Abstract
Background
Levosimendan can improve clinical symptoms and the cardiorenal rescue success rate, and stabilize hemodynamic parameters in individuals suffering from acute decompensated heart failure. In addition, Shenfu injection (SFI) has been shown to protect the ischemic heart and enhance myocardial contractility.
Methods
For this randomized control single-blind study, 101 patients with acute decompensated heart failure (ADHF) were enrolled and randomly assigned to control levosimendan (n = 51) and levosimendan + SFI injection (n = 50) groups. Attending physicians were not blinded for which arm the patients were allocated. Blood pressure, heart rate, the electrocardiogram, respiratory rate, fluid intake and urine output were all recorded 2 h and 24 h after drug infusions had commenced, and the cardiac index (CI) was monitored by ultrasonic cardiac output monitors.
Results
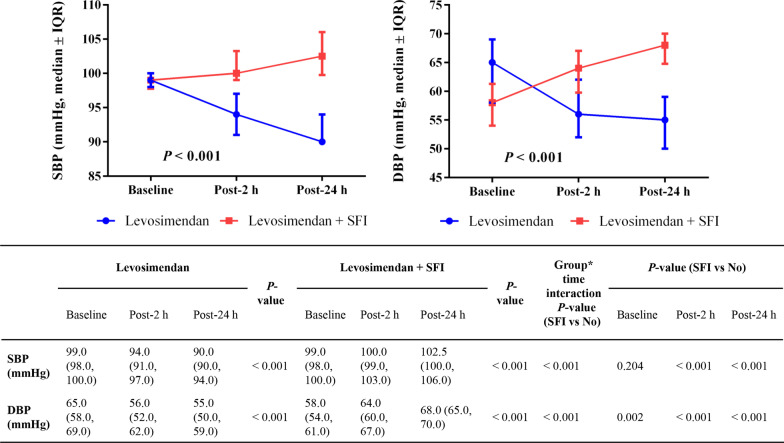
Median blood pressure was markedly increased in the levosimendan + SFI group after 2 h and 24 h from the initiation of infusions compared to levosimendan administration alone. Brain natriuretic peptide (BNP) concentrations were reduced after administrations of levosimendan + SFI or solely levosimendan (both P < 0.001). Alterations in BNP concentrations were not different in the combination and control groups. No differences were found between the 2 groups in heart rate or severe hypotension, but blood pressure (systolic blood pressure, diastolic blood pressure) and hemodynamic parameters including CI, cardiac output and stroke volume index responded better in the levosimendan + SFI group compared to the monotherapy levosimendan group.
Conclusions
Levosimendan + SFI was superior to treat ADHF patients compared to levosimendan monotherapy and produced significant improvements in hemodynamic parameters especially for ADHF patients with hypotension.
Trail registration The study was prospectively registered at Chinese Clinical Trial Registry with registration number [ChiCTR2000039385] (10/25/2020).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-022-02572-2.
Keywords: Acute decompensated heart failure, Brain natriuretic peptide, Cardiorenal rescue, Hemodynamic, Levosimendan, Shenfu injection
Background
Since 2000, levosimendan has been used to treat patients with acute decompensated heart failure (ADHF), because it produces safe hemodynamic stabilization [1]. Levosimendan promotes inotropy by sensitizing cardiac muscle troponin C (cTnC) to Ca2+ [2, 3]. In addition, it causes vasodilatation by activating ATP-dependent-K+ channels on smooth muscle. In a number of clinical trials, it was found that levosimendan elicited fewer adverse effects compared to other inotropic and vasoactive drugs; it is noteworthy that it still produced hypotension. One expert consensus report suggested the use of levosimendan in patients with pulmonary congestion. It is preferred to adrenoceptor agonists as first-line therapy for acute heart failure with acute coronary syndrome (ACS-AHF) patients on β-blocker medication, with unsatisfactory urine outputs after the administration of diuretics. It can be given as sole therapy or together with other inotropic or vasopressor drugs. However, patients should be closely monitored because of the risk of hypotension [4]. Lochner et al. [5] also reported that levosimendan combined with β-blockers or adrenergic inotropes did not inhibit the actions of levosimendan alone.
In patients with ADHF, levosimendan has been shown to significantly increase cardiac output (CO) and stroke and decrease pulmonary capillary wedge pressure (PCWP), mean blood, pulmonary artery and mean right atrial pressures, and total peripheral resistance [6]. For patients whose systolic blood pressure (SBP) is < 90 mmHg with hypoperfusion symptoms, it is necessary to raise CO, blood pressure and peripheral perfusion, to safeguard the functions of vital organs [7]. For acute heart failure (AHF) patients with low-output states, a drug that augments CO and increases the degree of vasodilatation would be expected to have better efficacy than one that augments CO alone.
