Summary
This is a highlight on the article ‘Extracellular vesicle formation in Lactococcus lactis is stimulated by prophage‐encoded holin‐lysin system’ by Yue Liu, Eddy Smid and Tjakko Abee.
This is a highlight on the article ‘Extracellular vesicle formation in Lactococcus lactis is stimulated by prophage‐encoded holin‐lysin system’ by Yue Liu, Eddy Smid, and Tjakko Abee.
![]()
Extracellular vesicles (EVs) are secreted proteinaceous lipid bilayers derived from the membranes of bacteria and eukaryotic cells. Although the production of outer membrane vesicles (OMVs) from Gram‐negative bacteria has been thoroughly investigated over the past few decades, the origin, mechanism of formation and release, and other characteristics of EVs produced by Gram‐positive bacteria is less well understood (Brown et al., 2015). The formation of Gram‐negative OMVs has been extensively investigated in mechanistic studies and is generally described as forming via blebbing from the outer membrane caused by a number of contributing factors including reduction in crosslinking to peptidoglycan (Jan, 2017). Conversely, EVs secreted by Gram‐positives encounter additional obstacles from their origin at the cytoplasmic membrane to their arrival in the extracellular environment, namely the proteoglycan cell wall and other surface features (Fig. 1).
Fig. 1.
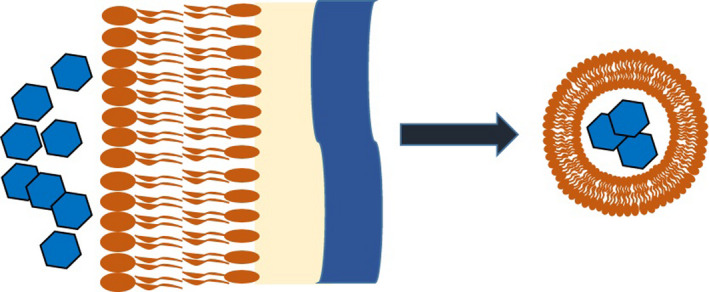
Schematic of extracellular vesicles produced by Gram‐positive bacteria.
The intricacies of vesiculogenesis in Gram‐positive have begun to be interrogated. Genetic control of EV production has been investigated in a number of species, where mutation of certain genes including broad gene regulators such as sigB and two‐component systems have been shown to significantly impact both EV production and characteristics (Briaud and Carroll, 2020). In Staphylococcus aureus, vesicle production was shown to depend on the production of amphipathic, alpha‐helical peptides called phenol‐soluble modulins that disrupt the membrane and promote EV formation (Wang et al., 2018). Release of EVs from the cell has been demonstrated in some species to depend on lipid composition, where dissimilarities between phospholipids in EVs and the parent bacteria suggest that production and release may occur at specific locations on the membrane. In many Gram‐positives, mass spectrometry proteomic analyses of EVs have shown the presence of penicillin‐binding proteins and autolysins suggesting their cell‐wall modification role is connected to vesicle release (Briaud and Carroll, 2020). Therefore, the current scheme for Gram‐positive EV production involves a complicated interplay of these mechanisms in addition to cell‐wall degrading enzymes allowing their release (Liu et al., 2018).
Despite the apparent hurdles to their production, a wide array of Gram‐positives have been demonstrated to produce EVs, including S. aureus, Listeria monocytogenes, Streptococcus pneumoniae, Clostridium perfringens and Bacillus anthracis as well as probiotic species Lactobacillus acidophilus and Lactobacillus plantarum among others (Dean et al., 2019; Bose et al., 2020). These EVs have begun to be well characterized over the past two decades, including early studies that reported proteomics profiling of the membrane‐bound and lumen‐carried proteins in various bacteria that have highlighted some potential mechanisms for Gram‐positive EV production, including the presence of peptidoglycan hydrolyase in EVs from S. aureus and phage‐associated endolysin from S. pneumoniae (Lee et al., 2009; Resch et al., 2016). In another interesting study, Toyofuku et al. (2017) found that Bacillus subtilis EV release was enabled by a prophage‐encoded endolysin.
The EV contents have begun to be viewed in the context of interaction with other cells and other functions in the Gram‐positive’s environment. In addition to proteins, Gram‐positive EVs have been shown to carry diverse cargo, including DNA that can be horizontally transferred between different species (Cao and Lin, 2021). In studies on their role in virulence, B. anthracis vesicles have shown to deliver their cargo – which include toxin components that are involved in cytotoxicity – to macrophages by phagocytosis or direct fusion (Wolf and Rivera, 2012). In opposition to the virulent effects of EVs originating from pathogens, the vesicles produced by probiotic bacteria have been implicated in protection against infection (Caruana and Walper, 2020), where, in one example, EVs of Lactobacillus species were shown to inhibit HIV‐1 infection of several human tissues ex vivo (Nahui Palomino et al., 2019). In another example, Lactobacillus paracasei vesicles significantly decreased the intestinal inflammatory response to lipopolysaccharide (Choi et al., 2020). This study is particularly interesting when considering the yet uncharacterized Gram‐positive EV architecture, as these EVs potentially maintain extracellular polysaccharides that have been shown to have health‐beneficial activities (Spangler et al., 2021). These anti‐inflammatory, anti‐infection and other positive effects of probiotic EVs are therefore of particular interest in the context of biotechnology and the emerging field of engineering probiotic bacteria (Spangler et al., 2021), where probiotics and the vesicles they produce can be engineered to further include therapeutic biomolecules or display characteristics favourable to vaccinations.
