Abstract
How animals sense the geomagnetic field remains a mystery today. A remarkable diversity has been revealed in animal magnetoreception and several sophisticated models have been put forward in the past few decades, but none have been commonly accepted yet. Cryptochrome (Cry) has been proposed in both the radical pair model and the MagR/Cry-based biocompass model. How exactly it participates in magnetic sensing is an ongoing discussion. Here we wish to suggest an intermolecular electron transport (ET) pathway conserved in evolution in the MagR/Cry complex, in which electrons travel stepwise along a flavin-tryptophan chain as described in the classic radical pair model, and further extends to iron-sulfur clusters in MagR via a series of stepping-stone amino acids as an ET bridge. The hypothesis we presented here may provide a solution to unite different models, and a feasible explanation for the intrinsic magnetic features of MagR, as well as a mechanism for signaling in animal magnetoreception, which are of considerable interest in both biology and physics.
Keywords: animal magnetoreception, radical pair model, biocompass model, intermolecular electron transport, conserved in evolution
The world as we know is perceived by senses, and we share most of our senses with other animals. However, a number of animals can perceive stimuli well beyond what we can. Magnetic sense, or magnetoreception, is intangible to us but is fundamentally important to many species that rely on the geomagnetic field to navigate thousands of miles. A long journey started from more than one and a half centuries ago in search of the underlying molecular mechanism of how animals perceive the geomagnetic field. Despite that significant progress has been made recently, magnetoreception still remains the least understood sense, which forces us to rethink what we know and what we don’t.
A search for unity in diversity
A remarkable diversity has been revealed in animal magnetoreception: the magnetic sense is phylogenetically widespread among animals, different geomagnetic cues including inclination, polarity, or even intensity can be perceived by different organisms, and animals respond to and utilize the geomagnetic field in different ways, including alignment and navigation. Correspondingly, several sophisticated mechanisms including the magnetite-based model, the radical pair model, and the recently suggested biocompass model, have been put forward in the past few decades in an attempt to resolve the mystery of animal magnetoreception from different perspectives.
Magnetite (Fe3O4 or iron (II, III) oxide) is the most common magnetic mineral found on Earth and has been “identified” in magnetotactic bacteria (MTB) and nearly every animal investigated.1, 2, 3 It is physically viable as a type of magnetic polarity and intensity sensor, although the biological relevance has never been established and potential contamination is hard to be excluded in experiments. The radical pair model was pioneered by Klaus Schulten in 1978, and was suggested as an inclination compass that could not distinguish between the magnetic North and South. It describes a quantum mechanical explanation on how an external magnetic field can alter chemical yields by interacting with the spin state of a pair of radicals , which is photochemically formed in a class of flavoproteins called cryptochromes (Cry) and stepwise transfer along a flavin-tryptophan chain stretches approximately 25 Å.4 While some animals navigate by the lines of the geomagnetic field with either polarity or inclination compasses, a few species of animals do use a combination of polarity and inclination as guidance cues for navigation. The recently proposed MagR/Cry-based biocompass model combines the concepts of ferrimagnetism and the involvement of Cry in magnetoreception to provide a potentially all-in-one solution.5 In this model, MagR, a highly conserved A-type iron-sulfur protein originally named IscA1, has been identified to interact with Cry in almost every animal species. It forms a 24 × 15-nm rod-like polymeric complex with Cry and exhibits an intrinsic magnetic moment of roughly 0.09 to 0.1 μB/f.u. in vitro. The MagR/Cry complex, therefore, may act as a biological compass to retrieve information from the geomagnetic field, such as polarity (as with a conventional compass), intensity, and inclination in a light- and magneto-coupled manner.5 However, lacking detailed mechanism of the magnetic moment of the MagR and MagR/Cry complex puts it under lively debate since the beginning. Regardless, several physical explanations have been proposed for the biocompass model, and various applications have been explored owing to the distinct magnetic property of MagR. All three models have accumulated credible theoretical and experimental evidence, but none have been widely accepted yet.
The great diversity of animal magnetoreception is unified by a common pattern: the geomagnetic field has existed for at least 3.5 billion years, and thus magnetoreception seems an unavoidable step for animal evolution and speciation. Crucial questions are then raised: How has magnetoreception evolved in the animal kingdom? The widespread distribution of organisms with magnetic sense suggests that this sensory modality evolved prior to the evolutionary radiations of the animal phyla.3 It is likely that phylogenetically distant animals may share a common origin and utilize a universal receptor to sense the geomagnetic fields and retrieve different guidance cues, but may interpret them differently to adapt to different environments.
