Abstract
Liver involvement is not an uncommon extraintestinal manifestation of inflammatory bowel disease (IBD). IBD-associated liver diseases may have a variety of etiopathogenetic origins (including shared autoimmune pathogenesis, the effect of chronic inflammatory status, and adverse effects of drugs). Nevertheless, acute granulomatous hepatitis in the setting of Crohn’s disease (CD) is a rare clinical entity. It warrants, however, a careful assessment as both clinical and pathological features of Crohn’s-associated granulomatous hepatitis closely mimic extrapulmonary hepatic sarcoidosis, with considerable overlaps between the 2 diseases, which certainly makes a definitive diagnosis quite challenging. It is crucial to exclude infectious etiologies during the evaluation of acute granulomatous hepatitis, as inappropriate immunosuppressive treatment may cause a systemic flare-up of an underlying liver infection. We report a rare case of a 35-year-old female with a history of CD who presented with recurrent fevers, acute abdominal pain, and cholestasis. She was found to have acute hepatitis with noncaseating granulomas on liver biopsy. A comprehensive diagnostic workup did not ultimately prove a specific etiological culprit. The patient was treated with oral corticosteroids, and she demonstrated a positive clinical and laboratory response to the treatment. Our case highlights the diagnostic dilemma of acute granulomatous hepatitis in the setting of co-existent CD with a multisystemic syndrome. Granulomatous hepatitis represents a relatively rare manifestation of both extraintestinal CD and extrapulmonary sarcoidosis, with potential difficulties discriminating between the 2 entities on many occasions. The case also demonstrates the value of an interdisciplinary approach in the context of multisystemic disease to achieve the best outcome.
Keywords: acute granulomatous hepatitis, extrapulmonary hepatic sarcoidosis, Crohn’s disease, extraintestinal manifestations
Introduction
Liver involvement is not an uncommon extraintestinal manifestation of inflammatory bowel disease (IBD), 1 and abnormal liver biochemical tests are present in up to 30% of patients with IBD. 2 IBD-associated liver diseases may have a variety of etiopathogenetic origins that include shared autoimmune pathogenesis, the effect of chronic inflammatory status, and adverse effects of some drugs. Nevertheless, granulomatous hepatitis represents an exceedingly rare extraintestinal manifestation of Crohn’s disease (CD) that has been reported only in a handful of few cases in the current literature.3,4 We present a case of a 35-year-old female with a history of CD, who presented with sepsis-like syndrome and cholestasis, and she was found to have acute noncaseating granulomatous hepatitis on liver biopsy. Possible infectious and other autoimmune etiologies of hepatic granuloma were subsequently ruled out, and the patient was eventually treated with oral corticosteroids and demonstrated a positive clinical and laboratory response.
The differential diagnosis of granulomatous hepatitis is briefly reviewed in this report with special emphasis on the diagnostic conundrum of granulomatous hepatitis in CD patients, in the setting of the extreme clinical and pathological similarities between extraintestinal CD and extrapulmonary sarcoidosis, with potential diagnostic difficulties in discriminating between the 2 entities.
Case Presentation
A 35-year-old Caucasian female with a history CD presented to the emergency department with low-grade fever, chills, nonspecific abdominal pain, and nonbilious emesis. There were no associated cough, dysuria, or skin rashes. The rest of the systems review was insignificant. Past medical history was significant for CD status postright hemicolectomy in 2013 for fistulating terminal ileum disease, adhesion-related small bowel obstruction status postadhesiolysis in 2020. The patient had been off disease-modifying therapy for the last 5 years prior to her index admission. She also had generalized anxiety disorders and bipolar disorders, but she was not on any regular medications.
On examination, the patient appeared ill with a toxic appearance. She was tachycardiac to 126 beats per minute, hypotensive to 80/50 mmHg, and febrile to 38.8°C. Abdominal examination revealed diffuse tenderness without focal guarding or rigidity. The rest of the physical examination was unremarkable.
