Abstract
Objective
Diabetes affected 463 million people globally in 2019, and this number is anticipated to reach 700 million by 2045. Diabetes results in lower limb amputation every 30 seconds. Egypt has a high prevalence of diabetic foot disease among patients with type 2 diabetes mellitus (T2DM). We aimed to identify high-risk patients for diabetic foot ulcers (DFUs) in Egypt.
Methods
We designed a cross-sectional study among adult patients with diabetes at Asyut University Hospital. Inlow’s 60-second diabetic foot screening tool was used to assess the risk of DFU. Neuropathy was assessed using the 10g monofilament test, and laboratory testing was performed to assess glycosylated hemoglobin (HbA1c) and diabetes control levels.
Results
Participants were aged 46.11 ± 9.18 years; 56% had T2DM and HbA1c levels >7%. In total, 47.9% of participants were at risk for DFUs. This risk was higher in patients who were older, male, widowed, working, illiterate, living in rural areas, and patients with diabetes duration >10 years, body mass index >32 kg/m2, uncontrolled blood glucose levels, on an insulin regimen, and smokers.
Conclusions
Increasing health care providers’ awareness and ability to identify high-risk patients is critical to prevent DFUs and reduce the risk of amputation.
Keywords: Diabetes, diabetic foot ulcer, Inlow’s 60-second diabetic foot screening tool, Egypt, risk factor, prevention
Introduction
In the past 30 years, the global incidence of diabetes has nearly quadrupled, with 422 million adults diagnosed, bringing the global prevalence of diabetes from 4.7% to 8.5%. 1 In Egypt, type 2 diabetes mellitus affects approximately 15.6% of the population.2,3 The annual incidence of diabetes-related foot disease has been estimated at 1% to 4% and as high as 25% in some studies.4,5 Diabetic foot ulcers (DFUs) are a leading cause of morbidity and mortality among adults with diabetes. 6 Approximately 25% of adults with diabetes will be affected by a DFU during their lifetime, 5 20% of whom will require either minor or major amputation. 7
It is estimated that between 1 and 3.5 million adults have a history of DFUs in the United States alone. 8 In Egypt, diabetes-related foot ulcers are estimated to affect 4% to 19% of people with diabetes. 9
According to the International Working Group on the Diabetic Foot, the annual incidence of DFUs is approximately 2%, with a lifetime incidence of 15% to 25%. 10 Because 85% of lower limb diabetes-related amputations are preceded by a DFU, early detection of DFUs is a crucial step in preventing lower limb amputation. Early identification of patients at high risk for DFUs is a top priority owing to the clinical and economic burden of diabetic foot complications. 11
In the present study, we aimed to identify patients with diabetes in Egypt who are at high risk for the development of diabetic foot ulceration using Inlow’s 60-second diabetic foot screening tool.
Methods
This was a cross-sectional study that included a convenience sample of adult patients with diabetes. The sample size was calculated using Epi Info, with a 10% margin of error and a 95% confidence interval, with the following inclusion criteria: diagnosis of diabetes mellitus and attending a diabetes follow-up clinic. We excluded any patients with an ulcer related to an accident or injury. The study was conducted at Asyut University Hospital, Egypt, from May 2018 to November 2018. Recorded study data included patient demographic characteristics such as age, sex, level of education, employment status, marital status, and residential area. We also collected diabetes-related patient medical profiles, including type and duration of diabetes, body mass index (BMI), smoking status assessed according to the World Health Organization Smoking and Tobacco Use Policy, treatment regimen, and level of glycemic control. Scores on Inlow’s 60-second diabetic foot screening test 12 were obtained to assess patients’ risk for DFUs. All patients underwent a complete foot examination; neuropathy was assessed using the 10g monofilament test, in which peripheral neuropathy was detected if sensation was lost at one or more locations. Laboratory testing was performed to assess glycosylated hemoglobin (HbA1c) and diabetes control levels.
To interpret the results of Inlow’s 60-second diabetic foot screening test, the highest score from the left or right foot was used, as follows: a score of 0 to 6 indicates no risk, a score of 7 to 12 mild risk, a score of 13 to 19 moderate risk, and a score of 20 to 25 indicates high risk.
