NSCLC patients. This review seeks to highlight these disparities with emphasis on racial disparity in NSCLC where the use of targeted therapy is most relevant.
Lung Cancer Treatment Disparities
Social Determinants of Health
Lung cancer treatment disparities are likely multifaceted, including access to high quality care and clinical trials that influence the racial disparity in lung cancer survival [11]. Importantly, disparities in lung cancer treatment may be attributed to social determinants of health. For example, (1) socioeconomic factors such as insurance status or the ability to pay out of pocket for treatment influences access to appropriate adjuvant therapies among the underinsured and poorer populations which include many Blacks [18, 19]. (2) Education level affects an individual’s treatment decision-making ability and adherence due to health literacy concerns that are more likely to occur among Blacks compared to other racial groups [20]. Low health literacy will likely affect lung cancer patients’ understanding of their disease and influence their ability to manage their treatment regimens. (3) Mistrust among Blacks caused by historical experiences with the health care system [21] also affects patient-provider communication/relationships that result in poor treatment choices by the patient. It has been suggested that negative surgical beliefs, fatalism, and mistrust may explain why some Black patients are less likely to comply with and receive recommended treatment [22]. Historical experiences and a lack of education concerning improvements of ethical standards governing healthcare fuels mistrust among Blacks. (4) With regard to neighborhood and physical environment, treatment disparities may be attributed to neighborhoods or communities with limited physical access to, or utilization of treatment. Transportation issues or living in rural vs. urban environments or high vs. low SES neighborhoods are factors related to these concerns.
Guideline-Concordant Treatment
Differences in Treatment Patterns
Despite clear differences in socially determinant factors, there are significant differences in treatment patterns that must be addressed. Black patients are less likely to receive guideline-concordant treatment for lung cancer than other racial groups, which is a major contributor to this survival disparity [16••]. Blom and colleagues [16••] examined the level of adherence to predefined, stage-specific, guideline-concordant treatment for different age and racial groups of patients with lung cancer in a large US dataset. Non-Hispanic black patients were less likely to receive guideline-concordant treatment (odds ratio = 0.82; 95% CI = 0.81–0.84) and this association persisted after adjusting for covariates (adjusted odds ratio = 0.78; 95% CI = 0.76–0.08) [16••].
Several studies previously demonstrated that Black patients are less likely to receive curative-intent surgery for early-stage lung cancer, even after accounting for confounding SES [23•, 24, 25]. However, another study demonstrated that this racial difference in surgical rate was eliminated for patients treated at Veterans Affairs (VA) facilities in 2010. This translated into no survival difference between races, suggesting equal access to healthcare systems plays a large role in guideline-concordant treatment and survival differences in early-stage disease [26]. In contrast, a more recent study of VA and SEER stage I NSCLC patients demonstrated that Black patients were, in fact, less likely to receive surgical treatment which was associated with inferior survival [27]. When treatment differences were accounted for, there were no racial differences in survival, supporting disparities in survival primarily being driven by treatment variability. Another study using the SEER-Medicare data set found the same pattern in patients with stage I NSCLC; Black patients were less likely to receive treatment (OR = 0.62, 95% CI = 0.53–0.73) and less likely to receive surgery when treated (OR = 0.7, 95% CI = 0.61–0.79), even when adjusting for clinical and demographic factors. Univariate survival was lower for Black than White patients, but when stratified by treatment type, differences in survival were not significant (adjusted HR = 0.98, 95% CI = 0.90–1.06) [28]. This is congruent with data reported by Dalwadi et al. [17•] which demonstrated that despite recent advances in radiotherapy and surgery, Black patients with early-stage NSCLC were less likely to receive surgery than White patients (55.9 vs. 66.7%, p < 0.0001) and continued to have inferior 2-year overall survival (65% vs. 70%, p < 0.0001) and cancer-specific survival (76% vs. 79%, p < 0.0001) than White patients [17•].
