Abstract
Background:
Diabetic foot ulcer (DFU) is one of the most terrifying diabetic complications for patients, due to the high mortality rate and risk for amputation. During the COVID-19 pandemic, many diabetic patients limited their visits to the hospital, resulting in delays for treatment especially in emergency cases.
Objective:
This study aimed to compare the characteristics of patients with DFU pre- and during COVID-19 pandemic period. Methods: This study was a retrospective cohort study using foot registry data. We compared our patients’ characteristics pre-COVID-19 pandemic period (1 March 2019-28 February 2020) and during COVID-19 pandemic period (1 March 2020-28 February 2021).
Results:
Cohorts of 84 and 71 patients with DFU pre- and during COVID-19 pandemic period, respectively, were included in this study. High infection grade (66.7% vs 83.1%, P = .032), osteomyelitis event (72.6% vs 87.3%, P = .04), leukocyte count (15 565.0/μL vs 20 280.0/μL, P = .002), neutrophil-to-lymphocyte ratio (7.7 vs 12.1, P = .008), waiting time-to-surgery (39.0 h vs 78.5 h, P = .034), and number of major amputation (20.2% vs 39.4%, P = .014) were significantly higher during the COVID-19 pandemic period.
Conclusion:
During the COVID-19 pandemic, patients with DFU had more severe infection, higher proportion of osteomyelitis, longer waiting time for getting surgical intervention, and higher incidence of major amputation.
Keywords: diabetic foot ulcer, COVID-19, infection, amputation, mortality, waiting time to surgery
Introduction
Diabetic foot is one of the most common and frightening complications of diabetes. 1 Around 15% to 25% of people with diabetes will experience foot ulcer in their life. The latest data shows that the risk may be up to 34%. 2 The duration of diabetic foot healing process is influenced by multiple factors3,4 and non-healing ulcer is a risk factor for major amputation. 5 At least half of all amputations occur in patients with diabetes, mainly caused by infected diabetic foot ulcer (DFU). 2
Previous study reported that 33% subjects with major lower-extremity amputation (LE) died within 1 year, and 65% died within 4 years after amputation. 6 Based on medical record data in 2017 from Dr. Cipto Mangunkusumo National General Hospital, a national referral hospital in Indonesia, reported that 33.9% of all hospitalized diabetes patients were due to DFU and gangrene, among whom 14.3% died, and 34.7% had major or minor amputation. 7 Furthermore, the proportion of re-amputation was 58.2%. 8 This might be due to the fact that most cases were hospitalized on an advanced state—which highlights the necessity of early diagnosis and prompt treatment. Unfortunately, the emergence of the coronavirus (COVID-19) pandemic, 9 since the first case of COVID-19 appearance in Indonesia on March 2, 2020, 10 might further hamper early access to care.
The COVID-19 pandemic has given challenges in the overall management of diabetes, especially for patients with foot ulcer complications. Changes in consultation mode from “face to face” to telemedicine and the patient’s fear of contracting virus both contributed to limitation of care as only a limited clinical examination could be performed. 11 A previous study reported that the average duration of ulcer was longer in patients admitted during the COVID-19 pandemic. 12 Furthermore, people with diabetes are more susceptible to become seriously ill and have higher glucose fluctuation due to COVID-19, 13 which might also influence the morbidity and mortality among those with DFU. A previous study indeed reported that the proportion of amputation was higher in patient during the COVID-19 lockdown compared to those admitted in the first 5 months of 2019. 12 In summary, the COVID-19 pandemic might influence the quality of care toward hospitalized patients with DFU, especially in a country with limited healthcare resources that has been overwhelmed by the surging cases and mortality due to COVID-19. 14 Our study aims to compare the clinical characteristics and mortality of diabetic foot patients hospitalized pre- and during the COVID-19 pandemic.
Method
This study was a retrospective cohort analyzing the diabetic foot registry from the Endocrinology, Metabolism, and Diabetes Division, Department of Internal Medicine, Dr. Cipto Mangunkusumo National General Hospital, a tertiary care hospital in Indonesia. We included subjects with DFU admitted during the 1 year pre- the COVID-19 pandemic (from 1 March 2019 to 28 February 2020) and 1 year during the COVID-19 pandemic (from 1 March 2020 to 28 February 2021).