Chemical analysis has shown that Shenfu injection mainly contains ginsenoside and higenamine [8, 9]. A Shenfu injection can increase arterial oxygen partial pressure and oxygen saturation [10, 11], increase hypoxia tolerance and anti-stress ability [12], reduce peripheral resistance and improve the microcirculation [13], increase myocardial contractility and cardiac output [14], dilate peripheral blood vessels [15], and improve the hypoxia-ischemic state of tissues and organs [16]. Ginsenosides can increase myocardial contractility, reduce small vascular resistance and increase cardiac, cerebral and renal perfusion [17]. Higenamine can not only improve myocardial contractility, but also dilate blood vessels and reduce cardiac load [18]. In the presence of ginsenosides, higenamine retains positive inotropic effects without positive frequency actions and thereby does not increase myocardial oxygen consumption [19]. In recent years, a number of clinical trials have shown that the Chinese patent medicine, Shenfu injection (SFI), greatly improved the symptoms of heart failure (HF) [7–9]. The mechanisms involved in the actions of SFI include a significant reduction in taurine, glutathione and phospholipids concentrations. This was shown in an ischemic heart failure rat model, when the distribution of these molecules in the non-infarct zone was markedly altered [20]. In clinical trials, SFI not only improved CO but also the vasodilator dimension [14, 21].
Therefore, in the present study, we hypothesized that levosimendan combined with SFI would improve cardiac functions without producing hypotension and improve the symptoms of patients with both ADHF and hypotension.
Methods
The study involved a cohort of 101 patients suffering from ADHF from 2019.12 to 2021.6 They were diagnosed according to the 2018 Chinese guidelines [22] and randomly assigned into control (levosimendan + placebo) (n = 51) and study (levosimendan + SFI) (n = 50) groups. Inclusion criteria were based on the New York Heart Association grading guidelines [22] and were: patients had grade III or IV; an left ventricular ejection fraction (LVEF) ≤ 40%; and a brain natriuretic peptide (BNP) level > 400 pg/mL. Some patients were also diagnosed with low cardiac output syndrome (LCOS). All enrolled patients were not allergic to traditional Chinese herbal medicines.
The study followed the Declaration of Helsinki principles and was approved by the Institutional Review Board of Tongren Hospital affiliated to Shanghai Jiao Tong University School of Medicine. All enrolled patients provided informed consent. The registered trial number was ChiCTR2000039385.
The exclusion criteria for patients were: of childbearing potential; HF due to restrictive or hypertrophic cardiomyopathy or stenotic valvular disease that was uncorrected; had acute myocardial infarction 14 days prior to the study or had refractory angina; sustained ventricular arrhythmia; severe liver and/or kidney insufficiency; severe infection; malignant tumor; systemic immune disease; those who would not cooperate with treatment; withdrawal from the study; or death.
Study procedure
The enrolled patients were randomized into 2 groups using randomization numbers generated by SPSS software. One group received a levosimendan infusion of 12 µg/kg in 0.9% sodium chloride and a placebo (5% GS 350 mL) and the other group received the same levosimendan infusion plus SFI (100 mL + 5% GS 250 mL). There were digital labels coded 1–100, enrolled patients blindly extracted labels, the patients with odd number were assigned into the levosimendan group, while patients with even numbers were assigned into the levosimendan + SFI group. The labels were discarded after each extraction, and newly enrolled patients extracted from the remaining labels, but attending physicians were not blinded for which arm the patient was allocated. This study enrolled 101 patients, the last patient extracted a label from the new digital labels which were coded 1–100 again.
Constant rate infusion of levosimendan was maintained for 24 h, unless the patient had a serious cardiovascular event, dose-limiting adverse events (AEs) or serious adverse events (SAEs), or required i.v. inotropic or vasodilator agents as rescue therapy. Standard clinical parameters included the electrocardiogram, blood pressure, heart and respiratory rates, fluid intake, output of urine were measured and recorded after 24 h infusion. The cardiac index (CI) was measured using ultrasonic cardiac output monitors (USCOM), which is a non-invasive CO monitor that employs transaortic or transpulmonary doppler flow tracing and valve area estimated using patient height to determine CO. The probe of USCOM was placed in the sternum or supraclavicular fossa to obtain the strongest signal. Three consecutive measurements were made with a deviation of no more than 10% each time, and the average CI was taken.
Assessments
Patients were evaluated at baseline (before treatment) and during treatment for variables including their medical histories, physical examinations, echocardiography and laboratory blood tests. The concentrations of BNP in plasma were measured again at 24 h after initiation of drug administration. AEs were evaluated and recorded by clinicians for 24 h.
Endpoints
The primary endpoint was the change in blood pressure (incidence of significant hypotension) when treatment was clinically effective 2 and 24 h after initiation of drug administration. For each measured variable, improvement was defined as a reduction in ≥ 1 grade from the baseline value.
Secondary endpoints included a decrease in the serum concentration of BNP from baseline and 24 h after the start of the infusion. In addition, heart rate (HR), CO, CI, stroke volume index (SVI) and systemic vascular resistance index (SVRI) parameters were evaluated 2 h and 24 h after initiation of drug administration and compared to baseline values.