Ethical considerations
Before beginning the study, the hospital where the research was conducted provided written consent, and the study was approved by the Research Ethics Committee (Decision No: 43) of the Faculty of Nursing, Damanhour University. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 13 Each eligible patient with type 2 diabetes mellitus received an explanation of the study purpose. First, verbal consent for voluntary participation was obtained from each patient. The researchers then explained the aim and nature of the study; confidentiality of the collected data was guaranteed, as was the opportunity to withdraw from the study at any time. Patients who agreed to participate in the study provided their written informed consent.
Statistical analysis
Data were entered and subsequently analyzed using IBM SPSS version 20 (IBM Corp., Armonk, NY, USA). Number, percentage, mean and standard deviation are used to describe quantitative data. We used the chi-square test to examine relationships between risk levels for categorical variables. Independent t-tests were used to examine connections between the amount of risk and quantitative factors for normally distributed data. Statistical significance was set at the 0.5% level.
Results
The current study comprised 200 adult patients with diabetes. The mean participant age was 46.11 ± 9.18 years; 53.5% of participants were male, 74% were married, 60% were illiterate, 14.5% had a university-level education, 59% were not working, and 80.5% of participants were living in rural areas. More than half of patients had type 2 diabetes and poor glycemic control (HbA1c levels >7%). Among the total, 47.9% of participants were at risk for the development of DFUs, according to the results of Inlow’s 60 second diabetic foot screen test.
Table 1 revealed that the highest incidence of diabetes was in the age group 50 to 65 years (39%). Table 2 shows that 56% of participants had type 2 diabetes and 44% had type 1 diabetes. The mean duration of diabetes was 13.14 ± 7.36 years, and mean BMI was 26.95 ± 6.75 kg/m2.
Table 2.
Distribution of participants according to medical profile (N = 200).
| Medical data | n | % |
|---|---|---|
| Diabetes type | ||
| Type 1 | 88 | 44 |
| Type 2 | 112 | 56 |
| Duration of diabetes, years | ||
| Mean ± standard deviation | 13.14 ± 7.36 | |
| Body mass index, kg/m2 | ||
| Mean ± standard deviation | 26.95 ± 6.75 | |
Table 1.
Distribution of participants according to sociodemographic characteristics (N = 200).
| Characteristics | n | % |
|---|---|---|
| Age group | ||
| 18–28 years | 9 | 4.5 |
| 29–39 years | 40 | 20.0 |
| 40–49 years | 72 | 36.0 |
| 50–65 years | 79 | 39.5 |
| Mean ± SD, years | 46.11 ± 9.18 | |
| Sex | ||
| Male | 107 | 53.5 |
| Female | 93 | 46.5 |
| Marital status | ||
| Single | 27 | 13.5 |
| Married | 148 | 74.0 |
| Divorced | 21 | 10.5 |
| Widowed | 4 | 2.0 |
| Level of education | ||
| Illiterate | 120 | 60.0 |
| Basic education | 46 | 23.0 |
| Secondary school | 5 | 2.5 |
| University | 29 | 14.5 |
| Employment status | ||
| Working | 82 | 41.0 |
| Not working | 118 | 59.0 |
| Residence | ||
| Urban | 39 | 19.5 |
| Rural | 161 | 80.5 |
Figure 1 shows that 52% of study participants were smokers. Fewer than half of participants were receiving insulin, 40% were receiving oral hypoglycemic agents, and 13.5% were receiving combined therapy (Figure 2). Figure 3 shows that 53% of the study population had poor glycemic control, 28% had fair control, and 19% had good glycemic control.
Figure 1.
