Abstract
Background
Shanghai, in east China, has one of the world’s highest burdens of Helicobacter pylori infection. While multidrug regimens can effectively eradicate H. pylori, the increasing prevalence of antibiotic resistance (AR) in H. pylori has been recognized by the WHO as ‘high priority’ for urgent need of new therapies. Moreover, the genetic characteristics of H. pylori AR in Shanghai is under-reported. The purpose of this study was to determine the resistance prevalence, re-substantiate resistance-conferring mutations, and investigate novel genetic elements associated with H. pylori AR.
Results
We performed whole genome sequencing and antimicrobial susceptibility testing of 112 H. pylori strains isolated from gastric biopsy specimens from Shanghai patients with different gastric diseases. No strains were resistant to amoxicillin. Levofloxacin, metronidazole and clarithromycin resistance was observed in 39 (34.8%), 73 (65.2%) and 18 (16.1%) strains, respectively. There was no association between gastroscopy diagnosis and resistance phenotypes. We reported the presence or absence of several subsystem protein coding genes including hopE, hofF, spaB, cagY and pflA, and a combination of CRISPRs, which were potentially correlated with resistance phenotypes. The H. pylori strains were also annotated for 80 genome-wide AR genes (ARGs). A genome-wide ARG analysis was performed for the three antibiotics by correlating the phenotypes with the genetic variants, which identified the well-known intrinsic mutations conferring resistance to levofloxacin (N87T/I and/or D91G/Y mutations in gyrA), metronidazole (I38V mutation in fdxB), and clarithromycin (A2143G and/or A2142G mutations in 23S rRNA), and added 174 novel variations, including 23 non-synonymous SNPs and 48 frameshift Indels that were significantly enriched in either the antibiotic-resistant or antibiotic-susceptible bacterial populations. The variant-level linkage disequilibrium analysis highlighted variations in a protease Lon with strong co-occurring correlation with a series of resistance-associated variants.
Conclusion
Our study revealed multidrug antibiotic resistance in H. pylori strains from Shanghai, which was characterized by high metronidazole and moderate levofloxacin resistance, and identified specific genomic characteristics in relation to H. pylori AR. Continued surveillance of H. pylori AR in Shanghai is warranted in order to establish appropriate eradication treatment regimens for this population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13099-022-00488-y.
Keywords: Helicobacter pylori, Antibiotic resistance, Outer membrane protein, Virulence, CRISPRs, Variation, Genetic characteristics
Introduction
Helicobacter pylori colonizes the gastric mucosa of 50–70% of the world’s population and increases the risk of developing chronic gastritis, peptic ulcer, gastric cancer, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma [1–3]. Currently, in China, the recommended quadruple regimens for H. pylori eradication therapy include a proton pump inhibitor (PPI) and bismuth, accompanied by two antibiotics, including clarithromycin (CLA), metronidazole (MTZ), levofloxacin (LEV), and amoxicillin (AMX) [4, 5], with the LEV-containing regimen recommended as an alternative for rescue therapy. Moreover, the choice of regimens should be based on the local H. pylori antibiotic resistance (AR) and history of personal medication. However, H. pylori exhibits a diverse genomic structure with a high mutation rate and, under selective antibiotic pressure, manifests growing AR, especially to MTZ and CLA [6]. The rates of resistance of H. pylori to MTZ, CLA, and LEV in China have previously been measured as 40–70%, 20–50% and 20–50%, respectively [7]. Increasing rates of resistance have also been reported in Europe and America [5, 8], and antibiotic-resistant H. pylori has been recognized by the World Health Organization (WHO) as a “high priority” bacterium for which new therapies are urgently needed [9]. Given the limited number of antibiotics that are appropriate for H. pylori eradication and the worldwide increase in AR, the need for new efforts to comprehensively understand the mechanisms underlying H. pylori resistance to existing antibiotics is significant.
The genomic diversity of H. pylori is crucial for the establishment of associations between genetic elements and AR. A well-known example of the intrinsic resistance mechanism of H. pylori has been elucidated, showing that the emergence of resistance occurs mainly due to mutations in antimicrobial target genes. In particular, the single-nucleotide mutations in the target genes of CLA (23S rRNA), MTZ (rdxA, frxA and fdxB), and LEV (gyrA and gyrB) enable H. pylori to evade the corresponding antibiotic activity by inhibiting bacterial protein synthesis, hampering intracellular reduction-activation of MTZ, and blocking bacterial DNA replication and transcription, respectively [10, 11]. In particular, for MTZ resistance, there are several relevant mutations in the genes specified, especially in rdxA, where insertions and deletions, as well as non-synonymous mutations, can occur to disrupt or hamper the function of the RdxA protein. Ciprofloxacin and levofloxacin resistance is caused primarily by non-synonymous mutations, leading to amino acid substitutions at positions 87 and 91 in the GyrA protein [10, 11]. However, AR in H. pylori is a complex and multifactorial problem. Several other possible mechanisms have been described, including high expression of outer membrane proteins (OMPs) that form a permeability barrier to reduce antibiotic uptake [12], loss of porins with narrow pore channels causing decreased uptake of extracellular antibiotics [13], increased efflux of antibiotics through efflux pump transporters [14, 15], passive tolerance to antimicrobials by biofilm formation [16], and sporadic mutations with synergistic effects in the loci of some important protein synthesis factors, such as translation initiation factor IF-2 and ribosomal protein L22 [17, 18]. Furthermore, many other whole-genome associations with AR have been established in recent years. For instance, a significant association has been found between the virulence factor dupA1 genotype and the A2147G CLA resistance mutation [19], and a correlation has been observed between resistant H. pylori strains and CagA-negative strains [20]. Additionally, in Gram-negative bacteria, most studies have suggested an inverse relationship of the clustered regularly interspaced short palindromic repeat (CRISPR) loci with antimicrobial resistance in Escherichia coli, Acinetobacter baumannii, and Klebsiella pneumoniae [21–24], although some inconsistent observations showed either a positive or negative correlation of CRISPR-Cas with AR genes (ARGs) in some clinically important species [25]. However, although H. pylori is indeed one of the few bacterial species constitutively competent for natural transformation and uptake extracellular DNA [26], little is known about the relationship between CRISPRs and AR and acquired resistance genes in H. pylori.
Recently, based on the tools and databases used in AR studies, a genome-wide ARG analysis provided unprecedented insights into the global distribution of potential AR determinants of many pathogenic bacteria [27], facilitating the discovery of the molecular bases of the association between AR and genetic diversity. The aim of this study was to determine the resistance prevalence, re-substantiate resistance-conferring mutations, and investigate novel genetic elements associated with AR by specific subsystem analysis (locus level) and genome-wide ARG analysis (variant level), within the 112 clinically isolated H. pylori strains from Shanghai.
Materials and methods
Patients and samples
A total of 112 H. pylori strains (H. pylori-Shi) from 112 individual patients were successfully isolated from 437 gastric biopsy specimens collected during endoscopy in patients with gastric diseases who resided in Shanghai. Patients who had received treatment for H. pylori with any antibiotics, H2 receptor blockers, or proton pump inhibitors within 4 weeks before the study were excluded. All the biopsy samples were identified as being positive for H. pylori by morphological analysis and immunohistochemistry. The 112 gastric biopsy samples positive for H. pylori included 19 chronic superficial gastritis (CSG) samples, 66 chronic atrophic gastritis (CAG) samples, 8 gastric ulcer (GU) samples, 18 duodenal ulcer (DU) samples, and 1 gastric carcinoma (GC) sample. The demographics and clinical characteristics (gastroscopy diagnosis) of the 112 patients are shown in Additional file 2: Table S1. This study was approved by the Ethics Committee for Human Studies of Fudan University Huadong Hospital, with written informed consent obtained from all subjects (Ethics Approval Number: 2020K080).
H. pylori isolation and culture
During endoscopy, two biopsy samples were obtained from the antrum, the 2–3 cm area in the front of the pylorus, of each patient. One sample was shipped immediately to a pathology centre for H. pylori detection in Huadong Hospital. The other sample was stored at − 80 °C and kept on dry ice during transfer. To culture H. pylori, the samples were homogenized and inoculated onto commercial H. pylori-selective plates (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan), followed by incubation for 3–7 days at 37 °C under microaerophilic conditions (5% O2, 10% CO2, and 85% N2). Small, transparent/translucent colonies typical of H. pylori (Additional file 1: Fig. S1) were subsequently subjected to Gram staining, the rapid urease test (RUT), antibiotic susceptibility testing, and genomic DNA extraction.
Antibiotic susceptibility testing
The AR of all the H. pylori strains to MTZ, CLA, LEV, and AMX was determined by both an E-test (Autobio, China) and the agar dilution method according to the protocols of the Clinical and Laboratory Standards Institute (Wayne, PA, USA). Briefly, after three subcultures of the individual strains isolated, the culture suspension turbidity of H. pylori was adjusted with saline to a McFarland opacity standard of 2.0 (approximately 6.0 × 109 CFU/ml), and the suspensions were inoculated onto a Mueller–Hinton II agar plate (Becton Dickinson, Sparks, MD, USA) supplemented with 10% horse blood. The four drug E-test strips were attached to the plate and incubated at 37 °C for 3–5 days under microaerophilic conditions. Agar dilution minimum inhibitory concentration (MIC) tests were performed by serial twofold dilution of MTZ, CLA, LEV, and AMX, with the concentration of each ranging from 0.0039 to 256 mg/L. All drugs were purchased from Wako Pure Chemical Industry (Osaka, Japan). H. pylori resistance phenotypes were determined following the guidelines of the Clinical and Laboratory Standards Institute (Wayne, PA, USA) [28]. The breakpoints used were MIC ≥ 1 µg/ml for CLA, MIC ≥ 8 µg/ml for MTZ, MIC ≥ 1 µg/ml for LEV, and MIC ≥ 2 µg/ml for AMX. H. pylori ATCC 43504 was used as the control strain. Based on the antibiotic susceptibility testing results, the H. pylori strains could be divided into different AR categories in two patterns: The resistance (R)- susceptibility (S) pattern (MTZ-S and -R, CLA-S and -R, LEV-S and -R) and nonoverlapping patterns (MTZ mono-R, CLA mono-R, LEV mono-R, MTZ and CLA dual-R, MTZ and LEV dual-R, CLA and LEV dual-R, multiple-drug resistant (MDR) and susceptible to four antibiotics).
H. pylori genomic DNA extraction and whole-genome sequencing
H. pylori strains were inoculated on Columbia agar (OXOID, Thermo Fisher Scientific Inc., Waltham, MA, USA) medium containing 8% sterile defibrinated sheep blood under microaerophilic conditions at 37 °C for 3–5 days. After three subcultures of the individual strains isolated, the total genomic DNA of H. pylori was extracted by the cetyltrimethyl ammonium bromide (CTAB) method [29]. All procedures were performed following the manufacturer’s instructions.
Genome sequencing was performed using the whole-genome shotgun (WGS) strategy based on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) by constructing paired-end DNA libraries. The DNA library (~ 400 base-pair insert size) for each strain was prepared using the TruSeq DNA Sample Preparation Kit (Illumina, CA, USA). Briefly, genomic DNA was randomly uniformly fragmented in the range of 300–500 bases by sonication (Diagenode Bioruptor UCD-200). Illumina adapters were ligated to each fragment. Quality control of the libraries was performed by an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) with a DNA 1000 chip according to the manufacturer’s instructions. The libraries were sequenced on an Illumina MiSeq sequencer using the TruSeq DNA Sample Preparation Kit (Illumina) with a paired-end 2 × 251-bp sequencing mode and a sequencing depth of more than 400× in all the strains. The quality of the raw reads was checked using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc). To obtain high-quality reads, adapter contamination was removed by AdapterRemoval (version 2.1.7) [30], and all reads were filtered on SOAPec (version 2.0) [31] with a Kmer of 17 to trim the low-quality bases with a Phred quality score (Q value) of less than 20. The filtered clean reads were assembled by A5-miseq (version 20160825) [32] into contigs and scaffolds. The draft genomes of the 112 H. pylori-Shi strains were submitted to the National Center for Biotechnology Information (NCBI). The accession number of each genome is shown in Additional file 3: Table S2.
H. pylori protein-coding gene, rRNA and CRISPR prediction
The genomic functional elements predicted included coding genes (CDSs), noncoding RNA, and CRISPRs. Glimmer software (version 3.0) [33] was used for gene prediction of the whole-genome sequence. The self-training gene prediction model was selected to extract the longest sequence in the assembled sequences, which was used for training the gene prediction model. Then, the gene prediction model constructed from this sequence was used for prediction of all gene sequences; the length of the open reading frame (ORF) was set to be no less than 110 bp, and the remaining parameters were the default settings of Glimmer 3.0. tRNA genes were identified by tRNAscan-SE (version 1.3.1) [34], and rRNA genes were identified by RNAmmer (version 1.2) [35]. CRISPRs were identified by CRISPRfinder (http://crispr.i2bc.paris-saclay.fr/Server/), and the direct repeats (DRs) and spacers in the whole genome were predicted by the CRISPR recognition tool [36].