An electron transport pathway conserved in evolution
In evolution, new functions were never built from scratch, but from pre-existing parts. Animal magnetoreception is certainly not an exception. The origin of this sensory modality remains a fundamental question in evolutionary biology. Magnetite biosynthesis by MTB may represent the earliest form of biogenic magnetic sensors on Earth. Could this iron-based system have later been carried-over and co-opted, either by direct symbiosis of MTB or by incorporation of MTB biomineralization genes into the eukaryotic genome as a magnetic sensor for animals? This possibility has been much discussed in detail widely in the literature,6,7 but relatively little work has been done regarding the origin and the connection of the radical pair model and the biocompass model. Two current protein magnetoreceptor candidates, MagR and Cry, were both evolutionarily ancient proteins participating in various essential biological processes. They have evolved over billions of years into specialized structures capable of diverse reactions. The original known function of MagR includes iron delivery, redox sensory, and electron transport in respiration, photosynthesis, nitrogen fixation, and DNA replication and repair, whereas Cry was widely known for playing essential roles in photomorphogenesis, photoreception, and maintenance of circadian rhythms, before their roles were proposed in animal magnetosensing.
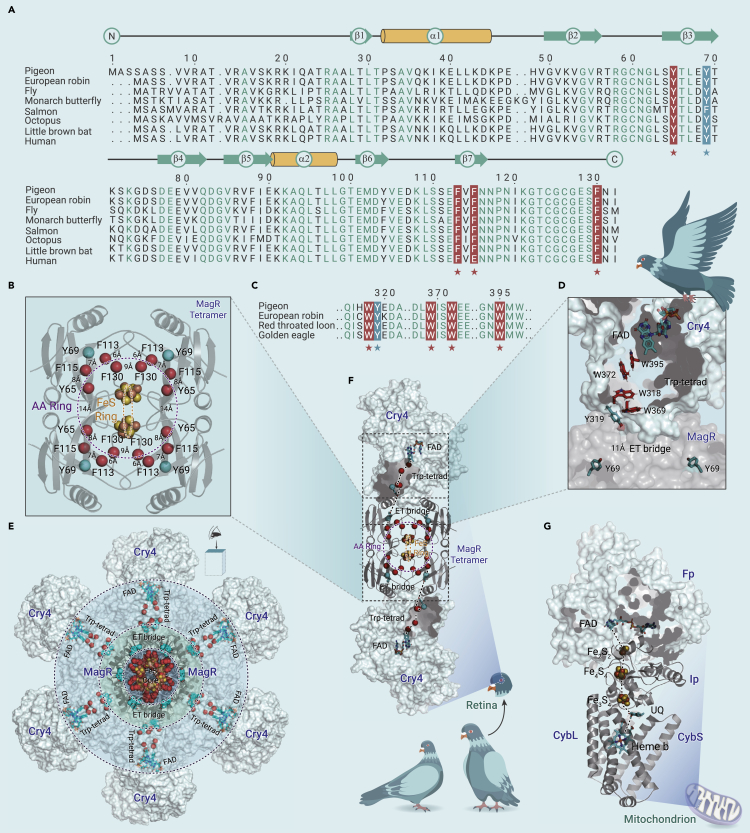
We traced the evolutionary origin of these magnetoreceptor candidates by analyzing the sequence and structure conservation. The amino acid sequences of MagR from over 130 species across all major phyla and the distribution of extremely conserved residues in the structural model were inspected. Some striking features of MagR emerged: four aromatic residues capable of electron transport (Y65, F113, F115, and F130 in the case of pigeon sequence) are 100% conserved during the evolution of MagR (Figure 1A). The location of these residues and the distance in between were marked on the structural model of the pigeon (Columba livia) MagR (clMagR) tetramer (Figure 1B). The ring-like arrangement of these 16 residues on the MagR tetramer structure, with distances in the physiological electron tunneling range from 6 Å to 14 Å between each two neighboring residues, indicates a possible role in the intermolecular electron transport within the disk-like MagR tetramer (Figure 1B, shown as a purple dotted circle and labeled as the “AA Ring”), based on the Marcus theory of electron transfer. Interestingly, this putative “electron transport ring” is perpendicular to the previously proposed “iron loop” formed by four Fe-S clusters5 (Figure 1B, shown as orange dotted circle and labeled as “FeS Ring”). Two additional residues (Y69 and Y104) are highly conserved, and Y69 is located closely to the interface between Cry and MagR tetramer, which may contribute to the relay of electrons to/from Cry (Figures 1D–1F).