There were several factors that we assessed in this study, which were duration of diabetes; duration, cause, location, and amount of lesion; history of ulcer; infections severity grades; osteomyelitis; laboratory measures; and comorbidities such as peripheral artery disease (PAD), hypertension, and dyslipidemia. In this study, we also assessed DFU and its surrounding infected areas—which hereinafter referred to as diabetic foot lesion.
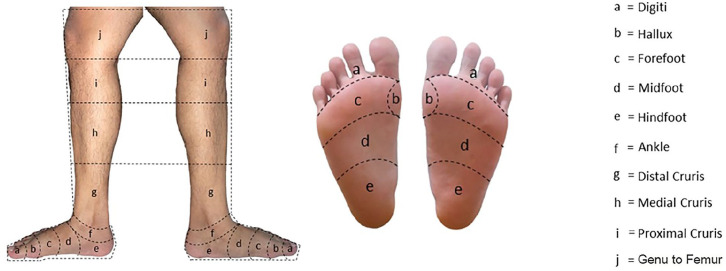
Location and number of lesion were assessed according to the 10 areas of the leg divided by imaginary lines (Figure 1), which were digiti, hallux, forefoot, midfoot, hindfoot, ankle, distal cruris, medial cruris, proximal cruris, and genu to femur. A subject could have multiple lesions in 1 leg, which represented the spread of infection, or in both legs. The number of lesion was categorized as 1 to 3, 4 to 6, or >6 areas. Infections severity grading based on the IWGDF guideline 2019. 15 Assessment of osteomyelitis based on positive bone prove test (with 0.87 sensitivity and 0.83 specificity) and/or bone exposure during physical examination, and/or periosteal erosion of bone around the wound based on X-ray. 15
Figure 1.
Location of lesions.
Laboratory variables, such as hemoglobin, leukocyte count, neutrophil lymphocyte ratio (NLR), thrombocyte count, random blood glucose (RBG), HbA1C, albumin, and eGFR, were conducted in a nationally accredited laboratory using venous blood samples in Dr. Cipto Mangunkusumo National General Hospital.
PAD was determined based on the ankle brachial index (ABI) score <0.9 or >1.3, and/or the vascular ultrasound, and/or CT angiography. Hypertension was defined as blood pressure above 140/90 or previous history of hypertension. Dyslipidemia is defined as abnormal lipid profile, described as triglyceride ≥150 mg/dL, LDL ≥ 100 mg/dL, or HDL ≤ 50 mg/dL or any history of dyslipidemia.
Outcomes being measured were time-to-surgery, amputation, and mortality proportion. Time-to-surgery, either for amputation or debridement, was defined as the waiting time for surgery since first admission in our emergency room. Amputation covered both major and minor amputation. Mortality was the number of all-cause death.
Statistical analyses was performed using the SPSS version 20. Normality test was assessed using the Kolmogorov–Smirnov. Numerical data were presented as mean (standard deviation; SD) or median (interquartile range; IQR). Analyses between variables were performed using the chi-square or fisher exact test for categorical variables and the independent t-test or Mann-Whitney test for continuous variables according to their distribution.
Result
There were 84 patients admitted due to DFU in 1 year pre- the COVID-19 pandemic and 71 patients in 1 year during the COVID-19 pandemic. There was no significant differences in term of age, duration of diabetes and duration of ulcer between both groups (Table 1). Similarly, there was no significance difference between both groups in term of history of ulcer, HbA1c, and albumin level. However, infection grade was more severe (66.7% vs 83.1%, P = .032) and there was higher cases of osteomyelitis (72.6% vs 87.3%, P = .04) in the pandemic group. Leukocyte count (15 565.0/μL vs 20 280.0/μL, P = .002) and NLR (7.7 vs 12.1, P = .008) were also significantly higher in the pandemic group. Table 2 showed the distribution of lesion according to the 10 areas of leg. We assessed 277 lesions pre-pandemic and 270 lesions during pandemic. Either in pre-pandemic or pandemic group, most of the lesions were located in the digiti, forefoot, and midfoot. There were also more subjects with >6 areas of lesion in the pandemic group (2.4% vs 15.5%).
Table 1.
