Abstract
Background
Ocular surface burns can be caused by chemicals (alkalis and acids) or by direct heat. Amniotic membrane transplantation (AMT) performed in the acute phase (day 0 to day 7) of an ocular surface burn is reported to relieve pain, accelerate healing and reduce scarring and blood vessel formation. The surgery involves applying a patch of amniotic membrane (AM) over the entire ocular surface up to the eyelid margins.
Objectives
To assess the effects of AMT on the eyes of people having suffered acute ocular surface burns.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2012, Issue 6), MEDLINE (January 1946 to June 2012), EMBASE (January 1980 to June 2012), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to June 2012), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 11 June 2012.
Selection criteria
We included randomised trials of medical therapy and AMT applied in the first seven days after an ocular surface burn compared to medical therapy alone.
Data collection and analysis
Two authors independently assessed the risk of bias of included studies and extracted relevant data. We contacted trial investigators for missing information. We summarised data using risk ratios (RRs) and mean differences (MDs) as appropriate.
Main results
We included one RCT of 100 participants with ocular burns that were randomised to treatment with AMT and medical therapy or medical therapy alone. A subset of patients (n = 68) who were treated within the first seven days of the injury met the inclusion criteria and were included in the analysis. The remaining 32 eyes were excluded. The included subset consisted of 36 moderate (Dua classification II‐III) and 32 severe (Dua classification IV‐VI) ocular burns from alkali, acid and thermal injuries. In the moderate category, 13/20 control eyes and 14/16 treatment eyes had complete epithelialisation by 21 days. The RR of failure of epithelialisation by day 21 was 0.18 in the treatment group (95% confidence interval (CI) 0.02 to 1.31; P = 0.09). Mean LogMAR final visual acuities were 0.06 (standard deviation (SD) 0.10) in the treatment group and 0.38 (SD 0.52) in the control group, representing a MD of ‐0.32 (95% CI ‐0.05 to ‐0.59). In the severe category, 1/17 treatment and 1/15 control eyes were epithelialised by day 21. The RR of failure of epithelialisation in the treatment group was 1.01 (95% CI 0.84 to 1.21; P = 0.93). Final visual acuity was 1.77 (SD 1.31) in the treated eyes and 1.64 (SD 1.48) in the control group (MD 0.13; 95% CI ‐0.88 to 1.14). The risks of performance and detection biases were high, because treating personnel and outcome assessors could not be masked to treatment. There was also a high risk of bias in the visual outcomes of the moderate category, since mean visual acuity was significantly worse at presentation in the control eyes. This reduced confidence in the study findings.
Authors' conclusions
Conclusive evidence supporting the treatment of acute ocular surface burns with AMT is lacking. Heterogeneity of disease presentation, variations in treatment, undefined criteria for treatment success and failure, and non‐uniform outcome measures are some of the factors complicating the search for clear evidence regarding this treatment.
Plain language summary
Amniotic membrane transplantation for the treatment of ocular burns
Ocular surface burns vary in severity from mild and self‐limiting to devastating injuries characterised by failure of regeneration of the ocular surface, leading to blindness and disfigurement. The historical use of amniotic membrane transplantation (AMT) to treat eye burns during the acute phase has re‐emerged in recent years, although its precise effects on the healing process have not been proven by randomised controlled trials (RCTs). One RCT conducted in India included a subset of patients who fulfilled the criteria for analysis in this review. The participants included 68 men and women of all ages with chemical or thermal burns to the ocular surface, who were randomised to treatment with conventional medical therapy alone or to medical therapy and AMT in the first seven days after injury. Conventional medical therapy included topical steroids, antibiotics, sodium ascorbate, sodium citrate, tear substitutes and cycloplegic drops, and oral vitamin C. Pressure‐lowering drops and/or oral acetazolamide were prescribed if required. Data from the RCT were analysed to compare corneal wound closure rates by the 21st day after the injury and visual outcomes at final follow‐up. The burns were classified as moderate or severe. In the moderate category, the AMT group had a higher proportion of eyes with complete epithelial closure by day 21 (not statistically significant) and significantly better visual acuity at final follow‐up. There was a high risk of bias resulting from the uneven characteristics of the control and treatment eyes at presentation and from the failure to mask personnel and outcome assessors involved in the study. This reduced confidence in the study findings.
Summary of findings
Background
Historical perspective
Amniotic membrane (AM) was first used as a biomaterial in ophthalmic surgery in 1938 (De Rötth 1940). It was applied as a replacement for the conjunctiva, the thin layer which covers the white of the eye, following the removal of scar tissue between the eye and the inner eyelids (symblepharon). Around the same time, AM dehydrated in 70% ethanol, called 'amnioplastin', was used as an adjunct in neurosurgery (Chao 1940). Amnioplastin subsequently featured in reports (Lavery 1946) and case series (Sorsby 1946; Sorsby 1947) describing temporary patching of acute burns to the cornea (the 'window' of the eye) and to the rest of the ocular surface. Washed in saline and potassium hydroxide rather than ethanol, amnioplastin patches were sutured directly onto superficial areas of damaged tissue, with apparently favourable outcomes.