Figure 1.
Sequence conservation and structural features of MagR and Cry revealed an intermolecular electron transport (ET) pathway to bridge different models of animal magnetoreception
(A) Sequence alignment of MagR in eight representative species. Secondary structures are shown in the upper lines. Conserved residues with ET properties are shown in the red and cyan background, indicated by stars. Other conserved residues are colored green.
(B) Structural model of a MagR tetramer. Two circles schematically illustrate the ET chain and spheres represent related residues. Fe-S clusters are shown as spheres and colored with orange (Fe) and yellow (S).
(C) Sequence alignment of Cry4 in four avian species showing the conserved Trp-tetrad (in red background) and the bridge residue Y319 (in cyan background).
(D) Structural model of MagR/Cry4 interface showing the FAD, Trp-tetrad residues, and the ET bridge formed by Y319 in Cry4 and Y69 in MagR.
(E) Multilayered architecture of MagR/Cry4 complex polymer (top view).
(F) Suggested intermolecular ET chain (shown as dotted lines) within the MagR/Cry complex.
(G) The structural model of mitochondrial respiratory complex II reveals a similar scheme for ET from FAD to a series of iron-sulfur clusters. Details are explained in the text.
Cry has been proposed in both the radical pair model and the MagR/Cry biocompass model.5,8 How exactly it participates in magnetoreception is an ongoing discussion. We previously suggested a possible electron transfer between MagR and Cry5; however, this likelihood was later challenged by Friis et al. because of the extraordinarily large edge-to-edge distance from flavin in Cry to iron-sulfur clusters within MagR.9
Essential knowledge regarding the partnership between MagR and Cry is still missing, which has hampered the effort to reveal the functional roles of Cry in both the radical pair model and the biocompass model. In a most recent study, we and collaborators suggested that Y319 in robin Cry4 could act as a stepping stone, which extends the electron transport chain from FAD and the Trp-tetrad (W395, W372, W318, and W369) farther to the protein surface,4 and allows the Trp-tetrad in the radical pair model to accept an electron from external partners, potentially MagR. Sequence alignment of Cry4 from several avian species (Figure 1C) and careful structural analysis of the MagR/Cry4 interface revealed that this conserved Y319 in Cry4, as well as Y69 in MagR, could form an electron transport bridge (ET bridge) to connect two proteins (Figures 1D–1F).
Conclusions and future prospects
Taken together, some striking features of both MagR and Cry4 emerged in this study, including a putative “electron transport ring” formed by conserved amino acids (AA Ring), an “iron loop” formed by iron-sulfur clusters (FeS Ring) in the MagR, and an “ET bridge” formed by two bridge residues (Y69 in MagR and Y319 in Cry4) located in the MagR/Cry4 interface. These findings justified the distance requirement of intermolecular electron transfer and highlighted a possible electron transport pathway in the MagR/Cry complex, which had not been previously revealed. In this picture, electron from flavin (FADH/FAD) in Cry4 travels through four conserved tryptophans (Trp-tetrad) as described in the radical pair model, and then along an “ET bridge” formed by two conserved tyrosines and a possible “AA Ring” to iron-sulfur clusters (FeS Ring or FeS Core) in the MagR tetramer (Figures 1D–1F). Flavin reduction and oxidation in Cry, two forms of iron-sulfur clusters binding in MagR10 and the existence of another iron-sulfur partner to oxidize or reduce MagR in vivo, could generate a potential difference inside of the MagR tetramer and in between the MagR/Cry complex to drive the electron transfer along the chain. Magnetic simulations based on this conceptual idea have been performed with an electron rate similar to that of respiratory systems and photosystems, and a maximum likely estimation of the instantaneous magnetic field can theoretically reach 0.1 to 0.6 Gauss (data not shown), providing insight into the origin of the magnetic moment of MagR, which is in agreement with the mechanism we proposed here. Further investigation and experimental validation are certainly required. The magnetic measurement of MagR/Cry mutants to abolish the ET pathway at an exceptional resolution and sensitivity in physiological environment could verify the origin of magnetism, and the detailed ET pathway and how it affects magnetic sensing in the MagR/Cry complex can be examined with well-developed time-resolved experimental electron spin resonance and nuclear magnetic resonance techniques.