Demographic and Ulcer Characteristic.
| Variable | N | Pre-pandemic | N | During pandemic | P |
|---|---|---|---|---|---|
| Men (n, %) | 84 | 44 (52.4) | 71 | 30 (42.3) | .27 |
| Age (years) (mean, SD) | 84 | 58.0 (10.3) | 71 | 57.3 (10.9) | .68 |
| Duration of diabetes (years) (median, IQR) | 84 | 10.0 (4.0-15.0) | 71 | 7.0 (3.0-13.0) | .26 |
| Duration of ulcer (days) (median, IQR) | 84 | 20.0 (7.7-45.0) | 71 | 21.0 (14.0-30.0) | .73 |
| Body Mass Index (kg/m2) (median, IQR) | 84 | 23.2 (21.3-26.9) | 71 | 25.1 (4.8) | .27 |
| Cause of ulcer | 84 | 71 | |||
| Spontaneous (n, %) | 40 (47.6) | 37 (52.1) | |||
| Mechanical trauma (n %) | 33 (39.3) | 29 (40.8) | |||
| Thermal trauma (n, %) | 5 (6.0) | 3 (4.2) | |||
| Chemical trauma (n, %) | 0 (0.0) | 1 (1.4) | |||
| Other (n, %) | 6 (7.1) | 1 (1.4) | |||
| Number of lesion | 84 | 71 | |||
| 1-3 (n, %) | 45 (53.6) | 36 (50.7) | .011* | ||
| 4-6 (n, %) | 37 (44.0) | 24 (33.8) | |||
| >6 (n, %) | 2 (2.4) | 11 (15.5) | |||
| History of ulcer (n, %) | 77 | 24 (31.2) | 62 | 25 (35.2) | .35 |
| Severe infection grade (n, %) | 84 | 56 (66.7) | 71 | 59 (83.1) | .032* |
| Osteomyelitis (n, %) | 84 | 61 (72.6) | 71 | 62 (87.3) | .04* |
| Laboratory | |||||
| Hemoglobin (g/dL) (mean, SD) | 84 | 10.2 (1.9) | 71 | 9.7 (1.9) | .10 |
| Leukocyte (/μL) (median, IQR) | 84 | 15 565.0 (11 207.5-23 405.0) | 71 | 20 280.0 (15 040.0-28 710.0) | .002* |
| NLR (median, IQR) | 83 | 7.7 (4.5-13.5) | 70 | 12.1 (7.3-18.3) | .008* |
| Thrombocyte (/μL) (mean, SD) | 84 | 437 202.4 (141 068.1) | 71 | 454 042.2 (173 471.5) | .51 |
| RBG (mg/dL) (median, IQR) | 83 | 240.7 (140.9) | 71 | 184.0 (129.0-315.0) | .78 |
| HbA1c (%) (median, IQR) | 66 | 8.0 (6.8-10.2) | 54 | 7.8 (6.6-11.1) | .77 |
| Albumin (g/dL) (mean, SD) | 73 | 2.8 (0.6) | 67 | 2.7 (0.6) | .35 |
| eGFR (mL/min/1.73 m2) (median, IQR) | 81 | 56.5 (21.7-97.0) | 70 | 48.5 (22.2-90.7) | .72 |
| Comorbidity | |||||
| Peripheral arterial disease (n, %) | 81 | 50 (61.7) | 67 | 32 (47.8) | .13 |
| Hypertension (n, %) | 84 | 57 (67.9) | 71 | 46 (64.8) | .82 |
| Dyslipidemia (n, %) | 51 | 48 (94.1) | 35 | 32 (91.4) | .68 |
Abbreviations: eGFR, estimated glomerular filtration rate; NLR, neutrophil lymphocyte ratio; RBG, random blood glucose.
Statistically significant.
Table 2.
Location of Lesions.