Functional annotation of predicted protein-coding genes in H. pylori
Functional annotations of all the predicted protein-coding genes were obtained using the Refseq database (NCBI-NR) (https://www.ncbi.nlm.nih.gov/refseq/) and SwissProt database (http://www.gpmaw.com/index.html). Each ORF was thus run through the two databases via blastall [37] with an E value less than 1e−6, at least 30% sequence identity and 70% query length coverage. The functional descriptions of the best hits in the databases were assigned to the corresponding protein-coding genes [38].
Functional classifications by clusters of orthologous groups (COGs) of proteins were analysed against the eggNOG (evolutionary genealogy of genes: Nonsupervised Orthologous Groups) database (version 5.0) [39]. The unique genes of the indicated categories were annotated using this database via blastp with a minimum E-value of 1e−6 and at least 70% for both sequence identity and query length coverage. The database assigned the best hits to the corresponding protein-coding genes, which were then classified into corresponding COG classes. The genes containing more than one domain from different categories were classified as multiple-class genes, whereas those that did not have a suitable hit against the database were classified as hypothetical protein-coding genes.
H. pylori genome-wide gene family analysis
The dataset of the predicted protein sequences with a minimum of 50 amino acids in all 112 H. pylori strains was used for further orthology analysis. An all-vs-all blastp analysis was performed on the dataset with an E value of 1e−10 and 70% protein coverage cut-off applied for the alignments. The orthologous gene clusters in the sequence alignment results were identified using OrthoMCL (version 2.0.8) [40], with the inflation (I) value set to 1.5.
H. pylori genomic subsystem analysis
Virulence factor-associated genes in the 112 H. pylori strains were predicted by aligning amino acid sequences in the Virulence Factor Database (VFDB) (http://www.mgc.ac.cn/VFs/) [41], which is a comprehensive database of pathogenic bacterial virulence factors containing 532 confirmed virulence factors from the literature and 2599 virulence-related genes from 74 genera of pathogenic bacteria, including Helicobacter. Each protein sequence was thus run through the database via blastp v.2.6.0+ to identify virulence-associated genes with an E-value less than 1e−5, greater than 60% identity, 70% coverage, and less than 10% gap length. To retrieve the given OMP family genes and efflux pump system genes, blastx v.2.5.0+ was used with an E-value less than 1e−6 and greater than 90% identity.
Phylogenetic analysis of H. pylori strains
Based on the whole-genome orthologue analysis of the gene families, the single-copy orthologous genes were selected for multiple sequence alignment and quality control using the MAFFT tool (http://mafft.cbrc.jp/alignment/software/) and Gblocks (version 0.91) (http://molevol.cmima.csic.es/castresana/Gblocks.html). The phylogenetic tree was constructed by the maximum likelihood method in phyml software (version 3.1). The reliability of the phylogenetic tree branches was validated using the bootstrapping method with 1000 replications.
Identification of acquired antimicrobial resistance genes
The command line version of ResFinder (version 4.1) with default parameters (90% identity and 60% coverage) was used to identify acquired antimicrobial resistance genes where resistance was conferred by a complete gene [42]. The ResFinder database is composed of 1649 acquired ARGs from 15 antibiotic drug classes.
Genome-wide ARG analysis in H. pylori
The genome-wide resistance genes of the 112 H. pylori strains were identified from databases and the literature (Additional file 4: Table S3). First, the AR genes in the genomes of the 112 H. pylori strains were identified using the Comprehensive Antibiotic Research Database (CARD) (http://arpcard.mcmaster.ca) via BLAST [43]. An E value of 1e−6, 60% identity, and 70% protein coverage cut-off were applied for the alignments. The database includes genes involved in antibiotic susceptibility and/or resistance. To specifically identify β-lactamase enzyme-encoding genes, the β-lactamase database (BLDB) (version 1.0.11) (http://bldb.eu/) [44], a newly updated manually curated database for β-lactamases, was used with an E value of 1e−6, 80% identity, and 70% protein coverage cut-off. Because no hits were obtained in the database, no β-lactamase genes were included among the final genome-wide resistance genes.
To more comprehensively identify resistance genes in the genome, we compiled a literature-supported list of genes whose variation, presence or absence was associated with AR in H. pylori, including antibiotic targets and other sporadically reported genes that were either known to confer AR to H. pylori or subsequently found to be associated with resistance in H. pylori.
Mapping and variant detection
To identify genetic alterations resulting in single-nucleotide polymorphisms (SNPs) and insertions or deletions (Indels), the sequencing reads of each strain were mapped to the H. pylori 26695 reference genome (NC_000915.1) using the Burrows–Wheeler Alignment Tool (version 0.7.12-r1039) [45]. The alignment files were subjected to local realignment and deduplication using Picard (version 1.107). The accuracy of variant calling was improved using the Genome Analysis Toolkit (GATK) [46]. Variant SNPs and Indels were called from each alignment file using GATK. Variant filtering was carried out by removing variants with mapping quality (MQ) < 40, quality depth (QD) < 2, and haplotype score > 13. The variants were annotated using ANNOVAR (Wang et al., 2010) to determine nonsynonymous SNPs (nsSNPs) and frameshift Indels (fsIndels). All variants identified in this study were manually inspected using the Integrative Genomics Viewer (version 2.3.86) [47].
Linkage disequilibrium among variants potentially associated with AR or antibiotic susceptibility
The linkage disequilibrium (LD) of SNPs/Indels potentially associated with MTZ, CLA, and LEV resistance and susceptibility (P < 0.05) was assessed by calculating r2 and D′ values to identify the cooccurring correlation strength between each pair of variant sites using plink (version 1.07) [48]. The value of D′ was between − 1 and 1; a D′ value of 0 represented the two variants in a state of complete linkage equilibrium, and a D′ value of 1 represented the two variants in a state of complete LD.
Crystal structures and functional domains of genome-wide resistance gene-encoding proteins with resistance-associated variations
The crystal structures of the proteins encoded by the indicated genes were obtained from SWISS-MODEL (https://swissmodel.expasy.org/) [49]. The structural model with sequence identity greater than 30%, Global Model Quality Estimation (GMQE) score greater than 0.6, and QMEAN Z score between − 4 and 0 was selected from the alignment results. The detailed model evaluation is shown in Additional file 1: Fig. S2. The functional domains of the indicated genes were identified using Pfam 34.0 (http://pfam.xfam.org/) [50]. All the significantly matched entries for each gene sequence were retained, and all nonsignificantly matched entries were discarded.
Statistical analysis
When comparing the mean gene numbers between groups, Student’s t test was used. When analysing the association of each individual genotype with AR, frequencies between groups were compared using Fisher’s exact tests, Chi-squared tests or correction for continuity according to the data type to obtain the P value or adjusted P value. The statistical analysis was performed via RStudio (R 4.0.2) or GraphPad Prism (version 8.0).
Results
Antibiotic susceptibility and general genomic features of the 112 H. pylori-Shi strains
A total of 112 H. pylori strains from patients with digestive diseases were categorized based on antibiotic susceptibility phenotypes. The patients’ mean age was 52.2 ± 14.8 (mean ± SD) years, and 54.5% (61/112) were male (Table 1). The prevalence of MTZ-resistance was the highest (65.2%), with 33.9% MTZ mono-resistance, followed by LEV-resistance (34.8%), with 4.5% LEV mono-resistance, and CLA-resistance (16.1%), with 2.7% CLA mono-resistance. No strain was resistant to AMX. Among dual-resistant strains, the majority (20.5%) were resistant to both MTZ and LEV. A total of eight strains were MDR strains that were simultaneously resistant to MTZ, CLA, and LEV, and 28 were susceptible to all four antibiotics (Table 1). Sex, age, and gastroscopy diagnosis were not significantly associated with resistance (P > 0.05).
Table 1.
Demographic information and antibiotic resistance rates
| Group | Total, n (%) | Antibiotic-Resistance Phenotypes, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amoxicillin-R/mono-R | Metronidazole-R/mono-R | Clarithromycin-R/mono-R | Levofloxacin-R/mono-R | Metronidazole-clarithromycin dual-R | Metronidazole-levofloxacin dual-R | Clarithromycin-levofloxacin dual-R | MDR | Susceptible to four antibiotics | ||
| Gender | ||||||||||
| Male | 61 (54.5) | 0 (0)/0 (0) | 40 (65.6)/23 (37.7) | 8 (13.1)/3 (4.9) | 19 (31.1)/4 (6.6) | 2 (3.3) | 12 (19.7) | 0 (0) | 3 (4.9) | 14 (23.0) |
| Female | 51 (45.5) | 0 (0)/0 (0) | 33 (64.7)/15 (29.4) | 10 (19.6)/0 (0) | 20 (39.2)/1 (2.0) | 2 (3.9) | 11 (21.6) | 3 (5.9) | 5 (9.8) | 14 (23.0) |
| Age | ||||||||||
| 19–29 | 8 (7.1) | 0 (0)/0 (0) | 5 (62.5)/2 (25.0) | 2 (25.0)/1 (12.5) | 3 (37.5)/0 (0) | 0 (0) | 2 (25.0) | 0 (0) | 1 (12.5) | 2 (25.0) |
| 30–39 | 18 (16.1) | 0 (0)/0 (0) | 8 (44.4)/7 (38.9) | 3 (16.7)/2 (11.1) | 3 (16.7)/3 (16.7) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 5 (27.8) |
| 40–49 | 17 (15.2) | 0 (0)/0 (0) | 12 (70.6)/6 (35.3) | 4 (23.5)/0 (0) | 6 (35.3)/0 (0) | 1 (5.9) | 3 (17.6) | 1 (5.9) | 2 (11.8) | 4 (23.5) |
| 50–59 | 29 (25.9) | 0 (0)/0 (0) | 20 (69.0)/10 (34.5) | 4 (13.8)/0 (0) | 9 (31.0)/0 (0) | 2 (6.9) | 7 (24.1) | 1 (3.4) | 1 (3.4) | 8 (27.6) |
| 60–69 | 27 (24.1) | 0 (0)/0 (0) | 20 (74.1)/11 (40.7) | 2 (7.41)/0 (0) | 11 (40.7)/1 (3.7) | 0 (0) | 8 (29.6) | 1 (3.7) | 1 (3.7) | 5 (18.5) |
| 70–79 | 13 (11.6) | 0 (0)/0 (0) | 6 (46.2)/2 (15.4) | 2 (15.4)/0 (0) | 6 (46.2)/1 (7.7) | 0 (0) | 2 (15.4) | 0 (0) | 2 (15.4) | 4 (30.8) |
| 80 < | 2 (1.8) | 0 (0)/0 (0) | 2 (100)/0(0) | 1 (50)/0(0) | 1 (50)/0(0) | 0 (0) | 1 (50) | 0 (0) | 1 (50) | 0 (0) |
| Gastroscopy diagnosis | ||||||||||
| Chronic superficial gastritis | 19 (17.0) | 0 (0)/0 (0) | 8(42.1)/5(26.3) | 2(10.5)/1(5.3) | 5(26.3)/2(10.5) | 0(0) | 2(10.5) | 0(0) | 1(5.3) | 8(42.1) |
| Chronic atrophic gastritis | 66 (58.9) | 0 (0)/0 (0) | 46(69.7)/20(30.3) | 13(19.7)/0(0) | 29(43.9)/3(4.5) | 3(4.5) | 16(24.2) | 3(4.5) | 7(10.6) | 14(21.2) |
| Gastric/Duodenal ulcer | 26 (23.2) | 0 (0)/0 (0) | 18(69.2)/12(46.2) | 3(11.5)/2(7.7) | 5(19.2)/0(0) | 1(3.8) | 5(19.2) | 0 (0) | 0 (0) | 6(23.1) |
| Gastric cancer | 1 (0.9) | 0 (0)/0 (0) | 1(100)/1(100) | 0 (0)/0 (0) | 0 (0)/0 (0) | 0 (0)/0 (0) | 0 (0)/0 (0) | 0 (0)/0 (0) | 0 (0)/0 (0) | 0 (0)/0 (0) |
| Total | 112 | 0 (0)/0 (0) | 73 (65.2)/38 (33.9) | 18 (16.1)/3 (2.7) | 39 (34.8)/5 (4.5) | 4 (3.6) | 23 (20.5) | 3 (2.7) | 8 (7.1) | 28 (25.0) |
The total sequence length and numbers of contigs, ORFs, and guanine-cytosine (GC) content for each genome of the 112 H. pylori strains grouped by AR phenotypes are shown in Additional file 3: Table S2. On average, the total length of contigs in the 112 H. pylori isolate genomes was 1.60 billion bp, encoding 1511–1624 genes per genome. A low average GC content, 38.7%, was identified, and the chromosome sizes ranged from 1.52 to 1.69 Mb. All sequenced genomes harboured 36 tRNA genes and an average of two rRNA genes.