It is important to point out that electron transfer both to and from MagR in this complex is possible, considering FADH can be fully oxidized as FAD to accept electrons from the Trp-tetrad in the radical pair mechanism.8,9 Theoretically, the intermolecular electron transfer between MagR and Cry4 could contribute to the magnetism of MagR and also to the modulation of the radical pair-based magnetoreceptive properties of Cry.
The ever-increasing desire to understand the fundamental principle of animal magnetoreception requires scientists to think both in physics and in biology, but not solely in physics or in biology. Previously with the identification of MagR and the MagR/Cry complex, we stated that “As complicated as biological systems are, they certainly obey and often make ingenious use of physical principles.”5 Several remarkable discoveries have been made to elucidate the quantum mechanism in the Cry-based radical pair model, but the connection between quantum mechanics and biological signaling has not been established yet, and a mechanism to understand coherent spin dynamics is currently not available in biological systems. With this perspective, we would like to provide a solution to bridge quantum chemistry and traditional biology in animal magnetoreception, and emphasize that as fascinating as quantum mechanics is, it certainly has to be understood by biological organisms.
In closing, we note that the MagR/Cry complex and the possible intermolecular electron transfer proposed here echo a similar electron transport pathway as in several biological processes, such as mitochondrial respiratory Complex II (Figure 1G), emphasizing that a similar architecture of the electron transport chain could be adopted in distant biological systems.
Acknowledgments
The author is deeply indebted to Dr. Aihua Xie, Dr. Peter Hore, Dr. Bing-Wu Wang, Dr. Qiu-Yun Tan, Dr. Kedong Wang, Dr. Maojun Yang, Dr. Haiguang Liu, and Dr. Tiantian Cai for critical discussions. The sequence alignments in this paper were completed by Dr. Tiantian Cai. Special thanks to Arden Xie for English editing, and to Haiguang Liu, Tiantian Cai, and Jingjing Xu for comments on the manuscript. The first draft of this manuscript and the initial model were finished at the Yu-Ni Resort in Jingmai Mountain, China, in February 2019, when and where the idea was inspired. The paper was thoroughly rewritten in Science Island, China, in December 2021, to represent the most recent progress with a refined model.
Declaration of interests
The author declares no competing interests.
Published Online: March 11, 2022
References
- 1.Li J., Liu P., Wang J., et al. Magnetotaxis as an adaptation to enable bacterial shuttling of microbial sulfur and sulfur cycling across aquatic oxic-anoxic interfaces. J. Geophys. Res. Biogeosci. 2020;125 e2020JG006012. [Google Scholar]
- 2.Johnsen S., Lohmann K.J. Magnetoreception in animals feature article. Phys. Today. 2008;61:29–35. [Google Scholar]
- 3.Kirschvink J.L., Walker M.M., Diebel C.E. Magnetite-based magnetoreception. Curr. Opin. Neurobiol. 2001;11:462–467. doi: 10.1016/s0959-4388(00)00235-x. [DOI] [PubMed] [Google Scholar]
- 4.Xu J., Jarocha L.E., Zollitsch T., et al. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature. 2021;594:535–540. doi: 10.1038/s41586-021-03618-9. [DOI] [PubMed] [Google Scholar]
- 5.Qin S., Yin H., Yang C., et al. A magnetic protein biocompass. Nat. Mater. 2016;15:217–226. doi: 10.1038/nmat4484. [DOI] [PubMed] [Google Scholar]
- 6.Natan E., Fitak R.R., Werber Y., Vortman Y. Symbiotic magnetic sensing: raising evidence and beyond. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020;375:20190595. doi: 10.1098/rstb.2019.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellinger M.R., Wei J., Hartmann U., et al. Conservation of magnetite biomineralization genes in all domains of life and implications for magnetic sensing. Proc. Natl. Acad. Sci. U S A. 2022;119 doi: 10.1073/pnas.2108655119. e2108655119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hore P.J., Mouritsen H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016;45:299–344. doi: 10.1146/annurev-biophys-032116-094545. [DOI] [PubMed] [Google Scholar]
- 9.Friis I., Sjulstok E., Solov’yov I.A. Computational reconstruction reveals a candidate magnetic biocompass to be likely irrelevant for magnetoreception. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-13258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Z., Xu S., Chen X., et al. Modulation of MagR magnetic properties via iron–sulfur cluster binding. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-03344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]