Several studies have described different patterns of employed curative-intent treatment, including surgery and radiation, between Black and White lung cancer patients. Lutfi et al. [29•] using The National Cancer Data Base to assess treatment patterns between 2004 and 2015 demonstrated that Black patients were less likely to receive surgery but more likely to receive External Beam Radiation Therapy (EBRT) and there was no racial difference in the rates of Stereotactic Ablative Radiotherapy (SABR), which has been shown to be more effective than EBRT for early-stage NSCLC [29•, 30]. While Black patients had an inferior 5-year overall survival, when adjusted for any definitive local therapy, Black patients had improved survival compared with White patients (HR = 0.97, 95% CI = 0.94–0.99) [29•]. Thus, improvement in the rates of surgery and definitive local radiation therapy is likely responsible for the narrowing survival disparity between Black and White early-stage NSCLC patients. In fact, a recent pragmatic trial implementing system-based, multifaceted, interventions including transparency of race-specific data feedback, real-time warnings derived from electronic health records, and patient-centered navigation reduced the Black-White treatment gap. There was significant improvement in the receipt of curative treatment for Black early-stage lung cancer patients and improved care for both races [31, 32]. Implementing programs such as this more broadly could have positive effects on the completion of curative treatment, equality of care and overall outcomes.
Black patients with advanced lung cancer are also less likely to receive guideline-concordant treatment than white patients; however, some of these treatment disparities continue to be influenced by social determinants. In the last decade, the advent of immunotherapy has immensely improved outcomes for both locally advanced NSCLC, with the use of consolidative durvalumab after definitive chemoradiation, as well as for first-line treatment of advanced NSCLC, with immune checkpoint inhibitors monotherapy or in combination with chemotherapy [33•, 34-37].
Centralization of Cancer Care
The relationship between hospital volume and cancer surgical outcomes was extensively studied in the past and this resulted in centralization of cancer care and encouraging patients to undergo cancer surgical procedures at high-volume (HV) hospitals [38, 39]. However, improvement in lung cancer outcomes was not shared equally among different racial groups [40]. A more recent study examined the effect of hospital centralization on closing the Black and White lung cancer surgical disparity gap and found some interesting patterns. The percentage of both Black and White patients using HV hospitals increased over the study period from 1995–2012. While the distance to the nearest HV hospital was significantly lower for Blacks compared to White patients, Black patients were less likely to use HV hospitals (adjusted OR = 0.26, 95% CI = 0.23–0.29) but more likely to use urban, teaching, and lower volume hospitals. Consequently, despite centralization increasing the use of HV hospitals across races, racial difference in access and utilization persists [41•].
Patient Response and Treatment Toxicities
There is conflicting data as to whether Black patients have an inferior treatment response or more severe toxicity after receiving platinum-based chemotherapy, which may impact survival along with inferior post-operative mortality rates. Unfortunately, Black patients are underrepresented in prospective clinical trials; Nationally, Black participants comprise 5% of cancer clinical trial enrollment, compared to 13% of the population (FDA Drug Trials Snapshots Summary Reports 2018–2019) [42]. Therefore, the information we have is through retrospective reviews, subject to additional bias. A study evaluating platinum metabolism in NSCLC patients who underwent neoadjuvant platinum-based chemotherapy, demonstrated that Black patients had significantly reduced expression of a platinum transporter CTR1 (p = 0.001), tissue platinum concentrations (p = 0.009) and tumor shrinkage (p = 0.016) compared to Whites [43]. Thus, CTR1 may be necessary for therapeutic efficacy and reduced expression may cause relative platinum resistance in Black lung cancer patients. However, a subset analysis of Blacks (10% of patients) included in the PointBreak trial, which compared combination of carboplatin and bevacizumab plus either pemetrexed or paclitaxel in advanced NSCLC patients, showed no significant difference in overall survival (OS) (HR = 1.125; p = 0.525) and toxicity profiles were similar in Black and White patients [44]. For early-stage patients, although postoperative cancer surgery mortality rates have improved across both Black and White cancer patients, including lung cancer patients, the racial disparity did not significantly narrow or widen between a study period of 2007 to 2016 (0.03%; 95% CI, – 0.03% to 0.08%; p = 0.36). The reduction in Black mortality was attributed to a within-hospital effect rather than a between-hospital effect, that is, differential care within the same institution rather than a shift in the number of Black patients from lower- to higher-quality hospitals [45]. Additional studies are needed to further examine differential treatment effects across races.