The idea of dressing the acutely burned eye with a biological membrane was not new. Denig 1918 had advocated patching with oral mucous membrane to separate the burned surfaces of the eye and inner eyelids. A method of applying rabbit peritoneal membrane to the entire ocular surface up to the lid margins was subsequently described in the management of severe lime burns (Brown 1941). Human AM was a convenient alternative to these membranes, and patching acutely burned eyes with AM soon became widespread as a means of preventing symblepharon (Shafto 1950). The treatment was soon to spread to the former Soviet Union (Uglova 1957). In 1965, in an address to the Ophthalmological Society of the United Kingdom, AM was mentioned as one of a number of membranes useful in the immediate surgical management of acute ocular burns (along with alternatives like mucous membranes, egg membrane, peritoneum and others) (Roper‐Hall 1965). At the same meeting, an easily replaceable stainless steel ring (Flieringa ring) wrapped in AM was proposed as a means of application. Thereafter, all mention of AM in ophthalmic surgery disappeared from Western (but not Soviet) medical literature for a period of almost 30 years. Treatment of eye burns with AM patching continued in Soviet bloc countries (Alberth 1971; Batmanov 1990).
Amniotic membrane in modern ophthalmic surgery
In Russia in the late 1980s, amniotic membrane transplantation (AMT) was shown to a visiting ophthalmic surgeon from Venezuela, who returned to his country with samples of the unidentified tissue (Dua 2004). Some of this was eventually passed to Dr. Juan Battle, who proceeded to identify it by histological methods and conducted clinical trials on AM. Once again, it was promoted as a conjunctival substitute (Battle 1993). The lead was soon taken up by Scheffer Tseng of the Bascom Palmer Institute in Miami. Following animal model experiments (Kim 1995a; Kim 1995b), Tseng and his co‐workers commercialised AM, freezing it at ‐80ºC in a 1:1 mix of glycerol and culture medium. They and others soon began to explore its potential in ocular surface surgery (Lee 1997; Shimazaki 1997). Although it was described as cryopreserved, there was no suggestion that its cells survived the freeze process. Today, both frozen and dried preparations of AM are available to ophthalmic surgeons. The durability, pliability and versatility of AM make it a useful adjunctive treatment in at least 20 ophthalmic procedures (see review by Saw 2007). In 2000, AM was once again proposed as a treatment for acute burns, either as a limited graft or as a patch investing the entire globe up to the lid margin (Meller 2000). In recent years, a number of reports on AM treatment of acute ocular burns have emerged (Arora 2005; Kheirkah 2008; Kobayashi 2003; Tandon 2011).
Description of the condition
Epidemiology of ocular burns
Approximately 1.6 million cases of blindness, 2.3 million cases of bilateral low vision and 19 million cases of unilateral blindness or low vision worldwide were caused by ocular surface injuries by 1998 (Négrel 1998). The annual incidence of monocular blindness worldwide, as a result of trauma and corneal ulceration, is approximately two million (Whitcher 2001). Between 11.5% (Wong 2000) and 22.1% (Loon 2009) of injuries are caused by chemical burns, the majority of victims being young and male. Worldwide, the commonest cause is exposure to alkali or acid from occupational exposure in industry and agriculture, domestic accidents and assault (Wagoner 1997). Thermal burns can be caused by a number of insults, including open flames, steam and molten metals. Munitions and firework injuries can produce complex thermal and mechanical damage to the eye (Shimazaki 2006). Chemical assaults and industrial accidents may cause ocular surface burns on a mass scale, a notable example being the Bhopal disaster of 1984, when methyl isocyanate was released into the atmosphere causing thousands of injuries.
Pathogenesis of ocular burns
The duration of exposure, depth of penetration, area of involvement and relative toxicity are all indicators of the severity of an ocular surface chemical burn (Wagoner 1997). Alkalis react with the cell membranes of the outermost protective layer (the epithelium), and are able to rapidly penetrate the front of the eye, causing extensive tissue damage. Acids tend to cause cell proteins to coagulate, which limits penetration. Hydrofluoric acid is an exception, being able to penetrate deeply and cause severe toxic effects (McCulley 1990). The immediate effects of a burn can include irreversible damage to multiple ocular tissues, including the cornea and conjunctiva. Destruction of the outer layers exposes the highly ordered corneal collagen bundles to chemical denaturation, leading to corneal opacity. Blood vessels around the cornea can become occluded (ischaemia), leading to tissue death in severe cases. Damage to deep corneal and intraocular structures can result in corneal fluid retention, cataract and a rise in intraocular pressure which in turn can lead to glaucoma. The clinical course of an ocular burn can be divided into immediate, acute (day 0 to 7), early repair (day 7 to 21) and late repair phases (McCulley 1987). In severe injuries, delayed corneal epithelial re‐growth (epithelialisation) can be seen during the early repair phase, and inflammatory sequelae can persist for many months after the injury.