Genome-wide gene family analysis and unique resistance genes of H. pylori strains
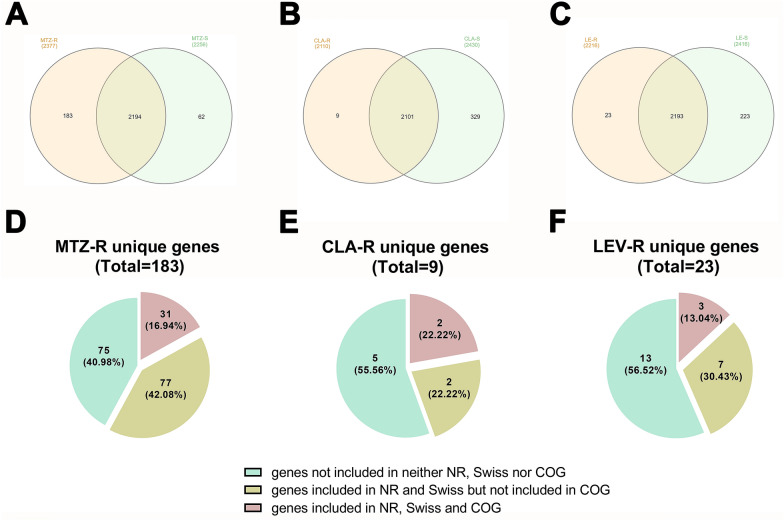
We initially conducted gene family analysis to define the shared genetic content of the resistant and susceptible categories and the unique gene pool for specific AR. Because different genomes possessed unique combinations of genes, the pan-genome size of the 112 genomes amounted to 2439, and the core-genome contains 1146 genes that were present in all genomes. A total of 2194 genes were shared between MTZ-S and MTZ-R, 2101 genes were shared between CLA-S and CLA-R, and 2193 genes were shared between LEV-S and LEV-R, accounting for 89.95%, 86.14% and 89.91% of the total genes, respectively. The shared genes between the corresponding resistant and susceptible categories here represented genes present in all and a subset of strains of both the resistant and susceptible categories but not those present in only the resistant or susceptible category. (Fig. 1A–C). Moreover, we identified 183, 9, and 23 unique genes in the MTZ, CLA, and LEV-R categories, respectively, including 75, 5, and 13 newly found genes that were not included in the NCBI-NR, SwissProt, or COG database (Fig. 1D–F, Additional file 5: Table S4). Among the remaining 108, 4 and 11 unique genes defined as unique resistance genes of the three AR categories (Additional file 6: Table S5), only 31, 2, and 3 genes could be assigned to various COG functional classes, respectively (Fig. 1D–F).
Fig. 1.
Genome-wide gene family analysis and functional classification of unique genes of antibiotic resistant categories. Venn diagrams showing the number of common and unique genes in MTZ- (A), CLA- (B) and LEV- (C) resistant (yellow) and susceptible (green) categories. The number in the parentheses indicates the total number of genes identified in each category. Pie diagrams revealing the status of unique genes of MTZ-R (D), CLA-R (E) and LEV-R (F) categories in NCBI-NR, SwissProt and COG databases
Distributions of OMP family genes in resistant and susceptible categories
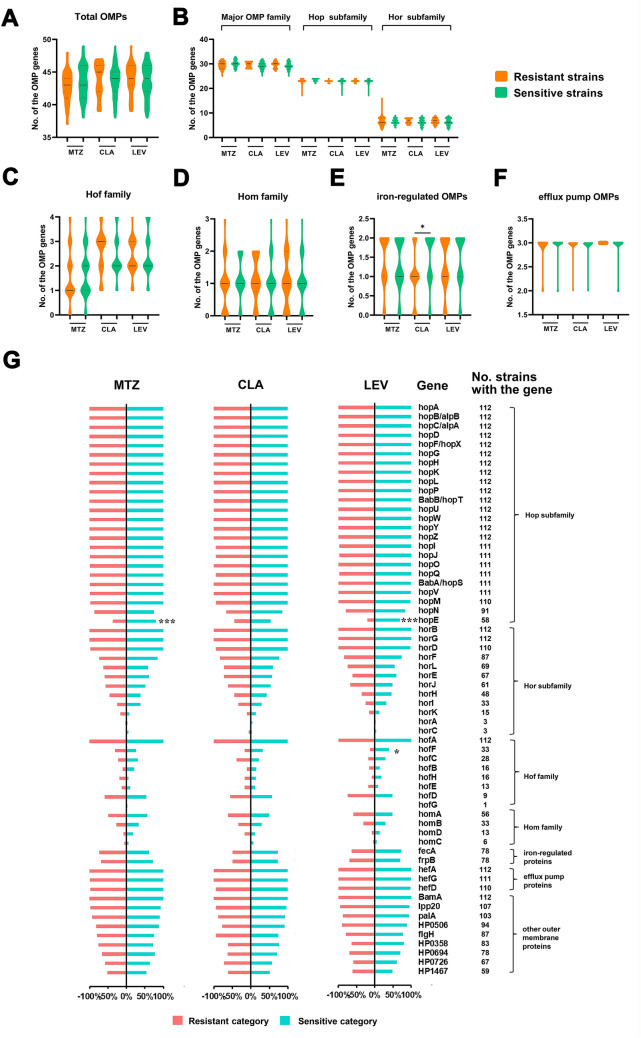
To investigate whether OMP family genes were related to specific AR, the presence and absence of genes predicted to encode OMPs in different categories were analysed, including the currently identified five paralogous gene families of the major OMP family, including the Hop subfamily and Hop-related (Hor) subfamily, Hof family, Hom family, iron-regulated OMPs, and efflux pump OMPs, ranging in size from 3 to 36 members, as well as 9 other putative OMPs not in paralogous families (Additional file 7: Table S6). We first compared the average numbers of OMP genes in the resistant and susceptible categories for the three antibiotics irrespective of the number of alleles of the genes. There were no significant differences in the average number of total OMP genes, the major OMP family genes, Hof and Hom family genes, and efflux pump OMP genes (Fig. 2A–F). Intriguingly, we found that the average frequency of the iron-regulated OMP genes (fecA and frpB) in the CLA-R category was significantly lower than that in the CLA-S category (P = 0.0103). Notably, the frequencies of these genes in the strains varied greatly. Nearly all the strains contained the majority of the Hop subfamily genes (not including hopE and hopN), the efflux pump OMP genes horB, horG, horD, and hofA belonging to the hor or hof subfamily, and the gene encoding a β-barrel assembly machinery (BAM) component BamA and murein lipoprotein 20 (Lpp20) (Fig. 2G). However, the horA, horC and hofG genes were present in very few strains. Remarkably, the proportion of strains carrying hopE in both the MTZ- and LEV-S categories was significantly higher than that in the corresponding resistant category (Fig. 2G). Another gene with a significantly lower frequency in the resistant category was hofF (LEV).
Fig. 2.
Analysis of OMP family genes in resistant and susceptible categories. Violin plots showing the distribution of the OMP genes in resistant and susceptible categories. The average presence levels of the total 62 OMP genes (A), the major OMP family genes along with two subfamilies genes (B), Hof family genes (C), Hom family genes (D), iron-regulated OMPs genes (F) and efflux pump OMP genes (F) in resistant and susceptible categories were compared, irrespective of the number of alleles of the genes. Solid lines indicate median levels and dotted lines indicate quartiles levels. G Proportion of strains with each OMP family gene in resistant and susceptible categories. Only genes with greater than 90% coverage were labelled as present. The number of strains containing the gene were listed on the right. A–G *P < 0.05, ***P < 0.001. Where there were no statistical analysis results labeled, there were no significant difference
Distributions of virulence-associated genes in the resistant and susceptible categories
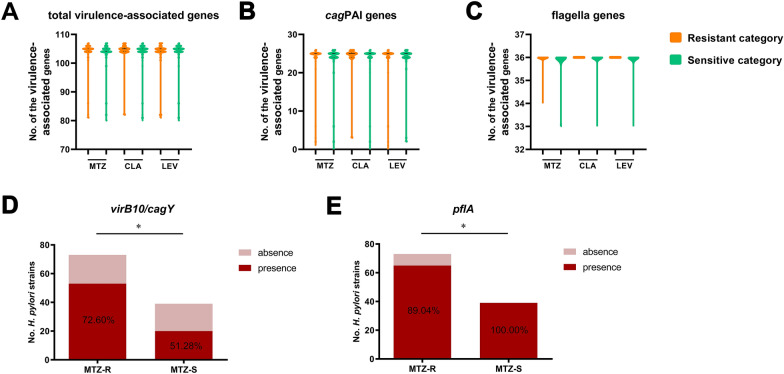
To investigate whether the occurrence of virulence factor genes carried by H. pylori was related to specific AR, the presence and absence of 108 predicted virulence-associated genes against the VFDB in the resistant and susceptible categories were analysed, including 26 cag pathogenicity island (cagPAI) genes, 36 flagellar genes, and 47 other virulence-associated genes (Additional file 8: Table S7). Similarly, we compared the average numbers of virulence-associated genes in the resistant and susceptible categories irrespective of the number of alleles of the genes. There were no significant differences in the number of total virulence-associated genes, cagPAI genes, or flagellar genes (Fig. 3A–C). Additionally, the frequencies of these genes in the resistant and susceptible categories were compared, and there was no significant difference in the distribution of most of the genes (Additional file 6: Table S5). Notably, the frequencies of cagY (HP0527) and pflA (HP1274) were significantly higher (P = 0.0240) and lower (P = 0.0488), respectively, in the MTZ-R category than in the MTZ-S category (Fig. 3D, E).
Fig. 3.
Analysis of virulence-associated genes in resistant and susceptible categories. Violin plots showing the distribution of the virulence-associated genes in resistant and susceptible categories. Comparisons of the average presence levels of the total virulence-associated genes (A), cagPAI genes (B) and flagellar genes (C) between resistant and susceptible categories were conducted, irrespective of the number of alleles of the genes. Solid lines indicate median levels and dotted lines indicate quartiles levels. Where there were no statistical analysis results labeled, there were no significant difference. D Two virulence-associated genes with significant different frequencies in MTZ-R and MTZ-S categories. *P < 0.05
Distributions of the efflux pump genes in the resistant and susceptible categories
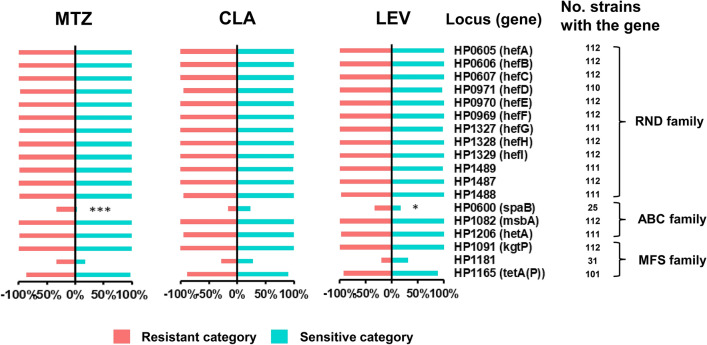
To investigate whether the efflux pump genes were related to specific AR, the presence and absence of 18 literature-based efflux pump genes were analysed, including 12 resistance-nodulation-cell division (RND) family genes, 3 ATP-binding cassette (ABC) family genes, and 3 major facilitator superfamily (MFS) family genes (Fig. 4). Among the 18 genes, 16 were present in almost all 112 H. pylori strains, and the remaining two genes, namely, spaB (HP0600) and HP1181, were absent in most of these strains, being detected in 25 and 31 strains, respectively. Nevertheless, the proportion of strains carrying spaB in the MTZ-R (P = 0.0002) and LEV-R (P = 0.0408) categories was obviously higher than that in the MTZ-S and LEV-S categories, respectively (Fig. 4).
Fig. 4.
Analysis of efflux pump genes in resistant and susceptible categories. Proportion of strains with each efflux pump gene in resistant and susceptible categories. The number of strains containing the gene were listed on the right. *P < 0.05, ***P < 0.001. Where there were no statistical analysis results labeled, there were no significant difference
Correlation analysis of CRISPRs and their components with H. pylori AR
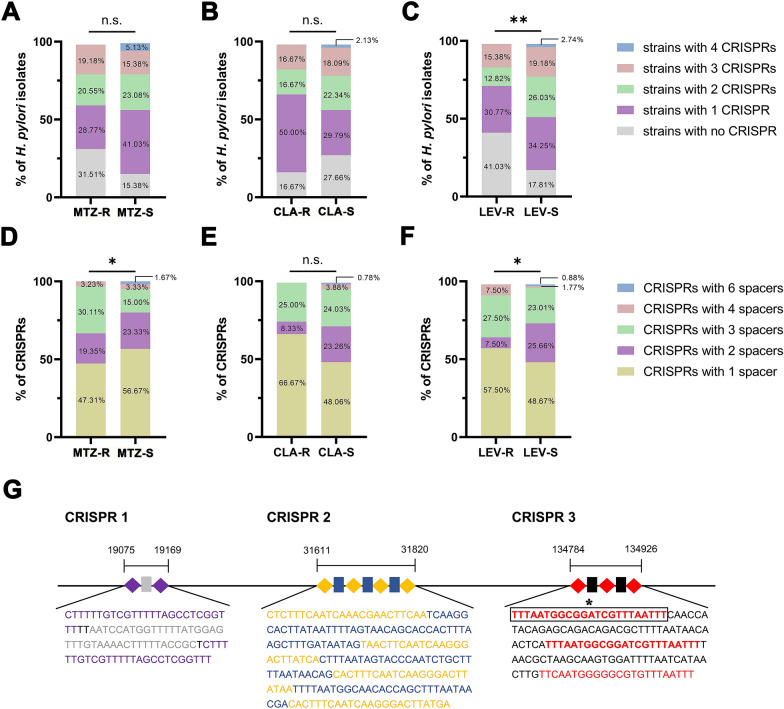
Given the natural function of the CRISPR-Cas system as an adaptive defence mechanism against foreign DNA and the controversial relationship between the CRISPR-Cas system and acquired ARGs in bacterial genomes [21–25], we conducted predictions for CRISPR-Cas systems and acquired ARGs where resistance was conferred by a complete gene rather than mutations, seeking to explore the relationship among CRISPR-Cas, acquired ARGs, and AR in H. pylori. Unfortunately, the results suggested that neither ARGs nor cas genes were found in the 112 H. pylori strains. However, a total of 153 CRISPR loci were predicted in 83 strains, ranging in length from 65 to 496 bp.