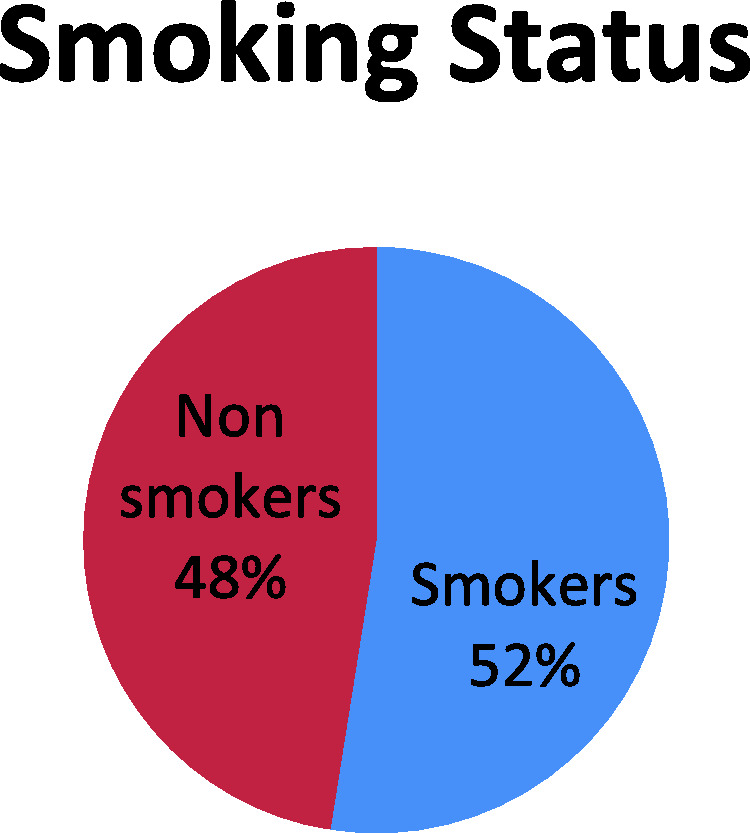
Distribution of participants according to smoking status.
Figure 2.
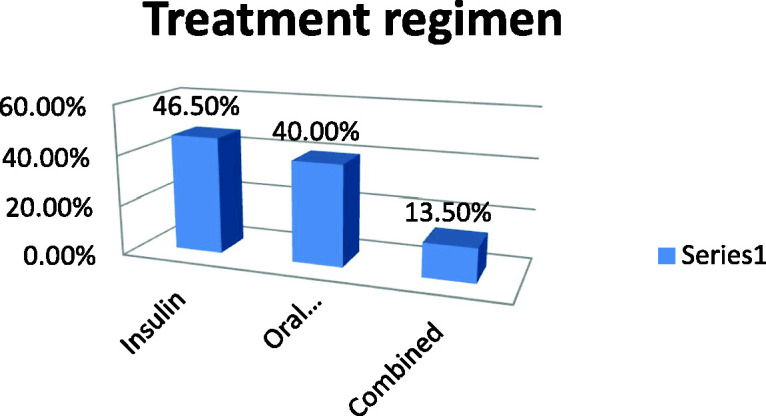
Distribution of participants according to treatment regimen.
Figure 3.
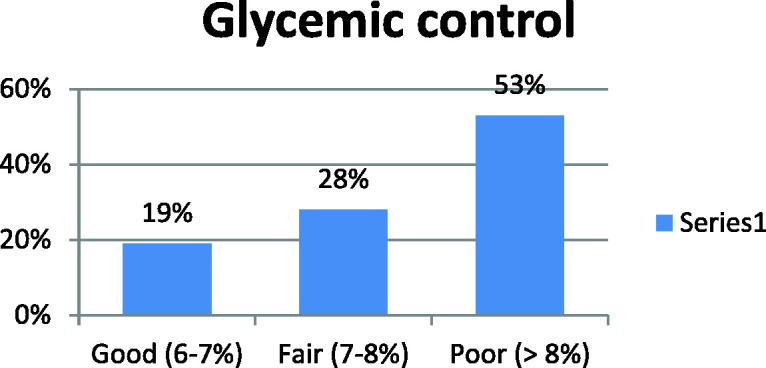
Distribution of participants according to glycemic control.
According to participants’ results on Inlow’s scale, the most notable findings were as follows: 22.6% of participants had dryness with fungus or light callus of the right foot. Nail assessment revealed that 40.5% of patients had unkempt and ragged toenails on the right foot. Sensation was lost in one or more sites of the left foot in 46.5% of participants. Pedal pulses were absent in the left foot among 19.4% of patients. The right foot was cool in 36.5% of patients; 7.5% of participants had amputation of the left foot and 5.5% had amputation of the right foot (Table 3).