Limbal stem cell failure
The extent of involvement of the corneal limbus (a strip of tissue circumscribing the cornea) and conjunctiva is of special significance, since it can be used to estimate the likelihood of failure of corneal epithelialisation, a key event in corneal wound healing. There is evidence that human corneal epithelial stem cells originate in crypts located in the subconjunctival space adjacent to the limbus (Dua 2005). Following a severe burn involving the limbus, a 'pannus' of scarred and inflamed conjunctival tissue may grow over areas of damaged cornea, resulting in an opaque and poorly seeing eye. This has been called a Type III healing pattern (Wagoner 1997). In contrast, Type I and II healing patterns are associated with total or subtotal recovery of the ocular surface. If there is insufficient residual conjunctiva in a severe injury, the denuded cornea becomes at risk of thinning and eventual perforation. In these cases, exposed collagens are destroyed by enzymes, which are both activated in the tissue and released by immune cells recruited from the tear film and blood vessels (Fini 1998). Collagen replacement is impaired by loss of keratocytes, cells which re‐supply the corneal tissue (stroma) with collagen, and by depletion of vitamin C levels, leading to progressive 'melting' (ulceration). This is known as a Type IV healing pattern (Wagoner 1997). Thus, the outcome in the long‐term depends on the severity of the initial injury, stem cell survival and the healing response (Shimmura 2008). It is possible to prevent stromal ulceration by restoring an epithelial surface to cover the denuded cornea, even if this is derived from opaque conjunctival tissue.
Classification of ocular burns
The extent of damage at initial assessment may help to predict the final visual outcome (prognosis) of chemical injuries. The 1965 Roper‐Hall classification of ocular burns (Table 3; Roper‐Hall 1965) concentrated on corneal involvement and limbal ischaemia to predict the anatomical and visual outcomes from the initial clinical presentation. Burns were graded from I (mild) to IV (severe). This benchmark classification for chemical burns takes into account the degree of corneal opacity, limbal ischaemia (a marker of presumed stem cell loss) and epithelial involvement at presentation. An updated understanding of stem cell distribution underscores the Dua classification of thermal and chemical ocular burns (Table 4; Dua 2001), further subdividing Roper‐Hall grade IV burns to IV, V and VI. The Dua classification is based on an estimate of limbal involvement (in clock hours) and the percentage of conjunctival damage. The prognosis is considered in the light of recent advances in the surgical rehabilitation of burned eyes, which have greatly improved outcomes. In a large randomised controlled trial (RCT) of acute burns, the Dua classification was confirmed to be superior to the Roper‐Hall classification in predicting the outcome of an ocular burn (Gupta 2011). However, the initial clinical appearance can be misleading, giving rise to unexpected results (Shimmura 2008). Moreover, the prognosis is altered by burned eyelids, which can delay or prevent healing (Malhotra 2009).
1. Roper‐Hall classification.
| Grade | Prognosis | Cornea | Conjunctiva |
| I | Good | Corneal epithelial damage | No limbal ischaemia |
| II | Good | Corneal haze, iris details visible | < 33% limbal ischaemia |
| III | Guarded | Total epithelial loss, stromal haze, iris details obscured | 33% to 50% limbal ischaemia |
| IV | Poor | Cornea opaque, iris and pupil obscured | > 50% limbal ischaemia |
2. Dua classification.
| Grade | Prognosis | Clinical findings | Conjunctival involvement | Analogue scale |
| I | Very good | 0 clock hours of limbal involvement | 0% | 0/0% |
| II | Good | < 3 clock hours of limbal involvement | < 30% | 0.1 to 3/1 to 29.9% |
| III | Good | 3 to 6 clock hours of limbal involvement | > 30% to 50% | 3.1 to 6/31 to 50% |
| IV | Good to guarded | 6 to 9 clock hours of limbal involvement | > 50% to 75% | 6.1 to 9/51 to 75% |
| V | Guarded to poor | 9 to < 12 clock hours of limbal involvement | > 75% to < 100% | 9.1 to 11.9/75.1 to 99.9% |
| VI | Very poor | Total limbus (12 clock hours) involved | Total conjunctiva (100%) involved | 12/100% |
*The analogue scale records accurately the limbal involvement in clock hours of affected limbus/percentage of conjunctival involvement. While calculating percentage of conjunctival involvement, only involvement of bulbar conjunctiva, up to and including the conjunctival fornices is considered.
Description of the intervention
Amniotic membrane transplantation + medical treatment
Amniotic membrane transplantation (AMT) is offered in some treatment centres as an adjunct to conventional medical therapies for acute eye burns. Conventional treatment includes removal of any residual corrosive particulate matter and continuous irrigation with phosphate‐free saline solutions until the pH is neutralised (Tuft 2009). Depending on the severity of the burn, immediate treatments typically consist of a combination of preservative‐free topical treatments including antibiotics, lubricants and steroids. Topical treatment may also include cycloplegic drops to relieve spasm, ascorbate drops and citrate drops. Ascorbate is required for collagen synthesis (Levinson 1976), whereas citrate drops chelate calcium required by inflammatory polymorphonuclear cells (Pfister 1984). Systemic medications may include oral ascorbate and tetracyclines, which may help to preserve corneal tissue by inhibiting matrix metalloproteinase activity (Smith 2004). In the event of elevated intraocular pressure as a result of the burn, pressure‐lowering medications are given.
Treatment with amniotic membrane (AM) consists of its application to a part or the whole of the burned ocular surface. There are no common adverse effects reported from using AM, but rare reports of serious complications of AMT can be found in the literature including fungal keratitis (Das 2009), uveitis (Srinivasan 2007) and corneal melt (Schechter 2005). A steroid‐responsive hypopyon (a collection of inflammatory cells in the eye) may appear after repeated exposure to AM from the same donor (Gabler 2000), since AM can stimulate an immune response in sensitised subjects (Hori 2006). AM used immediately after birth conveys a notional risk of transmission of an undiagnosed pathogen, such as human immunodeficiency virus (HIV). The risk of maternal transmission of infections through quarantined AM is thought to be extremely small, provided that testing for maternal infectious diseases is carried out, although there remains a theoretical risk of transmission of unknown infections (Rahman 2010).