Because of the different CRISPR contents of the strains, the proportions of strains with 0, 1, 2, 3, and 4 CRISPRs in the resistant and susceptible categories of MTZ, CLA and LEV were analysed (Fig. 5A–C). Notably, the proportion of the strains with no CRISPR in the LEV-R category was significantly higher than that in the LEV-S category (P = 0.0075, Fig. 5C). Because of the different spacer contents of the CRISPRs, the proportion of CRISPRs with 1, 2, 3, 4, and 6 spacers was also analysed (Fig. 5D–F). Interestingly, the proportion of CRISPRs with three spacers in the MTZ-R category was significantly higher than that in the MTZ-S category (P = 0.0331, Fig. 5D), and the proportion of CRISPRs with two spacers in the LEV-R category was significantly lower than that in the LEV-S category (P = 0.0152, Fig. 5F). Similarly, the proportion of CRISPRs with two or three spacers in the resistant category of the other two antibiotics was also higher or lower, respectively, than that in the corresponding susceptible category, although there was no significant difference (Fig. 5D–F). In addition, detailed analysis of the DR and spacer sequences showed that a CRISPR combination containing the three most common CRISPRs in H. pylori coexisted in seven MTZ mono-R strains (Fig. 5G). Of note, one of the DRs in the CRISPR combination occurred in the CRISPRs of 9 strains, which included CRISPRs in seven MTZ mono-R strains and 2 CRISPRs (Additional file 1: Fig. S3) in two other MTZ-R strains (a MTZ mono-R strain and a MTZ and CLA dual-R strain), leading to a significantly higher proportion of strains with the DR in the MTZ-R category than in the MTZ-S category (P = 0.0259, Fig. 5G, Additional file 9: Table S8).
Fig. 5.
Distribution and constitution of CRISPRs in resistant and susceptible categories and a previously unknown CRISPRs combination present exclusively in seven MTZ mono-R strains. The proportions of strains with 0, 1, 2, 3 and 4 CRISPRs in resistant and susceptible categories of MTZ (A), CLA (B) and LEV (C), respectively. **P < 0.01, n.s. not significant. The proportions of CRISPRs with 1, 2, 3, 4 and 6 spacers in resistant and susceptible categories of MTZ (D), CLA (E) and LEV (F), respectively. *P < 0.05, n.s. not significant. G The structural analysis of a previously unknown CRISPRs combination presented exclusively in seven MTZ mono-R strains, including DRs (purple, yellow and red diamonds in each CRISPR) and spacers (grey, blue and black rectangles in each CRISPR). The base sequences are shown below the CRISPR array. The DRs and spacers in the colored characters correspond with the colors of the respective diamonds and rectangles. The DR sequence in box, which exclusively presented in nine MTZ-R strains, indicated that there was a significant difference of the DR frequencies between MTZ-R and MTZ-S categories. *P < 0.05
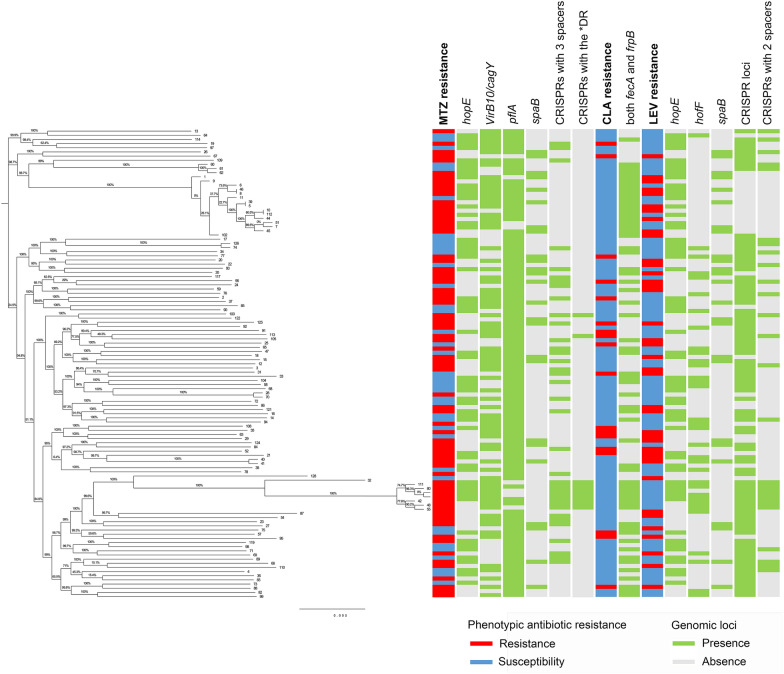
Phylogenetic analysis and relationship of AR patterns and specific genetic loci with strain relatedness in H. pylori strains
When constructing the maximum likelihood tree of the 112 H. pylori-Shi genomes based on the whole-genome level single-copy genes with respect to different resistance patterns and the profile of the observed differentially abundant subsystem genomic loci between the susceptible and resistant categories for the corresponding antibiotic, no strain relatedness was found to be associated with either each single antibiotic phenotype or the presence/absence of the differentially abundant loci, including genes encoding OMPs (hopE, hofF and iron-regulated OMPs), virulence-associated genes (virB10/cagY and pflA), efflux pump genes (spaB), and CRISPRs with 2 or 3 spacers or the indicated DR sequences (Fig. 6). Alternatively, these strains had distinct susceptibility and associated locus profiles regardless of genetic relatedness among strains.
Fig. 6.
Phylogenetic analysis with respect to antibiotic resistance patterns and specific genetic loci profiles in H. pylori strains. Maximum likelihood phylogenetic tree based on whole-genome level single-copy genes of the 112 H. pylori strains, the resistance patterns, and the significantly different genomic loci observed above between susceptible and resistant categories for the corresponding antibiotic. The susceptible and resistant patterns are denoted by blue and red rectangles, respectively. The presence and absence of the specific loci are denoted by green and grey rectangles, respectively
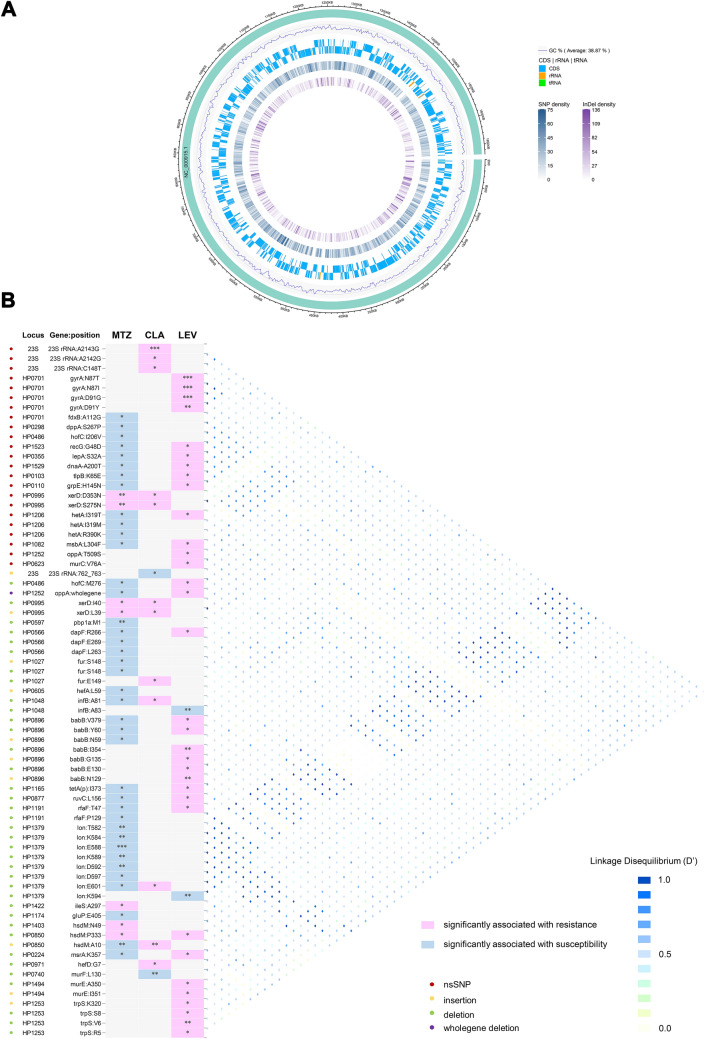
Genome-wide genetic variations and prevalence of variations in H. pylori ARGs
We called SNPs and Indels within the coding sequences and noncoding rRNAs and tRNAs from the genomes of all the H. pylori strains by comparison with the H. pylori 26695 reference genome (Fig. 7A), which showed high densities of the variations, which could be as high as 136 for Indel density and 75 for SNP density compared to the reference genome (Fig. 7A). Among these genetic variations, a total of 388 nsSNPs and 1718 fsIndels were identified (Additional file 10: Table S9).
Fig. 7.
Synoptic representation of the genetic variations in the genome-wide resistance genes and linkage disequilibrium analysis. A The densities of total SNPs and Indels identified in the 112 H. pylori strains against H. pylori 26695 genome are overviewed. The numbers next to the rulers represent the number of SNPs and Indels per unit length of H. pylori 26695 genome. B The proportions of the strains with the nsSNPs and fsIndels present in the H. pylori genome-wide resistant genes in the resistant and susceptible categories of MTZ, CLA and LEV were analyzed, respectively. Only nsSNPs and fsIndels with significantly higher (red cells) or lower (blue cells) frequencies in the resistant categories of MTZ, CLA and LEV were displayed. *P < 0.05, **P < 0.01, ***P < 0.001. Conversely, grey cells indicate the variations not significantly associated with resistance or susceptibility to the antibiotics. Loci, genes, and variations are reported in rows and antibiotics in columns. The nature of the variations is color-coded on the left. The right panel represents the extent of linkage disequilibrium between variations
We obtained a list of 80 genes defined as the H. pylori genome-wide resistance genes, whose variation, presence, or absence were either known to confer or subsequently found to be associated with H. pylori resistance to clinically used antibiotics, not limited to antibiotics with known phenotypes, in this study. To investigate the prevalence of the genetic variations in the resistance genes, we first assayed the variations in the six most common resistance genes, including the 23S rRNA, gyrA and gyrB genes and the rdxA, frxA, and fdxB genes, in which single-point mutations in specific regions are known to be responsible for CLA, LEV, and MTZ resistance, respectively (Tables 2, 3, 4). Of these SNPs, a subset was significantly associated with AR, including A2143G, A2142G and C148T in the 23S rRNA gene and N87T, N87I, and D91G, D91Y in gyrA, as well as I38V in fdxB. Additionally, the assay detected four Indels in 23S rRNA, three Indels in gyrA, and one in frxA. Unexpectedly, the prevalence of one of the Indels (762_763insT) in the 23S rRNA gene was significantly higher in the CLA-S category (53.19%) than in the CLA-R category (Table 2). Furthermore, we overviewed the distribution of the numbers of SNPs and Indels present in the remaining genome-wide resistance genes along with the AR profile of these genes (Additional file 1: Fig. S4). Among the remaining 74 H. pylori resistance genes, 174 variations were obtained, corresponding to 54 genes, including 29 genes containing nsSNPs, 36 containing fsIndels, and 10 containing both nsSNPs and fsIndels (Additional file 11: Table S10). Additionally, the maximum number of Indels (ranging from 0 to 15) contained in these genes was up to three times the maximum number of SNPs (ranging from 0 to 3), suggesting that Indel mutations make greater contributions to the high genetic diversity of genome-wide resistance genes than SNP mutations do (Additional file 1: Fig. S4).