Table 3.
Distribution of participants according to Inlow’s 60-second diabetic foot screening tool (N = 200).
| Parameters of Inlow’s 60-second diabetic foot screening tool | Left foot |
Right foot |
||
|---|---|---|---|---|
| n | % | n | % | |
| 1. Assessment for skin and nail changes | ||||
| Skin | ||||
| Intact and healthy | 113 | 56.5 | 118 | 59.2 |
| Dry with fungus or light callus | 42 | 21.0 | 45 | 22.6 |
| Heavy callus buildup | 22 | 10.8 | 20 | 9.8 |
| Open ulceration or history of previous ulcer | 23 | 11.7 | 17 | 8.4 |
| Nails | ||||
| Well-groomed and appropriate length | 98 | 49.0 | 92 | 45.8 |
| Unkempt and ragged | 68 | 34.0 | 81 | 40.5 |
| Thick, damaged, or infected | 34 | 17.0 | 27 | 13.7 |
| 2. Assessment for peripheral neuropathy/loss of protective sensation | ||||
| Sensation – Monofilament testing | ||||
| No: peripheral neuropathy was not discovered (there was sensation at all locations) | 107 | 53.5 | 108 | 54.1 |
| Yes: peripheral neuropathy discovered (sensation was lost at one or more sites) | 93 | 46.5 | 92 | 45.9 |
| Sensation – Four questions (Are the feet ever numb? Do they ever tingle? Do they ever burn? Do they ever feel like insects are crawling on them?) | ||||
| No to all questions | 114 | 57.1 | 110 | 54.8 |
| Yes to any of the questions | 86 | 42.9 | 90 | 45.2 |
| 3. Assessment for peripheral arterial disease | ||||
| Pedal pulses | ||||
| Present | 161 | 80.6 | 164 | 81.9 |
| Absent | 39 | 19.4 | 36 | 18.1 |
| Dependent rubor | ||||
| No | 175 | 87.6 | 175 | 87.3 |
| Yes | 25 | 12.4 | 25 | 12.7 |
| Cool foot | ||||
| No | 130 | 64.9 | 127 | 63.5 |
| Yes | 70 | 35.1 | 73 | 36.5 |
| 4. Assessment for bony deformity and footwear | ||||
| Deformity | ||||
| No deformity | 160 | 80 | 169 | 84.5 |
| Deformity | 20 | 10 | 15 | 7.5 |
| Amputation | 15 | 7.5 | 11 | 5.5 |
| Acute Charcot (+ warmth and erythema) | 5 | 2.5 | 5 | 2.5 |
| Range of motion | ||||
| Full range to hallux | 105 | 52.5 | 118 | 59.0 |
| Hallux limitus | 46 | 23 | 38 | 19 |
| Hallux rigidus | 34 | 17 | 33 | 16.5 |
| Hallux amputation | 15 | 7.5 | 11 | 5.5 |
| Footwear | ||||
| Appropriate | 100 | 50.0 | 96 | 48.0 |
| Inappropriate | 65 | 32.5 | 69 | 34.5 |
| Causing trauma | 35 | 17.5 | 35 | 17.5 |
To identify risk factors for the development of DFUs, the results for the identified parameters were categorized according to the International Working Group of the Diabetic Foot risk classification system. 14 Figure 4 illustrates that 47.9% of the study population was at risk for the development of DFUs, with 28.7% having mild risk, 14.5% moderate risk, and 4.7% high risk.
Figure 4.
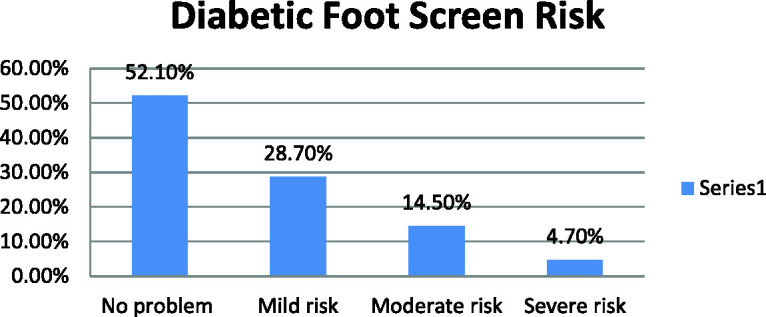
Distribution of participants according to risk of diabetic foot ulcer.