Complete blood count revealed low hemoglobin of 8.2 g/dl, leucopenic count (WCC) of 2.5 X 109/L, and platelets count of 76 X 109/l. Serum lactate was elevated (4.3 mmol/L) with raised C-reactive protein of 60.6 mg/l. Acute kidney injury was evident with a serum creatinine of 2.15 mg/dl (a normal baseline record was noted) with high serum calcium at 11.0 mg/dl. Liver function tests (LFTs) showed normal total bilirubin 0.7 mg/dl, slightly raised alanine transaminase (ALT) 66 U/l (normal ranges from 15-40), and abnormal alkaline phosphatase at 175 U/l (normal 45-115). Urinalysis was negative for leukocytes, nitrates, and blood, and the Chest X-rays were unremarkable.
The patient was admitted to the intensive care unit for presumed intra-abdominal sepsis secondary to acute cholecystitis or cholangitis and was treated with aggressive fluid resuscitation and empiric broad-spectrum antibiotics (vancomycin and piperacillin/tazobactam). A contrast-based computed tomography (CT) of the abdomen demonstrated mild gallbladder wall thickening with pericholecystic fluids but no calcified gallstones. There was no evidence of active Crohn’s flare-up. Magnetic resonance imaging (MRI) redemonstrated mild cholecystitis in the absence of gallbladder stones and a normal appearance of biliary ducts. The patient continued to spike fevers despite broad-spectrum antibiotics coverage, and she underwent an uneventful cholecystectomy for acalculous cholecystitis with a normal intraoperative cholangiogram. Histology of the removed gallbladder revealed mild nonspecific acalculous cholecystitis. However, postcholecystectomy Day 4; the liver biochemistry worsened significantly; ALT up trended to 240 U/L (6-times baseline levels), ALP rose to 200 U/L, and total bilirubin increased to 6.2 mg/dl with predominantly direct component (5.0 mg/dl). Imaging of the abdomen with contrast-based CT scan and MRI depicted normal postoperative changes with no collections and normal biliary ducts. An endoscopic retrograde cholangiopancreatography (ERCP) confirmed clearance of biliary ducts.
Viral serologies, hepatitis A/B/C, Epstein-Barr virus (EBV), cytomegalovirus (CMV), and human immunodeficiency virus (HIV) were nonreactive. The autoimmune screening was negative (including antinuclear antibodies, antimitochondrial antibodies, anti-smooth muscle antibodies, anti-myeloperoxidase antibodies, and serine protease 3). Total IgG, IgM, and IgA levels were within normal limits. Transferrin saturation, ferritin, and ceruloplasmin levels were all within the normal limits. A careful medication review didn’t reveal any known hepatotoxic medication.
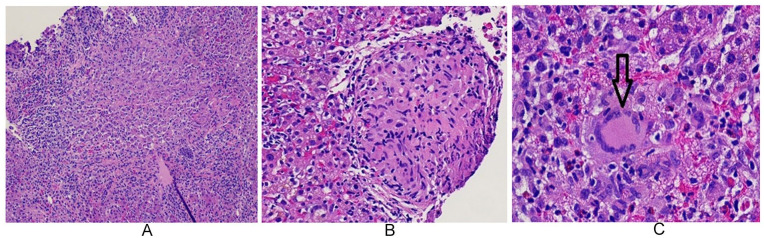
Hepatology consultation was obtained given unexplained persistent elevation in LFTs despite negative serial laboratory workup and extensive postoperative imaging. A liver biopsy was advised by the hepatology service, which revealed marked acute hepatitis, focal necrosis with noncaseating granulomas within the portal triads, and the presence of giant cells (Figure 1A-1C). No histopathological features to prove primary biliary cirrhosis, primary sclerosing cholangitis, or autoimmune hepatitis were demonstrated on revisiting the liver biopsy at a tertiary hepatobiliary center. The differential diagnosis for acute hepatic noncaseating granulomatous inflammation included infections (viral, fungal, and atypical bacterial infections), autoimmune disorders, and drug reactions.