Table 2.
nsSNPs and fsIndels in genes conferring resistance to CLA
| Gene | Nucleotide change | No. among CLA-R (n = 18) | No. among CLA-S (n = 94) | P |
|---|---|---|---|---|
| 23S rRNA | SNP | |||
| A2143G | 17 (94.44%) | 2 (2.13%) | < 0.0001 | |
| A2142G | 2 (11.11%) | 0 (0.00%) | 0.0250 | |
| T2182C | 6 (33.33%) | 31 (32.98%) | 0.9770 | |
| C148T | 2 (11.11%) | 0 (0.00%) | 0.0250 | |
| T1025C | 0 (0.00%) | 1 (1.06%) | 1.0000 | |
| G1027A | 14 (77.78%) | 52 (55.32%) | 0.0760 | |
| Indel | ||||
| 761delAA (GAA to G) | 16 (88.89%) | 84 (89.36%) | 1.0000 | |
| 762_763insT (A to AT) | 5 (27.78%) | 50 (53.19%) | 0.0482 | |
| 764_765insTTT (C to CTTT) | 8 (44.44%) | 54 (57.45%) | 0.3093 | |
| 1036_1037insC (A to AC) | 18 (100.00%) | 94 (100.00%) | 1.0000 | |
| 1515_1516insT (C to CT) | 3 (16.67%) | 9 (9.57%) | 0.4054 | |
| No change | 0 (0.00%) | 13 (13.83%) | 0.1232 |
Table 3.
nsSNPs and fsIndels in genes conferring resistance to LEV
| Gene | Sequence change | No. among LEV-R (n = 39) | No. among LEV-S (n = 73) | P | |
|---|---|---|---|---|---|
| Nucleotide position | Amino acid position | ||||
| gyrA | SNP | ||||
| C261A | N87T | 8 (20.51%) | 0 (0.00%) | 0.0001 | |
| C261G | N87I | 9 (23.08%) | 0 (0.00%) | < 0.0001 | |
| G271A | D91G | 12 (30.77%) | 0 (0.00%) | < 0.0001 | |
| G271T | D91Y | 5 (1.67%) | 0 (0.00%) | 0.0043 | |
| Indel | |||||
| 2455_2456insAT (AC to AATC) | T819fs | 32 (82.05%) | 57 (78.08%) | 0.6203 | |
| 2460_2463del (CTTCG to C) | S820fs | 34 (87.18%) | 62 (84.93%) | 0.7460 | |
| 2472delT (AT to A) | N824fs | 38 (97.44%) | 66 (90.41%) | 0.2577 | |
| No change | No change | 1 (2.56%) | 7 (9.59%) | 0.2577 | |
| gyrB | SNP | ||||
| C1801T | L601F | 37 (94.87%) | 73 (100.00%) | 0.3423 | |
| No change | No change | 2 (5.13%) | 0 (0.00%) | 0.1192 | |
Table 4.
nsSNPs and fsIndels in genes conferring resistance to MTZ
| Gene | Sequence change | No. among MTZ-R (n = 73) | No. among MTZ-S (n = 39) | P | |
|---|---|---|---|---|---|
| Nucleotide position | Amino acid position | ||||
| rdxA | No change | No change | 73 (100.00%) | 39 (100.00%) | NA |
| frxA | SNP | ||||
| G499A | D167N | 4 (5.48%) | 4 (10.26%) | 0.3497 | |
| T577A | C193S | 63 (86.30%) | 39 (100.00%) | 0.3238 | |
| Indel | |||||
| 653dupA (T to TA) | X218delinsX | 70 (95.89%) | 39 (100.00%) | 0.5503 | |
| No change | No change | 3 (4.11%) | 0 (0.00%) | 0.5503 | |
| fdxB | SNP | ||||
| A112G | I38V | 65 (89.04%) | 39 (100.00%) | 0.0488 | |
| No change | No change | 8 (10.96%) | 0 (0.00%) | 0.0488 | |
ins: insert, del: delete, fs: frameshift, NA: not available
Analysis of genetic variations in the H. pylori genome-wide ARGs
To explore MTZ, CLA, and LEV resistance-related or resistance-induced compensation-related variants, we compared the prevalence of the nsSNPs and fsIndels in the genome-wide ARGs between resistant and susceptible strains. We observed 23 nsSNPs and 48 fsIndels corresponding to 31 genes associated with the resistant or susceptible phenotypes. We found four variations within the integrase/recombinase gene xerD showing a significant association with both MTZ and CLA resistance. Both nsSNPs (xerD:D353N and xerD:S275N) were linked together but exhibited low LD with two xerD fsIndels. Similarly, two fsIndels (xerD:I40/120_121 delete GG and xerD:L39/116_117 insert G) were also linked together (Fig. 7B). A deletion within the site-specific DNA-methyltransferase gene hsdM (HP0850) (hsdM:P333/998_1002 delete AGCCG) was found to be related to both MTZ and LEV resistance, which were also in high LD with gyrA:N87I. Furthermore, we observed 16 variations within 15 genes simultaneously showing an association with MTZ susceptibility and LEV resistance, and many of these genes encoded membrane-related proteins (hetA, msbA, hofC, oppA, tetA(p), and babB) or proteins involved in recombination (recG and ruvC) (Fig. 7B). Moreover, several deletions within the ATP-dependent serine protease gene lon (especially the deletions at T582, K584 and E588) related to MTZ susceptibility exhibited high LD with more than half of the other variations, including the resistance-conferring nsSNPs 23S rRNA:A2143G, 23S rRNA:A2142G, 23S rRNA:C148T, gyrA:N87T, and some of the remaining variants, most of which were associated with MTZ resistance or susceptibility (Fig. 7B) (Additional file 12: Table S11).
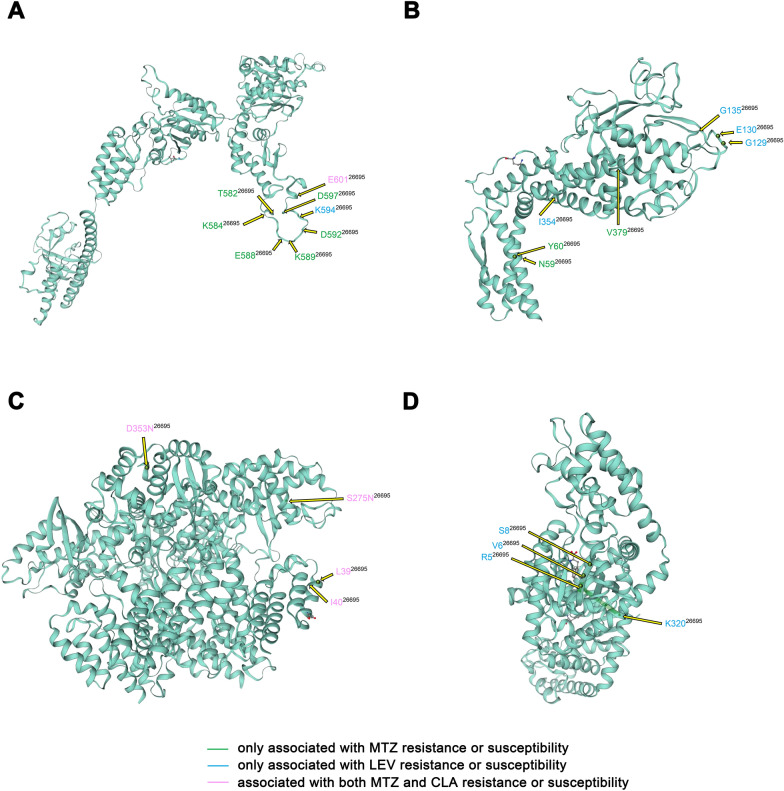
Resistance- or susceptibility-associated variations occurred in functionally important domains within the H. pylori genome-wide ARGs
For the purpose of relating the resistance- or susceptibility-related variations to specific protein domains, we further characterized these variations in the genes within the H. pylori genome-wide ARGs with corresponding protein structure data. The locations of the nsSNPs and fsIndels in the four proteins (Lon, BabB, XerD, and TrpS) with the largest number of phenotype-associated variations were summarized (Fig. 8), and the functional domains of all the resistance genes with phenotype-associated variations were identified. Eight variations in Lon and three in TrpS were closely located, although they were not in any domains (Fig. 8A and D, Additional file 1: Fig. S5). In BabB, two clusters of physically close variations located at L59, N60, G129, S130, and N135 were all in the sialic acid binding adhesin (SabA) N-terminal extracellular adhesion domain (Fig. 8B, Additional file 1: Fig. S5). Among four variations in XerD, only S275N was located in the phage integrase family domain that enables the protein to be covalently linked to DNA. Of the remaining 27 genes, 14 contained variations totally or partially located in their functional domains as follows: (1) three efflux pump transporter genes: dppA (S267P), hetA (R390K) and msbA (L304F); (2) three lipopolysaccharide (LPS) biosynthesis genes: murC (V76A), murE (with a deletion at A350 and an insertion at I351), and rfaF (with a deletion at P129); (3) two protein translation-related genes: lepA (S32A) and iles (with a deletion at A297); (4) the metalloregulator gene fur (with an insertion at S148 and a deletion at E149); (5) hofC (with I206V and a deletion at M276); (6) the stress-response protein coding gene grpE (H145N); (7) the chromosomal replication initiator gene dnaA (A200T); and (8) the restriction enzyme alleles hsdM_2 (with a deletion at P333) and hsdM_3 (with a deletion at N49) (Additional file 1: Fig. S5).
Fig. 8.
Localization of the nsSNPs and fsIndels in the four proteins with the largest number of resistance-associated variations within the H. pylori genome-wide resistant genes. The available crystal structure of each protein was used to indicate the sites with amino acid changes. A Lon, B BabB, C XerD, and D TrpS. The color scale represents the corresponding antibiotics of the resistance or susceptibility association
Discussion
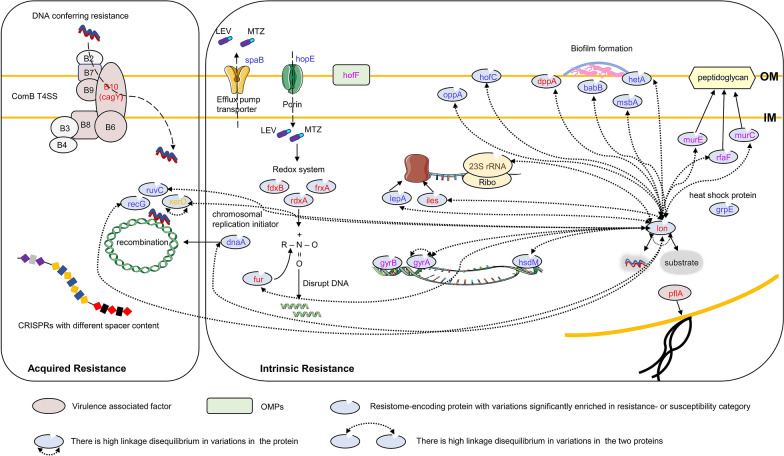
The emergence and rapid spread of antibiotic-resistant H. pylori has become a major obstacle to eradicating the associated infection and has become a great threat to human health. A sharp global decline in the efficacy of the recommended treatment, which used to be 90% effective, has led to an unacceptable threshold (< 80%) for H. pylori [4, 51–53]. The ability of H. pylori to develop resistance to antibiotics is usually genetically encoded, but only a few of these genetic features have been associated with specific AR. In this study, the clinically used antibiotic (MTZ, CLA, and LEV)-resistance-associated genetic features of 112 H. pylori-Shi strains were analysed to explore possible links between specific gene loci and variants with the resistance phenotype (Fig. 9).
Fig. 9.
Cartoon model showing the putative genetic characteristics likely related to H. pylori resistance- or susceptibility- to MTZ, CLA and LEV at locus- and variant-levels. The intrinsic resistance or resistance-associated compensation related genetic features involves the variations in the genome-wide resistant gene-encoding proteins including antibiotic targets, translation-associated factors, transporters, OMPs, proteins probably related to biofilm formation, and other sporadic factors including lon protease with variations in linkage disequilibrium with many others, as well as the presence or absence of a porin, and an efflux pump transporter. In the underlying acquired resistance related genetic elements, the presence or absence of a virulence-associated factor belonging to ComB T4SS and a certain CRISPRs, as well as variations in several recombination required proteins, were demonstrated. Different colors of the genetic element names denote they are in relation to resistance or susceptibility of different antibiotics. Red (MTZ), gold (CLA), pink (LEV), orange (MTZ and CLA), blue (MTZ and LEV), brown (CLA and LEV)
Initially, we determined the AR rates of the 112 H. pylori-Shi strains with four antibiotics. Our data demonstrated that both mono-resistance (65.2% and 34.8%) and dual-resistance (20.5%) to MTZ and LEV had the highest prevalence compared to the other resistance categories, while the prevalence of resistance to CLA (16.1%) was slightly lower than the resistance rate range of 20% to 50% reported previously in China [7]. Because of the rather low AMX resistance rate of 0% or < 5% worldwide [53] (zero for our 112 H. pylori-Shi strains), it was not possible to conduct further association analysis of the resistance mechanism of AMX. In addition, the H. pylori strains in this study were isolated from patients with four main gastric diseases, including chronic superficial gastritis, chronic atrophic gastritis, gastric/duodenal ulcer, and gastric cancer, which enabled us to examine potential differences in resistance rates in different specific gastrointestinal pathologies (except gastric cancer, because of insufficient sample number). The negative results implied that the intragastric conditions of patients with different H. pylori-linked gastric disorders may have no significant effect on the AR of the pathogen colonizing the gastric mucosa.