Differences in Molecular Testing and the Use of Targeted Therapies
Personalized therapy for lung cancer (i.e. next-generation sequencing (NGS)) has become a standard of care, with the development of precision cancer therapies permanently changing the treatment landscape for NSCLC. The clinical use of targeted kinase drugs has led to improved survival in sub-populations of patients with NSCLC who are candidates for these therapies [11, 12•]. For patients whose tumors lack targetable mutations, immunotherapy is now considered a standard component of first-line treatment of advanced NSCLC [34]. Immune checkpoint inhibitors are used as monotherapy or in combination with chemotherapy, according to the level of PDL-1 expression, among other factors [36, 37]. Overall, the clinical use of targeted kinase drugs and immunotherapy has improved the outlook for patients diagnosed with advanced NSCLC, with improved survival in sub-populations of patients with NSCLC who are candidates for these therapies. Five-year overall survival rates of 15–60% are now being seen for some patients with Stage IV disease [11, 12•, 46]. In view of the importance of targeted therapy in the management of lung cancer, the current NCCN guidelines have Category 1 recommendations for molecular testing for advanced/ metastatic NSCLC, including EGFR mutation, ALK rearrangement, and PDL-1 testing. Also recommended are testing for alterations in KRAS, ROS1, BRAF, NTRK1/2/3, METex14 skipping and RET, to be conducted as part of a broad molecular profiling [47]. Despite improvements in the last few decades, racial disparities in lung cancer survival persist [10].
As biomarker testing and broader molecular profiling are now key components in the improvement of care and outcome of patients with NSCLC, it is essential to consider the impact of race on biomarker testing and molecular profiling in lung cancer. Disparities in biomarker testing may help explain the racial disparity in outcome and be identified as a priority area for improvement as we aim to reduce cancer disparities. Disparities in guideline-concordant EGFR-testing have been noted for many years. As early as 2011, NCCN guidelines recommended EGFR mutation testing for all advanced NSCLC patients considered for first-line EGFR targeted therapy. While precision treatments were already recognized as advancements in NSCLC treatment, it was noted that utilization of some of these therapies was disproportionate across strata defined by race and SES [48•]. One early study showed that low-income patients, and those residing in high-poverty areas were less likely to have EGFR testing (or to receive treatment with the anti-EGFR therapy erlotinib). In terms of racial disparity, compared to Whites, in univariate analyses, Blacks were least likely to receive EGFR testing and also least likely to receive erlotinib after adjusting for demographic, clinical and SES [48•].
Other studies have focused on disparities in social determinant factors such as income and education and analyzed the association between likelihood to order an EGFR assay and the treating hospital’s institutional and regional characteristics. It was shown that hospitals located in areas with more high-income or more highly educated residents were more likely to order EGFR testing for patients with metastatic NSCLC [49]. Lynch et al. noted that delays in translating healthcare innovations such as EGFR testing and anti-EGFR therapies to community hospitals were a longstanding problem, with concern that these delays may widen the gap in cancer disparities [49]. A more recent study examined the predictors of EGFR and KRAS testing among Medicare beneficiaries with a new diagnosis of lung cancer and found that a reduced likelihood of testing was associated with factors suggesting lower socioeconomic status, including status as a Medicaid beneficiary and patient residence in a relatively low-income area [50•]. Of note, Hispanics and Black Hispanics were also less likely to be tested [50•].
