Highlights
-
•
GLP-1 improves peripheral glucose uptake in healthy dogs and cats.
-
•
GLP-1 analogues administration in diabetic cats reduces exogenous insulin requirement.
-
•
Dogs cardiomyocytes apoptosis is reduced by GLP-1-derived molecules action.
Keywords: GLP-1, Cats, Dogs, Diabetes mellitus, Obesity, Incretin
Abstract
Analogues of glucagon like peptide-1 (GLP-1) and other drugs that increase this peptide half-life are used worldwide in human medicine to treat type 2 diabetes mellitus (DM) and obesity. These molecules can increase insulin release and satiety, interesting effects that could also be useful in the treatment of domestic animals pathologies, however their use in veterinary medicine are still limited. Considering the increasing incidence of DM and obesity in cats and dogs, the aim of this review is to summarize the available information about the physiological and pharmacological actions of GLP-1 in domestic animals and discuss about its potential applications in veterinary medicine. In diabetic dogs, the use of drugs based on GLP-1 actions reduced blood glucose and increased glucose uptake, while in diabetic cats they reduced glycemic variability and exogenous insulin administration. Thus, available evidence indicates that GLP-1 based drugs could become alternatives to DM treatment in domestic animals. Nevertheless, current data do not provide enough elements to recommend these drugs widespread clinical use.
Graphical abstract
1. Introduction
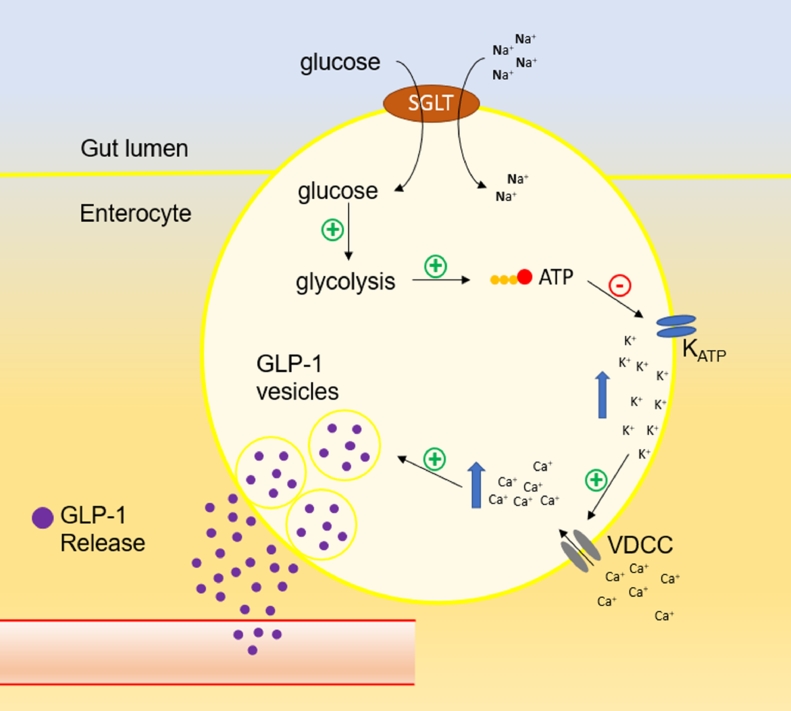
Glucagon like peptide-1 (GLP-1) is a peptide hormone synthetized by intestinal "L" cells, which are located mainly in the most distal intestinal regions, such as the ileum and colon. This peptide is also produced in the central nervous system (CNS), more specifically in solitary tract nucleus, and in pancreatic α cells (Chambers et al., 2017; Müller et al., 2019). The mechanisms that trigger GLP-1 release is quite like that of insulin Fig. 1. Increase in intracellular glucose leads to higher ATP synthesis, which in turn interacts with ATP-sensitive K+ channels (KATP), closing them. This leads to cell depolarization and the opening of voltage dependent calcium channels (VDCC), increasing this cation concentration in the cells. Finally, Ca++ interacts with vesicles containing GLP-1 (or insulin in β cells) and promotes its exocytosis (Reimann & Gribble, 2002; Rowlands, Heng, Newsholme & Carlessi, 2018; Tolhurst, Reimann & Gribble, 2009).
Fig. 1.
Schematic illustration of GLP-1 release after glucose uptake in enteroendocrine L cells. SGLT: sodium-glucose linked transporter. KATP: ATP-sensitive K+ channels. VDCC: voltage dependent calcium channels.
After its release by L cells, GLP-1 enters the portal circulation and reach the pancreatic islets, where it exerts the so-called incretin effect (Baggio & Drucker, 2007; Nauck & Meier, 2018). The incretin effect is described as a higher insulin release resulting from oral than intravenous glucose administration, despite similar blood glucose levels. (Baggio & Drucker, 2007; Nauck & Meier, 2018). GLP1 has an extremely short half-life due to rapid degradation by dipeptidylpeptidase 4 (DPP4), also known as CD26, a serine peptidase produced in the vascular endothelium (Deacon, 2018; Nauck & Meier, 2005). Recent data indicates that GLP-1 originated in α cells also has a central role in the stimulation of insulin release by β cells, in an intra-islet paracrine action (Chambers et al., 2017; Smith et al., 2014).
Regardless of its origin, islet α cells or enteroendocrine L cells, when GLP1 reaches the β cells and binds to its receptor (GLP-1R), a series of events that culminate in insulin release amplification are triggered (Fig. 2) (Müller et al., 2019). When GLP-1R, a G protein-coupled receptor, is activated, adenylate cyclase is activated resulting in ATP convertion to cAMP (Meloni, DeYoung, Lowe & Parkes, 2013). To stimulate the insulin release, this second messenger activates mainly two pathways: protein kinase A (PKA) and the exchange protein directly activated by cAMP (Epac) (Kang, Leech, Chepurny, Coetzee & Holz, 2008; Skelin & Rupnik, 2011). PKA phosphorylates the KATP channels, sensitizing them to ATP and facilitating their closure, thus promoting cell depolarization and opening the VDCC, leading to an increase in intracellular Ca++ (Bünemann, Gerhardstein, Gao & Hosey, 1999; MacDonald et al., 2003; Rowlands et al., 2018). PKA also inhibits voltage-dependent K+ channels, delaying repolarization of β cells (MacDonald et al., 2003). In addition to this rapid stimulation of insulin release, PKA also interacts with pancreatic and duodenal homeobox 1 (Pdx-1), leading to stimulation of the proinsulin gene transcription, which increases the stability of its mRNA, thus promoting insulin synthesis (D. J. Drucker, Philippe, Mojsov, Chick & Habener, 1987; Müller et al., 2019; Wang et al., 1995). Epac also acts sensitizing the KATP channels, but its main function is to facilitate the opening of ryanodine receptor calcium release channels (RYR) on the endoplasmic reticulum, amplifying the intracellular Ca++ increase (Holz, 2004; Kang et al., 2008). It is important to note that the actions of PKA and Epac potentiates and enhances β cells response to the increase in circulating glucose, but without concomitant increase in intracellular ATP, therefore, GLP-1 signal is a week insulin release promoter (Müller et al., 2019).
Fig. 2.
Schematic illustration of the intracellular signaling cascade by which GLP-1 stimulates insulin release in β cells. AC: adenylate cyclase. Epac: exchange protein directly activated by cAMP. PKA: protein kinase A. KATP: ATP-sensitive K+ channels. VDCC: voltage dependent calcium channels. KV: voltage-dependent K+ channels. PDX-1: pancreatic and duodenal homeobox 1. RYR: ryanodine receptor calcium release channels.
Besides its acute effects, GLP-1 also has long-term effects on β cells, that are mainly related to apoptosis inhibition and cellular multiplication (J. Buteau et al., 2004; Jean Buteau, Foisy, Joly & Prentki, 2003; Kapodistria, Tsilibary, Kotsopoulou, Moustardas & Kitsiou, 2018; Shimoda et al., 2011; Tsunekawa et al., 2007). However, studies with diabetic human patients have not shown an increase in β cells proliferation (Smits et al., 2017). Pancreatic islets α and δ cells also express GLP-1R, therefore GLP1 binding can also inhibits glucagon and stimulates somatostatin release (de Heer, Rasmussen, Coy & Holst, 2008; Ørskov, Holst & Nielsen, 1988; Richards et al., 2014).
Much of the interest in GLP-1 is due to its action on pancreatic islets, however, it was described in rodents and humans that several other tissues express GLP-1R (Müller et al., 2019; Rowlands et al., 2018). GLP-1R has already been identified in the stomach, skeletal muscle, smooth muscle, atrial cardiac muscle, kidneys, lungs, adipose tissue and in several areas of the central nervous system (CNS) (Bullock, Heller & Habener, 1996; Challa et al., 2012; Delgado et al., 1995; Richards et al., 2014). Some authors have already reported the presence of GLP-1R in the liver, however the most recent results indicate that these findings are mainly due to findings with non-specific antibodies, and that hepatocytes of mice, humans and monkeys (Macaca mulatta and Macaca fascicularis) do not express GLP-1R (Drucker, 2018; Knudsen & Lau, 2019; Pyke et al., 2014). To our best knowledge, GLP-1R presence in dogs and cats’ liver was not evaluated yet.