Statistical analysis
SAS ver. 9.2 was utilized for all estimations of sample sizes and analyses. Normally distributed continuous variables were analyzed using Student’s t-test or ANOVA with the Kruskal–Wallis test for significant differences between them and expressed as mean ± SD, while abnormally distributed continuous variables were analyzed using the Mann–Whitney U test or the Wilcoxon rank sum test and provided as median with interquartile range [IQR]). A χ2 or Fisher’s exact test was employed to look for differences between categorical variables. A P value < 0.05 was deemed to be a significant finding.
Results
Clinical characteristics of patients and baseline values
A total of 101 patients with AHF were screened between 2019.12 and January 2021.6 having met the inclusion criteria (vide supra). The median age (IQR) of the levosimendan group was 73 (67.00, 80.00) years and for the levosimendan combined SFI group 73 (69.00, 80.00) years. The general demographic characteristics and baseline data of the two groups were comparable with no significant differences between most parameters. Hemoglobin values were somewhat less in the SFI group, but still within the physiological range. Patients in both groups had hypotension and diabetes comorbidities and received various drugs including angiotensin system inhibitors, β-blockers and diuretics including spironolactone and torsemide before enrollment; it is noteworthy that > 90% of patients were taking β-blockers. Torsemide doses were higher in the solely levosimendan group (Table 1).
Table 1.
Characteristics and baseline values of patients
| Levosimendan (N = 51) |
Levosimendan + SFI (N = 50) |
P value | |
|---|---|---|---|
| Gender n (%) Female | 15 (29.40) | 11 (22.00) | 0.394 |
| Male | 36 (70.60) | 39 (78.00) | |
| Age (yr), median (IQR) | 73.00 (67.00, 80.00) | 73.00 (69.00, 80.00) | 0.618 |
| BMI (kg/m2), median (IQR) | 23.89 (22.04, 25.39) | 22.59 (20.43, 25.10) | 0.108 |
| Hemoglobin (g/L), mean ± SD | 132.39 ± 18.34 | 123.76 ± 20.87 | 0.030 |
| Creatinine (μmoI/L), median (IQR) | 94.10 (76.30, 116.60) | 104.20 (83.10, 118.70) | 0.443 |
| eGFR (mL/min/1.73 m2), median (IQR) | 68.00 (57.40, 83.87) | 59.78 (52.43, 82.57) | 0.237 |
| LVEF (%), median (IQR) | 38.00 (33.30, 39.00) | 38.00 (32.00, 39.00) | 0.624 |
| BNP (ng/L), median (IQR) | 1169 (482.42, 2168.33) | 1415.81 (501.80, 3056.70) | 0.220 |
| Hypotension, n (%) | 37 (72.55) | 38 (76.00) | 0.692 |
| Etiology of heart failure, n (%) | 0.364 | ||
| Hypertrophic cardiomyopathy | 1 | 0 | |
| Alcoholic cardiomyopathy | 0 | 1 | |
| Hypertensive heart disease | 1 | 2 | |
| Coronary heart disease | 36 | 38 | |
| Dilated cardiomyopathy | 10 | 4 | |
| Valvular heart disease | 3 | 5 | |
| Previous admission with heart failure, n (%) | 44 (86.27) | 44 (88.00) | 1.000 |
| Comorbidities, n (%) | |||
| Hypertension | 37 (72.55) | 41 (82.00) | 0.344 |
| Diabetes mellitus | 26 (50.98) | 29 (58.00) | 0.479 |
| Cerebral stroke | 5 (9.80) | 7 (14.00) | 0.554 |
| COPD | 3 (5.88) | 6 (12.00) | 0.318 |
| Hyperlipidemia | 5 (9.80) | 7 (14.00) | 0.554 |
| Pre-admission medication | |||
| ACEI/ARB/ARNI, n (%) | 45 (88.24) | 43 (86.00) | 0.775 |
| Sacubitril/Valsartan | 43 (84.31) | 43 (86.00) | |
| Valsartan | 1 (1.96) | 0 | |
| Enalapril | 1 (1.96) | 0 | |
| β-blocker, n (%) | 46 (90.20) | 49 (98.00) | 0.205 |
| Metoprolol | 43 (84.31) | 44 (88.00) | |
| Bisoprolol | 2 (3.90) | 1 (2.00) | |
| Carvedilol | 1 (1.96) | 4 (2.00) | |
| Spironolactone, n (%) | 42 (82.35) | 40 (80.00) | 0.762 |
| Loop diuretic | 37 (72.55) | 39 (78.00) | 0.141 |
| Torsemide | 32 (62.75) | 39 (78.00) | |
| Furosemide | 5 (9.80) | 0 | |
| Pre-admission medication dose | |||
| ACEI/ARB/ARNI | |||
| Sacubitril/Valsartan (mg, bid), median (IQR) | 50 (50, 50) [n = 43] | 50 (25, 50) [n = 43] | 0.303 |
| Valsartan (mg, qd) | 80 [n = 1] | – [n = 0] | – |
| Enalapril (mg, qd) | 10 [n = 1] | – [n = 0] | – |
| β-blocker | |||
| Metoprolol (mg, qd), median (IQR) | 47.50 (23.75, 47.50) [n = 43] | 47.50 (23.75, 47.50) [n = 44] | > 0.999 |
| Bisoprolol (mg, qd) | 5 [n = 2] | 5 [n = 1] | – |
| Carvedilol (mg, bid), median | 10 [n = 1] | 10 [n = 4] | – |
| Spironolactone (mg, qd) | 20 [n = 42] | 20 [n = 40] | – |
| Loop diuretic | |||
| Torsemide (mg, qd), median (IQR) | 20 (20, 20) [n = 32] | 10 (10, 10) [n = 39] | < 0.001 |
| Furosemide (mg, qd) | 20 [n = 5] | – [n = 0] | – |
ACEI angiotensin-converting enzyme inhibitors, ARB angiotensin-receptor blocker, ARNI angiotensin receptor neprilysin inhibitor, BMI body mass index, BNP brain natriuretic peptide, COPD chronic obstructive pulmonary disease, eGFR estimated glomerular filtration rate, LVEF left ventricular ejection fraction
Medications during admission are listed in Additional file 1: Table 1. There was no significant difference between the 2 groups.