How the intervention might work
Physical properties of amniotic membrane
Many of the useful effects of AM are due to its gross physical properties. AM is a thin, pliable membrane (about 50 microns thick). It was recognised early on that the application of a patch of AM to the uncomfortable, burned ocular surface had the effect of improving patient comfort (Shafto 1950), presumably by reducing eyelid friction over the injured surface whilst allowing oxygen transfer to take place (Baum 2002). The effect of AM on pain relief is generally accepted (Dua 2004). The mechanical separation of inflamed tissues may also prevent symblepharon. AM may also provide a barrier to white blood cells, physically trapping them on the stromal side and causing cell death through as yet unclear mechanisms (Shimmura 2001).
Biological properties of amniotic membrane
It has been claimed that AM contains biological factors that are capable of influencing the tissue response (Meller 2000; Tseng 2004). Amniotic membrane transplantation (AMT) is reported to dampen inflammation, promote epithelialisation, prevent scarring and neovascularisation through these factors (see review by Dua 2004). This concept has been reinforced by laboratory and animal model findings, of which the following are three of many examples in the literature.
Content of AM: preserved AM contains ribonucleic acid (RNA) for growth factors and the factors themselves (Koizumi 2000b).
Effects on cells: supernatants from AM cultures suppressed immune cells in mice, an action attributed to soluble inhibitory factors (Ueta 2002).
Effect on animal models of burns: patching of rabbit eyes following alkaline burns reduced infiltration of inflammatory cells (Kim 2000).
Many of the studies of AM treatment of acute burns make reference to its pro‐epithelialising, anti‐inflammatory, anti‐fibrotic and anti‐neovascular effects (Meller 2000). It has been suggested that these effects can help prevent perforation and melting (Arora 2005; Zhou 2004). Others have suggested that AM restores and preserves limbal stem cell function (Prabhasawat 2007; Tejwani 2007) and that it reduces the need for subsequent limbal stem cell transplantation (da Silva Ricardo 2009; Kheirkah 2008).
The presumed mediators of these effects may become degraded by preservation techniques. They are mostly present in minimal quantities in therapeutic AM, mitigating against the likelihood of biological effects from certain short‐acting secreted factors, such as cytokines. Nonetheless, important regulatory proteins such as transforming growth factor β1 (TGF‐β1) are retained in AM processed for therapeutic use (Hopkinson 2006). While these may have a role in wound healing, they may presumably also cause undesirable effects, such as scarring. Dua 2004 has outlined the numerous biological factors in AM and their functions; some of these have conflicting activities.
Alternative surgical management of acute ocular burns
Alternative surgical approaches to protect the ocular surface can include advancing the vascularised conjunctiva and the subconjunctival Tenon's layer over the burned tissue to the limbus (Tenon‐plasty) (Kuckelkorn 1995). Tenon‐plasty can only be carried out if there is sufficient residual vascularised tissue. Autologous conjunctival patches from the uninjured fellow eye have also been used to treat eye burns (Thoft 1977). Similar to oral and nasal mucosal grafts, these may fail to vascularise, and become necrotic (Kuckelkorn 1995). One advantage of AM is that, in contrast to autologous conjunctiva, it is avascular and generally easily available.
In recent years, attention has turned to treating acute burns with tissue‐engineered constructs of cultivated corneal epithelial cells (Koizumi 2001) and oral mucosal epithelial cells (Ma 2009; Nakamura 2004) expanded on denuded AM (Koizumi 2000a). Experimental treatments have included mesenchymal stem cell sheets (Ye 2006). Synthetic hydrogel preparations have emerged recently as possible alternatives to tissue‐based treatments (Pratoomsoot 2008). In a rabbit model of chemical injuries, recombinant collagen implants have been used to replace corneal tissue two months after a burn (Hackett 2011).
Why it is important to do this review
Standardisation of treatment
Ophthalmic surgeons need to know which grades of burns to treat and when, which products and techniques to use and what outcomes may be expected (Panda 2002). Because of its early promise, the clinical application of AM has proceeded in spite of the lack of RCTs (Bouchard 2004). Consequently, AM has been used in excess of its true potential (Dua 2004). High quality evidence of the benefits of this treatment is lacking, suggesting a pressing need for a systematic review to highlight which characteristics are needed for future RCTs. A systematic review can encourage high quality trials to be conducted (Jüni 2001). The application of AM for burns is not standardised (Dua 2010). The following reasons can account for the lack of standardisation.
The tissue is heterogeneous, with both biochemical (Gicquel 2009; Hopkinson 2006) and physical (Connon 2007; Connon 2010; von Versen‐Höynck 2004; von Versen‐Hoeynck 2008) variations between donors, and even between areas of one donor membrane. These variations may be increased by tissue handling during processing.
Variability may be accentuated by different methods of preserving AM. In the majority of studies on AM patching of burns, AM was frozen ('cryopreserved') for a quarantine period of six months to preclude transmission of maternal infections. In a small number of studies, unpreserved AM was used on the eye within days of childbirth (Chen 2000; Uçakhan 2002; Zhou 2004). To our knowledge, there are no reports of dried AM being used to treat burns.