GLP-1 has several important extra pancreatic functions, and most of them depend on actions in the CNS. It increases satiety and thermogenesis, inhibits blood pressure rising, gastric emptying and water intake, is involved in the reward pathway and in the hypothalamic adrenal pituitary (HPA) axis (Beiroa et al., 2014; Hayes, Skibicka & Grill, 2008; Herman, 2018; I˙meryüz et al., 1997; Kooijman et al., 2015; Krieger et al., 2016; Pacheco et al., 2011; Rüttimann, Arnold, Hillebrand, Geary & Langhans, 2009; Sirohi, Schurdak, Seeley, Benoit & Davis, 2016; Tang-Christensen et al., 1996; Wettergren, Wojdemann, Meisner, Stadil & Holst, 1997). GLP-1 also acts on the cardiovascular system, either directly binding to GLP-1R in cardiomyocytes and vascular smooth muscle cells, and indirectly, by its CNS actions (Baggio et al., 2017; Ban et al., 2008). Considering the increasing incidence of diabetes melitus (DM) and obesity in cats and dogs, the aim of this review is to summarize the available information about the physiological and pharmacological actions of GLP-1 in domestic animals and discuss about its potential applications in veterinary medicine.
2. Material and methods
To find the studies that evaluated the action of drugs based on GLP-1 in dogs and cats, we used the platforms PubMed/NCBI, Scopus, Web of Science and Google Scholar to search for the terms "dog and GLP-1″, "canine and GLP-1″, "cat and GLP-1″ "feline and GLP-1″, "pet and GLP-1″ in addition to their plurals. We performed the same searches again but replacing the term "GLP-1″ with "glucagon like peptide-1″ and then with the term "incretin". These searches were carried out between May and June 2021, no restrictions on publication date were imposed. Only papers in English were found and considered. All authors performed searches independently. Afterwards, all papers were gathered (disregarding replicates) and two individuals independently evaluated which ones should be included. As the inclusion criterion was any article that addressed the proposed topic, there were no discrepancies in the results obtained by the reviewers. From 30 papers found, all that addressed these topics were included in the discussion below, with the exception of one (Padrutt, Lutz, Reusch & Zini, 2015). This study was excluded because the authors did not perform a statistical analysis of the data.
3. Physiological actions of GLP-1 in dogs and cats
Most of the studies cited so far, which allow us to understand GLP-1 physiological importance and its pharmacological potential, have been carried out using rats, mice, human volunteers and cell lines of these three species. However, some studies with dogs and cats demonstrate GLP-1 species-specific characteristics. In dogs, GLP-1 is also synthetized and released by intestinal L cells, especially in the jejunum (Damholt, Kofod & Buchan, 1999). However, the classic form of GLP-1 release, shown in Fig. 1, does not seem to occur in dogs, with at least part of GLP-1 release being stimulated by GIP´s (from K cells) paracrine action (Damholt et al., 1999; Damholt, Buchan & Kofod, 1998; Sugiyama et al., 1994). Also, while glucose is the main inducer of GLP-1 release in humans and rodents, in dogs high fat diets stimulate GLP-1 release, while carbohydrate-rich diets do not have the same effect (Fig. 3) (Lubbs et al., 2010; Schauf et al., 2018; Van Citters et al., 2002).
Fig. 3.
Comparative summary of the origin and main outcomes of the incretin effect in humans, rodents, dogs, and cats.
As well as in other species, GLP-1 stimulates insulin release in dogs (Ohneda et al., 1991). GLP-1 administration directly into the pancreatic artery stimulates insulin and inhibits glucagon release in dogs, however, when GLP-1 is administered in venous circulation, it had no effect on pancreatic hormones. (Elahi et al., 2014; Ionut et al., 2006; V. Ionut, Hucking, Liberty & Bergman, 2005; Johnson et al., 2007, 2008; Ohneda et al., 1991). The administration of DPP4 inhibitors in dogs leads to an increase in GLP-1, but it does not alter insulinemia. (Deacon, Wamberg, Bie, Hughes & Holst, 2002; Edgerton et al., 2009). Even in experiments without changes in pancreatic hormones, GLP-1 is able to stimulate glucose uptake and hepatic glycogen synthesis (Dardevet et al., 2005; Elahi et al., 2014; Johnson et al., 2007, 2008). In animals with diabetes mellitus (DM), GLP-1 does not alter blood glucose, and in pancreatectomized dogs it has no effects when administered alone, but improves glucose utilization and the antilipolytic action promoted by exogenous insulin. (Freyse et al., 1999; Sandhu et al., 1999). These findings suggest that GLP-1 may have an indirect action on peripheral tissues, increasing insulin sensitivity.
The incretin effect is also observed in cats (Gilor et al., 2011b; Nishii et al., 2014). Different GLP-1R agonists increase plasma insulin concentration in cats as well as in rodents and humans (Gilor etal., 2011a; Hall et al., 2015; Rudinsky et al., 2015). There are evidences that GLP-1 degradation by DPP4 in cats is also similar to other species (Mori et al., 2016; Nishii et al., 2014). As in dogs, glucose also does not seem to be the main stimulus for the release of GLP-1 in cats. After amino acids rich meals, there is a higher increase in plasmatic GLP-1 than after meals rich in carbohydrates or lipids, with no difference between the last two. The diets used in these studies were caloric and volume equivalent (Gilor et al., 2011b; McCool, Rudinsky, Parker, Herbert & Gilor, 2018). The number of meals can also affect plasma GLP-1 concentration, with cats that received 1 meal per day having higher values than those that received 4 meals (Camara et al., 2020). However, dietary caloric changes over 16 weeks were not able to alter the plasmatic GLP-1 (McCool et al., 2018).
4. GLP-1 pharmacology
The known physiological actions of GLP-1 indicate a great potential for its pharmacological use in obesity and DM treatment. However, some factors turn its use unfeasible. The main issues about GLP-1 use as a treatment are its short half-life (around 1 – 2 min) and the fact that its administration in high doses causes gastrointestinal complications such as nausea, vomiting and diarrhea (Baggio & Drucker, 2002; Nauck & Meier, 2005). To overcome these limitations, two general types of drugs that use the incretin mechanism of action have been developed, the synthetic GLP1 agonists (GLP-1As) and the DPP4 inhibitors (DPP4-is) (Nauck & Meier, 2005). Although these drugs have been developed for use in humans, both types have already been tested in dogs and cats (Gilor, Rudinsky & Hall, 2016; Ionut et al., 2016; Kim et al., 2016). One of the main advantages of using DPP4-is or GLP-1As is that they have few side effects, being highly safe (Aziz et al., 2020; Scheen, 2018).
4.1. GLP-1 pharmacology in cats
The similarity between human type 2 DM and feline DM pathogenesis makes natural that drugs successfully used in humans treatment be evaluated for their use in cats (Gilor, Niessen, Furrow & DiBartola, 2016). Although the same reasoning is valid for drugs used to treat obesity in humans, there are very few studies evaluating drugs based on GLP-1 in obese cats (Table 1).
Table 1.
GLP-1-based drug studies in cats.
| Breed | Age (years) | sex | Castration | "n" per group | Condition | Drug | Brant / Manufacturer | Dosage | Treatment Duration | Complications | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NI | 8.0 ± 1.1 | F/M | All | 2 - 3 | healthy | sitagliptin | Januvia/ Banyu Pharmaceutical | 25 – 50 mg.kg−1 | acute | NO | (Nishii et al., 2014) |
| NI | 1 to 4 | F/M | All | 5 | healthy | sitagliptin | Januvia/ Merck & Co. Inc | 4.2 mg* | 1 week | diarrhea | (Mori et al., 2016) |
| DSH | 1.3 to 2 | M | All | 6 | healthy | NVP-DPP728 | NI/ Novartis Pharma | 0.5 - 1 mg.kg−1 | acute | NO | (Furrer et al., 2010) |
| DSH, BSH, Burmilla and mixed breeds | above 3 | F/M | All | 6 | obese | exenatide | Byetta/Eli Lilly | 0.5 – 1 μg.kg−1 | 12 weeks | vomiting, diarrhea and hypoglycemia | (Hoelmkjaer et al., 2016) |
| NI | 2 to 8 | F/M | All | 6 | healthy | exenatide | Byetta/Eli Lilly | 0.1 – 0.24 – 1 μg.kg−1 | acute | NO | (Seyfert et al., 2012) |
| purpose-bred | 4.5 (3.1 – 4.8) | F/M | All | 9 | healthy | exenatide | Byetta/Amylin Pharmaceuticals | 1.04 ± 0.18 µg.kg−1 | acute | NO | (Gilor etal., 2011a) |
| purpose-bred | 3 | M | All | 6 | healthy | exenatide ER | Bydureon/Amylin Pharmaceuticals | 0.13 mg.kg−1 | 21 days | NO | (Rudinsky et al., 2015) |
| purpose-bred | 3 | F/M | All | 8 | healthy | liraglutide | Victoza/ Novo Nordisk | 0.112 ± 0.019 mg.kg−1 | 8 days | vomiting, diarrhea and anorexia | (Hall et al., 2015) |
| DSH | 1 to 4 | M | NI | 6 | healthy | exenatide monthly | synthesized by the research group | 1.2 – 4.6 µmol | 10 weeks | vomiting and diarrhea | (Schneider et al., 2020) |
| DSH, DLH⁎⁎ | 9.4 ± 3.7 | F/M | 14 | 15 | DM | exenatide ER | Bydureon/ Amylin Pharmaceuticals | 200 µg.kg−1 | 16 weeks | vomiting and diarrhea | (Riederer et al., 2016) |
| DSH, DMH and Siamese cross | 12 (6 – 15) | F/M | All | 8 | DM | exenatide | Byetta/Eli Lilly | 1 µg.kg−1 | 6 weeks | anorexia and hypoglycemia | (Scuderi et al., 2018) |
| DSH, DLH, Maine Coons, NFC, Exotic | 9.3 (4.3 – 14) | F/M | 14 | 15 | DM | exenatide ER | Bydureon/ Amylin Pharmaceuticals | 200 µg.kg−1 | 16 weeks | NI | (Krämer et al., 2020) |
NO: not observed. NI not informed. DM: diabetes mellitus. DSH: Domestic shorthair. BSH: British shorthair. DLH: Domestic longhair. DMH: Domestic mediumhair. NFC: Norwegian forest cat. ER: extended release.
the authors reported only the total administered.