Endpoints
Primary endpoint (clinical effects)
The blood pressure including SBP and diastolic blood pressure (DBP) after SFI combined with levosimendan were significantly increased at different time points, but were still within the normal range [median: 102.5 (IQR: 100.0, 106.0)/median: 68.0 (IQR: 65.0, 70.0)]. The SBP and DBP of levosimendan were significantly decreased [median: 90.0 (IQR: 90.0, 94.0)/median: 55.0 (IQR: 50.0, 59.0)] 24 h after the infusion initiation (Fig. 1).
Fig. 1.
The change in blood pressure 2 h and 24 h after initiation of drug administration. Note: All data are shown as median (IQR). Abbreviations: DBP diastolic blood pressure, SBP systolic blood pressure
Secondary endpoints
The BNP values in both groups significantly decreased compared to baseline values, the change in the concentrations of BNP were not different in the combination and control groups (Fig. 2).
Fig. 2.
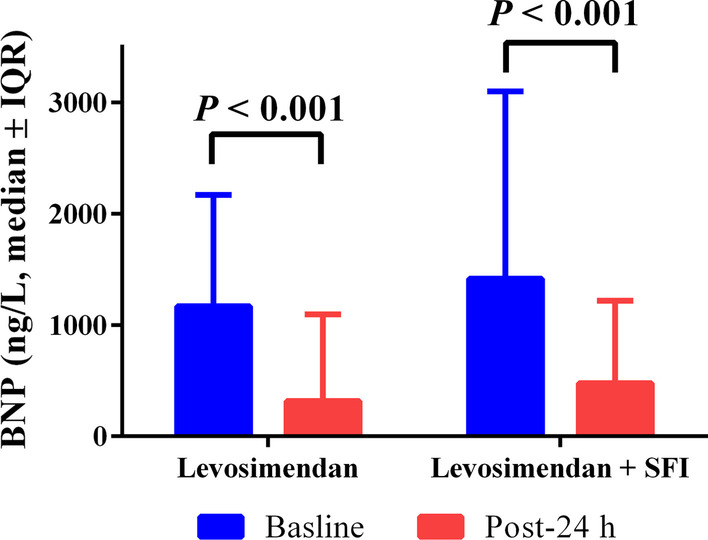
Changes in BNP before and after therapy. Note: All data are shown as median (IQR). Abbreviations: BNP brain natriuretic peptide, SFI Shenfu injection
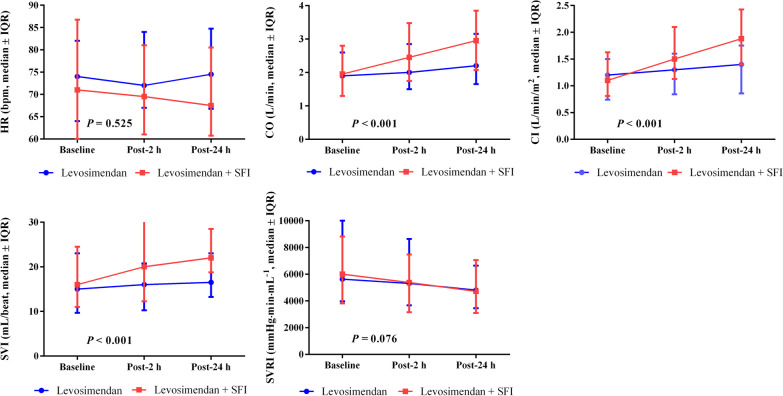
In addition, with regard to heart rate (HR) there were no significant differences between the two groups. Hemodynamic parameters including CI, CO and SVI were superior improved in the levosimendan combined with SFI group than in the levosimendan monotherapy group. Similarly, although the SVRI at 24 h appeared to be different in both groups compared to baseline, but statistical significance was not reached (P = 0.076) for the group differences over time.
The differences in the changes of CO, CI and SVI hemodynamic parameters over time were all significantly enhanced in the levosimendan combined with SFI group (P < 0.05) (Fig. 3, Table 2).
Fig. 3.