The timing, surgical application and extent of treatment vary. AM can be applied from a few hours (Kobayashi 2003) to weeks (Sridhar 2000) after an ocular surface burn. AM is usually placed with the stromal side in direct contact with the ocular surface, but it may be placed epithelial‐side down (Meller 2000). Suturing of AM patches to the lids can be performed with interrupted sutures or with running sutures for easy removal (Arora 2005; Kobayashi 2003). Alternative methods of application include fitting a purpose‐made plastic ring conformer (symblepharon ring) wrapped in AM (Arora 2005; Kheirkah 2008; Tamhane 2005) and gluing with fibrin‐based adhesives (Sekiyama 2007). Although AM is typically applied as a removable patch (Kheirkah 2008; Kobayashi 2003), the tissue can be grafted onto the damaged ocular surface and left until it degrades (Meller 2000).
AMT has been used to treat a wide range of grades of burn, from mild (Kheirkah 2008) to severe (da Silva Ricardo 2009; Joseph 2001; Sridhar 2000). Since these injuries have very different natural histories, the effects of treatment are obscured. Consequently, the criteria for success and failure of AMT are unclearly defined, and there is confusion regarding the purpose and objectives of treatment (Maharajan 2007).
Objectives
To assess the effects of amniotic membrane (AM) patching of moderate and severe ocular burns in the acute phase (day 0 to 7).
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) of amniotic membrane (AM) patching of eyes with acute ocular burns. The minimum length of follow‐up was six months.
Types of participants
Participants of all ages were eligible and included those presenting for emergency eye care with chemical ocular surface burns of grade II or worse, or ocular surface thermal injuries of similar severity. Exclusion criteria included pre‐existing ocular surface disease or visual loss. Participants who received AM treatment after day seven were not considered eligible. We considered the following two subgroup populations.
Moderate burns ‐ patients who had ocular burns of grade II and III (Roper‐Hall and Dua classifications).
Severe burns ‐ patients who had ocular burns of grade IV (Roper‐Hall)/grades IV to VI (Dua).
Types of interventions
We included trials in which AM patching or grafting of whole or part of the ocular surface (including the fornices but not the external lids) was performed within seven days of injury, combined with all types of medical therapy together. Studies in which AM extracts or suspensions were applied as drops were not eligible. As Tenon‐plasty and autologous conjunctival patching are not commonly reported, we could not perform a comparison between surgical treatments.
The control patients received all types of medical therapy together, with no surgical intervention other than manual lysis of mechanical adhesions between the eyelids and the globe during the repair phases. The medical therapy used was defined for each included study. This normally consists of antibiotics, steroids, lubricants, oral and topical vitamin C, topical sodium citrate and oral doxycycline.
Types of outcome measures
We collected data on primary and secondary outcome measures, and included dichotomous, continuous, ordinal and time‐to‐event data.
Primary outcomes
The proportion of eyes with complete corneal epithelialisation 21 days after the burn injury. A persistent epithelial defect after 21 days of an ocular surface burn carries an increased risk of corneal stromal melting, and is therefore clinically meaningful.
Visual outcomes at final follow‐up.
Secondary outcomes
The proportion of eyes with symblepharon. Symblepharon limits normal movement, and is an index of severity.
The proportion of eyes with new vessels in the cornea (corneal neovascularisation). The extent of new vessels is an index of oxygen deprivation to the tissues. The number of quadrants of neovascularisation was expressed as an ordinal outcome from 0 to 4.
The proportion of eyes with adverse events, including rare incidences of corneal infections and immune reactions to AMT.
The time‐to‐complete corneal epithelialisation.
The proportion of eyes with vascularised scarring on the cornea (fibrovascular pannus), a cause of disfigurement and visual loss.
The proportion of patients who report pain reduction after AMT.
Follow‐up
For the primary outcome, the critical point for follow‐up was 21 days after the injury, because in the absence of an intact epithelium at the end of the early repair phase (McCulley 1987), the chances of requiring later ocular surface reconstruction are significant. Therefore, any difference between treatment and control groups at this stage can be considered to be clinically significant, and it is more likely to reflect a difference in treatment in the acute phase than would be the case after several more weeks. By 21 days, attrition rates of follow‐up appointments would not be high, and secondary surgeries would not yet have been performed. For most secondary outcomes (except pain reduction, which would be immediate), measurement time points reflected the healing process during the late repair phase. The exact time points were less critical, and could be multiple (e.g. six and 12 months), depending on outcome reporting.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 6, part of The Cochrane Library. www.thecochranelibrary.com (accessed 11 June 2012), MEDLINE (January 1946 to June 2012), EMBASE (January 1980 to June 2012), Latin American and Caribbean Health Sciences (LILACS) (January 1982 to June 2012), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 11 June 2012.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), mRCT (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We assessed references cited in studies identified as relevant and contacted the authors of included studies to find out about ongoing trials.
Data collection and analysis
Selection of studies
We screened the search results to assess relevant titles and abstracts and categorised them as 'included', 'excluded' or 'unclear'. Two review authors (GC and HS) independently screened the titles and abstracts. We retrieved published articles to identify and link multiple publications from the same study and to review the study characteristics of all included and unclear studies. We designed an eligibility (screening) form containing general information and eligibility criteria to justify inclusion or otherwise. We recorded search results on a flow sheet, and resolved discrepancies through consultation with a third author (HD). We listed studies that did not meet the inclusion criteria in the 'Characteristics of excluded studies' table and provided a reason for exclusion.