Table 4 illustrates a statistically significant difference between patients’ sociodemographic characteristics and their level of risk for DFUs. Patients with diabetes who were male, married, aged 50–65 years, illiterate, working, and those living in rural areas had a high risk for DFUs (all P = 0.001).
Table 4.
Relationship between risk of DFUs and patient sociodemographic characteristics.
| Level of risk |
P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No risk (n = 104) |
Mild risk (n = 57) |
Moderate risk (n = 29) |
High risk (n = 10) |
||||||
| Variables | N | % | N | % | N | % | N | % | |
| Age (years) | |||||||||
| 18–28 | 18 | 17.5 | 4 | 6.3 | 2 | 8.3 | 1 | 10.6 | 0.001* |
| 29–39 | 22 | 21.3 | 8 | 13.6 | 3 | 11.0 | 3 | 27.7 | |
| 40–49 | 23 | 21.9 | 15 | 26.8 | 7 | 23.4 | 2 | 17.0 | |
| 50–65 | 41 | 39.3 | 30 | 53.3 | 17 | 57.2 | 4 | 44.7 | |
| Sex | |||||||||
| Male | 46 | 43.8 | 33 | 57.8 | 13 | 43.4 | 7 | 72.3 | 0.001* |
| Female | 58 | 56.2 | 24 | 42.2 | 16 | 56.6 | 3 | 27.7 | |
| Marital status | |||||||||
| Single | 13 | 12.7 | 7 | 12.9 | 1 | 3.4 | 1 | 10.0 | 0.001* |
| Married | 44 | 42.4 | 38 | 65.2 | 17 | 60.0 | 1 | 10.0 | |
| Divorced | 18 | 17.7 | 5 | 9 | 4 | 13.8 | 1 | 10.0 | |
| Widowed | 28 | 27.3 | 7 | 12.9 | 7 | 22.8 | 7 | 70.0 | |
| Education level | |||||||||
| Illiterate | 42 | 40.5 | 30 | 52.3 | 15 | 51.7 | 5 | 50.0 | 0.001* |
| Basic education | 38 | 37.1 | 19 | 33.1 | 11 | 37.9 | 2 | 20.0 | |
| Secondary school | 12 | 11.2 | 5 | 9.4 | 2 | 6.9 | 2 | 20.0 | |
| University | 12 | 11.2 | 3 | 5.2 | 1 | 3.5 | 1 | 10.0 | |
| Employment status | |||||||||
| Not working | 42 | 39.4 | 25 | 43.8 | 13 | 44.8 | 2 | 20.0 | 0.001* |
| Working | 58 | 60.6 | 32 | 56.2 | 16 | 55.2 | 8 | 80.0 | |
| Area of residence | |||||||||
| Rural | 47 | 45.1 | 31 | 54.3 | 20 | 68.9 | 6 | 60.0 | 0.001* |
| Urban | 53 | 54.9 | 26 | 45.7 | 9 | 31.1 | 4 | 40.0 | |
The risk of DFUs was increased in patients with the following characteristics; type 2 diabetes, longer duration of diabetes, mean BMI 28.02 ± 4.39, smokers, receiving insulin therapy, and patients with uncontrolled blood glucose levels (all P = 0.001), as shown in Table 5.
Table 5.