Comparison of the differences in the changes of hemodynamic parameters over time between the two groups (groups * time interaction). Abbreviations: CI cardiac index, CO cardiac output, HR heart rate, SFI Shenfu injection, SVI stroke volume index, SVRI systemic vascular resistance index
Table 2.
Comparison of hemodynamic parameters of the patients between levosimendan combined with Shenfu injection and levosimendan alone
| Levosimendan | P value | Levosimendan + SFI | P value |
P value (SFI vs No) |
P value (SFI vs No) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-2 h | Post-24 h | Baseline | Post-2 h | Post-24 h | Baseline | Post-2 h | Post-24 h | ||||
| HR (bpm) | 74.0 (64.0, 82.0) | 72.0 (67.0, 84.0) | 74.5 (67.0, 84.0) | 0.833 | 71.0 (60.0, 86.0) | 69.5 (61.0, 81.0) | 67.5 (61.0, 80.0) | 0.927 | 0.525 | 0.265 | 0.210 | 0.042 |
| CO (L/min) | 1.9 (1.3, 2.6) | 2.0 (1.5, 2.8) | 2.2 (1.7, 3.0) | 0.420 | 1.95 (1.3, 2.8) | 2.45 (1.77, 3.45) | 2.95 (2.1, 3.8) | 0.001 | < 0.001 | 0.676 | 0.084 | 0.019 |
| CI (L/min/m2) | 1.2 (0.74, 1.5) | 1.3 (0.85, 1.5) | 1.4 (0.86, 1.7) | 0.347 | 1.1 (0.82, 1.6) | 1.5 (1.15, 2.1) | 1.88 (1.4, 2.4) | < 0.001 | < 0.001 | 0.889 | 0.009 | 0.003 |
| SVI (mL/beat) | 15.0 (9.7, 23.0) | 16.0 (10.5, 20.5) | 16.5 (13.5, 23.0) | 0.516 | 16.0 (11.0, 24.0) | 20.0 (12.5, 30.5) | 22.0 (19.0, 28.0) | 0.004 | < 0.001 | 0.501 | 0.024 | 0.003 |
| SVRI (mmHg·min·mL−1) | 5633.0 (3969.0, 10,000.0) | 5315.0 (3773.0, 8274.0) | 4816.0 (3591.0, 6217.0) | 0.214 | 6001.5 (3895.0, 8817.0) | 5379.0 (3200.0, 7454.0) | 4722.5 (3141.0, 6867.0) | 0.250 | 0.076 | 0.763 | 0.320 | 0.705 |
All data are shown as median (IQR)
CO cardiac output, CI cardiac index, SVI stroke volume index, SVRI systemic vascular resistance index
It became also evident from inspection of Table 3 that the changes elicited by levosimendan combined with SFI and levosimendan alone mainly improved cardiac function, and therefore blood pressure. It was more beneficial for patients with AHF and hypotension included in the present study.
Table 3.
Comparison the difference of changes in hemodynamic parameters of patients from post 24 h to baseline between levosimendan combined with Shenfu injection and levosimendan alone groups
| Levosimendan | Levosimendan + SFI | P value | |
|---|---|---|---|
| Changes of post 24 h-baseline |
Changes of post 24 h-baseline |
||
| BNP (ng/L) | − 908.68 (− 1849.92, − 446.57) | − 932.64 (− 1906.30, − 247.74) | 0.741 |
| SBP (mmHg) | − 8.00 (− 10.00, − 4.00) | 4.00 (2.00, 9.00) | < 0.001 |
| DBP (mmHg) | − 9.00 (− 12.00, − 4.00) | 9.00 (6.00, 12.00) | < 0.001 |
| HR (bpm) | 0.50 (− 4.00, 7.00) | 0.50 (− 6.00, 7.00) | 0.525 |
| CO (L/min) | 0.10 (− 0.20, 0.50) | 0.70 (0.50, 1.20) | < 0.001 |
| CI (L/min/m2) | 0.10 (− 0.10, 0.40) | 0.61 (0.21, 0.80) | < 0.001 |
| SVI (mL/beat) | 0.09 (− 2.00, 3.40) | 6.00 (3.00, 9.00) | < 0.001 |
| SVRI (mmHg·min·mL−1) | − 862.00 (− 2704.00, 51.00) | − 494.50 (− 2249.00, 780.00) | 0.076 |
All the data are shown as median (IQR)
BNP brain natriuretic peptide, CI cardiac index, CO cardiac output, DBP diastolic blood pressure, HR heart rate, SBP systolic blood pressure, SFI Shenfu injection, SVI stroke volume index, SVRI systemic vascular resistance index
Kidney and liver functions
Table 4 shows that there were no significant differences in serum creatinine (Scr), blood urea nitrogen (BUN), alanine transaminase (ALT) and aspartate aminotransferase (AST), respectively between the 2 groups over 24 h (all P > 0.05).
Table 4.