Genomic testing is often a criterion for clinical trial enrollment for NSCLC targeted therapy trials; hence, disparity in broad molecular profiling/NGS testing could be an important reason for differences in clinical trial enrollment between racial groups. The questions of whether racial disparities exist in biomarker testing utilization, and if comprehensive genomic testing is associated with clinical trial enrollment were recently addressed. Bruno et al. studied a contemporary series of patients diagnosed with NSCLC between 2017 and 2020 [51••]. Biomarker testing, use of targeted therapy, and clinical trial enrollment were compared between Black and White racial groups. While there was no difference in the rates of EGFR mutations and ALK rearrangement between races, Black patients were significantly less likely to be ever tested for any biomarker when compared to White counterparts. There was a notable difference in the rates of comprehensive genomic testing utilizing NGS. Patients who self-identified as Black were less likely to have had NGS testing when compared to patients who were White (39.8% vs. 50.1%, p < 0.0001). Black patients also underwent significantly less comprehensive genomic testing prior to initiation of first-line therapy.
There was a difference in clinical trial participation according to race, with twice as much clinical trial participation among White compared to Black patients. Importantly, patients whose tumors had NGS sequencing were significantly more likely to participate in a clinical trial. Having biomarker testing before start of first-line therapy, as well as ever having NGS testing, increased the likelihood of clinical trial enrollment more than twofold, while Black lung cancer patients were 55% less likely to be included in clinical trials [51••]. We agree with Bruno et al. who noted that while multiple factors are known to impact health care disparities, access to appropriate biomarker testing may be an attainable goal to ensure equal access to quality care [51••].
Disparities in molecular testing in advanced NSCLC can translate into poorer outcomes due to underutilization of targeted therapies which have been shown to improve survival, and by contributing to reduced likelihood of clinical trial enrollment. As the use of targeted therapies expands into the early-stage lung cancer space, for example with the approval of adjuvant osimertinib for patients with resected EGFR-mutated NSCLC [52•], it will be important to assess the impact of testing and use of targeted therapies in earlier stages of the disease.
Conclusion
We have shown that there are a number of social determinants of health factors that contribute to racial disparities in lung cancer treatment, thus translating to poor survival rates for Black NSCLC patients. We have also shown that sub-optimal application of guideline-concordant treatment and access to HV hospitals could contribute to the poorer survival of Blacks with lung cancer compared to other ethnic groups. Furthermore, in the era of personalized medicine, the identification of actionable mutations has resulted in improved outcomes. Racial disparities in NSCLC survival may therefore be partly explained by under-utilization of biomarker testing according to standard guidelines [9]. Guideline discordant practice, specifically under-testing in Black and other sub-populations, will put these patients at a distinct disadvantage as appropriate targeted therapies may not be offered if the presence of a targetable mutation is not known. As shown, lack of genomic testing can also reduce clinical trial enrollment opportunities, which can contribute to reduced survival for patients, and perpetuate the issue of underrepresentation of Blacks in clinical trials. Therefore, one way to circumvent this disparity in lung cancer survivorship among Blacks and Whites is to ensure equal representation in research at all levels that will provide insight on interventions that will address social determinants of health, differences in treatment patterns, molecular testing, and clinical trial involvement.
Funding
This work is supported in part by 5P30CA006927, by TUF-CCC/HC Regional Comprehensive Cancer Health Disparity Partnership, Award Number U54 CA221704(5) from the National Cancer Institute of National Institutes of Health (NCI/NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI/NIH.
Footnotes
Conflict of Interest The authors declare no competing interests.