| Pre-pandemic (N = 277 lesions) | During pandemic (N = 270 lesions) | |
|---|---|---|
| Digiti, n (%) | 55 (19.8) | 43 (15.9) |
| Hallux, n (%) | 30 (10.8) | 27 (10.0) |
| Forefoot, n (%) | 57 (20.6) | 44 (16.3) |
| Midfoot, n (%) | 57 (20.6) | 50 (18.6) |
| Hindfoot, n (%) | 32 (11.5) | 37 (13.7) |
| Ankle, n (%) | 20 (7.2) | 31 (11.5) |
| Distal cruris, n (%) | 14 (5.1) | 18 (6.7) |
| Medial cruris, n (%) | 8 (2.9) | 12 (4.4) |
| Proximal cruris, n (%) | 3 (1.1) | 6 (2.2) |
| Genu to femur, n (%) | 1 (0.4) | 2 (0.7) |
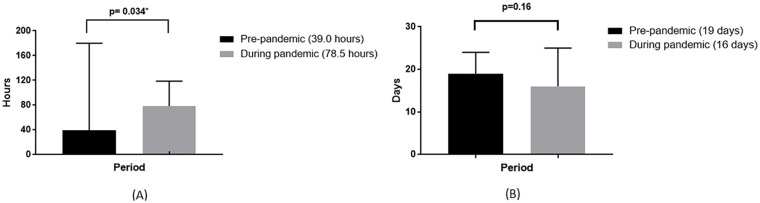
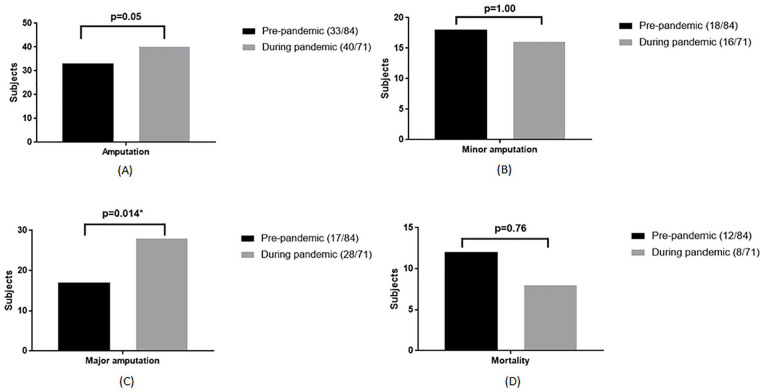
A significantly longer waiting time-to-surgery was observed in the pandemic group (39.0 h vs 78.5 h, P = .034, Figure 2A). While major amputations were also higher in the pandemic group (20.2% vs 39.4%, P = .014, Figure 3C), no differences in length of stay (19 (12-24) days vs 16 (11-25) days, P = .16, Figure 2B) and mortality (14.3% vs 11.3%, P = .76, Figure 3D) were observed between pre- and during the pandemic group. During the pandemic, those with COVID-19 infection (7/71, 9.8%) had more severe infections, worse kidney function, longer waiting time-to-surgery, and higher mortality (Table 3).
Figure 2.
Characteristics of diabetic foot ulcer patients. (A) Median of waiting time to surgery. (B) Median of length of stay.
Figure 3.
Outcome of diabetic foot ulcer patients during hospitalization. (A) Total amputation. (B) Minor amputation. (C) Major amputation. (D) Mortality.
Table 3.
Clinical Characteristic of Patients During the Pandemic.
| Variable | N | COVID-19 | N | Non COVID-19 |
|---|---|---|---|---|
| Severe infection grade (n, %) | 7 | 7 (100.0) | 64 | 52 (81.3) |
| Peripheral arterial disease (n, %) | 6 | 4 (66.7) | 61 | 28 (45.9) |
| Osteomyelitis (n, %) | 7 | 7 (100.0) | 64 | 55 (85.9) |
| HbA1c (%) (mean, SD) | 7 | 7.7 (3.6) | 64 | 7.9 (6.7-11.4) |
| Leukocyte (/μL) (mean, SD) | 7 | 29 925.7 (11 397.2) | 64 | 21 913.4 (10 874.4) |
| eGFR (mL/min/1.73 m2) (mean, SD) | 7 | 40.9 (21.4) | 64 | 58.1 (38.0) |
| NLR (mean, SD) | 7 | 25.9 (17.4) | 64 | 11.2 (6.7-17.4) |
| Waiting time-to-surgery (h) (median, IQR) | 7 | 95.6 (30.9) | 64 | 72 (38.0-118.0) |
| Amputation (n, %) | 7 | 4 (57.1) | 64 | 36 (56.3) |
| Minor amputation (n, %) | 7 | 2 (28.6) | 64 | 14 (21.9) |
| Major amputation (n, %) | 7 | 2 (28.6) | 64 | 26 (40.6) |
| Mortality (n, %) | 7 | 1 (14.3) | 64 | 7 (10.9) |
Abbreviations: eGFR, estimated glomerular filtration rate; NLR, neutrophil lymphocyte ratio.