Data extraction and management
Two review authors (GC and HS) extracted data independently onto a data extraction form to obtain details of methodology and outcomes. General information entered included demographic data, diagnostic criteria and the grades and causative agent of the injury, and the timing and method of application of AM. We used the form to confirm eligibility for the review. In the event of missing data, we contacted the trial authors for more details. GC entered data into Review Manager 5 (RevMan 2011) for data analysis, and HS checked this. For dichotomous and stratified ordinal outcomes, we entered the proportion of patients in each intervention arm having the outcome of interest. We recorded continuous outcomes with all relevant statistical data. We separately processed time‐to‐event data in GraphPad Prism statistical software (La Jolla, California). We attached the curves as additional figures.
Assessment of risk of bias in included studies
We conducted a domain‐based evaluation of the risk of bias within each study, using an assessment tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). GC and HS independently assessed the risk of bias in each domain (see 'Risk of bias' table in 'Characteristics of included studies').
Measures of treatment effect
Primary measures of treatment effect
The primary measures of treatment effect included the relative risk of dichotomous outcomes (e.g. persistent epithelial defect at 21 days) and mean differences (MDs) of continuous outcomes (e.g. visual acuity). This was calculated using the Mantel‐Haenszel statistical method assuming a fixed‐effect, and reported with the 95% confidence interval (CI) and P value. We converted visual acuity measurements to a logarithmic scale to calculate the MD in the visual acuities at final follow‐up.
Secondary measures of treatment effect
We calculated the number‐needed‐to treat (NNT) for one additional patient to benefit (NNTB) or harm (NNTH) with its 95% CI using the Newcombe‐Wilson hybrid score without a continuity correction (Newcombe 1998). In cases of non‐significance (i.e. where the CI of the difference between the control event rate and the experimental event rate does not exclude 0), we quoted the estimated CIs for NNT using the method described by Altman 1998.
We calculated the relative risk for dichotomous data. We stratified ordinal data on corneal neovascularisation into three categories: 0 quadrants, 1 to 2 quadrants and 3 to 4 quadrants. We calculated the relative risk between each stratum and the combined remainder. For moderate burns, we calculated the risk of developing 1 to 2 quadrants of new vessels, while for severe burns, we calculated the risk of developing 3 to 4 quadrants. We summarised time‐to‐event data by survival analysis methods and expressed them as a hazard ratio where possible.
Unit of analysis issues
Primary outcomes were eye‐related rather than patient‐related. In cases of patients being burned in both eyes, we assessed the method of randomising the treatments. If one eye was chosen for bilateral patients, we documented the method of selection in order to detect possible biases. Analyses based on a single eye per individual conveniently allow standard statistical methods to be employed, although information for the fellow eye is lost (Murdoch 1998).
If both eyes were included and treatment to one eye influenced the treatment to the other, we considered this as a source of bias. In the case of both eyes being treated similarly, the likely correlation between fellow eyes was assumed to result in a possible cluster effect. Therefore, we considered analyses based on both injured eyes permissible if the proportion of bilateral eye injuries was small (less than 1%).
Dealing with missing data
We assessed whether or not there were any patients for whom outcomes were not assessed. In the case of missing outcome data, we set out to compare the characteristics of patients for whom there was missing data with those of patients with complete data in order to detect possible bias. We checked whether or not intent‐to‐treat analyses had been conducted, as a failure to do so could introduce bias.
Assessment of heterogeneity
We assessed heterogeneity between studies by review of study characteristics. We had planned to assess statistical heterogeneity in effect estimates across studies using the Chi2 test (P = 0.10). We had planned to assess inconsistency between studies by examination of the I2 statistic with CIs, using 75% as a cut‐off.
Assessment of reporting biases
To mitigate publication bias, we extended our search to unpublished studies and trial registers. We did not impose any language restrictions.
Data synthesis
We had planned to conduct a meta‐analysis in the event of three or more suitable RCTs being identified. We will incorporate new studies identified by future updates into this review, according to the protocol described (Clare 2011).
Subgroup analysis and investigation of heterogeneity
We performed analyses on moderate and severe subgroups of acute burns to investigate differential outcomes of AMT. We grouped together the causative agents of acute burns, rather than separately analysing them.
Sensitivity analysis
We planned to conduct sensitivity analyses based on the risk of bias (low, unclear, high) concerning the reported treatment effect on each outcome measure in different studies.
Summary of findings table
We consulted Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a) for the completion of a 'Summary of findings' table of included studies. We constructed this with particular reference to two outcomes of interest: the proportion of eyes with complete epithelial closure by the 21st day after the injury, and visual acuity at final follow‐up in patients with moderate burns and severe burns (Table 1; Table 2). We used the GRADEprofiler software (GRADEpro) to assess the quality of evidence for each treatment effect from high to very low in accordance with guidance in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011b).