Kidney and liver function indicators in the two groups
| Levosimendan | Levosimendan + SFI | P value | |
|---|---|---|---|
| Changes in post 24 h-baseline |
Changes in post 24 h-baseline |
||
| Scr (μmoI/L) | 3.45 (− 2.95, 21.25) | 5.30 (− 8.00, 23.50) | 0.752 |
| BUN (mmol/L) | 0.60 (− 1.73, 2.40) | − 0.04 (− 3.41, 3.45) | 0.251 |
| ALT (U/L) | − 2.50 (− 7.50, 4.50) | − 2.00 (− 10.00, 7.00) | 0.788 |
| AST (U/L) | 0.00 (− 10.50, 10.00) | 0.00 (− 19.00, 5.00) | 0.363 |
All data are shown as median (IQR)
ALT alanine transaminase, AST aspartate aminotransferase, BUN blood urea nitrogen, Scr serum creatinine, SFI Shenfu injection
Discussion
In the present study, we confirmed that levosimendan combined with SFI effectively increased blood pressure, which was reduced in patients with AHF due to insufficient peripheral blood volume. AHF refers to an attack or aggravation of the functions of the left heart, mainly due to reduced myocardial contractility, an increase in cardiac load, and pressure in the pulmonary circulation, and raised resistance of the peripheral circulation. Pulmonary congestion and edema, together with poor organ perfusion and cardiogenic shock are the most common clinical syndromes caused by pulmonary circulation congestion [23, 24]. Therefore, AHF has a relatively high in-hospital mortality rate of 3%, and 3- and 5-year mortality rates of 30% and 60%. The pathogenesis of AHF is complex, but most studies have demonstrated that it is related to hemodynamic disorders [25, 26].
The results of epidemiological investigation have shown that the incidence of AHF has been increasing in recent years in China, and has seriously affected people's physical and mental health and their quality of life [27]. Our results strongly suggest that the addition of an adjuvant significantly improved the hemodynamic indicators CO, CI, SVI and SVRI, which will naturally improve the survival rate and prognosis of patients.
Both levosimendan and SFI are relatively common drugs used to treat HF in China. Among them, levosimendan is a positive inotropic drug, which can bind with troponin C after drug action, increasing the sensitivity of contractile protein to Ca2+, thus improving myocardial contractility and reducing cardiac load. However, the effect of this drug alone is not ideal [28] for ADHF patients with hypotension. We also use SFI in clinical practice. Ginsenosides and aconitine alkaloids in SFI are the main active components [29]. Ginsenoside in red ginseng can reduce myocardial oxygen consumption and enhance myocardial contractility, while normethylidene alkaloid in aconitine alkaloids, has the effect of anti-myocardial ischemia and heart strengthening [30].
From ex vivo experiments in a septic shock rabbit model, we found that SFI could increase mean arterial pressure (MAP), decrease serum lipopolysaccharide (LPS), lactate dehydrogenase (LDH) and AST concentrations, and improve the tissue morphology of the heart, liver and kidney. In addition, SFI can re-increase the concentrations of ATP and taurine while reducing the concentration of AMP in cardiac muscle during septic shock [29].
Much research has been carried out on cardiac functions in patients with ADHF who have been treated with levosimendan or levosimendan combination drugs. Many studies have confirmed that levosimendan can significantly improve CO, reduce the BNP concentration and increase LVEF in patients with ADHF [31, 32]. In the present study, it was clear that similar effects of levosimendan combined with SFI and levosimendan therapies decreased the BNP concentration, although addition of SFI did not significantly improve the heart rate.
Levosimendan-nesiritide combination therapy produced the most pronounced improvements during the early stages of treatment, which gradually declined to the same levels produced by monotherapies at day 9 [33]. Therefore, for patients with ADHF, combination therapies achieved clinical efficacies faster than respective monotherapies, but improvements in the long term may well be similar.
Levosimendan and SFI have different mechanisms of action; levosimendan is a positive inotropic drug which does not raise the free intracellular Ca2+ concentration. In theory, a combination of these two drugs should produce synergistic effects greater than those produced by administration of only one of the drugs.
Study limitations
The results of this study should not be regarded as definitive regarding whether an SFI infusion affected BNP concentrations. Although BNP has a brief half-life, there was a delay of up to 48 h from cessation of drug administration to the determination of the BNP concentration. Due to the lack of measurements of proANP and aldosterone in laboratory tests, the BNP concentrations were only assessed 24 h after drug administration. In an ideal world, however, it would be desirable if all the concentrations (BNP, proBNP and aldosterone) were included. In addition, it is possible that a single-center study may introduce bias. Our study had a relatively small sample size, which would weaken the primary endpoint. Larger clinical trials are needed to confirm the improvement in hemodynamic parameters of SFI combined with levosimendan. Furthermore, SFI may not be available outside China, which leads to an issue of generalizability.
Conclusion
Intravenous infusion of levosimendan and SFI to acute decompensated heart failure patients with hypotension was a superior treatment compared to levosimendan monotherapy with regard to hemodynamic parameters.
Supplementary Information
Additional file 1: Clinical study protocol.
Additional file 2: Supplementary Table 1. Medications used during the admission.
Acknowledgements
None.