Because different phenotypes of H. pylori are considered to be associated with unique sets of genetic features [54, 55], we conducted genome-wide gene family analysis to compare the resistant and susceptible categories to obtain unique gene pools and their functional enrichment, which has rarely been investigated in H. pylori. Putatively affected by differences in the strain number of each resistant and susceptible category, the level of the unique gene pools of the three antibiotics varied greatly. Nevertheless, nearly half or more than half of them were newly found genes in H. pylori with resistance to MTZ (75/183, 40.98%), CLA (5/9, 55.56%), and LEV (13/23, 56.52%), indicating the extensive intraspecies diversity and genetic heterogeneity of H. pylori [56].
In our analysis, despite porin gene hopE was absent in nearly half of the 112 strains, the gene displayed significant enrichment in the susceptible categories of both MTZ and LEV compared with the resistant population. Similar situations have been reported in a large number of publications showing the loss of porins in clinical strains [57]. It is assumed that bacterial OMPs influence the influx of small-molecule antimicrobials into bacterial cells, and there have been many reports of AR acquired through loss or functional change of porins in a large number of organisms [58]. For example, in E. coli, loss of the porins OmpC and OmpF contributes to quinolone resistance [59, 60]. The A. baumannii porins OmpA, CarO, Omp33-36, and OprD have been associated with resistance to cephalothin and carbapenem [61–64]. The loss of the OmpK35 and OmpK36 porins in K. pneumoniae has been related to resistance to cephalosporins and increased MIC of meropenem [58]. The Hop member HopE, one of the first identified porins in H. pylori, is a major nonselective porin that is homologous to the E. coli OmpF porin [65, 66]. In our study, the significant loss of hopE in the MTZ- and LEV-resistant populations may indicate the underlying effect of hopE loss on the development of resistance to the two antibiotics with small molecular weights rather than CLA, which has a large molecular weight.
Regarding the relationship of virulence factors and AR, our analysis suggested the existence of a generally irrelevant relationship between most of the predicted virulence-associated genes and the phenotypes at the “locus” level, including the extensively studied H. pylori virulence factors CagA and VacA. This observation is consistent with some reports showing no correlation between AR and the presence of either the cagA or vacA gene [20, 67], but it is in contrast to many other findings showing that the cagA-negative strains and vacA-containing strains were associated with CLA resistance [68, 69]. These results remain inconclusive, as they showed a variable pattern, most likely due to low sample numbers in individual studies or variations in geographical regions and evolution. Moreover, we first reported the apparent differences in cagY and pflA frequencies in MTZ-resistant and MTZ-susceptible H. pylori. CagY is an orthologue of VirB10 and is an essential gene in the ComB type IV secretion system (T4SS) [70]. Rather than the canonical type IV (pseudo) pilus used by other Gram-negative bacteria, H. pylori employs the ComB system for initial DNA uptake during natural transformation [71]. Thus, the significantly higher frequency of cagY in the MTZ resistance category suggests that the functional ComB system with intact CagY enables the entry of resistance-associated DNA via transformation, which promotes the development of MTZ resistance. Further research is needed to identify and better understand the specific mechanisms behind this observation.
Previous reports have demonstrated that the AcrAB-TolC efflux pump belonging to the RND family encoding hefABC, hefDEF, and hefGHI is the most important efflux system in H. pylori, mainly mediating resistance to several antibiotics, such as MTZ and CLA, under laboratory conditions [11, 15]. It has been reported that AcrAB-TolC is associated with the development of resistance to CLA represented by the reduced lowest inhibitory concentration observed when using RND pump inhibitors [72]. Moreover, the RND pumps may contribute to MTZ resistance in H. pylori because the expression of the TolC homologous genes was elevated in MTZ-resistant isolates [73]. However, our analysis showed that the frequencies of the RND family efflux pump genes in the resistant and susceptible categories were not significantly different. We also reported here that the ABC family member spaB showed a difference in frequency between the resistant and susceptible categories of both MTZ and LEV, which is consistent with a previous report by Yang et al. from China showing that the expression level of spaB in MDR strains was significantly higher than that in susceptible strains and that the sensitivity of spaB-knockout H. pylori to four antibiotics (LEV, CLA, chloramphenicol and rifampicin) was significantly higher than that of wild-type strains (reference not provided). Similar studies on spaB have not been reported in the rest of the world. Whether the transporter is involved in H. pylori resistance remains to be determined.
Studies investigating the characteristics of CRISPR-Cas systems in relation to AR in clinical H. pylori strains are lacking. These are significant gaps in knowledge because the CRISPR-Cas system is hypothesized to be a natural impediment to horizontal gene transfer (HGT) in bacteria [74] and because HGT disseminates AR-associated genetic elements among competent pathogens in vivo [75], and it is essential to identify strategies to reduce the spread of these elements. Nevertheless, our analysis failed to predict any acquired ARGs where resistance was conferred by a complete gene. Given the high rate of the naturally competent transformation of H. pylori [76], it is plausible that the transfer of single-nucleotide mutations conferring AR rather than complete ARGs plays a prominent role in the horizontally acquired resistance of H. pylori, which has been supported by a series of reports [26, 71, 77]. Furthermore, we found that LEV resistance and possession of complete CRISPR loci were inversely related. Similar associations showing that the CRISPR system was more likely to be found in antimicrobial-susceptible species such as E. coli and Enterococci have also been reported [74, 78, 79]. Additionally, our correlation of a different number of spacers with resistance or susceptibility to LEV or MTZ, as well as the interesting finding of a CRISPR combination and a DR sequence occurring exclusively in MTZ-resistant strains, provided an initial foundation for potentially utilizing the spacer content or DR sequence to rapidly determine the phenotype of H. pylori strains.
In the phylogenetic analysis, we noticed that a subset of 8 isolates in a subclade showed high separation, indicating that these strains had considerable differences in genetic similarity and relatively distant genetic relationships compared with the genomes of other strains.
Similar to other bacteria with small genomes, the responses of H. pylori to environmental pressure rely mainly on variations rather than a complex regulatory network [80]. Next-generation sequencing is a powerful tool to compare whole genomes and analyse the variations found within AR-associated genes. When compiling the H. pylori genome-wide resistance gene list based on databases, no β-lactamase genes were found in the 112 H. pylori strains, which was consistent with a previous report showing that the ß-lactamase gene could be detected in AMX-resistant H. pylori but not in AMX-susceptible strains [81]. Regarding the antibiotic target genes, not only the well-known mutations in gyrA (N87T/I and D91G/Y) for LEV resistance and mutations in 23S rRNA (A2143G and A2142G) for CLA resistance but also several other single-nucleotide mutations and Indels associated with AR or antibiotic susceptibility were detected, and the latter could be compensatory mutations to mitigate the deleterious effects of the specific functions compromised by the resistance mutations [82]. In the other variations in the genome-wide resistance genes associated with AR or antibiotic susceptibility, we noticed MTZ susceptibility-associated variations in fur. MTZ belonging to the nitroimidazole class is a prodrug that is activated by nitroreductases, and the reduction products can disrupt DNA. It was suggested that the resistance stemming from inactive nitroreductases can be eliminated by mutations in the fur protein [83]. The LD analysis among these variations revealed several notable deletion mutations in lon that were found to be in high LD with more than half of the other variations, including the resistance-conferring nsSNPs. Given that the lon-encoded protease mediates the selective degradation of mutants and abnormal proteins [84, 85], we speculated that deletion mutations at specific sites of lon indirectly participate in the resistance mechanisms of H. pylori by influencing the effect of lon in degrading mutants or proteins encoded by mutants potentially related to the AR or antibiotic susceptibility of H. pylori, which may explain their linked co-occurrence to a certain extent. Further structure and functional domain analysis of genome-wide resistance genes containing resistance- or susceptibility-associated variations better indicated their probable involvement in the regulation of AR. For example, five Indels were present in the SabA N-terminal extracellular adhesion domain of BabB. This domain is conserved among SabA orthologues and BabA and is known to function as a sugar-binding adhesion domain with conserved disulfide bonds [86]. Smiley et al. previously reported that the transmembrane protein BabB was upregulated in CLA-resistant H. pylori [12]. The other functional domains in the genome-wide resistance genes containing variations included regions important for efflux (dppA, hetA and msbA), LPS biosynthesis (murC, murE and rfaF) and stress response (grpE). It has been reported that adhesins (especially OMPs from the Hop and Hom families) [87–89], LPS [87, 89, 90], efflux pumps [14, 87, 91] and proteins regulating a stress response [87, 88] are all associated with the biofilm formation of H. pylori. Thus, it may be the case that biofilm formation influenced by these genetic mutants is an important mechanism in the AR of H. pylori, which needs to be substantiated via extensive experiments.
Conclusion
Our study demonstrated that H. pylori strains isolated from Shanghai exhibited multidrug resistance and correctly identified the well-known mutations conferring intrinsic resistance to MTZ, CLA and LEV. Furthermore, we found that AR phenotypes were associated with a number of novel locus- and variant-level genetic elements. The underlying co-occurrence relationships among them were also revealed. These findings provide a data basis for further resistance-associated research. Moreover, thorough detection and characterization of AR in H. pylori strains is necessary to develop efficacious treatment regimens. Continued monitoring of AR using susceptibility assays and genotyping is warranted in Shanghai.
Supplementary Information
Additional file 1: Figure S1. Colony morphology of sub-cultured H. pylori strains on the selective plate. Figure S2. Local quality estimates and comparison plots of the established protein structure models. The Local Qualit Estimate shows, for each residue of the model (reported on the x-axis), the expected similarity to the native structure (y-axis). Typically, residues showing a score below 0.6 are expected to be of low quality. Different model chains are shown in different colous. (A) Lon, (C) BabB, (E) XerD, (G) TrpS. Generally, model quality scores of individual models are related to scores obtained for experimental structures of similar size. In the Comparison plot, the x-axis shows protein length (number of residues). The y-axis is the normalized QMEAN score. Every dot represents one experimental protein structure. Black dots are experimental structures with a normalized QMEAN score within 1 standard deviation of the mean (|Z-score| between 0 and 1), experimental structures with a |Z-score| between 1 and 2 are grey. Experimental structure that are even further from the mean are light grey. The actual model is represented as a red star. (B) Lon, (D) BabB, (F) XerD, (H) TrpS. Figure S3. The structural analysis of two other CRISPRs containing the DR exclusively presenting in nine MTZ-R strains. (A, B) DRs are shown as red diamonds and spacers are shown as green and blue rectangles in each CRISPR. The base sequences are shown below the CRISPR array. The DRs and spacers in the colored characters correspond with the colors of the respective diamonds and rectangles. The DR sequence exclusively presenting in nine MTZ-R strains is in box. Figure S4. Heatmap of the numbers of the variations presented in the genes of the H. pylori resistome (in addition to 23S rRNA, gyrA, gyrB, rdxA, frxA and fdxB genes) in the 112 strains. Heatmaps showing the distribution of the numbers of the nsSNPs (A) and the fsIndels (B) presented in the remaining genes of the H. pylori resistome in the 112 isolates categorized by phenotypes of three antibiotics involved in this study. The genes and the corresponding loci as well as the antibiotic resistant profile of the genes are listed on the right. Gens within the resistome with no SNPs or Indels are not included in the heatmaps. The different numbers of the nsSNPs or the fsIndels are represented by different colors displayed on the right panel. Figure S5. Functionally important domains of genes containing the resistance- or susceptibility-associated variations within the H. pylori resistome. The length of each bar indicates the size of the gene. The regions with different colors represent the corresponding functional domains. The red indicator line represents the variation site, with the position information labeld above it.
Additional file 2: Table S1. Demographics, clinical characteristics and antibiotic resistance phenotypes.
Additional file 3: Table S2. General genomic features for the 112 H. pylori isolates.
Additional file 4: Table S3. The databases- and literature- supported H. pylori resistome.
Additional file 5: Table S4. Source data of the inclusion situation of the resistanceunique genes in the NCBI-NR, SwissProt and COG databases.
Additional file 6: Table S5. List of unique genes of MTZ-R, CLA-R and LEV-R categories.
Additional file 7: Table S6. Distribution of the outer membrane protein family genes in the resistant and sensitive categories.
Additional file 8: Table S7. Distribution of the predicted virulence-associated genes in the resistant and sensitive categories.
Additional file 9: Table S8. Source data of the CRISPRs and DR sequences in the 112 H. pylori strains.
Additional file 10: Table S9. Source data of the nsSNPs and fsIndels identified in the 112 H. pylori strains.
Additional file 11: Table S10. The 173 variations found in the 54 genes within the H. pylori resistome.
Additional file 12: Table S11. Source data of the LD analysis.
Acknowledgements
Not applicable.