4 cats were identified as "purebred" by the authors.
Despite the similarities in the pathogenesis of obesity, the relationship between this disease and incretins in felines seems to be different from that observed in humans and rodents. In these species obesity leads to an increase in GIP and decrease in GLP-1 (Chia & Egan, 2020). Diabetic cats were shown to have lower insulin levels than healthy and obese animals, but both GIP and GLP-1 were increased (McMillan, Zapata, Chelikani, Snead & Cosford, 2016). Unlike in humans, there was no difference in GIP and GLP-1 between healthy and obese felines. Even insulin was not altered between obese and healthy cats, something that has already been reported in other studies. (Appleton, Rand & Sunvold, 2001; Hoenig et al., 2013; McMillan et al., 2016). We found no studies evaluating treatment of obese cats with DPP4-is and just one in which GLP-1A was used for treatment in obese cats. In this study, the animals received the GLP-1A exenatide (0.5 – 1 μg.kg−1 BID) for 12 weeks, but the treatment did not resulted in differences in total weight, percentage of body fat, glycemia, leptin, adiponectin, glucagon and in glucose and insulin tolerance tests (GTT and ITT) (Hoelmkjaer et al., 2016).
In addition to the lack of studies about DPP4-is in obese cats, we did not find any reports in diabetic felines, only healthy animals. A single application of the DPP4i NVP-DPP728 (0.5 – 2.5 mg.kg−1) increased insulin and reduced glucagon release in a GTT. The authors attribute these results to the increase in GLP-1, although it was not directly measured (Furrer, Kaufmann, Tschuor, Reusch & Lutz, 2010). In another study with a DPP4i, sitagliptin (25 – 50 mg.kg−1) was administered along with oral glucose or with standard food. GLP-1 increased in cats that received sitagliptin regardless of diet. On the other hand, blood glucose only increased in cats who received oral glucose. Insulinemia increased in animals that received standard food regardless of whether or not sitagliptin was administered, but those that received glucose only showed an increase in insulin concentrations when the DPP4i was also administered (Nishii et al., 2014).
Mori et al. (2016) evaluated sitagliptin administration (4.2 mg) alone, acarbose (disaccharide digestion inhibitor) alone, or both together in healthy cats. After two weeks of treatment, the cats were fed a maltose-rich meal. Blood glucose was not altered in any of the groups, insulin decreased in all treatments, GLP-1 increased in groups with sitagliptin (alone or with acarbose) while GIP decreased in these same groups. Regardless of the type of food or another drug concomitant administration, the DPP4i evaluated was able to increase GLP-1 in the studied animals. However, both glycemia and insulinemia did not show such a clear response to sitagliptin treatment, leading the authors themselves to raise doubts about this drug value in DM cats treatment (Nishii et al., 2014).
GLP-1As have already been studied in both healthy and DM felines. In healthy individuals, GLP-1A has been evaluated for its short and long-term effects. In DM cats it was tested only together with insulin therapy. Exenatide acute actions were evaluated in two different studies with similar doses (1.04 ± 0.18 µg.kg−1 and 0.1 / 0.24 / 1 µg.kg−1), using isoglycemic and hyperglycemic clamps, respectively. In both, the treatment increased insulin secretion, but the glucose infusion required to maintain the blood glucose was not altered (Gilor etal., 2011a; Seyfert, Brunker, Maxwell, Payton & McFarlane, 2012).
Longer-term treatments were performed with GLP-1As, daily liraglutide applications or with a single administration of extended release exenatide. In an 8-day treatment with daily applications of liraglutide (0.6 mg (0.112 ± 0.019) mg.kg−1) two hyperglycemic clamps were evaluated, one performed before and the other after treatment. As occurred in the acute studies, the GLP-1A did not lead to differences in the infused glucose concentration needed to maintain glycemia, however insulin release increased while glucagon release decreased after the treatment. Another interesting result, but which should be evaluated with caution, was that the animals lost weight after 8 days receiving liraglutide. Although this is a promising result, it is necessary to emphasize that 8 of the 9 cats used in the study had at least one episode of vomiting or diarrhea, which may have influenced weight loss (Hall et al., 2015).
Different types of long acting exenatide have been tested for their efficacy and pharmacological characteristics in cats. Two hyperglycemic clamps, performed 7 days before and 21 days after the application of a single extended-release exenatide dose (0.13 mg.kg−1), were compared. Initial blood glucose was lower after treatment, while initial insulin and glucagon concentrations did not change. Glucose administered to maintain hyperglycemia was higher after exenatide, while insulin release increased and glucagon release decreased (Rudinsky et al., 2015). A recently developed exenatide formulation that allows for monthly applications had similar pharmacological and pharmacodynamic characteristics to traditional exenatide (1.2 – 4.6 µmol) and was able to stimulate insulin release in an ivGTT, although it did not change glucose concentrations (Schneider et al., 2020).
In diabetic cats, no GLP-1A has been studied as a single treatment, but it has been tested with insulin therapy, presenting inconsistent results. Diabetic cats were treated with insulin glargine plus exenatide (1 µg.kg−1 BID) or saline for 6 weeks. There was no difference in blood glucose, hematological and biochemical parameters between treatments, however animals that received exenatide lost more weight than those who received saline and needed lower insulin doses to maintain blood glucose (Scuderi et al., 2018). DM cats newly diagnosed were treated with insulin glargine alone or together with long-acting exenatide (weekly application of 200 µg.kg−1). After 16 weeks no significant difference was observed in plasma biochemistry (including glucose and glycated fructosamine) and in the insulin dose required to maintain blood glucose. The only difference observed was an increased weight of animals that received just insulin, while it did not occur in those treated with insulin and exenatide (Riederer et al., 2016). In a similar experiment Krämer et al. (2020) also compared newly diagnosed diabetic cats that received insulin glargine alone or with long-acting exenatide (weekly application of 200 µg.kg−1 for 16 weeks). Again, there were no differences in glycemia, however animals that received exenatide had less variability in this parameter and reached normoglycemia earlier, after 6 weeks, while the placebo group reached normoglycemia after 16 weeks. Because in all three studies glycemia was controlled by insulin treatment, it is not surprising that this parameter was not different between insulin and insulin plus exenatide groups. However, unlike what was observed with DPP4-is, the use of GLP-1As showed improvements in animal treatment by reducing the required insulin dose and glycemic variability. Even though a larger body of evidence is needed to reach conclusions about the effectiveness of GLP-1A treatments in cats, the results obtained so far demonstrate that these drugs may be used, at least as adjuvants, in the DM treatment.
4.2. GLP-1 pharmacology in dogs
Regarding the modulation of endogenous GLP-1 and DM, no articles were found related to DDP4i use in diabetic dogs (Table 2). This absence is expected considering the expressive loss of pancreatic β cells in canine DM (Deacon et al., 2002; Edgerton et al., 2009; Ionut et al., 2006). However, considering that GLP-1 effects are not necessarily restricted to the pancreas, it could be evaluated in DM dogs (Edgerton et al., 2009; Ionut et al., 2006; Johnson et al., 2007). In healthy dogs, DPP4i administration showed contradictory results (Table 2). The DPP4i vildagliptin increased hepatic glucose uptake regardless of changes in circulating insulin or glucagon levels (Edgerton et al., 2009). Consistently, gemigliptin improved the oral GTT (oGTT) profile compared to control, increased GLP-1, and decreased glucagon after glucose load (Kim et al., 2016). However, sitagliptin did not improve the oGTT curve (Hitomi Oda et al., 2014). Although the three studies used equal drug doses (1 mg.kg−1) and similar sample sizes (4 - 6), variations in results may be due to differences in dogs´ breeds, sexes, and treatment duration.
Table 2.