Figure 1.
Liver biopsy (low, medium, and high magnifications) showing marked acute inflammation changes and non-caseating granulomas within portal triads (A, B and C). Vertical arrow in 1C demonstrating multi-nucleated giant cells typical of granulomatous inflammation.
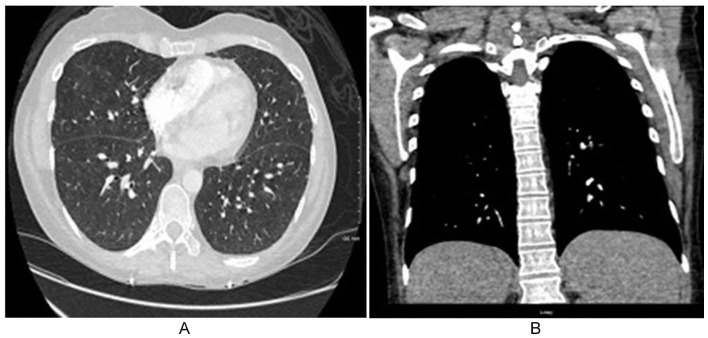
Infectious workup was recommended to exclude an underlying cause for the granulomas. Acid-alcohol fast bacilli (AAFP) and Giemsa fungal staining of the liver biopsy were consecutively negative. QuantiFERON gold assay, Coccidiosis, Brucella and Bartonella, and Coxiella (Q-fever) screening serology returned negative, same as urine screening for Histoplasma antigens. Hypercalcemia was rapidly responsive to initial aggressive fluid therapy, and renal function gradually normalized. Parathyroid hormones (PTH) were slightly below normal (<6 U/L). PTH-related peptide, Vitamin D3 1,25-OH, and angiotensin-/ (ACE) levels were within normal ranges. A Chest CT scan did not reveal pulmonary sarcoidosis or other significant abnormalities apart from small nonspecific ground-glass pulmonary nodules without associated hilar or mediastinal lymphadenopathy (Figure 2A and 2B).
Figure 2.
Axial and coronal computed tomography (CT) scan of the chest demonstrating scattered small-sized bilateral lung nodules in the setting of sepsis-like multi-organ syndrome and cholestasis (A and B).
Discontinuation of antibiotics treatment on Day 14 was recommended by the infectious disease team, with a plan of re-institution if the patient re-spikes fevers during cautious clinical monitoring, as serial results from all potential infectious sources were repeatedly negative, and the patient’s recurrent fevers were deemed to represent a systemic manifestation of an autoimmune process. Interdisciplinary discussions concluded that hepatic granulomas possibly represent either an extraintestinal manifestation of CD or extrapulmonary sarcoidosis in the setting of the multisystemic clinical syndrome given the fact that the diagnostic workup for infectious, other autoimmune, and vasculitis etiologies proved no definitive cause. Oral steroids were instituted as per Rheumatology consultation. The patient was discharged on a prednisone taper to complete an 8-week course of steroids. Over a 2-month serial follow-up, the patient remained clinically well, and a remarkable normalization of ALT (to 70 U/l) and ALP (to 69 U/l) was observed over the same follow-up period.