Discussion
Our study observed that during the COVID-19 pandemic, patients with DFU significantly had higher infection grade, leukocyte count, and NLR; higher number of osteomyelitis and major amputation; and longer waiting time-to-surgery compared to the pre-pandemic group. Fortunately, the mortality between groups did not differ. We also observed that DFU patients with confirmed COVID-19 had more severe infection, longer time-to-surgery, and mortality compared to those without the COVID-19.
According to our study, the COVID-19 pandemic forced hospitals to modify their services and management, such as limiting elective procedures, re-distributing health workers, and reducing patient visits. During the COVID-19 pandemic, hospitals would allocate most of their workers to handle the COVID-19 wards. Moreover, as a part of the patient and healthcare worker safety approach, hospitals also obliged patients to provide a negative COVID-19 PCR result before surgery. 16 This policy indeed would extend waiting time-to-surgery. 17 With high numbers of patients requiring PCR examination, the time needed to obtain results of COVID-19 PCR examination at that time was longer in Indonesia, 17 which would obviously delay surgery. 11
Not only did hospital policies change, many available healthcare workers have been confirmed to be COVID-19 positive, which reduced the number of available workers even more. A previous study conducted in Indonesia and Portugal showed that positivity rate in medical workers during March-June 2020 was about 5.8% and 6.8%, respectively.18,19 In addition, many patients also limit their visit to the hospital due to risk of COVID-19 infection and limit access to transportation. Consent for surgery also could not be obtained immediately from the patient due to several of the factors mentioned above. Patients with confirmed cases of COVID-19 would experience even longer waiting time-to-surgery, due to limited operating rooms allocated for COVID-19 cases.
In the present study, patients visited during the pandemic tend to have more severe and more extensive infection compare to the pre-pandemic group, marked by higher leukocyte count (15 565.0/μL vs 20 280.0/μL), NLR (7.7 vs 12.1), and severe infection grade (66.7% vs 83.1%). NLR is an inflammatory marker, in which higher NLR indicates more severe inflammation. 20 Increased neutrophils will trigger the release of reactive oxygen species that can induce DNA damage mediated by antibody dependent cells (ADCC).21,22 Increased neutrophils may also trigger the release of IL-6, IL-8, and TNF-α.21,22
Even though adequate antibiotics have been given, severe infection may still occur due to several factors. First, infection could occur because insulin resistance could inhibit insulin signaling, leading to immune cells dysfunction. 23 Second, blood glucose control can affect immunity. Hyperglycemia would cause deletion of SOCS-1 that produced interferons (IFNs), which were responsible to assist normal phagocytosis. In addition, there was a decrease in STAT-1 activity in suppressing inflammatory process. As the result, inflammatory reaction through the JAK/STAT inhibitor pathway was increasing and overall immunity was decreasing. 24 According to COVID-19 status, our study showed that patient with COVID-19 had more severe manifestation, marked by higher leukocyte count, NLR, and infection grade. Study by Atri 25 showed that COVID-19 infection induced coagulopathy, altered vascular flow, and potentially worsen foot ulcers. Patients with COVID-19 had elevated ICAM-1 level, which could aggravate the pre-existing inflammation in patient with DFU. 26
During the pandemic, we also found that number of patients with osteomyelitis was higher than the pre-pandemic (72.6% vs 87.3%). Osteomyelitis could occur when infection from wound site spread contiguously to the bone. 15 Factors that could influence the development of osteomyelitis were low immunity, location of infected wounds, types of pathogens, diabetes, PAD, malnutrition, and infection grade according to IWGDF/IDSA.27,28 According to COVID-19 status, we found that all of our patients with confirmed COVID-19 had osteomyelitis. We suggest that immune system was inhibited in COVID-19 patients, causing infection to spread much faster from soft tissue to the bone. 29
In this study, we found that the proportion of amputation was higher during the pandemic (39.3% vs 56.3%) and the proportion of major amputation was almost twice higher during the pandemic than the pre-pandemic (20.2% vs 39.4%). We associated this finding with higher infection severity grade in the pandemic group compared to the pre-pandemic group. Quilici et al 30 showed that 1 point increase in Wagner ulcer classification criteria was associated with a 65% increase of amputation risk. Caruso et al 12 also found that there was threefold increase of amputation in patients with diabetic foot during the COVID-19 pandemic. Obviously, lockdown regulation due to COVID-19 had detrimental effects on amputation risk due to sudden disruption of DFU care and lower limb preservation pathways, resulting in delayed diagnosis and treatment. 12
There was no significant difference in the duration of ulcer between the 2 groups, even though infection severity was higher in the pandemic group. In fact, more severe infection usually occurred in patients with longer duration of ulcer. This finding might be due to higher patients’ concern when they deal with open wound. With an open wound that could be seen visibly with bare eyes, patients would have higher determination to seek for treatment, as patients fear amputation more than they fear death. 31 The thought of amputation itself often gave psychological burden for patients as it may reduce their quality of life, sense of well-being, ability to work, and, ultimately, social confidence, and cognitive impairment.32-34 Since we obtained this data from interview, there might be recall bias.