GLP-1-based drug studies in dogs.
| Breed | Age (years) | Sex | Castration | "n" per group | Condition | Drug | Brant / Manufacturer | Dosage | Treatment Duration | Complications | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NI | NI | NI | NI | 6 | healthy | vildagliptin | NI | 1 mg.kg−1 | acute | NI | (Edgerton et al., 2009) |
| Beagle | NI | M | NI | 4 – 5 | healthy | gemigliptin | NI/ LG Life Sciences | 1 mg.kg−1 | acute | NI | (Kim et al., 2016) |
| Beagle | 3 to 5 | F/M | All | 5 | healthy | sitagliptin | Januvia/ MSD K.K. | 1 mg.kg−1 | 7 days | NI | (Oda et al., 2014) |
| Mongrel | NI | F/M | NI | 6 | depancreatized | GLP-1 (7–36) amide | NI/ Bachem | 1.5 pmol.kg−1.min−1 | acute | NI | (Sandhu et al., 1999) |
| ASDI-strain | 2,8 ± 0,4 | F/M | NI | 9 | DM | GLP-1 (7–36) amide | NI/ Saxon Biochemicals | 10 pmol.kg−1.min−1 | acute | NI | (Freyse et al., 1999) |
| Beagle – MDH | 3 to 11 | F/M | 6 (of 9) | 4 – 5 | DM – healthy | liraglutide | Victoza/ Novo Nordisk | 15 µg.kg−1 | acute | NI | (Oda et al., 2013) |
| Mongrel | NI | F/M | NI | 4 – 5 | healthy | lixisenatide | NI/ Sanofi | 1.5 µg.kg−1 | acute | vomiting | (Moore et al., 2013) |
| Mongrel | NI | F/M | NI | 7 – 8 | healthy | GLP-1 (7–36) amide | NI/ Sigma-Aldrich | 0.9 – 5.1 – 10 – 20 pmol.kg−1.min−1 | acute | NO | (Nishizawa et al., 2003) |
| Mongrel | 3.2 ± 0.7 | F/M | NI | 5 | healthy | GLP1 (32–36) amide | NI/ Massachusetts General Hospital | 30 pmol.kg−1.min−1 | acute | NI | (Elahi et al., 2014) |
| Beagle | 3 to 4 | M | NI | 2 – 3 | healthy | ZY15557 | NI/ Zydus Research center | 0.5 – 3 mg.k − 1 | acute | NI | (Jain et al., 2017) |
| Beagle | adults | M | NI | 2 – 6 | healthy | ZYDPLA1 | NI/ Zydus Research center | 0.5 – 2 mg.k − 1 | acute | NI | (Jain et al., 2015) |
| Beagle | NI | M | NI | 4 | healthy | DBPR108 | NI/ Ryss Laboratory | 0.01, 0.05, 0.1, 0.3, 1 and 3 mg.kg−1 | acute | NI | (Yeh et al., 2021) |
| Mongrel | 1 to 2 | M | NI | 7 | pre-DM model | exenatide | NI/ Amylin Pharmaceuticals | 10 μg* | 12 weeks | NI | (Ionut et al., 2016) |
| Beagle | adults | F | NI | 6 | ischemia-reperfusion model | alogliptin | NI/ Takeda Pharmaceuticals | 3 mg.kg−1 | 4 days | NI | (Ihara et al., 2015) |
| Beagle | 3 – 10 to 12⁎⁎ | NI | NI | 7 – 9 | cardiomyopathy | GLP −1 (7–36) amide | NI/ Massachusetts General Hospital | 2.5 pmol.kg−1.min−1 | 7 weeks | NI | (Chen et al., 2014) |
| Mongrel | NI | F/M | NI | 7 – 9 | cardiomyopathy | GLP −1 (7–36) and (9–36) amide | NI/ Massachusetts General Hospital | 1.5 pmol.kg−1.min−1 for both | 48 h | NI | (Nikolaidis et al., 2005) |
| Beagle | adults | F | NI | 3 – 4 | cardiomyopathy | liraglutide | Victoza/ Novo Nordisk | 150 µg.kg−1.day−1 | 24 days | NI | (Nakamura et al., 2019) |
NO: not observed. NI not informed. DM: diabetes mellitus. MDH: miniature Dachshund.
the authors reported only the total administered.
the animals were divided into groups according to age into young (3 years) and old (10 to 12 years).
Early studies with GLP-1 or its analogues in diabetic dogs also showed conflicting results. In depancreatized dogs, glucose utilization increased, while circulating free fatty acids and glycerol reduced when GLP-1 (7–36) amide (1.5 pmol.kg−1.min−1) was administered along with insulin Sandhu et al., (1999). In the same year, Freyse et al. (1999) performed a normoglycemic clamp in DM dogs with combined therapy of insulin and GLP-1(7–36) amide (10 pmol.kg−1.min−1). The administration of GLP-1 did not change insulin, glucose, and glucagon infusions rate. There were also no differences in metabolic glucose clearance, alanine turnover, urea synthesis and glucose formation by gluconeogenesis using alanine as precursor. This investigation line was resumed only in 2013. In healthy and DM dogs, liraglutide treatment (15 µg.kg−1) reduced the area under the curve in an oGTT. Also, in diabetic dogs the circulating glucose decreased by 66.5% with liraglutide compared to dogs treated with insulin alone, revealing its potential use in DM. The authors correlate the suppression of glucagon secretion with the observed effect (Oda et al., 2013).
Few studies with GLP-1, GLP-1A or DPP4i treatment have been conducted in DM dogs, but the outcomes in healthy animals point to a potential utilization of these drugs. Lixisenatide (1.5 µg.kg−1), a GLP-1A, decreased glucose and insulin areas under the curves (Moore, Werner, Smith, Farmer & Cherrington, 2013). GLP-1 (7–36) amide intraportal infusion (0.9, 5.1, 10, 20 pmol.kg−1.min−1) increased glucose uptake (hepatic and peripheral) and the glucose infusion rate necessary to maintain blood glucose levels (Nishizawa et al., 2003). In hyperglycemic clamp, dogs treated with of GLP-1 (32–36) amide cleavage-derived pentapeptide (30 pmol.kg−1.min−1) increased glucose uptake regardless of insulin and glucagon changes (Elahi et al., 2014). Taken together, these studies reinforce the hypothesis that unlike rodents and humans, in dogs, the main action of GLP-1 is to stimulate glucose uptake instead of insulin release, although this action still needs to be better understood, especially the molecular mechanisms involved. Initially, this may be a promising finding, after all, GLP-1 action would be less dependent on β cells, which are highly damaged in canine DM (Gilor et al., 2016). However, unlike what occurs in feline DM, insulin resistance in dogs is not a primary factor in DM development (Gilor et al., 2016). Thus, it is possible to speculate what is the real benefit of this treatment for diabetic dogs. Besides, there are still many aspects to be explored until its applicability could be encouraged or dropped.
Although numerous molecules related to GLP-1 actions are available, clinical studies focused on obesity or overweight treatment with this drugs in dogs are limited to pharmacokinetic (Jain et al., 2015, 2017; Yeh et al., 2021). Exenatide effects were evaluated in a pre-diabetes canine model (high-fat diet plus streptozotocin injection). The animals without treatment developed increased body weight, reduced glucose tolerance and higher fasting blood glucose, but insulin sensitivity was not altered. Exenatide treatment (10 µg per dog BID for 12 weeks) led to weight loss, but did not affect food consumption, fasting glucose, insulin, glycated hemoglobin or OGTT. In the same study, exenatide increased insulin secretion in vitro (Ionut et al., 2016). Although this model, prediabetes, reproduces a human condition of low occurrence in dogs, the results indicates that GLP-1As may become one alternative to canine obesity treatment (Gilor et al., 2016; Ionut et al., 2016).
Another focus of investigations are the effects of GLP-1 on canine cardiovascular diseases. In an ischemia-reperfusion model, the DPP4i alogliptin (3 mg.kg−1), suppressed apoptosis pathways in heart extracts (Ihara et al., 2015). In a model of rapid atrial pacing, liraglutide treatment (150 µg.kg−1) was able to decrease the refractory period and lead to increased conduction velocity after 2 or 3 weeks (Nakamura et al., 2019). Pretreatment with GLP-1 (7–36) amide (2.5 pmol.kg−1.min−1) in a model of dilated cardiomyopathy decreased FFA levels and improved cardiac insulin resistance. In addition, GLP-1 treatment in elderly dogs increased latency for heart disease development and reduced mortality (Chen, Angeli, Shen & Shannon, 2014). In dogs with induced cardiomyopathy, the metabolite GLP-1 (9–36) amide improved left ventricular function. GLP-1 (9–36) amide also improved glucose uptake and cardiac output, without modifying insulin levels, presenting an insulinomimetic effect without insulinotropic action (Nikolaidis, Elahi, Shen & Shannon, 2005). Although there are still few studies and the knowledge on the subject is not sufficiently depth, the available data shows a potential beneficial effect of using GLP-1 mimetics in different models of cardiac insult.
5. Concerns and perspectives
The studies so far do not report serious side effects related to GLP-1As use, however, in high doses they can lead to gastrointestinal problems, such as nausea, vomiting and diarrhea (Hall et al., 2015; Moore et al., 2013). In humans, GLP-1As have already been related to an increase in neoplasms development, such as pancreatic adenocarcinoma and thyroid C-cell tumor, but recent meta-analyses of randomized controlled trials failed to show any significant increase in cancer risk (Cao, Yang & Zhou, 2019; Monami et al., 2017; Vangoitsenhoven, Mathieu & Van der Schueren, 2012). Studies evaluating GLP-1A administration and the risk of tumor development in domestic animals are still lacking.
A possible risk of DPP4i treatments stems from the fact that DPP4 (also known as CD26) is a T cell stimulator, so theoretically its inhibition could disrupt immune system regulation (Anz et al., 2014; Ohnuma, Dang & Morimoto, 2008). However, there is essentially no evidence that DPP4i cause deficiencies in the immune response. (Anz et al., 2014). The possibility that DPP4i may be harmful to animals with immune deficiency still needs to be clarified.
Human studies also show that DPP4i could slightly increase pancreatitis risk (from 0.067 to 0.117), which may be of particular concern for dogs (Aziz et al., 2020; DeVries & Rosenstock, 2017). Although the primary cause of DM in dogs often remains unknown, it is possible that a large percentage is due to complications from pancreatitis that lead to β-cell damage (Gilor et al., 2016). Yet, some studies demonstrate that GLP-1R activation in β cells inhibits apoptosis enhancing these cells survival (Aziz et al., 2020; DeVries & Rosenstock, 2017; Li et al., 2003). Thus, understanding how drugs based on GLP-1 act in the pancreas of dogs, protecting β cells or facilitating the development of pancreatitis, is a fundamental step towards their inclusion in the treatment of canine DM.