Relationship between risk of DFUs and patient’s medical profile.
| Level of risk |
P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | No risk (n = 104) |
Mild risk (n = 57) |
Moderate risk (n = 29) |
High risk (n = 10) |
|||||
| N | % | N | % | N | % | N | % | ||
| Type of diabetes | |||||||||
| Type 1 | 39 | 37.5 | 28 | 49.5 | 15 | 51.7 | 3 | 30.0 | 0.001 |
| Type 2 | 65 | 62.5 | 29 | 50.5 | 14 | 48.3 | 10 | 70.0 | |
| Duration of diabetes | |||||||||
| Mean ± standard deviation | 9.85 ± 5.87 | 12.80 ± 6.93 | 12.48 ± 6.27 | 11.15 ± 4.87 | 0.001 | ||||
| Body mass index | |||||||||
| Mean ± standard deviation | 29.39 ± 7.06 | 28.57 ± 3.93 | 28.44 ± 3.32 | 28.02 ± 4.39 | 0.001 | ||||
| Smoking | |||||||||
| Smoker | 39 | 37.5 | 23 | 40.3 | 10 | 34.4 | 7 | 70.0 | 0.001 |
| Nonsmoker | 65 | 62.5 | 34 | 59.7 | 19 | 65.6 | 3 | 30.0 | |
| Treatment | |||||||||
| Oral hypoglycemic agent | 32 | 30.7 | 15 | 26.3 | 4 | 13.7 | 1 | 10.0 | 0.001 |
| Insulin | 64 | 61.5 | 38 | 66.6 | 20 | 68.9 | 6 | 60.0 | |
| Combined | 8 | 7.8 | 4 | 7.1 | 5 | 17.4 | 3 | 30.0 | |
| Blood glucose level | |||||||||
| Controlled | 67 | 64.5 | 19 | 33.4 | 4 | 13.8 | 10 | 10.0 | 0.001 |
| Uncontrolled | 33 | 35.5 | 38 | 66.7 | 25 | 86.2 | 9 | 90.0 | |
Discussion
We conducted the present study to assess the risk of DFUs among patients with diabetes (N = 200). DFUs are a common but avoidable condition in individuals with diabetes. Loss of sensation owing to somatic neuropathy, vascular impairment, structural foot deformity, and poor glycemic management are all risk factors for DFUs,15,16 as is a history of ulceration.15,17 In this study, 47.9% of the study population was at risk for the development of DFUs, with 28.7% having mild risk, 14.5% moderate risk, and 4.7% high risk. This result was consistent with that of Jbour et al. 18 There was a significant difference between patients’ sociodemographic characteristics and the risk for DFUs. Male patients with diabetes, those aged from 50 to 65 years, smokers, and patients living in rural areas had a high risk for DFUs. This result is in accordance with the findings of Assaad-Khalila et al., 19 who reported a significantly higher prevalence of foot ulceration among men than women (14.1% and 9.7%, respectively; P = 0.002).
The risk for DFU development was higher among patients living in rural areas, which might be attributable to the dry climate in Upper Egypt where the study was conducted, as well as the tradition of walking barefoot. This result was in concordance with the findings of another study. 20 Patients' smoking habits are another important risk factor for DFU development. According to Xia et al., 21 smoking is an important risk factor for peripheral vascular disease, which is linked to DFUs.
The impact of sex on lower extremity morbidity could be influenced by a variety of circumstances. These may include activity levels, smoking habits, hormonal variation, degree of treatment compliance, level of denial regarding a diabetes diagnosis, social support mechanisms, and educational level. 22 In this study, age was a significant risk factor, with higher risk for DFUs among patients aged 50–65 years. This could be because older people often live alone and have impaired vision, other health issues, and a reduced ability to care for their feet. A significant difference was found between the risk for DFUs and patients’ medical profile (P = 0.001), with a high risk for DFUs among patients with type 2 diabetes, those with increased duration of diabetes, patients with a mean BMI 28.02 ± 4.39 kg/m2, smokers, patients receiving insulin therapy, and those with uncontrolled blood glucose levels.
We found a statistically significant association between having diabetes for a longer period and the risk of developing DFUs (mean diabetes duration 13.14 ± 7.36 years). This is most likely owing to additional risk factors that develop over time, such as peripheral neuropathy and peripheral artery disease.