In our study, we assess glycemic control according to RBG and HbA1C. However, their level in both groups was high (RBG 240 mg/dL vs 184 mg/dL, HbA1C 8.0% vs 7.8%) and hence did not differ significantly. Previous studies showed that mortality rates increase in patients with admission RBG >200 mg/dL.35,36 Similarly, mortality rate in an ICU was significantly increased in patients with RBG level >140 mg/dL, even in patients without diabetes. 37 Another study by Palta et al 38 reported that HbA1C level ≥8.0% would increase mortality rate in diabetic patients (HR 2.2 vs 1.6). All in all, those studies came to a conclusion that high blood glucose level could increase production of reactive oxygen species, which may induce cell damage and hence mortality. In our study, glycemic control pre- and during the pandemic was quite similar, which might be one of the factors that cause no significant difference in the mortality pre- and during the pandemic. We noted that HbA1C examination might be bias because of anemia (Hb 10.2 g/dL vs 9.7 g/dL) and hemoglobinopathy. Considering that about 50% Indonesian population had hemoglobinopathy trait, 39 we could not rule out this possibility. RBG level also did not differ significantly, perhaps because our subjects already may have consumed anti-diabetic drugs before arriving at the hospital.
Mortality in the pandemic group did not differ from pre-pandemic group in this study. Research conducted by Rastogi et al 40 showed a similar result, in which the mortality rate in diabetic foot patients did not differ before and during the COVID-19 pandemic (3.3% vs 3.8%). This finding might be due to the fact that glycemic control between the 2 groups in our study also did not differ significantly. Nevertheless, we found that mortality in both of our groups was higher compared to other studies. 12 We suggested that it was partly due to the severe infection in both groups. 41 Because of more severe infection and osteomyelitis, debridement or amputation was more preferred to accelerate infection source control so mortality could be reduced.42,43
This is the first study that compares patients with DFU characteristics pre- and during the pandemic in one of tertiary referral hospitals in Indonesia. Despite our study covering patients with various characteristics, it may only represent more severe DFU cases. Furthermore, we were unable to perform further analyses in terms of direct and indirect contributing factors such as blood glucose variations, availability of operating theaters, and patient’s perspectives.
Conclusion
Our study observed that during the COVID-19 pandemic, patients with diabetic foot ulcer were hospitalized with more severe infections, higher proportion of osteomyelitis, and longer waiting time-to-surgery, which led to higher events of major amputation, but not mortality. It is suggested that future studies are needed to develop a modified diabetic foot ulcer care model in critical situations to increase early detection and timely treatment, thus preventing amputation.
Acknowledgments
The author would like to thank all parties that have been involved in setting up the diabetic foot registry.
Footnotes
Author Contributions: Idea and study design: EY; Data collection: EI, AS; Data analysis: EI; Article draft writing: EY, TJET, EI, AS, EVC, DW, DT; Draft revision: EY, TJET, WW, DP, FK, MR, FA, AM, DT; Writing supervision: EY, DT.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Clearance: This study was approved by The Ethical Committee of Faculty of Medicine Universitas Indonesia (KET-1153/UN2.F1/ETIK/PPM.00.02/2021).