Castration is another factor that needs to be better evaluated. Recent studies have shown that sex steroids, especially testosterone and estradiol, modulates GLP-1 response in different tissues (El Bekay et al., 2016; Handgraaf, Dusaulcy, Visentin, Philippe & Gosmain, 2018; Maske, Jackson, Terrill, Eckel & Williams, 2017; Navarro et al., 2016). Although these are still initial data, there are indications that GLP-1 response of castrated males is lower than that of intact animals (El Bekay et al., 2016; Model et al., 2021b). On the other hand, castrated females would not only respond similarly to intact ones, but treatment with GLP-1As could reverse part of the metabolic problems associated with estrogens reduction (Handgraaf et al., 2018; Model et al., 2021a). Even though these data do not come from domestic animals, they indicate an important factor to be evaluated in future studies.
Currently, the body of evidence available in the literature indicates GLP-1As and DPP4-is as possible alternatives to DM treatment in domestic animals, but at present there is not enough information to recommend these drugs widespread clinical use. Many of the experiments described in this review have limitations that must be overcomed. The experimental amostral number (N), usually between 2 and 5, must be increased to avoid erroneous inferences (Callegari-Jacques, 2003). Still, the sample selection must be more careful, reducing the inherent variability of breed, sex, age, castration, among others. However, experimental replication is crucial, even in well-designed controlled studies, which reinforces the need for further studies evaluating the role of GLP-1 in physiological and pathophysiological conditions, and its therapeutic potential. Finally, after checking the effects in controlled studies, it would be reasonable to expand to studies in patients, with more heterogeneous samples, but with experimental N significantly higher than that of the laboratory experiments, as well as those used in epidemiological studies, to again avoid incorrect inferences (Porsani et al., 2020).
6. Conclusions
The data collected so far demonstrate that GLP-1 may have a species-specific action in dogs, being more insulinomimetic than insulinotropic, although the latter has already been observed. In cats, the physiological actions of GLP-1 seem like those considered classic in humans and rodents. In diabetic dogs, the use of drugs based on GLP-1 actions led to reduction in blood glucose and higher glucose uptake, while in diabetic cats there was a reduction in glycemic variability and in the need of exogenous insulin administration. Thus, available evidence indicates that GLP-1 based drugs could become alternatives to DM treatment in domestic animals. However, there are still many open points, and current data are surprisingly limited, not providing enough elements to recommend these drugs widespread clinical use.
Funding
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Pró-reitoria de Pesquisa (PROPESQ) of Universidade Federal do Rio Grande do Sul (UFRGS), Brazil.
Authors contributions
JFAM: Writing - original draft,Visualization,Writing - review & editing. DSR: Writing - original draft. ACF: Data Curation. ASV: Visualization, Writing - Review & Editing.
Declaration of Competing Interest
None
References
- Abd El Aziz M., Cahyadi O., Meier J.J., Schmidt W.E., Nauck M.A. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: A meta-analysis based on cardiovascular outcomes trials. Diabetes, Obesity and Metabolism. 2020;22(4):699–704. doi: 10.1111/dom.13924. 10.1111/dom.13924. [DOI] [PubMed] [Google Scholar]
- Anz D., Kruger S., Haubner S., Rapp M., Bourquin C., Endres S. The dipeptidylpeptidase-IV inhibitors sitagliptin, vildagliptin and saxagliptin do not impair innate and adaptive immune responses. Diabetes, Obesity and Metabolism. 2014;16(6):569–572. doi: 10.1111/dom.12246. 10.1111/dom.12246. [DOI] [PubMed] [Google Scholar]
- Appleton D., Rand J., Sunvold G. Insulin Sensitivity decreases with obesity, and lean cats with low insulin sensitivity are at greatest risk of glucose intolerance with weight gain. Journal of Feline Medicine and Surgery. 2001;3(4):211–228. doi: 10.1053/jfms.2001.0138. 10.1053/jfms.2001.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio L.L., Drucker D.J. Harnessing the therapeutic potential of glucagon-like peptide-1. Treatments in Endocrinology. 2002;1(2):117–125. doi: 10.2165/00024677-200201020-00005. 10.2165/00024677-200201020-00005. [DOI] [PubMed] [Google Scholar]
- Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Baggio L.L., Ussher J.R., McLean B.A., Cao X., Kabir M.G., Mulvihill E.E., et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Molecular Metabolism. 2017;6(11):1339–1349. doi: 10.1016/j.molmet.2017.08.010. 10.1016/j.molmet.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban K., Noyan-Ashraf M.H., Hoefer J., Bolz S.S., Drucker D.J., Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117(18):2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- Beiroa D., Imbernon M., Gallego R., Senra A., Herranz D., Villarroya F., et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63(10):3346–3358. doi: 10.2337/db14-0302. 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- Bullock B.P., Heller R.S., Habener J.F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137(7):2968–2978. doi: 10.1210/endo.137.7.8770921. 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- Bünemann, M., Gerhardstein, B.L., .Gao, T., & Hosey, M.M. (1999). Functional regulation of l-type calcium channels via protein kinase A-mediated phosphorylation of the β2 subunit. Journal of Biological Chemistry, 274(48), 33851–33854. 10.1074/jbc.274.48.33851. [DOI] [PubMed]
- Buteau J., El-Assaad W., Rhodes C.J., Rosenberg L., Joly E., Prentki M. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004;47(5):806–815. doi: 10.1007/s00125-004-1379-6. 10.1007/s00125-004-1379-6. [DOI] [PubMed] [Google Scholar]
- Buteau Jean, Foisy S., Joly E., Prentki M. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52(1):124–132. doi: 10.2337/diabetes.52.1.124. 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- Callegari-Jacques, S.M. (2003). Bioestatística: Princípios e aplicações. Porto Alegre: ARTMED.
- Camara A., Verbrugghe A., Cargo-Froom C., Hogan K., DeVries T.J., Sanchez A., et al. The daytime feeding frequency affects appetite-regulating hormones, amino acids, physical activity, and respiratory quotient, but not energy expenditure, in adult cats fed regimens for 21 days. PloS one. 2020;15(9) doi: 10.1371/journal.pone.0238522. 10.1371/journal.pone.0238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Yang S., Zhou Z. GLP-1 receptor agonists and risk of cancer in type 2 diabetes: An updated meta-analysis of randomized controlled trials. Endocrine. 2019;66(2):157–165. doi: 10.1007/s12020-019-02055-z. 10.1007/s12020-019-02055-z. [DOI] [PubMed] [Google Scholar]
- Challa T.D., Beaton N., Arnold M., Rudofsky G., Langhans W., Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. Journal of Biological Chemistry. 2012;287(9):6421–6430. doi: 10.1074/jbc.M111.310342. 10.1074/jbc.M111.310342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A.P., Sorrell J.E., Haller A., Roelofs K., Hutch C.R., Kim K.-.S., et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metabolism. 2017;25(4):927–934. doi: 10.1016/j.cmet.2017.02.008. e3. 10.1016/j.cmet.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Angeli F.S., Shen Y.tang, Shannon R.P. GLP-1 (7-36) amide restores myocardial insulin sensitivity and prevents the progression of heart failure in senescent beagles. Cardiovascular Diabetology. 2014;(1):13. doi: 10.1186/s12933-014-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia C.W., Egan J.M. Incretins in obesity and diabetes. Annals of the New York Academy of Sciences. 2020;1461(1):104–126. doi: 10.1111/nyas.14211. 10.1111/nyas.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damholt A.B., Buchan A.M.J., Kofod H. Glucagon-like-peptide-1 secretion from canine l-cells is increased by glucose-dependent-insulinotropic peptide but unaffected by glucose. Endocrinology. 1998;139(4):2085–2091. doi: 10.1210/endo.139.4.5921. [DOI] [PubMed] [Google Scholar]
- Damholt A.B., Kofod H., Buchan A.M.J. Immunocytochemical evidence for a paracrine interaction between GIP and GLP-1-producing cells in canine small intestine. Cell and Tissue Research. 1999;298(2):287–293. doi: 10.1007/s004419900093. 10.1007/s004419900093. [DOI] [PubMed] [Google Scholar]
- Dardevet D., Moore M.C., DiCostanzo C.A., Farmer B., Neal D.W., Snead W., et al. Insulin secretion-independent effects of GLP-1 on canine liver glucose metabolism do not involve portal vein GLP-1 receptors. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005;289(5) doi: 10.1152/ajpgi.00121.2005. G806–G814. 10.1152/ajpgi.00121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heer J., Rasmussen C., Coy D.H., Holst J.J. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51(12):2263–2270. doi: 10.1007/s00125-008-1149-y. 10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- Deacon C.F. Peptide degradation and the role of DPP-4 inhibitors in the treatment of type 2 diabetes. Peptides. 2018;100:150–157. doi: 10.1016/j.peptides.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Deacon C., Wamberg S., Bie P., Hughes T., Holst J. Preservation of active incretin hormones by inhibition of dipeptidyl peptidase IV suppresses meal-induced incretin secretion in dogs. Journal of Endocrinology. 2002;172(2):355–362. doi: 10.1677/joe.0.1720355. 10.1677/joe.0.1720355. [DOI] [PubMed] [Google Scholar]