Consent for Publication The authors grant consent for publication.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Pineros M, et al. Global cancer observatory: cancer today. Lyon: International Agency For Research On Cancer; 2020. Available From: https://Gco.Iarc.Fr/Today. [Google Scholar]
- 2.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016:893:1–19. [DOI] [PubMed] [Google Scholar]
- 3.Schabath MB, Cote MI. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev. 2019:28(10):1563–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stram DO, Park SL, Haiman CA, Murphy SE, Patel Y, Hecht SS, et al. Racial/ethnic differences in lung cancer incidence in the multiethnic cohort study: an update. J Natl Cancer Inst. 2019;111(8):811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackman E, Ashing K, Gibbs D, Kuo YM, Andrews A, Ramakodi M, et al. The Cancer Prevention Project of Philadelphia: preliminary findings examining diversity among the African diaspora. Ethn Health. 2021:5:659–75. 10.1080/13557858.2018.1548695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer Statistics For African Americans, 2019. Ca Cancer J Clin. 2019;69(3):211–33. [DOI] [PubMed] [Google Scholar]
- 7.Park ER, Japuntich SJ, Traeger L, Cannon S, Pajolek H. Disparities between Blacks and Whites in tobacco and lung cancer treatment. Oncologist. 2011;16(10):1428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lake M, Shusted CS, Juon HS, Mcintire RK, Zeigler-Johnson C, Evans NR, et al. Black patients referred to a lung cancer screening program experience lower rates of screening and longer time to follow-up. BMC Cancer. 2020;20(1):561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United States Cancer Statistics: 1999 - 2017, Wonder Online Database. United States Department Of Health And Human Services, Centers For Disease Control And Prevention And National Cancer Institute. 2020. 2019. Available From: http://Wonder.Cdc.Gov/Cancer-V2016.html. [Google Scholar]
- 10.Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The Effect Of Advances In Lung-Cancer Treatment On Population Mortality. N Engl J Med. 2020;383(7):640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin JJ, Cardarella S, Lydon CA, Dahlberg SE, Jackman DM, Janne PA, et al. Five-year survival in egfr-mutant metastatic lung adenocarcinoma treated with Egfr-Tkis. J Thorac Oncol. 2016:11(4):556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.•. Pacheco JM, Gao D, Smith D, Purcell T, Hancock M, Bunn P, et al. Natural history and factors associated with overall survival in stage Iv Alk-rearranged non-small cell lung cancer. J Thorac Oncol. 2019:14(4):691–700. This publication highlighted findings from a clinical trial involving stage Iv lung cancer patients treated with an Alk inhibitor. It was found that patients had better overall survival; however, there was no indication of the racial make up of the study population. However, given the fact that blacks are usually less likely to be involved in clinical trials, it is possible that the benefit of this therapy is unknown for Black patients.
- 13.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. Seer Cancer Statistics Review, 1975–2017. Bethesda, Md, Based On November 2019 Seer Data Submission, Posted To The Seer Web Site, April 2020: National Cancer Institute; 2020. [Google Scholar]
- 14.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 15.••. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25–33. This paper highlighted the effects of social determinants of health on the observed racial/ethnic disparities. Social deteminants of health impacts the survival disparitiy that exist between Black and White patients.
- 16.••. Blom EF, Ten Haaf K, Arenberg DA, De Koning HJ. Disparities in receiving guideline-concordant treatment for lung cancer in the United States. Ann Am Thorac Soc. 2020;17(2):186–94. This reference outlined the different guideline-concordant treatments recommended for non-small cell lung cancer and the level of adherence to these treatments in the United States. It demonstrated that there are racial disparities with Black patients being one of the disadvantaged groups.
- 17.•. Dalwadi SM, Lewis GD, Bernicker EH, Butler EB, Teh BS, Farach AM. Disparities in the treatment and outcome of stage I non-small-cell lung cancer in the 21st century. Clin Lung Cancer. 2019:20(3):194–200. This reference focuses on guideline-concordant treatment but emphasized the observed disparity that black patients diagnosed with early-stage lung cancer were less likely to be given the surgery option despite this being shown to have improved outcomes among White lung cancer patients.