ORCID iDs: Em Yunir  https://orcid.org/0000-0002-2004-9050
https://orcid.org/0000-0002-2004-9050
Eunike Vania Christabel  https://orcid.org/0000-0002-8998-3386
https://orcid.org/0000-0002-8998-3386
References
- 1. Apelqvist J, Bakker K, Van Houtum W, Schaper N. International Working Group of Diabetic Foot. International Consensus on the Diabetic Foot. International Diabetes Federation; 2007. [Google Scholar]
- 2. Boulton A, Armstrong D, Kirsner R, et al. Diagnosis and Management of Diabetic Foot Complications. American Diabetes Association; 2018. [PubMed] [Google Scholar]
- 3. Soewondo P, Suyono S, Sastrosuwignyo MK, Harahap AR, Sutrisna B, Makmun LH. Prediction of wound healing in diabetic foot ulcers: an observational study in Tertiary Hospital in Indonesia. Acta Med Indones. 2017;49(1):41-45. [PubMed] [Google Scholar]
- 4. Marzoq A, Shiaa N, Zaboon R, Baghlany Q, Alabbood M. Assessment of the outcome of diabetic foot ulcers in Basrah, southern Iraq: a cohort study. Int J Diab Metab. 2019;25:33-38. [Google Scholar]
- 5. Uccioli L, Izzo V, Meloni M, Vainieri E, Ruotolo V, Giurato L. Non-healing foot ulcers in diabetic patients: general and local interfering conditions and management options with advanced wound dressings. J Wound Care. 2015;24(4 Suppl):35-42. [DOI] [PubMed] [Google Scholar]
- 6. Cascini S, Agabiti N, Davoli M, et al. Survival and factors predicting mortality after major and minor lower-extremity amputations among patients with diabetes: a population-based study using health information systems. BMJ Open Diabetes Res Care. 2020;8(1):e001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yunir E. Diabetic Foot Problem in Cipto Mangunkusumo Hospital 2007. Kyoto Foot Meeting 2008: Training of diabetic foot care for young doctors; 5–7 March 2008: Kyoto. (Unpublished data). [Google Scholar]
- 8. Sitompul Y, Budiman B, Soebardi S, Abdullah M. Profil Pasien Kaki Diabetes yang Menjalani Reamputasi di Rumah Sakit Cipto Mangunkusumo Tahun 2008 -2012. Jurnal Penyakit Dalam Indonesia. 2015;2(1):9-14. [Google Scholar]
- 9. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020. Accessed July 1, 2021. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
- 10. Ministry of Health of Indonesia. Situasi Terkini Perkembangan Coronavirus Disease (COVID-19) 2 Maret 2020. 2020. Accessed September 22, 2021. https://infeksiemerging.kemkes.go.id/situasi-infeksi-emerging/situasi-terkini-perkembangan-coronavirus-disease-covid-19-2-maret-2020
- 11. Boulton AJM. Diabetic foot disease during the COVID-19 pandemic. Medicina. 2021;57(2):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caruso P, Longo M, Signoriello S, et al. Diabetic foot problems during the COVID-19 pandemic in a Tertiary Care Center: the emergency among the emergencies. Diabetes Care. 2020;43:e123-e4. [DOI] [PubMed] [Google Scholar]
- 13. International Diabetes Federation. Covid-19 and diabetes. 2021. Accessed August 13, 2021. https://www.idf.org/aboutdiabetes/what-is-diabetes/covid-19-and-diabetes/1-covid-19-and-diabetes.html [PubMed]
- 14. COVID-19 Response Acceleration Task Force. Analisis Data Covid Indonesia. 2021. Accessed May 5, 2021. https://covid19.go.id/p/berita/analisis-data-covid-19-indonesia-update-3-januari-2021 [Google Scholar]
- 15. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchlife RJ, Lipsky BA. IWGDF practical guidelines on the prevention and management of diabetic foot disease. 2019. Accessed June 2, 2021. https://iwgdfguidelines.org/wp-content/uploads/2019/05/01-IWGDF-practical-guidelines-2019.pdf [DOI] [PubMed] [Google Scholar]
- 16. Al-Muharraqi MA. Testing recommendation for COVID-19 (SARS-CoV-2) in patients planned for surgery - continuing the service and ‘suppressing’ the pandemic. Br J Oral Maxillofac Surg. 2020;58(5):503-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sucahya PK. Barriers to covid-19 RT-PCR testing in Indonesia: a health policy perspective. J Indones Health Policy Adm. 2020;5(2):36-42. [Google Scholar]
- 18. Adelina M, Dwijayanti F. The infection of COVID-19 among health care workers in Dharmais Cancer Hospital. Indones J Cancer. 2021;15(1):1-3. [Google Scholar]
- 19. Mendonça-Galaio L, Sacadura-Leite E, Raposo J, et al. The COVID-19 impact in hospital healthcare workers: development of an occupational health risk management program. Port J Public Heal. 2020;38(Suppl. 1):26-31. [Google Scholar]