- Delgado E., Luque M.A., Alcántara A., Trapote M.A., Clemente F., Galera C., et al. Glucagon-like peptide-1 binding to rat skeletal muscle. Peptides. 1995;16(2):225–229. doi: 10.1016/0196-9781(94)00175-8. 10.1016/0196-9781(94)00175-8. [DOI] [PubMed] [Google Scholar]
- DeVries J.H., Rosenstock J. DPP-4 inhibitor–related pancreatitis: rare but real! Diabetes care. 2017;40(2):161–163. doi: 10.2337/dci16-0035. [DOI] [PubMed] [Google Scholar]
- Drucker D.J., Philippe J., Mojsov S., Chick W.L., Habener J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proceedings of the National Academy of Sciences. 1987;84(10):3434–3438. doi: 10.1073/pnas.84.10.3434. 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker Daniel J. The ascending GLP-1 road from clinical safety to reduction of cardiovascular complications. Diabetes. 2018;67(9):1710–1719. doi: 10.2337/dbi18-0008. [DOI] [PubMed] [Google Scholar]
- Edgerton D.S., Johnson K.M.S., Neal D.W., Scott M., Hobbs C.H., Zhang X., et al. Inhibition of dipeptidyl peptidase-4 by vildagliptin during glucagon-like peptide 1 infusion increases liver glucose uptake in the conscious dog. Diabetes. 2009;58(1):243–249. doi: 10.2337/db08-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bekay R., Coín-Aragüez L., Fernández-García D., Oliva-Olivera W., Bernal-López R., Clemente-Postigo M., et al. Effects of glucagon-like peptide-1 on the differentiation and metabolism of human adipocytes. British Journal of Pharmacology. 2016;173(11):1820–1834. doi: 10.1111/bph.13481. 10.1111/bph.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi D., Angeli F.S., Vakilipour A., Carlson O.D., Tomas E., Egan J.M., et al. GLP-1(32–36)amide, a novel pentapeptide cleavage product of GLP-1, modulates whole body glucose metabolism in dogs. Peptides. 2014;59:20–24. doi: 10.1016/j.peptides.2014.06.004. 10.1016/j.peptides.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyse E.-.J., Knospe S., Becher T., El Hag O., Göke B., Fischer U. Glucagon-like peptide-1 has no insulin-like effects in insulin-dependent diabetic dogs maintained normoglycemic and normoinsulinemic. Metabolism. 1999;48(1):134–137. doi: 10.1016/s0026-0495(99)90023-9. 10.1016/S0026-0495(99)90023-9. [DOI] [PubMed] [Google Scholar]
- Furrer D., Kaufmann K., Tschuor F., Reusch C.E., Lutz T.A. The dipeptidyl peptidase IV inhibitor NVP-DPP728 reduces plasma glucagon concentration in cats. The Veterinary Journal. 2010;183(3):355–357. doi: 10.1016/j.tvjl.2008.11.017. 10.1016/j.tvjl.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Gilor C., Graves T.K., Gilor S., Ridge T.K., Rick M. The GLP-1 mimetic exenatide potentiates insulin secretion in healthy cats. Domestic Animal Endocrinology. 2011;41(1):42–49. doi: 10.1016/j.domaniend.2011.03.001. 10.1016/j.domaniend.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Gilor C., Graves T.K., Gilor S., Ridge T.K., Weng H.-.Y., Dossin O. The incretin effect in cats: Comparison between oral glucose, lipids, and amino acids. Domestic Animal Endocrinology. 2011;40(4):205–212. doi: 10.1016/j.domaniend.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Gilor C., Niessen S.J.M., Furrow E., DiBartola S.P. What's in a name? Classification of diabetes mellitus in veterinary medicine and why it matters. Journal of Veterinary Internal Medicine. 2016;30(4):927–940. doi: 10.1111/jvim.14357. 10.1111/jvim.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilor C., Rudinsky A.J., Hall M.J. New approaches to feline diabetes mellitus. Journal of Feline Medicine and Surgery. 2016;18(9):733–743. doi: 10.1177/1098612X16660441. 10.1177/1098612X16660441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M.J., Adin C.A., Borin-Crivellenti S., Rudinsky A.J., Rajala-Schultz P., Lakritz J., et al. Pharmacokinetics and pharmacodynamics of the glucagon-like peptide-1 analog liraglutide in healthy cats. Domestic Animal Endocrinology. 2015;51:114–121. doi: 10.1016/j.domaniend.2014.12.001. 10.1016/j.domaniend.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Handgraaf S., Dusaulcy R., Visentin F., Philippe J., Gosmain Y. 17-β Estradiol regulates proglucagon-derived peptide secretion in mouse and human α- and L cells. JCI Insight. 2018;(7):3. doi: 10.1172/jci.insight.98569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M.R., Skibicka K.P., Grill H.J. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149(8):4059–4068. doi: 10.1210/en.2007-1743. 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P. Regulation of hypothalamo-pituitary-adrenocortical responses to stressors by the nucleus of the solitary tract/dorsal vagal complex. Cellular and Molecular Neurobiology. 2018;38(1):25–35. doi: 10.1007/s10571-017-0543-8. 10.1007/s10571-017-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelmkjaer K.M., Wewer Albrechtsen N.J., Holst J.J., Cronin A.M., Nielsen D.H., Mandrup-Poulsen T., et al. A Placebo-controlled study on the effects of the glucagon-like peptide-1 mimetic, exenatide, on insulin secretion, body composition and adipokines in obese, client-owned cats. PloS one. 2016;11(5) doi: 10.1371/journal.pone.0154727. 10.1371/journal.pone.0154727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig M., Pach N., Thomaseth K., Le A., Schaeffer D., Ferguson D.C. Cats differ from other species in their cytokine and antioxidant enzyme response when developing obesity. Obesity. 2013 doi: 10.1002/oby.20306. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Holz G.G. Epac : A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes. 2004;53(1):5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I˙meryüz N., Yeğen B.Ç., Bozkurt A., Coşkun T., Villanueva-Peñacarrillo M.L., Ulusoy N.B. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1997;273(4):G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- Ihara M., Asanuma H., Yamazaki S., Kato H., Asano Y., Shinozaki Y., et al. An interaction between glucagon-like peptide-1 and adenosine contributes to cardioprotection of a dipeptidyl peptidase 4 inhibitor from myocardial ischemia-reperfusion injury. American Journal of Physiology-Heart and Circulatory Physiology. 2015;308(10) doi: 10.1152/ajpheart.00835.2014. H1287–H1297. 10.1152/ajpheart.00835.2014. [DOI] [PubMed] [Google Scholar]
- Ionut V., Hucking K., Liberty I.F., Bergman R.N. Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia. 2005;48(5):967–975. doi: 10.1007/s00125-005-1709-3. 10.1007/s00125-005-1709-3. [DOI] [PubMed] [Google Scholar]
- Ionut V., Liberty I.F., Hucking K., Lottati M., Stefanovski D., Zheng D., et al. Exogenously imposed postprandial-like rises in systemic glucose and GLP-1 do not produce an incretin effect, suggesting an indirect mechanism of GLP-1 action. American Journal of Physiology-Endocrinology and Metabolism. 2006;291(4):E779–E785. doi: 10.1152/ajpendo.00106.2005. 10.1152/ajpendo.00106.2005. [DOI] [PubMed] [Google Scholar]
- Ionut V., Woolcott O.O., Mkrtchyan H.J., Stefanovski D., Kabir M., Iyer M.S., et al. Exenatide treatment alone improves β-cell function in a canine model of pre-diabetes. PloS one. 2016;11(7) doi: 10.1371/journal.pone.0158703. 10.1371/journal.pone.0158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M.R., Joharapurkar A.A., Bahekar R.H., Patel H., Jadav P., Kshirsagar S.G., et al. Pharmacological characterization of ZYDPLA1, a novel long-acting dipeptidyl peptidase-4 inhibitor. Journal of Diabetes. 2015;7(5):708–717. doi: 10.1111/1753-0407.12233. 10.1111/1753-0407.12233. [DOI] [PubMed] [Google Scholar]
- Jain M.R., Joharapurkar A.A., Kshirsagar S.G., Patel V.J., Bahekar R.H., Patel H.V., et al. ZY15557, a novel, long acting inhibitor of dipeptidyl peptidase-4, for the treatment of Type 2 diabetes mellitus. British Journal of Pharmacology. 2017;174(14):2346–2357. doi: 10.1111/bph.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.M.S., Edgerton D.S., Rodewald T., Scott M., Farmer B., Neal D., et al. Intraportal GLP-1 infusion increases nonhepatic glucose utilization without changing pancreatic hormone levels. American Journal of Physiology-Endocrinology and Metabolism. 2007;293(4) doi: 10.1152/ajpendo.00275.2007. E1085–E1091. 10.1152/ajpendo.00275.2007. [DOI] [PubMed] [Google Scholar]
- Johnson K.M.S., Edgerton D.S., Rodewald T., Scott M., Farmer B., Neal D., et al. Intraportally delivered GLP-1, in the presence of hyperglycemia induced via peripheral glucose infusion, does not change whole body glucose utilization. American Journal of Physiology-Endocrinology and Metabolism. 2008;294(2):E380–E384. doi: 10.1152/ajpendo.00642.2007. 10.1152/ajpendo.00642.2007. [DOI] [PubMed] [Google Scholar]