Discussion
A granuloma is a circumscribed lesion resulting from a specific entity of chronic inflammatory reactions characterized by a central accumulation of mononuclear cells, primarily macrophages, with a surrounding peripheral rim consisting of lymphocytes and fibroblasts. 5 Histologically, there are 4 variants of hepatic granulomas. Noncaseating granulomas are often seen in sarcoidosis, caseating granulomas in tuberculosis, fibrin-ring variants in some infections, as well as vasculitis, and lastly, lipogranulomas variants in the context of ingested mineral oil. 5 Activated macrophages release cytokines that may cause systemic symptoms (fever, anorexia, night sweats) or direct hepatic injury. 6
Clinically, hepatic granulomas are associated with a myriad of disorders. 5 The most frequent causes in the United States are sarcoidosis, tuberculosis, primary biliary cirrhosis, and drug reactions, which collectively account for approximately 50% to 75% of all cases. 6 Hence, a careful approach should be considered during evaluating acute hepatic granulomatous inflammation, as it could be the only clue to a multisystemic disease. 4 It is also critical to exclude any infectious etiology of granulomatous hepatitis, as inappropriate steroids or immunosuppressive therapy may cause systemic dissemination of an underlying liver infection.4,5
Granulomatous hepatitis is a rare association with IBD,3,7 with a prevalence of less than 1%. It has been observed more frequently with CD. 7 It could also be related to the adverse effects of drugs like mesalamine and sulfasalazine. 7 Notably, our patient was not on any medications for CD. Acute granulomatous hepatitis usually presents with a triad of fever, hepatomegaly, and elevated alkaline phosphatase.4,7,8 The clinical course is typically unrelated to Crohn’s intestinal disease activity, in keeping with the presentation of this reported patient. 4
As per our literature review, we identified only 3 cases of acute granulomatous hepatitis in patients with CD that resembled our case’s clinical presentation as sepsis-like syndrome with cholestasis and posed a diagnostic challenge.4,8,9 Two of these cases reported by Patedakis Litvinov et al, 4 and Kahana et al 8 shared a further common finding with ours, as acalculous cholecystitis was the presumed sepsis source on the initial evaluation. However, in the case described by Kahana et al, granulomatous inflammation was present in both the liver and gallbladder in disagreement with our patient, whereas granulomatous inflammation was confined to only the liver with nonspecific inflammatory changes of the gallbladder. In fact, acalculous cholecystitis has been reported as a long-standing complication of CD due to possible associated gallbladder motility dysfunction. 8
Interestingly, the patient described by Patedakis Litvinov et al represents an extreme mimicker of ours in many folds. The multisystemic nature of the clinical syndrome, presence of pulmonary nodules and hypercalcemia, the uncertainty of the definitive etiology that would equivocally represent an extraintestinal phenomenon of CD or hepatic sarcoidosis in sitting of extrapulmonary disease, and the demonstrable clinical and laboratory response to steroids treatment. 4
The pulmonary nodules noted in our patient and the similar one reported by Patedakis Litvinov et al 4 are less likely to be considered as pulmonary sarcoidosis given the lack of typical radiological features of the latter. Very rarely, these lung nodules may represent necrobiotic nodules, an unusual pulmonary manifestation of extraintestinal CD. 10 Furthermore, histopathological findings of hepatic sarcoidosis that encompass periportal fibrosis and chronic intrahepatic cholestasis are not pathognomonic for sarcoidosis and can also be present in CD-associated granulomatous hepatitis,4,5 thus making a remarkable distinction between the 2 entities quite challenging.4,11
The co-occurrence of sarcoidosis and CD is a rare phenomenon, though it has been described in the available literature.12,13 Therefore, the possibility of concurrent sarcoidosis cannot be entirely excluded in our patient, although it may be very much unlikely. In fact, the shared pathogenetic theory of co-existence of the 2 diseases may be supported by a few observations that include, for instance, co-occurrence of sarcoidosis and CD among different members of the same family, the shared response of the 2 diseases to anti-tissue necrosis factor (TNF) therapy, and evidence of some identified genetic polymorphism that is common to both diseases. 4
The mainstay therapy for granulomatous hepatitis, after exclusion of other infectious etiologies, is systemic corticosteroids. Relapses may occur after corticosteroid discontinuation, necessitating repeated courses. 4 Second-line therapy for steroid-resistant cases includes immunomodulators (azathioprine and methotrexate) and anti-TNFα. 14 Surprisingly, some reports reported paradoxical granulomatous hepatitis and extrapulmonary sarcoidosis in patients with CD who were treated with anti-TNFα. 15
Conclusion
The occurrence of acute granulomatous hepatitis in a CD patient with multi-organ syndrome can pose a diagnostic dilemma, as demonstrated in our case. Extraintestinal CD may strongly resemble extrapulmonary sarcoidosis with significant clinicopathological similarities between the 2 diseases. Exclusion of infectious etiologies of acute granulomatous hepatitis is crucial, as inappropriate steroids therapy may provoke systemic dissemination of an underlying liver infection.