In agreement with the current study results, elevated blood glucose levels are linked to the development of diabetic foot ulceration, as reported previously. 23 The Diabetes Control and Complications Trial of type 1 diabetes mellitus and the UK Prospective Diabetes Study of type 2 diabetes mellitus demonstrated that intensive diabetes management minimizes the risk of neuropathy and other problems associated with DFUs.24,25 Additionally, the administration of insulin therapy for the treatment of diabetes was identified as a high-risk factor for DFUs in the Seattle Diabetic Foot Study. It is possible that this factor reflects diabetes severity. Moreover, studies have shown an association between elevated BMI and a higher risk of foot ulceration. 26
Peripheral sensory neuropathy was found in the left foot among 46.5% of participants and in the right foot among 45.9%. This is comparable to other studies reporting that patients with DFUs have a prevalence of peripheral sensory neuropathy of 70% to 100%. 5 In agreement with the current results, several studies have found that patients with a history of ulceration or amputation are more likely to develop DFUs. Apelqvist and colleagues 27 found ulceration recurrence rates of 34% and 70% after 1 year and 5 years, respectively. Murray et al. 28 reported a 56.8% relative risk of developing an ulcer on the site of a previous ulcer. Furthermore, the factor most closely connected with the development of new ulceration is a history of ulceration or amputation; after a successful major amputation, the likelihood of limb amputation on the opposite side is 12% in the first year and more than 50% after 3 years. 29
Limitations of the study
In this study, we assessed both types of diabetes. Therefore, some factors such as insulin use and distal neuropathy, which are related to the future development of DFU, might be different between patients with type 1 and type 2 diabetes mellitus. Another limitation of this study is its cross-sectional nature, making it impossible to prove cause and effect.
Conclusion
In this study, we found that 47.9% of patients with diabetes were at risk for the development DFUs. Older age, male sex, being illiterate, having type 2 diabetes and longer diabetes duration, being on an insulin regimen, uncontrolled glucose level, and smoking were the most common risk factors detected in our study.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221087815 for Screening for identification of patients at high risk for diabetes-related foot ulcers: a cross-sectional study by Mohammed Al-Mohaithef, Sahar A Abdelmohsen, Magda Algameel and Amal Y Abdelwahed in Journal of International Medical Research
Acknowledgements
The authors wish to express their gratitude to the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University, and Saudi Electronic University for their assistance with this work.
Availability of data and materials: On reasonable request, the corresponding author will provide the datasets used/or analyzed in the current work.
Declaration of conflicting interest: The author declares that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
ORCID iD: Mohammed Al-Mohaithef https://orcid.org/0000-0002-8312-1005
References
- 1.World Health Organization. Global Report on Diabetes.; 2019. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf.
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2014 Estimates of Diabetes and Its Burden in the Epidemiologic estimation methods. Natl Diabetes Stat Rep. Published online 2014:2009–2012. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- 3.Hegazi R, El-Gamal M, Abdel-Hady N, et al. Epidemiology of and Risk Factors for Type 2 Diabetes in Egypt. Ann Glob Heal 2015; 81: 814–820. doi:10.1016/j.aogh.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Adler A, Boyko E, Ahroni J, et al. Lower-Extremity Amputation in Diabetes. Diabetes Care 1999; 22: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 5.May K. Preventing foot ulcers. Aust Prescr 2008; 31: 94–96. doi:10.18773/austprescr.2008.055. [Google Scholar]
- 6.Brownrigg JRW, Davey J, Holt PJ, et al. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: A meta-analysis. Diabetologia 2012; 55: 2906–2912. doi:10.1007/s00125-012-2673-3. [DOI] [PubMed] [Google Scholar]
- 7.Lavery LA, Armstrong DG, Wunderlich RP, et al. Diabetic Foot Syndrome Evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care 2003; 26: 1435–1438. doi:10.1024/0040-5930/a001201. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med 2017; 376: 2367–2375. doi:10.1056/nejmra1615439. [DOI] [PubMed] [Google Scholar]
- 9.Abbas ZG, Archibald LK. Epidemiology of the diabetic foot in Africa. Med Sci Monit 2005; 11: 262–270. [PubMed] [Google Scholar]
- 10.Van Netten JJ, Price PE, Lavery LA, et al . Prevention of foot ulcers in the at-risk patient with diabetes: a systematic review. Diabetes Metab Res Rev 2016; 1: 84–98. doi:10.1002/dmrr.2701. [DOI] [PubMed] [Google Scholar]
- 11.Adem AM, Andargie AA, Teshale AB, et al. Incidence of Diabetic Foot Ulcer and Its Predictors Among Diabetes Mellitus Patients at Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia. Diabetes Metab Syndr Obes 2020: 13: 3703–3711. 10.2147/DMSO.S280152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inlow S. The 60-second foot exam for people with diabetes. Wound Care Canada 2004; 2: 10–11. [Google Scholar]
- 13.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 14.IDF Clinical Practice Recommendations on the Diabetic Foot 2017. https://www.idf.org/e-library/guidelines/119-idf-clinical-practice-recommendations-on-diabetic-foot-2017.html.