- Kang G., Leech C.A., Chepurny O.G., Coetzee W.A., Holz G.G. Role of the cAMP sensor Epac as a determinant of K ATP channel ATP sensitivity in human pancreatic β-cells and rat INS-1 cells. The Journal of Physiology. 2008;586(5):1307–1319. doi: 10.1113/jphysiol.2007.143818. 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapodistria K., Tsilibary E., Kotsopoulou E., Moustardas P., Kitsiou P. Liraglutide, a human glucagon-like peptide-1 analogue, stimulates AKT-dependent survival signalling and inhibits pancreatic β-cell apoptosis. Journal of Cellular and Molecular Medicine. 2018;22(6):2970–2980. doi: 10.1111/jcmm.13259. 10.1111/jcmm.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-.H., Jung E., Yoon M.K., Kwon O.H., Hwang D.-.M., Kim D.-.W., et al. Pharmacological profiles of gemigliptin (LC15-0444), a novel dipeptidyl peptidase-4 inhibitor, in vitro and in vivo. European Journal of Pharmacology. 2016;788:54–64. doi: 10.1016/j.ejphar.2016.06.016. 10.1016/j.ejphar.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Knudsen L.B., Lau J. The discovery and development of liraglutide and semaglutide. Frontiers in Endocrinology. 2019;10 doi: 10.3389/fendo.2019.00155. 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman S., Wang Y., Parlevliet E.T., Boon M.R., Edelschaap D., Snaterse G., et al. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia. 2015;58(11):2637–2646. doi: 10.1007/s00125-015-3727-0. 10.1007/s00125-015-3727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer A.L., Riederer A., Fracassi F., Boretti F.S., Sieber-Ruckstuhl N.S., Lutz T.A., et al. Glycemic variability in newly diagnosed diabetic cats treated with the glucagon-like peptide-1 analogue exenatide extended release. Journal of Veterinary Internal Medicine. 2020;34(6):2287–2295. doi: 10.1111/jvim.15915. 10.1111/jvim.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J.-.P., Arnold M., Pettersen K.G., Lossel P., Langhans W., Lee S.J. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34–43. doi: 10.2337/db15-0973. 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- Li Y., Hansotia T., Yusta B., Ris F., Halban P.A., Drucker D.J. Glucagon-like peptide-1 receptor signaling modulates β cell apoptosis. Journal of Biological Chemistry. 2003;278(1):471–478. doi: 10.1074/jbc.M209423200. 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- Lubbs D.C., Vester Boler B.M., Ridge T.K., Spears J.K., Graves T.K., Swanson K.S. Dietary macronutrients and feeding frequency affect fasting and postprandial concentrations of hormones involved in appetite regulation in adult dogs. Journal of Animal Science. 2010;88(12):3945–3953. doi: 10.2527/jas.2010-2938. 10.2527/jas.2010-2938. [DOI] [PubMed] [Google Scholar]
- MacDonald P.E., Wang X., Xia F., El-kholy W., Targonsky E.D., Tsushima R.G., et al. Antagonism of rat β-cell voltage-dependent K+ currents by exendin 4 requires dual activation of the cAMP/protein kinase A and phosphatidylinositol 3-kinase signaling pathways. Journal of Biological Chemistry. 2003;278(52):52446–52453. doi: 10.1074/jbc.M307612200. 10.1074/jbc.M307612200. [DOI] [PubMed] [Google Scholar]
- Maske C.B., Jackson C.M., Terrill S.J., Eckel L.A., Williams D.L. Estradiol modulates the anorexic response to central glucagon-like peptide 1. Hormones and Behavior. 2017;93:109–117. doi: 10.1016/j.yhbeh.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool K.E., Rudinsky A.J., Parker V.J., Herbert C.O., Gilor C. The effect of diet, adiposity, and weight loss on the secretion of incretin hormones in cats. Domestic Animal Endocrinology. 2018;62:67–75. doi: 10.1016/j.domaniend.2017.10.004. 10.1016/j.domaniend.2017.10.004. [DOI] [PubMed] [Google Scholar]
- McMillan C.J., Zapata R.C., Chelikani P.K., Snead E.C.R., Cosford K. Circulating concentrations of glucagon-like peptide 1, glucose-dependent insulinotropic peptide, peptide YY, and insulin in client-owned lean, overweight, and diabetic cats. Domestic Animal Endocrinology. 2016;54:85–94. doi: 10.1016/j.domaniend.2015.10.001. 10.1016/j.domaniend.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Meloni A.R., DeYoung M.B., Lowe C., Parkes D.G. GLP-1 receptor activated insulin secretion from pancreatic β-cells: Mechanism and glucose dependence. Diabetes, Obesity and Metabolism. 2013;15(1):15–27. doi: 10.1111/j.1463-1326.2012.01663.x. 10.1111/j.1463-1326.2012.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model J.F.A., Lima M.V., Ohlweiler R., Lopes Vogt É., Rocha D.S., Souza S.K.de.…Vinagre A.S. Liraglutide improves lipid and carbohydrate metabolism of ovariectomized rats. Molecular and Cellular Endocrinology. 2021;524 doi: 10.1016/j.mce.2021.111158. [DOI] [PubMed] [Google Scholar]
- Model J.F.A., Lima M.V., Ohlweiler R., Sarapio E., Vogt É.L., Rocha D.S., et al. Liraglutide treatment counteracts alterations in adipose tissue metabolism induced by orchiectomy in rats. Life Sciences. 2021;278 doi: 10.1016/j.lfs.2021.119586. [DOI] [PubMed] [Google Scholar]
- Monami M., Nreu B., Scatena A., Cresci B., Andreozzi F., Sesti G., et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes, Obesity and Metabolism. 2017;19(9):1233–1241. doi: 10.1111/dom.12926. 10.1111/dom.12926. [DOI] [PubMed] [Google Scholar]
- Moore M.C., Werner U., Smith M.S., Farmer T.D., Cherrington A.D. Effect of the glucagon-like peptide-1 receptor agonist lixisenatide on postprandial hepatic glucose metabolism in the conscious dog. American Journal of Physiology-Endocrinology and Metabolism. 2013;305(12):E1473–E1482. doi: 10.1152/ajpendo.00354.2013. 10.1152/ajpendo.00354.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A., Ueda K., Lee P., Oda H., Ishioka K., Arai T., et al. Effect of acarbose, sitagliptin and combination therapy on blood glucose, insulin, and incretin hormone concentrations in experimentally induced postprandial hyperglycemia of healthy cats. Research in Veterinary Science. 2016;106:131–134. doi: 10.1016/j.rvsc.2016.04.001. 10.1016/j.rvsc.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Müller T.D., Finan B., Bloom S.R., D'Alessio D., Drucker D.J., Flatt P.R., et al. Glucagon-like peptide 1 (GLP-1) Molecular Metabolism. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Niwano S., Niwano H., Fukaya H., Murakami M., Kishihara J., et al. Liraglutide suppresses atrial electrophysiological changes. Heart and Vessels. 2019;34(8):1389–1393. doi: 10.1007/s00380-018-01327-4. 10.1007/s00380-018-01327-4. [DOI] [PubMed] [Google Scholar]
- Nauck M.A., Meier J.J. Glucagon-like peptide 1 and its derivatives in the treatment of diabetes. Regulatory Peptides. 2005;128(2):135–148. doi: 10.1016/j.regpep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Nauck M.A., Meier J.J. Incretin hormones: Their role in health and disease. Diabetes, Obesity and Metabolism. 2018;20:5–21. doi: 10.1111/dom.13129. October 201710.1111/dom.13129. [DOI] [PubMed] [Google Scholar]
- Navarro G., Xu W., Jacobson D.A., Wicksteed B., Allard C., Zhang G., et al. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metabolism. 2016;23(5):837–851. doi: 10.1016/j.cmet.2016.03.015. 10.1016/j.cmet.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis L.A., Elahi D., Shen Y.-.T., Shannon R.P. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. American Journal of Physiology-Heart and Circulatory Physiology. 2005;289(6) doi: 10.1152/ajpheart.00347.2005. H2401–H2408. 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- Nishii N., Takashima S., Iguchi A., Murahata Y., Matsuu A., Hikasa Y., et al. Effects of sitagliptin on plasma incretin concentrations after glucose administration through an esophagostomy tube or feeding in healthy cats. Domestic Animal Endocrinology. 2014;49:14–19. doi: 10.1016/j.domaniend.2014.04.006. 10.1016/j.domaniend.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Moore M.C., Shiota M., Gustavson S.M., Snead W.L., Neal D.W., et al. Effect of intraportal glucagon-like peptide-1 on glucose metabolism in conscious dogs. American Journal of Physiology-Endocrinology and Metabolism. 2003;284(5):E1027–E1036. doi: 10.1152/ajpendo.00503.2002. 10.1152/ajpendo.00503.2002. [DOI] [PubMed] [Google Scholar]
- Oda H., Mori A., Lee P., Saeki K., Arai T., Sako T. Preliminary study characterizing the use of sitagliptin for glycemic control in healthy beagle dogs with normal gluco-homeostasis. Journal of Veterinary Medical Science. 2014;76(10):1383–1387. doi: 10.1292/jvms.13-0590. 10.1292/jvms.13-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H., Mori A., Lee P., Saeki K., Ishioka K., Arai T., et al. Characterization of the use of liraglutide for glycemic control in healthy and Type 1 diabetes mellitus suffering dogs. Research in Veterinary Science. 2013;95(2):381–388. doi: 10.1016/j.rvsc.2013.04.003. 10.1016/j.rvsc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Ohneda A., Ohneda K., Ohneda M., Koizumi F., Ohashi S., Kawai K., et al. The structure-function relationship of GLP-1 related peptides in the endocrine function of the canine pancreas. The Tohoku Journal of Experimental Medicine. 1991;165(3):209–221. doi: 10.1620/tjem.165.209. 10.1620/tjem.165.209. [DOI] [PubMed] [Google Scholar]