Abbreviations
- ACS-AHF
Acute heart failure with acute coronary syndrome
- ADHF
Acute decompensated heart failure
- AEs
Adverse events
- AHF
Acute heart failure
- ALT
Alanine transaminase
- AST
Aspartate aminotransferase
- BNP
Brain natriuretic peptide
- BUN
Blood urea nitrogen
- CI
Cardiac index
- cTnC
Cardiac muscle troponin C
- CO
Cardiac output
- DBP
Diastolic blood pressure
- HF
Heart failure
- HR
Heart rate
- LCOS
Low cardiac output syndrome
- LDH
Lactate dehydrogenase
- LVEF
Left ventricular ejection fraction
- LPS
Lipopolysaccharide
- MAP
Mean arterial pressure
- PCWP
Pulmonary capillary wedge pressure
- SBP
Systolic blood pressure
- SAEs
Serious adverse events
- Scr
Serum creatinine
- SFI
Shenfu injection
- SVI
Stroke volume index
- SVRI
Systemic vascular resistance index
- USCOM
Ultrasonic cardiac output monitors
Author contributions
Conception and design: MML, YZ, QLW and FY. Data collection: MML, YZ, YOL and TZQ. Analysis and interpretation: MML, YZ and FY. Statistical analysis: MML and YZ. Writing the article: MML, YZ, YOL and TZQ. Critical revision: MML, YZ, QLW and FY. Final approval: all authors.
Funding
This study was supported by the Integrated Thinking Research Fund of China International Medical Foundation (Grant No. 20201630). The funder had no role in the study design, data collection or analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study followed the Declaration of Helsinki principles and was approved by the Institutional Review Board of Tongren Hospital affiliated to Shanghai Jiao Tong University School of Medicine. All enrolled patients provided informed consent. The registered trial number was ChiCTR2000039385.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Miaomiao Li and Yue Zhang contributed equally to this work.
References
- 1.Pashkovetsky E, Gupta CA, Aronow WS. Use of levosimendan in acute and advanced heart failure: short review on available real-world data. Ther Clin Risk Manag. 2019;15:765–772. doi: 10.2147/TCRM.S188761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gencer E, Doğan V, Öztürk MT, Nadir A, Musmul A, Cavuşoğlu Y. Comparison of the effects of levosimendan dobutamine and vasodilator therapy on ongoing myocardial injury in acute decompensated heart failure. J Cardiovasc Pharmacol Ther. 2017;22(2):153–158. doi: 10.1177/1074248416657612. [DOI] [PubMed] [Google Scholar]
- 3.Haikala H, Kaivola J, Nissinen E, Wall P, Levijoki J, Lindén IB. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. J Mol Cell Cardiol. 1995;27(9):1859–1866. doi: 10.1016/0022-2828(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 4.Nieminen MS, Buerke M, Cohen-Solál A, Costa S, Édes I, Erlikh A, et al. The role of levosimendan in acute heart failure complicating acute coronary syndrome: a review and expert consensus opinion. Int J Cardiol. 2016;218:150–157. doi: 10.1016/j.ijcard.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Lochner A, Colesky F, Genade S. Effect of a calcium-sensitizing agent, levosimendan, on the postcardioplegic inotropic response of the myocardium. Cardiovasc Drugs Ther. 2000;14(3):271–281. doi: 10.1023/A:1007878523663. [DOI] [PubMed] [Google Scholar]
- 6.Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, et al. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36(6):1903–1912. doi: 10.1016/S0735-1097(00)00961-X. [DOI] [PubMed] [Google Scholar]
- 7.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 8.Song Y, Zhang N, Shi S, Li J, Zhang Q, Zhao Y, et al. Large-scale qualitative and quantitative characterization of components in Shenfu injection by integrating hydrophilic interaction chromatography, reversed phase liquid chromatography, and tandem mass spectrometry. J Chromatogr A. 2015;1407:106–118. doi: 10.1016/j.chroma.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Li B-Z, Liu H. Pharmacological action and clinical application of Shen Fu injection. Med Recapitul. 2008;8:1247–1250. [Google Scholar]
- 10.Lin J-G, Lyu J, Sun M-H, Liao X, Xie Y-M. Systematic review and meta-analysis of shenfu injection on treating acute exacerbation of chronic obstructive pulmonary disease. World J Tradit Chin Med. 2020;6(3):276–283. doi: 10.4103/wjtcm.wjtcm_45_20. [DOI] [Google Scholar]
- 11.Qin H, Liu G, Zhang F, Shen J, Sun H. Clinical observation on Shenfu Zhusheye in the treatment of patients with acute exacerbation chronic obstructive pulmonary disease. Chin J Clin Med. 2010;17(5):659–660. [Google Scholar]
- 12.Xu J, Lou H, Lou Y, He W, Xu X, Xu D. Research advance in pharmacological action of "Shenfu Injection". Shanghai J Tradit Chin Med. 2008;42(10):87. [Google Scholar]