- 18.Verma V, Haque W, Cushman TR, Lin C, Simone CB 2nd, Chang JY, et al. Racial And insurance-related disparities in delivery of immunotherapy-type compounds in the United States. J Immunother. 2019;42(2):55–64. [DOI] [PubMed] [Google Scholar]
- 19.Farrow NE, An SJ, Speicher PJ, Harpole DH Jr, D’amico TA, Klapper JA, et al. Disparities in guideline-concordant treatment for node-positive, non-small cell lung cancer following surgery. J Thorac Cardiovasc Surg. 2020;160(1):261–71 E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutner M, Greenberg E, Jin Y, Boyle B, Hsu Y-C, Dunleavy E. Literacy in Everyday Life: Results from the 2003 National Assessment Of Adult Literacy. 2007. Available From: http://Nces.Ed.Gov/Pubsearch/Pubsinfo.Asp?Pubid=2007480. [Google Scholar]
- 21.Kennedy BR, Mathis CC, Woods AK. African Americans and their distrust of the health care system: healthcare for diverse populations. J Cult Divers. 2007;14(2):56–60. [PubMed] [Google Scholar]
- 22.Lin JJ, Mhango G, Wall MM, Lurslurchachai L, Bond KT, Nelson JE, et al. Cultural factors associated with racial disparities in lung cancer Care. Ann Am Thorac Soc. 2014:11(4):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.•. Check DK, Albers KB, Uppal KM, Suga JM, Adams AS, Habel LA, et al. Examining the role of access to care: racial/ethnic differences in receipt of resection for early-stage non-small cell lung cancer among integrated system members and non-members. Lung Cancer. 2018:125:51–6. This reference examined the effect of access to care on the racial disparities between Black and White patients. The study was based in California, so it cannot be extrapolated to all US states but it is a good example of what may exists elsewhere.
- 24.Lathan CS. Lung cancer care: the impact of facilities and area measures. Transl Lung Cancer Res. 2015;4(4):385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coughlin SS, Matthews-Juarez P, Juarez PD, Melton CE, King M. Opportunities to address lung cancer disparities among African Americans. Cancer Med. 2014:3(6):1467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams CD, Salama JK, Moghanaki D, Karas TZ, Kelley MJ. Impact of race on treatment and survival among U.S. veterans with early-stage lung cancer. J Thorac Oncol. 2016;11(10):1672–81. [DOI] [PubMed] [Google Scholar]
- 27.Williams CD, Alpert N, Redding TST, Bullard AJ, Flores RM, Kelley MJ, et al. Racial differences in treatment and survival among veterans and non-veterans with stage i nsclc: an evaluation of veterans affairs and seer-medicare populations. Cancer Epidemiol Biomarkers Prev. 2020:29(1):112–8. [DOI] [PubMed] [Google Scholar]
- 28.Wolf A, Alpert N, Tran BV, Liu B, Flores R, Taioli E. Persistence of racial disparities in early-stage lung cancer treatment. J Thorac Cardiovasc Surg. 2019;157(4):1670–9 E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.•. Lutfi W, Martinez-Meehan D, Sultan I, Evans N 3rd, Dhupar R, Luketich JD, et al. Racial disparities in local therapy for early-stage non-small-cell lung cancer. J Surg Oncol. 2020;122(8):1815–20. This study investigated the treatment disparities in early-stage lung cancer, including trends over time of surgery and two types of radiation therapy between Black and White patients.
- 30.Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (Trog 09.02 Chisel): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20(4):494–503. [DOI] [PubMed] [Google Scholar]
- 31.Cykert S, Eng E, Walker P, Manning MA, Robertson LB, Arya R, et al. A system-based intervention to reduce black-white disparities in the treatment of early-stage lung cancer: a pragmatic trial at five cancer centers. Cancer Med. 2019:8(3):1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potera C. Intervention reduces racial disparity in care of lung cancer patients. Am J Nurs. 2019;119(5):13. [DOI] [PubMed] [Google Scholar]
- 33.•. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–50. The reference reported the findings of a phase III radomized trial involving durvalumab as therapy for stage III non-small cell lung cancer patients. However, race/ethnicity was not indicated for the patient population. Therefore, it is unclear whether or not Black patients will benefit from this therapy because they are less likely to be involved in clinical trials such as this.