Authors’ contributions
HZ and YZ conceived of and designed the study. DJ, JZ and XP conducted sample collection. WC, LD, TL and FZ conducted the bacteria culture and antibiotic susceptibility testing. YF and JZ performed data reduction. SW and FY conducted manual inspection of the data. YL performed analysis and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Shanghai Science and Technology Committee (Grant No: 18411960600, 16411968000, 18411950800), National High Technology Research and Development Program of China (Grant No: 2015AA021107-019), Shanghai Shenkang Hospital Development Center “New Frontier Technology Joint Research Project” (Grant No: SHDC12015107), and the Shanghai Health and Family Planning Commission Youth Project of Scientific Research Subject (Grant No: 20184Y0032). Funding was also received from Shanghai “Rising Stars of Medical Talent” Outstanding Youth Development Program of 2018 and “Huadong Hospital project” (Grant No: 2019jc019).
Availability of data and materials
The Whole Genome Shotgun project of the 112 H. pylori-Shi strains has been deposited at DDBJ/ENA/GenBank (BioProject number PRJNA690879) under the accessions shown in Additional file 3: Table S2. Additional raw data is available in Additional files 4–12: Table S3–S11.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee for Human Studies of Fudan University Huadong Hospital, with written informed consent from all subjects (Ethics Approval Number: 2020K080).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yixin Liu and Su Wang have contributed equally to this work
Contributor Information
Yanmei Zhang, Email: 15618653286@163.com.
Hu Zhao, Email: ZH13701618011@163.com.
References
- 1.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55(10):2111–2115. [PubMed] [Google Scholar]
- 2.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15(3):306–316. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Kuipers EJ, Perez-Perez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87(23):1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 4.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 5.Nezami BG, Jani M, Alouani D, Rhoads DD, Sadri N. Helicobacter pylori mutations detected by next-generation sequencing in formalin-fixed, paraffin-embedded gastric biopsy specimens are associated with treatment failure. J Clin Microbiol. 2019 doi: 10.1128/JCM.01834-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in world health organization regions. Gastroenterology. 2018;155(5):1372–1382 e1317. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH, et al. Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Helicobacter. 2018;23(2):e12475. doi: 10.1111/hel.12475. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Cunningham SA, Cole NC, Kohner PC, Mandrekar JN, Patel R. Phenotypic and molecular antimicrobial susceptibility of Helicobacter pylori. Antimicrob Agents Chemother. 2017 doi: 10.1128/AAC.02530-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 10.Alba C, Blanco A, Alarcon T. Antibiotic resistance in Helicobacter pylori. Curr Opin Infect Dis. 2017;30(5):489–497. doi: 10.1097/QCO.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 11.Gong Y, Yuan Y. Resistance mechanisms of Helicobacter pylori and its dual target precise therapy. Crit Rev Microbiol. 2018;44(3):371–392. doi: 10.1080/1040841X.2017.1418285. [DOI] [PubMed] [Google Scholar]
- 12.Smiley R, Bailey J, Sethuraman M, Posecion N, Showkat Ali M. Comparative proteomics analysis of sarcosine insoluble outer membrane proteins from clarithromycin resistant and sensitive strains of Helicobacter pylori. J Microbiol. 2013;51(5):612–618. doi: 10.1007/s12275-013-3029-5. [DOI] [PubMed] [Google Scholar]
- 13.Co EM, Schiller NL. Resistance mechanisms in an in vitro-selected amoxicillin-resistant strain of Helicobacter pylori. Antimicrob Agents Chemother. 2006;50(12):4174–4176. doi: 10.1128/AAC.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Y, Wang C, Chen Z, Xu Z, Li H, Li W, Sun Y. Transporters HP0939, HP0497, and HP0471 participate in intrinsic multidrug resistance and biofilm formation in Helicobacter pylori by enhancing drug efflux. Helicobacter. 2020;25(4):e12715. doi: 10.1111/hel.12715. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto A, Tanahashi T, Okada R, Yoshida Y, Kikuchi K, Keida Y, Murakami Y, Yang L, Yamamoto K, Nishiumi S, et al. Whole-genome sequencing of clarithromycin resistant Helicobacter pylori characterizes unidentified variants of multidrug resistant efflux pump genes. Gut Pathog. 2014;6:27. doi: 10.1186/1757-4749-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krzyżek P, Grande R, Migdał P, Paluch E, Gościniak G. Biofilm formation as a complex result of virulence and adaptive responses of Helicobacter pylori. Pathogens. 2020 doi: 10.3390/pathogens9121062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miftahussurur M, Syam AF, Nusi IA, Makmun D, Waskito LA, Zein LH, Akil F, Uwan WB, Simanjuntak D, Wibawa ID, et al. Surveillance of Helicobacter pylori antibiotic susceptibility in indonesia: different resistance types among regions and with novel genetic mutations. PLoS ONE. 2016;11(12):e0166199. doi: 10.1371/journal.pone.0166199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miftahussurur M, Shrestha PK, Subsomwong P, Sharma RP, Yamaoka Y. Emerging Helicobacter pylori levofloxacin resistance and novel genetic mutation in Nepal. BMC Microbiol. 2016;16(1):256. doi: 10.1186/s12866-016-0873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussein NR, Tunjel I, Majed HS, Yousif ST, Aswad SI, Assafi MS. Duodenal ulcer promoting gene 1 (dupA1) is associated with A2147G clarithromycin-resistance mutation but not interleukin-8 secretion from gastric mucosa in Iraqi patients. New Microbes New Infect. 2015;6:5–10. doi: 10.1016/j.nmni.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fasciana T, Cala C, Bonura C, Di Carlo E, Matranga D, Scarpulla G, Manganaro M, Camilleri S, Giammanco A. Resistance to clarithromycin and genotypes in Helicobacter pylori strains isolated in Sicily. J Med Microbiol. 2015;64(11):1408–1414. doi: 10.1099/jmm.0.000163. [DOI] [PubMed] [Google Scholar]
- 21.Li HY, Kao CY, Lin WH, Zheng PX, Yan JJ, Wang MC, Teng CH, Tseng CC, Wu JJ. Characterization of CRISPR-Cas systems in clinical Klebsiella pneumoniae isolates uncovers its potential association with antibiotic susceptibility. Front Microbiol. 2018;9:1595. doi: 10.3389/fmicb.2018.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangas EL, Rubio A, Alvarez-Marin R, Labrador-Herrera G, Pachon J, Pachon-Ibanez ME, Divina F, Perez-Pulido AJ. Pangenome of Acinetobacter baumannii uncovers two groups of genomes, one of them with genes involved in CRISPR/Cas defence systems associated with the absence of plasmids and exclusive genes for biofilm formation. Microb Genom. 2019 doi: 10.1099/mgen.0.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touchon M, Charpentier S, Pognard D, Picard B, Arlet G, Rocha EP, Denamur E, Branger C. Antibiotic resistance plasmids spread among natural isolates of Escherichia coli in spite of CRISPR elements. Microbiology. 2012;158(12):2997–3004. doi: 10.1099/mic.0.060814-0. [DOI] [PubMed] [Google Scholar]
- 24.Touchon M, Cury J, Yoon EJ, Krizova L, Cerqueira GC, Murphy C, Feldgarden M, Wortman J, Clermont D, Lambert T, et al. The genomic diversification of the whole Acinetobacter genus: origins, mechanisms, and consequences. Genome Biol Evol. 2014;6(10):2866–2882. doi: 10.1093/gbe/evu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shehreen S, Chyou TY, Fineran PC, Brown CM. Genome-wide correlation analysis suggests different roles of CRISPR-Cas systems in the acquisition of antibiotic resistance genes in diverse species. Philos Trans R Soc Lond B Biol Sci. 2019;374(1772):20180384. doi: 10.1098/rstb.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corbinais C, Mathieu A, Kortulewski T, Radicella JP, Marsin S. Following transforming DNA in Helicobacter pylori from uptake to expression. Mol Microbiol. 2016;101(6):1039–1053. doi: 10.1111/mmi.13440. [DOI] [PubMed] [Google Scholar]
- 27.Jaillard M, van Belkum A, Cady KC, Creely D, Shortridge D, Blanc B, Barbu EM, Dunne WM, Jr, Zambardi G, Enright M, et al. Correlation between phenotypic antibiotic susceptibility and the resistome in Pseudomonas aeruginosa. Int J Antimicrob Agents. 2017;50(2):210–218. doi: 10.1016/j.ijantimicag.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria (M45). 3rd ed. 2016. [DOI] [PubMed]
- 29.Jin Y, Deng J, Liang J, Shan C, Tong M. Efficient bacteria capture and inactivation by cetyltrimethylammonium bromide modified magnetic nanoparticles. Colloids Surf B Biointerfaces. 2015;136:659–665. doi: 10.1016/j.colsurfb.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Schubert M, Lindgreen S, Orlando L. AdapterRemoval v2: rapid adapter trimming, identification, and read merging. BMC Res Notes. 2016;9:88. doi: 10.1186/s13104-016-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1(1):18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coil D, Jospin G, Darling AE. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics. 2015;31(4):587–589. doi: 10.1093/bioinformatics/btu661. [DOI] [PubMed] [Google Scholar]
- 33.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999;27(23):4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35(9):3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bland C, Ramsey TL, Sabree F, Lowe M, Brown K, Kyrpides NC, Hugenholtz P. CRISPR recognition tool (CRT): a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinformatics. 2007;8:209. doi: 10.1186/1471-2105-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 38.Blake JD, Cohen FE. Pairwise sequence alignment below the twilight zone. J Mol Biol. 2001;307(2):721–735. doi: 10.1006/jmbi.2001.4495. [DOI] [PubMed] [Google Scholar]
- 39.Powell S, Forslund K, Szklarczyk D, Trachana K, Roth A, Huerta-Cepas J, Gabaldon T, Rattei T, Creevey C, Kuhn M, et al. eggNOG v4.0: nested orthology inference across 3686 organisms. Nucleic Acids Res. 2014;42(Database issue):D231–239. doi: 10.1093/nar/gkt1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer S, Brunk BP, Chen F, Gao X, Harb OS, Iodice JB, Shanmugam D, Roos DS, Stoeckert CJ. Using OrthoMCL to assign proteins to OrthoMCL-DB groups or to cluster proteomes into new ortholog groups. Vol 12. In: Goodsell DS, editor. Current protocols in bioinformatics. Hoboken: Wiley; 2011. pp. 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Xiong Z, Sun L, Yang J, Jin Q. VFDB 2012 update: toward the genetic diversity and molecular evolution of bacterial virulence factors. Nucleic Acids Res. 2012;40(Database issue):D641–645. doi: 10.1093/nar/gkr989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, Philippon A, Allesoe RL, Rebelo AR, Florensa AF, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75(12):3491–3500. doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McArthur AG, Waglechner N, Nizam F, Yan A, Azad MA, Baylay AJ, Bhullar K, Canova MJ, De Pascale G, Ejim L, et al. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother. 2013;57(7):3348–3357. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naas T, Oueslati S, Bonnin RA, Dabos ML, Zavala A, Dortet L, Retailleau P, Iorga BI. Beta-lactamase database (BLDB)—structure and function. J Enzyme Inhib Med Chem. 2017;32(1):917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu P, He L, Li Y, Huang W, Xi F, Lin L, Zhi Q, Zhang W, Tang YT, Geng C, et al. Correction: OTG-snpcaller: an optimized pipeline based on TMAP and GATK for SNP calling from ion torrent data. PLoS ONE. 2015;10(9):e0138824. doi: 10.1371/journal.pone.0138824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25(21):2865–2871. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46(W1):W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer ELL, Tosatto SCE, Paladin L, Raj S, Richardson LJ, et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 2021;49(D1):D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26(3):343–357. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 52.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence consensus report. Gut. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 53.Megraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badr MT, Omar M, Hacker G. Comprehensive integration of genome-wide association and gene expression studies reveals novel gene signatures and potential therapeutic targets for Helicobacter pylori-induced gastric disease. Front Immunol. 2021;12:624117. doi: 10.3389/fimmu.2021.624117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunaletchumy SP, Seevasant I, Tan MH, Croft LJ, Mitchell HM, Goh KL, Loke MF, Vadivelu J. Helicobacter pylori genetic diversity and gastro-duodenal diseases in Malaysia. Sci Rep. 2014;4:7431. doi: 10.1038/srep07431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, Blaser MJ, Graham DY, Vacher S, Perez-Perez GI, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299(5612):1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 57.Masi M, Winterhalter M, Pages JM. Outer membrane porins. Subcell Biochem. 2019;92:79–123. doi: 10.1007/978-3-030-18768-2_4. [DOI] [PubMed] [Google Scholar]