In the highlighted paper by Liu et al., they report investigations into the questions of vesiculogenesis in Lactococcus lactis EVs. In looking at the EVs produced by the lysogenic L. lactis strain FM‐YL11 they saw that prophage‐inducing conditions increased the production of EVs by greater than 10‐fold. They confirmed the role of the holin‐lysin system by creation of a prophage‐encoded holin‐lysin knock‐out mutant and prophage‐cured mutant which showed stable low‐level of vesicle production. Following further experimentation, including proteomic analysis of the EVs and amazing transmission electron microscopic images showing the presence of phage heads within the L. lactis vesicles, the group clearly demonstrated the function of the prophage‐encoded holin‐lysin system. Critically, the described system can be applied in the production of vesicles from other Gram‐positives, including other probiotic species (Liu and Smid, 2021). In the context of probiotic engineering, the findings by Liu et al. are particularly exciting as they enable both hypervesiculation and controlled production of the EVs, supplying a valuable engineering tool for a field where tools are currently lacking.
Future work stemming from the work of Liu et al. and other groups will likely further elucidate the questions surrounding Gram‐positive vesiculogenesis, the contents of EVs and their functions. The mechanism reported by Liu et al. could be widely applied among Gram‐positives, and probiotic bacteria in particular, and therefore deserves further investigation. These results may serve as a starting point for synthetic biologists to work on a means of control for EV production from probiotic species such as L. lactis and species of Lactobacillus. An engineered controllable, inducible system could allow for increased production of EVs for therapeutic or other biotechnology purposes, depending on their contents. While mysteries concerning the underlying mechanisms of Gram‐positive EVs remain unexplained, especially in the area of EV biogenesis, explanations of this component of the process, such as this report by Liu et al., will likely further encourage interesting new work that has clear applications in medicine and biotechnology.
Conflict of interest
None declared.
Microbial Biotechnology (2022) 15(4), 1055–1057
Funding Information
U.S. Naval Research Laboratory (Grant/Award Number: 'WU# MA041–06–41').
Contributor Information
Scott N. Dean, Email: scott.dean@nrl.navy.mil.
Joseph R. Spangler, Email: joseph.spangler@nrl.navy.mil.
References
- Bose, S. , Aggarwal, S. , Singh, D.V. , and Acharya, N. (2020) Extracellular vesicles: an emerging platform in gram‐positive bacteria. Microb Cell 7: 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briaud, P. , and Carroll, R. K. (2020) Extracellular vesicle biogenesis and functions in gram‐positive bacteria. Infect Immun 88: e00433‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, L. , Wolf, J.M. , Prados‐Rosales, R. , and Casadevall, A. (2015) Through the wall: extracellular vesicles in Gram‐positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13: 620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , and Lin, H. (2021) Characterization and function of membrane vesicles in Gram‐positive bacteria. Appl Microbiol Biotechnol 105: 1795–1801. [DOI] [PubMed] [Google Scholar]
- Caruana, J.C. , and Walper, S.A. (2020) Bacterial membrane vesicles as mediators of microbe ‐ microbe and microbe ‐ host community interactions. Front Microbiol 11: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J.H. , Moon, C.M. , Shin, T.‐S. , Kim, E.K. , McDowell, A. , Jo, M.‐K. , et al. (2020) Lactobacillus paracasei‐derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp Mol Med 52: 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, S.N. , Leary, D.H. , Sullivan, C.J. , Oh, E. , and Walper, S.A. (2019) Isolation and characterization of Lactobacillus‐derived membrane vesicles. Sci Rep 9: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan, A.T. (2017) Outer Membrane Vesicles (OMVs) of gram‐negative bacteria: a perspective update. Front Microbiol 8: 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, E.‐Y. , Choi, D.‐Y. , Kim, D.‐K. , Kim, J.‐W. , Park, J.O. , Kim, S. , et al. (2009) Gram‐positive bacteria produce membrane vesicles: proteomics‐based characterization of Staphylococcus aureus‐derived membrane vesicles. Proteomics 9: 5425–5436. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Defourny, K.A.Y. , Smid, E.J. , and Abee, T. (2018) Gram‐positive bacterial extracellular vesicles and their impact on health and disease. Front Microbiol 9: 1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Smid, E.J. , and Abee, T. (2021) Extracellular vesicle formation in Lactococcus lactis is stimulated by prophage‐encoded holin‐lysin system. Microb Biotechnol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ñahui Palomino, R.A. , Vanpouille, C. , Laghi, L. , Parolin, C. , Melikov, K. , Backlund, P. , et al. (2019) Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV‐1 infection of human tissues. Nat Commun 10: 5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch, U. , Tsatsaronis, J. A. , Le Rhun, A. , Stubiger, G. , Rohde, M. , Kasvandik, S. , et al. (2016) A two‐component regulatory system impacts extracellular membrane‐derived vesicle production in group A Streptococcus. mBio 7: e00207‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler, J.R. , Caruana, J.C. , Medintz, I.L. , and Walper, S.A. (2021) Harnessing the potential of Lactobacillus species for therapeutic delivery at the lumenal‐mucosal interface. Future Sci OA 7: FSO671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku, M. , Cárcamo‐Oyarce, G. , Yamamoto, T. , Eisenstein, F. , Hsiao, C.‐C. , Kurosawa, M. , et al. (2017) Prophage‐triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis . Nat Commun 8: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Thompson, C.D. , Weidenmaier, C. , and Lee, J.C. (2018) Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat Commun 9: 1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, J.M. , Rivera, J. , and Casadevall, A. (2012) Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell Microbiol 14: 762–773. [DOI] [PubMed] [Google Scholar]