- Ohnuma K., Dang N.H., Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends in Immunology. 2008;29(6):295–301. doi: 10.1016/j.it.2008.02.010. 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Ørskov C., Holst J.J., Nielsen O.V. Effect of truncated glucagon-like peptide-1 [Proglucagon-(78–107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology. 1988;123(4):2009–2013. doi: 10.1210/endo-123-4-2009. 10.1210/endo-123-4-2009. [DOI] [PubMed] [Google Scholar]
- Pacheco B.P.M., Crajoinas R.O., Couto G.K., Davel A.P.C., Lessa L.M., Rossoni L.V., et al. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. Journal of Hypertension. 2011;29(3):520–528. doi: 10.1097/HJH.0b013e328341939d. 10.1097/HJH.0b013e328341939d. [DOI] [PubMed] [Google Scholar]
- Padrutt I., Lutz T.A., Reusch C.E., Zini E. Effects of the glucagon-like peptide-1 (GLP-1) analogues exenatide, exenatide extended-release, and of the dipeptidylpeptidase-4 (DPP-4) inhibitor sitagliptin on glucose metabolism in healthy cats. Research in Veterinary Science. 2015;99:23–29. doi: 10.1016/j.rvsc.2014.12.001. 10.1016/j.rvsc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Porsani M.Y.H., Teixeira F.A., Oliveira V.V., Pedrinelli V., Dias R.A., German A.J., et al. Prevalence of canine obesity in the city of São Paulo, Brazil. Scientific Reports. 2020;10(1):14082. doi: 10.1038/s41598-020-70937-8. 10.1038/s41598-020-70937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke C., Heller R.S., Kirk R.K., Ørskov C., Reedtz-Runge S., Kaastrup P., et al. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280–1290. doi: 10.1210/en.2013-1934. 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- Reimann F., Gribble F.M. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51(9):2757–2763. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- Richards P., Parker H.E., Adriaenssens A.E., Hodgson J.M., Cork S.C., Trapp S., et al. Identification and characterization of GLP-1 receptor–expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. doi: 10.2337/db13-1440. 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer A., Zini E., Salesov E., Fracassi F., Padrutt I., Macha K., et al. Effect of the glucagon-like peptide-1 analogue exenatide extended release in cats with newly diagnosed diabetes mellitus. Journal of Veterinary Internal Medicine. 2016;30(1):92–100. doi: 10.1111/jvim.13817. 10.1111/jvim.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands J., Heng J., Newsholme P., Carlessi R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Frontiers in Endocrinology. 2018;9:1–23. doi: 10.3389/fendo.2018.00672. (November)10.3389/fendo.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudinsky A.J., Adin C.A., Borin-Crivellenti S., Rajala-Schultz P., Hall M.J., Gilor C. Pharmacology of the glucagon-like peptide-1 analog exenatide extended-release in healthy cats. Domestic Animal Endocrinology. 2015;51:78–85. doi: 10.1016/j.domaniend.2014.12.003. 10.1016/j.domaniend.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Rüttimann E.B., Arnold M., Hillebrand J.J., Geary N., Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–1181. doi: 10.1210/en.2008-1221. 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu H., Wiesenthal S.R., MacDonald P.E., McCall R.H., Tchipashvili V., Rashid S., et al. Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes. 1999;48(5):1045–1053. doi: 10.2337/diabetes.48.5.1045. 10.2337/diabetes.48.5.1045. [DOI] [PubMed] [Google Scholar]
- Schauf S., Salas-Mani A., Torre C., Jimenez E., Latorre M.A., Castrillo C. Effect of feeding a high-carbohydrate or a high-fat diet on subsequent food intake and blood concentration of satiety-related hormones in dogs. Journal of Animal Physiology and Animal Nutrition. 2018;102(1):e21–e29. doi: 10.1111/jpn.12696. 10.1111/jpn.12696. [DOI] [PubMed] [Google Scholar]
- Scheen A.J. The safety of gliptins : Updated data in 2018. Expert Opinion on Drug Safety. 2018;17(4):387–405. doi: 10.1080/14740338.2018.1444027. 10.1080/14740338.2018.1444027. [DOI] [PubMed] [Google Scholar]
- Schneider E.L., Reid R., Parkes D.G., Lutz T.A., Ashley G.W., Santi D.V. A once-monthly GLP-1 receptor agonist for treatment of diabetic cats. Domestic Animal Endocrinology. 2020;70 doi: 10.1016/j.domaniend.2019.07.001. 10.1016/j.domaniend.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Scuderi M.A., Ribeiro Petito M., Unniappan S., Waldner C., Mehain S., McMillian C.J., et al. Safety and efficacy assessment of a GLP-1 mimetic: Insulin glargine combination for treatment of feline diabetes mellitus. Domestic Animal Endocrinology. 2018;65:80–89. doi: 10.1016/j.domaniend.2018.04.003. 10.1016/j.domaniend.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Seyfert T.M., Brunker J.D., Maxwell L.K., Payton M.E., McFarlane D. Effects of a glucagon-like peptide-1 mimetic (exenatide) in healthy cats. International Journal of Applied Research in Veterinary Medicine. 2012;10(2):147–156. [Google Scholar]
- Shimoda M., Kanda Y., Hamamoto S., Tawaramoto K., Hashiramoto M., Matsuki M., et al. The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia. 2011;54(5):1098–1108. doi: 10.1007/s00125-011-2069-9. 10.1007/s00125-011-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S., Schurdak J.D., Seeley R.J., Benoit S.C., Davis J.F. Central & peripheral glucagon-like peptide-1 receptor signaling differentially regulate addictive behaviors. Physiology & Behavior. 2016;161:140–144. doi: 10.1016/j.physbeh.2016.04.013. 10.1016/j.physbeh.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Skelin M., Rupnik M. cAMP increases the sensitivity of exocytosis to Ca2+ primarily through protein kinase A in mouse pancreatic beta cells. Cell calcium. 2011;49(2):89–99. doi: 10.1016/j.ceca.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Smith E.P., An Z., Wagner C., Lewis A.G., Cohen E.B., Li B., et al. The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metabolism. 2014;19(6):1050–1057. doi: 10.1016/j.cmet.2014.04.005. 10.1016/j.cmet.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M.M., Tonneijck L., Muskiet M.H.A., Kramer M.H.H., Pieters-van den Bos I.C., Vendrik K.E.W., et al. Pancreatic effects of liraglutide or sitagliptin in overweight patients with type 2 diabetes: A 12-week randomized, placebo-controlled trial. Diabetes care. 2017;40(3):301–308. doi: 10.2337/dc16-0836. 10.2337/dc16-0836. [DOI] [PubMed] [Google Scholar]
- Sugiyama K., Manaka H., Kato T., Yamatani K., Tominaga M., Sasaki H. Stimulation of truncated glucagon-like peptide-1 release from the isolated perfused canine ileum by glucose absorption. Digestion. 1994;55(1):24–28. doi: 10.1159/000201118. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M., Larsen P.J., Goke R., Fink-Jensen A., Jessop D.S., Moller M., et al. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1996;271(4) doi: 10.1152/ajpregu.1996.271.4.R848. R848–R856. 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- Tolhurst G., Reimann F., Gribble F.M. Nutritional regulation of glucagon-like peptide-1 secretion. The Journal of Physiology. 2009;587(1):27–32. doi: 10.1113/jphysiol.2008.164012. 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunekawa S., Yamamoto N., Tsukamoto K., Itoh Y., Kaneko Y., Kimura T., et al. Protection of pancreatic β-cells by exendin-4 may involve the reduction of endoplasmic reticulum stress; in vivo and in vitro studies. Journal of Endocrinology. 2007;193(1):65–74. doi: 10.1677/JOE-06-0148. 10.1677/JOE-06-0148. [DOI] [PubMed] [Google Scholar]
- Van Citters G.W., Kabir M., Kim S.P., Mittelman S.D., Dea M.K., Brubaker P.L., et al. Elevated glucagon-like peptide-1-(7–36)-amide, but not glucose, associated with hyperinsulinemic compensation for fat feeding. The Journal of Clinical Endocrinology & Metabolism. 2002;87(11):5191–5198. doi: 10.1210/jc.2002-020002. 10.1210/jc.2002-020002. [DOI] [PubMed] [Google Scholar]
- Vangoitsenhoven R., Mathieu C., Van der Schueren B. GLP1 and cancer: Friend or foe? Endocrine-Related Cancer. 2012;19(5) doi: 10.1530/ERC-12-0111. F77–F88. 10.1530/ERC-12-0111. [DOI] [PubMed] [Google Scholar]
- Wang Y., Egan J.M., Raygada M., Nadiv O., Roth J., Montrose-Rafizadeh C. Glucagon-like peptide-1 affects gene transcription and messenger ribonucleic acid stability of components of the insulin secretory system in RIN 1046-38 cells. Endocrinology. 1995;136(11):4910–4917. doi: 10.1210/endo.136.11.7588224. 10.1210/endo.136.11.7588224. [DOI] [PubMed] [Google Scholar]
- Wettergren A., Wojdemann M., Meisner S., Stadil F., Holst J.J. The inhibitory effect of glucagon-like peptide-1 (GLP-1) 7-36 amide on gastric acid secretion in humans depends on an intact vagal innervation. Gut. 1997;40(5):597–601. doi: 10.1136/gut.40.5.597. 10.1136/gut.40.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh K.-.C., Yeh T.-.K., Huang C.-.Y., Hu C.-.B., Wang M.-.H., Huang Y.-.W., et al. DBPR108, a novel dipeptidyl peptidase-4 inhibitor with antihyperglycemic activity. Life Sciences. 2021;278 doi: 10.1016/j.lfs.2021.119574. [DOI] [PubMed] [Google Scholar]