- 58.Beceiro A, Tomas M, Bou G. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev. 2013;26(2):185–230. doi: 10.1128/CMR.00059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bekhit A, Fukamachi T, Saito H, Kobayashi H. The role of OmpC and OmpF in acidic resistance in Escherichia coli. Biol Pharm Bull. 2011;34(3):330–334. doi: 10.1248/bpb.34.330. [DOI] [PubMed] [Google Scholar]
- 60.Liu YF, Yan JJ, Lei HY, Teng CH, Wang MC, Tseng CC, Wu JJ. Loss of outer membrane protein C in Escherichia coli contributes to both antibiotic resistance and escaping antibody-dependent bactericidal activity. Infect Immun. 2012;80(5):1815–1822. doi: 10.1128/IAI.06395-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catel-Ferreira M, Nehme R, Molle V, Aranda J, Bouffartigues E, Chevalier S, Bou G, Jouenne T, De E. Deciphering the function of the outer membrane protein OprD homologue of Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56(7):3826–3832. doi: 10.1128/AAC.06022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.del Mar TM, Beceiro A, Perez A, Velasco D, Moure R, Villanueva R, Martinez-Beltran J, Bou G. Cloning and functional analysis of the gene encoding the 33- to 36-kilodalton outer membrane protein associated with carbapenem resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2005;49(12):5172–5175. doi: 10.1128/AAC.49.12.5172-5175.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Limansky AS, Mussi MA, Viale AM. Loss of a 29-kilodalton outer membrane protein in Acinetobacter baumannii is associated with imipenem resistance. J Clin Microbiol. 2002;40(12):4776–4778. doi: 10.1128/JCM.40.12.4776-4778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sugawara E, Nikaido H. OmpA is the principal nonspecific slow porin of Acinetobacter baumannii. J Bacteriol. 2012;194(15):4089–4096. doi: 10.1128/JB.00435-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bina J, Bains M, Hancock RE. Functional expression in Escherichia coli and membrane topology of porin HopE, a member of a large family of conserved proteins in Helicobacter pylori. J Bacteriol. 2000;182(9):2370–2375. doi: 10.1128/jb.182.9.2370-2375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doig P, Exner MM, Hancock RE, Trust TJ. Isolation and characterization of a conserved porin protein from Helicobacter pylori. J Bacteriol. 1995;177(19):5447–5452. doi: 10.1128/jb.177.19.5447-5452.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boehnke KF, Valdivieso M, Bussalleu A, Sexton R, Thompson KC, Osorio S, Novoa Reyes I, Crowley JJ, Baker LH, Xi C. Antibiotic resistance among Helicobacter pylori clinical isolates in Lima. Peru Infect Drug Resist. 2017;10:85–90. doi: 10.2147/IDR.S123798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brennan DE, Dowd C, O'Morain C, McNamara D, Smith SM. Can bacterial virulence factors predict antibiotic resistant Helicobacter pylori infection? World J Gastroenterol. 2018;24(9):971–981. doi: 10.3748/wjg.v24.i9.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haddadi MH, Negahdari B, Asadolahi R, Bazargani A. Helicobacter pylori antibiotic resistance and correlation with cagA motifs and homB gene. Postgrad Med. 2020;132(6):512–520. doi: 10.1080/00325481.2020.1753406. [DOI] [PubMed] [Google Scholar]
- 70.Barrozo RM, Hansen LM, Lam AM, Skoog EC, Martin ME, Cai LP, Lin Y, Latoscha A, Suerbaum S, Canfield DR, et al. CagY is an immune-sensitive regulator of the Helicobacter pylori type IV secretion system. Gastroenterology. 2016;151(6):1164–1175 e1163. doi: 10.1053/j.gastro.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damke PP, Di Guilmi AM, Varela PF, Velours C, Marsin S, Veaute X, Machouri M, Gunjal GV, Rao DN, Charbonnier JB, et al. Identification of the periplasmic DNA receptor for natural transformation of Helicobacter pylori. Nat Commun. 2019;10(1):5357. doi: 10.1038/s41467-019-13352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirata K, Suzuki H, Nishizawa T, Tsugawa H, Muraoka H, Saito Y, Matsuzaki J, Hibi T. Contribution of efflux pumps to clarithromycin resistance in Helicobacter pylori. J Gastroenterol Hepatol. 2010;25(Suppl 1):S75–S79. doi: 10.1111/j.1440-1746.2009.06220.x. [DOI] [PubMed] [Google Scholar]
- 73.Mehrabadi JF, Sirous M, Daryani NE, Eshraghi S, Akbari B, Shirazi MH. Assessing the role of the RND efflux pump in metronidazole resistance of Helicobacter pylori by RT-PCR assay. J Infect Dev Ctries. 2011;5(2):88–93. doi: 10.3855/jidc.1187. [DOI] [PubMed] [Google Scholar]
- 74.Price VJ, McBride SW, Hullahalli K, Chatterjee A, Duerkop BA, Palmer KL. Enterococcus faecalis CRISPR-Cas is a robust barrier to conjugative antibiotic resistance dissemination in the murine intestine. mSphere. 2019 doi: 10.1128/mSphere.00464-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lerminiaux NA, Cameron ADS. Horizontal transfer of antibiotic resistance genes in clinical environments. Can J Microbiol. 2019;65(1):34–44. doi: 10.1139/cjm-2018-0275. [DOI] [PubMed] [Google Scholar]
- 76.Moore ME, Lam A, Bhatnagar S, Solnick JV. Environmental determinants of transformation efficiency in Helicobacter pylori. J Bacteriol. 2014;196(2):337–344. doi: 10.1128/JB.00633-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levine SM, Lin EA, Emara W, Kang J, DiBenedetto M, Ando T, Falush D, Blaser MJ. Plastic cells and populations: DNA substrate characteristics in Helicobacter pylori transformation define a flexible but conservative system for genomic variation. FASEB J. 2007;21(13):3458–3467. doi: 10.1096/fj.07-8501com. [DOI] [PubMed] [Google Scholar]
- 78.Aydin S, Personne Y, Newire E, Laverick R, Russell O, Roberts AP, Enne VI. Presence of Type I-F CRISPR/Cas systems is associated with antimicrobial susceptibility in Escherichia coli. J Antimicrob Chemother. 2017;72(8):2213–2218. doi: 10.1093/jac/dkx137. [DOI] [PubMed] [Google Scholar]
- 79.Palmer KL, Gilmore MS. Multidrug-resistant enterococci lack CRISPR-cas. mBio. 2010 doi: 10.1128/mBio.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kraft C, Suerbaum S. Mutation and recombination in Helicobacter pylori: mechanisms and role in generating strain diversity. Int J Med Microbiol. 2005;295(5):299–305. doi: 10.1016/j.ijmm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Tseng YS, Wu DC, Chang CY, Kuo CH, Yang YC, Jan CM, Su YC, Kuo FC, Chang LL. Amoxicillin resistance with beta-lactamase production in Helicobacter pylori. Eur J Clin Invest. 2009;39(9):807–812. doi: 10.1111/j.1365-2362.2009.02166.x. [DOI] [PubMed] [Google Scholar]
- 82.Hanafi A, Lee WC, Loke MF, Teh X, Shaari A, Dinarvand M, Lehours P, Megraud F, Leow AH, Vadivelu J, et al. Molecular and proteomic analysis of levofloxacin and metronidazole resistant Helicobacter pylori. Front Microbiol. 2016;7:2015. doi: 10.3389/fmicb.2016.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu Y, Zhang M, Lu B, Dai J. Helicobacter pylori and antibiotic resistance, a continuing and intractable problem. Helicobacter. 2016;21(5):349–363. doi: 10.1111/hel.12299. [DOI] [PubMed] [Google Scholar]
- 84.Andrianova AG, Kudzhaev AM, Abrikosova VA, Gustchina AE, Smirnov IV, Rotanova TV. Involvement of the N domain residues E34, K35, and R38 in the functionally active structure of Escherichia coli Lon protease. Acta Naturae. 2020;12(4):86–97. doi: 10.32607/actanaturae.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tzeng SR, Tseng YC, Lin CC, Hsu CY, Huang SJ, Kuo YT, Chang CI. Molecular insights into substrate recognition and discrimination by the N-terminal domain of Lon AAA+ protease. Elife. 2021 doi: 10.7554/eLife.64056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pang SS, Nguyen STS, Perry AJ, Day CJ, Panjikar S, Tiralongo J, Whisstock JC, Kwok T. The three-dimensional structure of the extracellular adhesion domain of the sialic acid-binding adhesin SabA from Helicobacter pylori. J Biol Chem. 2014;289(10):6332–6340. doi: 10.1074/jbc.M113.513135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hathroubi S, Zerebinski J, Ottemann KM. Helicobacter pylori biofilm involves a multigene stress-biased response, including a structural role for flagella. mBio. 2018 doi: 10.1128/mBio.01973-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krzyzek P, Grande R, Migdal P, Paluch E, Gosciniak G. Biofilm formation as a complex result of virulence and adaptive responses of Helicobacter pylori. Pathogens. 2020 doi: 10.3390/pathogens9121062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong EH, Ng CG, Chua EG, Tay AC, Peters F, Marshall BJ, Ho B, Goh KL, Vadivelu J, Loke MF. Comparative genomics revealed multiple helicobacter pylori genes associated with biofilm formation in vitro. PLoS ONE. 2016;11(11):e0166835. doi: 10.1371/journal.pone.0166835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Servetas SL, Doster RS, Kim A, Windham IH, Cha JH, Gaddy JA, Merrell DS. ArsRS-dependent regulation of homB contributes to helicobacter pylori biofilm formation. Front Microbiol. 2018;9:1497. doi: 10.3389/fmicb.2018.01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yonezawa H, Osaki T, Hojo F, Kamiya S. Effect of Helicobacter pylori biofilm formation on susceptibility to amoxicillin, metronidazole and clarithromycin. Microb Pathog. 2019;132:100–108. doi: 10.1016/j.micpath.2019.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Colony morphology of sub-cultured H. pylori strains on the selective plate. Figure S2. Local quality estimates and comparison plots of the established protein structure models. The Local Qualit Estimate shows, for each residue of the model (reported on the x-axis), the expected similarity to the native structure (y-axis). Typically, residues showing a score below 0.6 are expected to be of low quality. Different model chains are shown in different colous. (A) Lon, (C) BabB, (E) XerD, (G) TrpS. Generally, model quality scores of individual models are related to scores obtained for experimental structures of similar size. In the Comparison plot, the x-axis shows protein length (number of residues). The y-axis is the normalized QMEAN score. Every dot represents one experimental protein structure. Black dots are experimental structures with a normalized QMEAN score within 1 standard deviation of the mean (|Z-score| between 0 and 1), experimental structures with a |Z-score| between 1 and 2 are grey. Experimental structure that are even further from the mean are light grey. The actual model is represented as a red star. (B) Lon, (D) BabB, (F) XerD, (H) TrpS. Figure S3. The structural analysis of two other CRISPRs containing the DR exclusively presenting in nine MTZ-R strains. (A, B) DRs are shown as red diamonds and spacers are shown as green and blue rectangles in each CRISPR. The base sequences are shown below the CRISPR array. The DRs and spacers in the colored characters correspond with the colors of the respective diamonds and rectangles. The DR sequence exclusively presenting in nine MTZ-R strains is in box. Figure S4. Heatmap of the numbers of the variations presented in the genes of the H. pylori resistome (in addition to 23S rRNA, gyrA, gyrB, rdxA, frxA and fdxB genes) in the 112 strains. Heatmaps showing the distribution of the numbers of the nsSNPs (A) and the fsIndels (B) presented in the remaining genes of the H. pylori resistome in the 112 isolates categorized by phenotypes of three antibiotics involved in this study. The genes and the corresponding loci as well as the antibiotic resistant profile of the genes are listed on the right. Gens within the resistome with no SNPs or Indels are not included in the heatmaps. The different numbers of the nsSNPs or the fsIndels are represented by different colors displayed on the right panel. Figure S5. Functionally important domains of genes containing the resistance- or susceptibility-associated variations within the H. pylori resistome. The length of each bar indicates the size of the gene. The regions with different colors represent the corresponding functional domains. The red indicator line represents the variation site, with the position information labeld above it.
Additional file 2: Table S1. Demographics, clinical characteristics and antibiotic resistance phenotypes.
Additional file 3: Table S2. General genomic features for the 112 H. pylori isolates.
Additional file 4: Table S3. The databases- and literature- supported H. pylori resistome.
Additional file 5: Table S4. Source data of the inclusion situation of the resistanceunique genes in the NCBI-NR, SwissProt and COG databases.
Additional file 6: Table S5. List of unique genes of MTZ-R, CLA-R and LEV-R categories.
Additional file 7: Table S6. Distribution of the outer membrane protein family genes in the resistant and sensitive categories.
Additional file 8: Table S7. Distribution of the predicted virulence-associated genes in the resistant and sensitive categories.
Additional file 9: Table S8. Source data of the CRISPRs and DR sequences in the 112 H. pylori strains.
Additional file 10: Table S9. Source data of the nsSNPs and fsIndels identified in the 112 H. pylori strains.
Additional file 11: Table S10. The 173 variations found in the 54 genes within the H. pylori resistome.
Additional file 12: Table S11. Source data of the LD analysis.
Data Availability Statement
The Whole Genome Shotgun project of the 112 H. pylori-Shi strains has been deposited at DDBJ/ENA/GenBank (BioProject number PRJNA690879) under the accessions shown in Additional file 3: Table S2. Additional raw data is available in Additional files 4–12: Table S3–S11.