- 15.Boulton AJM, Armstrong DG, Albert SF, et al . Comprehensive fool examination and risk assessment: A report of the task force of the foot care interest group of the American diabetes association, with endorsement by the American association of clinical endocrinologists. Diabetes Care 2012; 88: 1437–1443. doi:10.2337/dc08-9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy CA, Laforet K, Da Rosa P, et al. Reliability and predictive validity of Inlow’s 60-second diabetic foot screen tool. Adv Ski Wound Care 2012; 25: 261–266. doi:10.1097/01.ASW.0000415343.45178.91. [DOI] [PubMed] [Google Scholar]
- 17.Bokan V. Risk Factors for Diabetic Foot Ulceration-Foot Deformity and Neuropathy. Acta Medica Median 2010; 49: 19–22. [Google Scholar]
- 18.Jbour AKS, Jarrah NS, Radaideh ARM, et al. Prevalence and predictors of diabetic foot syndrome in type 2 diabetes mellitus in Jordan. Saudi Med J 2003; 24: 761–764. [PubMed] [Google Scholar]
- 19.Assaad-Khalil SH, Zaki A, Rehim AA, et al . Prevalence of diabetic foot disorders and related risk factors among Egyptian subjects with diabetes. Prim Care Diabetes 2015; 9: 297–303. doi:10.1016/j.pcd.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 20.El-Nahas MR, Gawish HM, Tarshoby MM, et al. The prevalence of risk factors for foot ulceration in Egyptian diabetic patients. Pr Diabetes Int 2008; 25: 362–366. [Google Scholar]
- 21.Xia N, Morteza A, Yang F, et al. Review of the role of cigarette smoking in diabetic foot. J Diabetes Investig 2019; 10: 202–215. 10.1111/jdi.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavery LA, Armstrong DG, Vela SA, et al. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med 1998; 158: 157–162. doi:10.1001/archinte.158.2.157. [DOI] [PubMed] [Google Scholar]
- 23.Parchman ML, Pugh JA, Wang CP, et al. Glucose control, self-care behaviors, and the presence of the chronic care model in primary care clinics. Diabetes Care 2007; 30: 2849–2854. doi: 10.2337/dc06-2516. [DOI] [PubMed] [Google Scholar]
- 24.Nathan DM, Genuth S, Lachin J, et al. DCCT research trial. N Engl J Med 1993; 329. [DOI] [PubMed] [Google Scholar]
- 25.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 26.Boyko Beward J, Jessie H, Ahroni VS, et al. A Prospective Study of Risk Factors for Erectile Dysfunction. Diabetes Care 1999; 22: 1036–1042. doi:/doi.org/10.2337/diacare.22.7.1036. [DOI] [PubMed] [Google Scholar]
- 27.Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med 1993; 233: 485–491. doi:10.1111/j.1365-2796.1993.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 28.Murray HJ, Young MJ, Hollis S, et al. The Association Between Callus Formation, High Pressures and Neuropathy in Diabetic Foot Ulceration. Diabet Med 1996; 13: 979–982. doi:10.1002/(sici)1096-9136(199611)13:11<979::aid-dia267>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Alexiadou K, Doupis J. Management of Diabetic Foot Ulcers. Diabetes Ther 2012; 3: 4. doi:10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221087815 for Screening for identification of patients at high risk for diabetes-related foot ulcers: a cross-sectional study by Mohammed Al-Mohaithef, Sahar A Abdelmohsen, Magda Algameel and Amal Y Abdelwahed in Journal of International Medical Research