- 20. Liu C-C, Ko HJ, Liu W-S, et al. Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Medicine. 2019;98(43):e17537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang A-P, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16(5):442-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sachithanandan N, Graham KL, Galic S, et al. Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes. 2011;60(8):2023-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atri A, Kocherlakota CM, Dasgupta R. Managing diabetic foot in times of COVID-19: time to put the best ‘foot’ forward. Int J Diabetes Dev Ctries. 2020;40:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oley MH, Oley MC, Kepel BJ, et al. ICAM-1 levels in patients with covid-19 with diabetic foot ulcers: a prospective study in southeast Asia. Ann Med Surg. 2021;63:102171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panteli M, Giannoudis PV. Chronic osteomyelitis: what the surgeon needs to know. EFORT Open Rev. 2016;1(5):128-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang N, Yang B-H, Wang G, et al. A meta-analysis of the relationship between foot local characteristics and major lower extremity amputation in diabetic foot patients. J Cell Biochem. 2019;120(6):9091-9096. [DOI] [PubMed] [Google Scholar]
- 29. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quilici MT, Del Fiol Fde S, Vieira AE, Toledo MI. Risk factors for foot amputation in patients hospitalized for diabetic foot infection. J Diabetes Res. 2016;2016:8931508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wukich DK, Raspovic KM, Suder NC. Patients with diabetic foot disease fear major lower-extremity amputation more than death. Foot Ankle Spec. 2018;11(1):17-21. [DOI] [PubMed] [Google Scholar]
- 32. Coffey L, Mahon C, Gallagher P. Perceptions and experiences of diabetic foot ulceration and foot care in people with diabetes: a qualitative meta-synthesis. Int Wound J. 2019;16(1):183-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crocker RM, Palmer KNB, Marrero DG, Tan T-W. Patient perspectives on the physical, psycho-social, and financial impacts of diabetic foot ulceration and amputation. J Diabetes Complications. 2021;35(8):107960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shaikh FA, Bhuvan K, Htar TT, Gupta M, Kumari Y. Cognitive Dysfunction in Diabetes Mellitus. Type 2 Diabetes-From Pathophysiology to Modern Management. IntechOpen; 2019. [Google Scholar]
- 35. Cheung NW, Li S, Ma G, Crampton R. The relationship between admission blood glucose levels and hospital mortality. Diabetologia. 2008;51(6):952-955. [DOI] [PubMed] [Google Scholar]
- 36. Mcgrade P, Yang S, Nugent K. The association between admission glucose levels and outcomes in adults admitted to a tertiary care hospital. J Community Hosp Intern Med Perspect. 2019;9(3):195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Madhumathi R, Rajiv E, Amogh D, Kavya ST, Srinivasa V. The relationship between admission blood glucose levels and hospital mortality. J Evol Med Dent Sci. 2013;2(27):4872-4876. [Google Scholar]
- 38. Palta P, Huang ES, Kalyani RR, Golden SH, Yeh H-C. Hemoglobin a1c and mortality in older adults with and without diabetes: results from the National Health and Nutrition Examination Surveys (1988-2011). Diabetes Care. 2017;40(4):453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Surjawan Y, Tan HL, Setiabudy RD, Rositawati W. Early screening of hemoglobinopathy in Indonesia using erythrocyte indices. Indones Biomed J. 2017;9(2):99-105. [Google Scholar]
- 40. Rastogi A, Hiteshi P, Bhansali A, Jude EB. Virtual triage and outcomes of diabetic foot complications during covid-19 pandemic: a retro-prospective, observational cohort study. PLoS One. 2021;16(5):e0251143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chammas NK, Hill RL, Edmonds ME. Increased mortality in diabetic foot ulcer patients: the significance of ulcer type. J Diabetes Res. 2016;2016:2879809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mavrogenis AF, Megaloikonomos PD, Antoniadou T, et al. Current concepts for the evaluation and management of diabetic foot ulcers. EFORT Open Rev. 2018;3(9):513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]