Summary of findings for the main comparison. AMT during acute phase of moderate ocular burns.
| AMT during acute phase of moderate ocular burns | ||||||
|
Patient or population: patients with moderate acute ocular burns
Settings: ophthalmic hospital
Intervention: AMT and medical therapy Control: medical therapy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | AMT | |||||
| Epithelial defect on day 21 post‐injury Image analysis of digital photographs Follow‐up: 6 months to 24 months | 350 per 1000 | 63 per 1000 (7 to 458) | RR 0.18 (0.02 to 1.31) | 36 (1 study) | ⊕⊕⊝⊝ low1 | |
| Visual acuity at final follow‐up LogMAR. Scale from: 0 to 3 Follow‐up: 6 months to 24 months | The mean visual acuity at final follow‐up in the control groups was 0.38 | The mean visual acuity at final follow‐up in the intervention groups was 0.32 lower (0.09 to 0.55 lower) | 36 (1 study) | ⊕⊝⊝⊝ very low2,3,4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) AMT: amniotic membrane transplantation; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Epithelial defect assessed on day of clinic review, not daily. Defect was assessed under partially opaque membrane. This suggests possible imputation of data. 2 High risk of performance and detection biases, as not possible to mask personnel and outcome assessors. 3 Baseline imbalance in visual acuities between treatment and control groups. Mean visual acuity in control eyes significantly worse than treatment eyes at presentation. Improvement in visual acuity was greater in control group. Follow‐up was significantly longer in treatment group. In a number of control eyes, outcome was very poor, suggesting possible misclassification. These factors could have skewed findings in favour of treatment. 4 Visual acuity measured at final follow‐up rather than at a fixed interval.
Summary of findings 2. AMT during acute phase of severe ocular burns.
| AMT during acute phase of severe ocular burns | ||||||
|
Patient or population: patients with severe acute ocular burns
Settings: ophthalmic hospital
Intervention: AMT and medical therapy Comparison: medical therapy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | AMT | |||||
| Epithelial defect on day 21 post‐injury Image analysis of digital photographs Follow‐up: 6 months to 24 months | 933 per 1000 | 943 per 1000 (784 to 1000) | RR 1.01 (0.84 to 1.21) | 32 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Visual acuity at final follow‐up LogMAR. Scale from: 0 to 3 Follow‐up: 6 months to 24 months | The mean visual acuity at final follow‐up in the control groups was 1.64 | The mean visual acuity at final follow‐up in the intervention groups was 0.13 higher (0.84 lower to 1.10 higher) | 32 (1 study) | ⊕⊕⊝⊝ low1,3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) AMT: amniotic membrane transplantation; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High risk of detection and performance biases related to difficulties in masking personnel and outcome assessors. 2 Possible imputation of data due to non‐daily clinic attendance of patients and assessment of defect under partially opaque membrane. 3 Visual acuity measured at final follow‐up rather than at a fixed interval.
Results
Description of studies
Results of the search
The electronic searches identified 88 records (Figure 1). We screened the title and abstracts to identify RCTs; of the results, 10 were recent retrospective case series studies of ocular burns (Chen 2000; da Silva Ricardo 2009; Joseph 2001; Kheirkah 2008; Kobayashi 2003; Prabhasawat 2007; Sridhar 2000; Tejwani 2007; Uçakhan 2002; Zhou 2004). We screened the search results and rejected 80 records as not eligible for inclusion in the review. We obtained full‐text copies of eight reports for further assessment. We excluded five prospective studies (Arora 2005; López‐García 2006; López‐García 2007; Meller 2000; Muraine 2001) and one randomised study by Tamhane 2005 (see 'Characteristics of excluded studies' for reasons for exclusion). One citation (NCT00370812), concerned a clinical trial of amniotic membrane transplantation (AMT) for acute chemical burns. To our knowledge, no results have yet been published. Repeated efforts to contact the principal investigator proved unsuccessful. We classified the study as ongoing, and we will include the study in the review if data become available. We included one study Tandon 2011 in the review. Two additional citations (Chew 2011; Gupta 2011) concerned the RCTs identified by the search (Tamhane 2005; Tandon 2011). The original RCTs were performed by Tamhane 2005 and by Tandon 2011. Gupta 2011 compared the predictive value of the Roper‐Hall and Dua classifications using data from Tandon 2011. The article by Chew 2011 provides an assessment of the Tandon 2011 and Tamhane 2005 studies.
1.
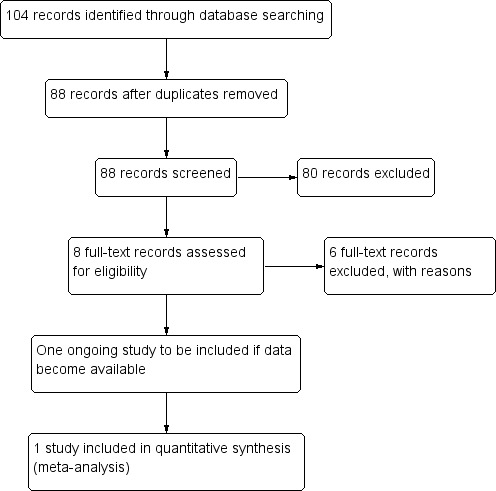
Results from searching for studies for inclusion in the review.
Included studies
A subset of patients from one RCT (Tandon 2011) met the inclusion criteria for this review. Due to the paucity of adequate RCTs, we could not conduct a meta‐analysis; instead, we have analysed the data on the subset of RCT participants. The RCT data were provided by the study authors.
AMT was carried out within two days of presentation by draping the stromal side over the entire ocular surface up to the lid margins and securing it in place with vicryl sutures. The original data reported outcomes for 100 patients (see Figure 2). Of these, 68 were treated in the first seven days and were therefore suitable for inclusion in this review. No missing data were reported. In cases of bilateral injuries, the right eye was selected.
2.

Flow chart showing categories of injured eyes.