- 13.Wu J, Li C, Yuan W. Effects of Shenfu injection on macrocirculation and microcirculation during cardiopulmonary resuscitation. J Ethnopharmacol. 2016;180:97–103. doi: 10.1016/j.jep.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Wen-Ting S, Fa-Feng C, Li X, Cheng-Ren L, Jian-Xun L. Chinese medicine shenfu injection for heart failure: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2012;2012:713149. doi: 10.1155/2012/713149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Liu J. Clinical application of Shenfu Injection. Chin Pharm Affairs. 2010;24(5):510–513. [Google Scholar]
- 16.Jiang Y, Zhang R-R, Yang L-J, Wu Z, Chen H-P, Tian Z-F. Shenfu injection provides protection for perinatal asphyxia in neonates. Bangladesh J Pharmacol. 2016;11(1):236–239. doi: 10.3329/bjp.v11i1.25124. [DOI] [Google Scholar]
- 17.Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38(3):161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang N, Lian Z, Peng X, Li Z, Zhu H. Applications of Higenamine in pharmacology and medicine. J Ethnopharmacol. 2017;196:242–252. doi: 10.1016/j.jep.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L. Curative effect of Shenfu injection on chronic heart failure. Med J West China. 2011;23(1):75–76. [Google Scholar]
- 20.Wu H, Dai Z, Liu X, Lin M, Gao Z, Tian F, et al. Pharmacodynamic evaluation of Shenfu injection in rats with ischemic heart failure and its effect on small molecules using matrix-assisted laser desorption/ionization-mass spectrometry imaging. Front Pharmacol. 2019;10:1424. doi: 10.3389/fphar.2019.01424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YH, Yu B, Duan ZZ, Akinyi OM, Yu JH, Zhou K, et al. The coronary dilation effect of Shen Fu injection was mediated through NO. PLoS ONE. 2014;9(3):e92415. doi: 10.1371/journal.pone.0092415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.[Chinese guidelines for the diagnosis and treatment of heart failure 2018]. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46(10):760–89. [DOI] [PubMed]
- 23.Kurmani S, Squire I. Acute heart failure: definition, classification and epidemiology. Curr Heart Fail Rep. 2017;14(5):385–392. doi: 10.1007/s11897-017-0351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan F, Zhao L, Wang J, Zhang W, Li X, Qiu XB, et al. PITX2c loss-of-function mutations responsible for congenital atrial septal defects. Int J Med Sci. 2013;10(10):1422–1429. doi: 10.7150/ijms.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Liu H, Song M, Fang W, Yuan F. Clinical effect of cardiac shock wave therapy on myocardial ischemia in patients with ischemic heart failure. J Cardiovasc Pharmacol Ther. 2016;21(4):381–387. doi: 10.1177/1074248415616189. [DOI] [PubMed] [Google Scholar]
- 26.Yuan F, Qiu XB, Li RG, Qu XK, Wang J, Xu YJ, et al. A novel NKX2-5 loss-of-function mutation predisposes to familial dilated cardiomyopathy and arrhythmias. Int J Mol Med. 2015;35(2):478–486. doi: 10.3892/ijmm.2014.2029. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Liu R, Jiang C, Du X, Huffman MD, Lam CSP, et al. Assessing the evidence-practice gap for heart failure in China: the Heart Failure Registry of Patient Outcomes (HERO) study design and baseline characteristics. Eur J Heart Fail. 2020;22(4):646–660. doi: 10.1002/ejhf.1630. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Zhang Y, Yan Y. Effect of levosimendan on the levels of NT-proBNP and inflammatory markers in patients with acute heart failure. J Hainan Med Univ 2013;19(11):1515–7,20.
- 29.Liu X, Liu R, Dai Z, Wu H, Lin M, Tian F, et al. Effect of Shenfu injection on lipopolysaccharide (LPS)-induced septic shock in rabbits. J Ethnopharmacol. 2019;234:36–43. doi: 10.1016/j.jep.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Zheng S, Wu H, Yu S, Duo W, Ren J, Wang S, et al. Intervention study of Shenfu Injection on cardiomyocyte apoptosis in experimental heart failure rats. China J Tradit Chin Med Pharm. 2012;27(11):2972–2975. [Google Scholar]
- 31.Jia Z, Guo M, Zhang YQ, Liang HQ, Zhang LY, Song Y. Efficacy of intravenous levosimendan in patients with heart failure complicated by acute myocardial infarction. Cardiology. 2014;128(2):195–201. doi: 10.1159/000357864. [DOI] [PubMed] [Google Scholar]
- 32.Silva-Cardoso J, Ferreira J, Oliveira-Soares A, Martins-de-Campos J, Fonseca C, Lousada N, et al. Effectiveness and safety of levosimendan in clinical practice. Rev Port Cardiol. 2009;28(2):143–154. [PubMed] [Google Scholar]
- 33.Jia Z, Guo M, Zhang LY, Zhang YQ, Liang HQ, Song Y. Levosimendan and nesiritide as a combination therapy in patients with acute heart failure. Am J Med Sci. 2015;349(5):398–405. doi: 10.1097/MAJ.0000000000000461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Clinical study protocol.
Additional file 2: Supplementary Table 1. Medications used during the admission.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.