- 34.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 35.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. [DOI] [PubMed] [Google Scholar]
- 36.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for Pd-L1-positive non-small-cell lung cancer. N Engl J Med. 2016:375(19):1823–33. [DOI] [PubMed] [Google Scholar]
- 37.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, Pd-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (Keynote-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019:393(10183):1819–30. [DOI] [PubMed] [Google Scholar]
- 38.Learn PA, BACH PB. A decade of mortality reductions in major oncologic surgery: the impact of centralization and quality improvement. Med Care. 2010:48(12):1041–9. [DOI] [PubMed] [Google Scholar]
- 39.Stitzenberg KB, Sigurdson ER, Egleston BI, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27(28):4671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neighbors CJ, Rogers ML, Shenassa ED, Sciamanna CN, Clark MA, Novak SP. Ethnic/racial disparities in hospital procedure volume for lung resection for lung cancer. Med Care. 2007;45(7):655–63. [DOI] [PubMed] [Google Scholar]
- 41.•. Lieberman-Cribbin W, Liu B, Leoncini E, Flores R, Taioli E. Temporal trends in centralization and racial disparities in utilization of high-volume hospitals for lung cancer surgery. Medicine (Baltimore). 2017;96(16):E6573. This article reported on the racial disparity which exists for the utilization of high volume hospitals which have been developed to centralize centain services to bridge the disparity gap between Blacks and Whites. It was found that even in instances where high volume hospitals are accessible black patients still prefer to go to smaller facilities for care.
- 42.FDA US Food And Drug Administration. 2018 Drug Trials Snapshots Summary Report. 2019. http://www.fda.gov.
- 43.Kim ES, Tang X, Peterson DR, Kilari D, Chow CW, Fujimoto J, et al. Copper transporter Ctr1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer. 2014:85(1):88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds CH, Patel JD, Garon EB, Olsen MR, Bonomi P, Govindan R, et al. Exploratory subset analysis of African Americans from the pointbreak study: pemetrexed-carboplatin-bevacizumab followed by maintenance pemetrexed-bevacizumab versus paclitaxel-carboplatin-bevacizumab followed by maintenance bevacizumab in patients with stage IIIB/IV nonsquamous non-small-cell lung cancer. Clin Lung Cancer. 2015:16(3):200–8. [DOI] [PubMed] [Google Scholar]
- 45.Lam MB, Raphael K, Mehtsun WT, Phelan J, Orav EJ, Jha AK, et al. Changes in racial disparities in mortality after cancer surgery in the US, 2007–2016. JAMA Netw Open. 2020;3(12):E2027415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: results from the phase I Keynote-001 study. J Clin Oncol. 2019:37(28):2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nccn Clinical Practice Guidelines In Oncology (Nccn Guidelines): Non-Small Cell Lung Cancer Version 5.2021.
- 48.•. Palazzo LL, Sheehan DF, Tramontano AC, Kong CY. Disparities and trends in genetic testing and erlotinib treatment among metastatic non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2019;28(5):926–34. This retrospective study reported that race was associated with patients receiving genetic testing to inform erlotinib treatment, as Black patients are less likely to undergo genetic testing therefore perpetuating the racial disparity.
- 49.Lynch JA, Khoury MJ, Borzecki A, Cromwell J, Hayman LL, Ponte PR, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the united states: a case study of T3 translational research. Genet Med. 2013;15(8):630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.•. Lynch JA, Berse B, Rabb M, Mosquin P, Chew R, West SL, et al. Underutilization and disparities in access to egfr testing among medicare patients with lung cancer from 2010–2013. BMC Cancer. 2018;18(1):306. This article outlined the racial disparity that exists with the utilization of molecular testing, specifically tumor testing for Egfr i mutations. It was found that zip code was a large predictor of Egfr testing. Blacks and Hispanics were less likely to have Egfr testing and therefore are not benefiting from the improvements in patient's outcome.
- 51.••. Bruno DS, Hess LM, Li X, Su EW, Zhu YE, Patel M. Racial disparities in biomarker testing and clinical trial enrollment in non-small cell lung cancer (NSCLC). J Clin Oncol. 2021;39(15_Suppl):9005. This article explains the persistence of the racial disparities due to the relatively small numbers of blacks who are involved in clinical trials.
- 52. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib In resected egfr-mutated non-small-cell lung cancer. N Engl J Med. 2020:383(18):1711–23 (This article presented another standard of care which is now available for previously untreated epidermal growth factor receptor (Egfr) mutation-positive advanced non-small-cell lung cancer (NSCLC) patients. Black patients are unlikely to have Egfr testing and therefore will more likely not benefit from the observed improvements in patients overall survival that was found after treatment with osimertinib).