Footnotes
Authors Contributions: The authors contributed equally to the conceptualization and designing of the report, writing first manuscript, and critical review. All authors reviewed the last draft and agreed for submission.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Abdalaziz Awadelkarim  https://orcid.org/0000-0002-8366-4114
https://orcid.org/0000-0002-8366-4114
References
- 1. Uko V, Thangada S, Radhakrishnan K. Liver disorders in inflammatory bowel disease. Gastroenterol Res Pract. 2012;2012:642923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ozaras R, Tahan V, Mert A, et al. The prevalence of hepatic granulomas in chronic hepatitis C. J Clin Gastroenterol. 2004;38:449-452. [DOI] [PubMed] [Google Scholar]
- 3. Janiak M, Jablonska A, Skrobot K, Perdyan A, Pieńkowska J, Adrych K. Hepatic granulomas as an extraintestinal manifestation of Crohn’s disease. Pol Arch Intern Med. 2020;130:240-241. [DOI] [PubMed] [Google Scholar]
- 4. Patedakis Litvinov BI, Pathak AP. Granulomatous hepatitis in a patient with Crohn’s disease and cholestasis. BMJ Case Rep. 2017;2017:bcr-2017-220988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drebber U, Kasper HU, Ratering J, et al. Hepatic granulomas: histological and molecular pathological approach to differential diagnosis–a study of 442 cases. Liver Int. 2008;28:828-834. [DOI] [PubMed] [Google Scholar]
- 6. Culver EL, Watkins J, Westbrook RH. Granulomas of the liver. Clin Liver Dis (Hoboken). 2016;7:92-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rojas-Feria M, Castro M, Suarez E, Ampuero J, Romero-Gómez M. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol. 2013;19:7327-7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahana D, Jiyamapa J, Vasinrapee P, French S, Gershman G. P-0011: granulomatous hepatitis and acalculus cholecystitis in an adolescent male with newly diagnosed Crohn’s disease: a case report. Inflamm Bowel Dis. 2009;15:S7-S8. [Google Scholar]
- 9. Hilzenrat N, Lamoureux E, Sherker A, Cohen A. Cholestasis in Crohn’s disease: a diagnostic challenge. Can J Gastroenterol. 1997;11:35-37. [DOI] [PubMed] [Google Scholar]
- 10. Garg C, Shrimanker I, Goel S, Mclaughlin J, Nookala V. Extraintestinal manifestations of Crohn’s disease in the form of pulmonary nodules: a case report. Cureus. 2020;12:e7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devaney K, Goodman ZD, Epstein MS, Zimmerman HJ, Ishak KG. Hepatic sarcoidosis. Clinicopathologic features in 100 patients. Am J Surg Pathol. 1993;17:1272-1280. [PubMed] [Google Scholar]
- 12. Fries W, Grassi SA, Leone L, et al. Association between inflammatory bowel disease and sarcoidosis. Report of two cases and review of the literature. Scand J Gastroenterol. 1995;30:1221-1223. [DOI] [PubMed] [Google Scholar]
- 13. McCormick PA, O’Donoghue DP, FitzGerald MX. Crohn’s colitis and sarcoidosis. Postgrad Med J. 1986;62:951-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kennedy PT, Zakaria N, Modawi SB, et al. Natural history of hepatic sarcoidosis and its response to treatment. Eur J Gastroenterol Hepatol. 2006;18:721-726. [DOI] [PubMed] [Google Scholar]
- 15. Decock A, Van Assche G, Vermeire S, et al. Sarcoidosis-like lesions: another paradoxical reaction to anti-TNF therapy? J Crohns Colitis. 2017;11:378-383. [DOI] [PubMed] [Google Scholar]