Types of participants
The 68 participants included 36 with moderate and 32 with severe ocular burns. In the moderate burns group, there were 34 males and 2 females, with a mean age of 22.3 years (standard deviation (SD) 10.1) (median 20.5, range 4 to 52). The burns were caused by alkali in 22 cases, acid in eight, and direct heat in four. The severe category consisted of 24 males and eight females with ocular burns; 23 caused by alkali, seven by acid, and two by direct heat. The mean age was 19.3 years (SD 15.8) (median 12.5, range 6 to 61). The length of follow‐up of the included patients was six to 24 months for moderate burns, and nine to 24 months for severe burns.
Types of intervention
All patients were randomised to receive AMT and medical therapy or medical therapy alone. This consisted of topical prednisolone acetate (1%) every six hours, ofloxacin (0.3%) every six hours, sodium ascorbate (10%) every four hours, sodium citrate (10%) every four hours, preservative‐free tear substitutes every two hours, homatropine drops (2%) twice daily and oral vitamin C (500 mg) every six hours for two to four weeks. Pressure‐reducing therapy included timolol maleate drops (0.5%) and/or oral acetazolamide, if required.
Types of outcome measure
The primary outcome measure of the included RCT was the rate of healing of epithelial defect. The study authors provided information on the proportion of eyes with incomplete epithelialisation by day 21 post‐injury. The secondary outcome measures were visual outcomes at final follow‐up, the extent of corneal clarity and vascularisation, and the proportion of eyes with symblepharon. No data were available concerning the proportion of eyes with fibrovascular pannus or the reduction of pain.
Excluded studies
Five citations concerned non‐randomised prospective studies (Arora 2005; López‐García 2006; López‐García 2007; Meller 2000; Muraine 2001). These were definitively excluded after checking the full‐text report against the eligibility criteria. One RCT (Tamhane 2005) did not meet the inclusion criteria as the intervention was performed outside seven days, and the required data were no longer available (we contacted the co‐authors).
Risk of bias in included studies
A summary of The Cochrane Collaboration's tool for assessing risk of bias in the included study (Tandon 2011) is shown for two outcomes: epithelial closure at 21 days and final visual acuity (Characteristics of included studies). The risk of bias is summarised across domains for both study‐level entries, such as allocation, and outcome‐specific entries such as masking. The following points illustrate the reasoning behind the judgements made. A 'Risk of bias' summary figure is shown in Figure 3.
3.
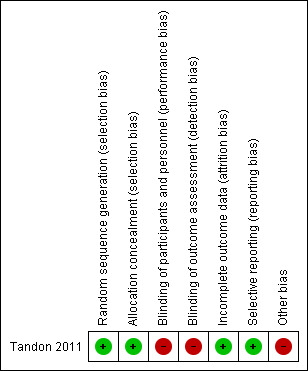
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The patients were randomised to a treatment assignment list prepared from a random numbers table. Serial numbers were given to the cases, and the randomly allocated treatment decision was concealed by using sealed envelopes. This suggests that adequate measures were taken to prevent foresight of treatment at the point of enrolment. The overall risk of allocation bias is classified as low.
Blinding
For practical reasons, it was not possible to mask study participants and personnel from knowing which treatment was received, suggesting a high risk of performance bias. Although digital photographs were used for assessment of outcomes by masked observers, the outcome assessors would have known whether AMT had been allocated since the AM persists for several weeks. This indicates a high risk of detection bias (Higgins 2011).
Incomplete outcome data
Since no participants in the study were lost to follow‐up, the overall risk of attrition bias is classified as low.
Selective reporting
The possibility of selective reporting bias in the study was considered to be low, i.e. the outcomes reported were not chosen because they were significant.
Other potential sources of bias
The risk of bias from other causes in the included study was considered to be high. Several potential sources of bias are identified below. For each reason, the risk of bias is shown in brackets. Cases in which the direction of bias favoured a treatment effect were graded as having a high risk of bias.
The data presented concern a subset of the patients recruited in the included RCT. This may have introduced bias, since the patients treated within seven days of injury may have had different characteristics to the remaining patients (unclear).
Of the 68 patients included in the study, 24 went on to have secondary procedures, such as limbal transplantation, in the months following the initial injury. The confounding effects of the secondary procedures on the measured outcomes (e.g. vision) are not clear. These eyes are likely to have suffered more serious injuries, and any treatment effect of AM is therefore likely to be smaller (unclear).
There was a significant difference in baseline visual acuity between treatment and control groups in the moderate burns category. The mean LogMAR visual acuity at presentation was 0.45 in the treatment group (SD 0.29) and 0.92 in the controls (SD 0.88) (independent samples t test, P = 0.04, equal variances assumed). This could have skewed the final visual outcomes in favour of a treatment effect (high).
Although final visual acuities were better in the AMT group (0.06, SD 0.10 versus 0.38, SD 0.52), the mean difference (MD) in visual acuity before and after treatment was greater in the control eyes (AMT ‐0.39, 95% CI ‐0.23 to ‐0.55, P = 0.001 versus controls ‐0.54, 95% CI ‐0.08 to ‐1.00, P < 0.001). The implication that final visual acuity was improved by AMT is therefore misleading (high).
The poor outcomes in the control eyes are not fully explained. In the moderate category, three control eyes had a time‐to‐epithelial closure of 90 days, while in the treatment group only one eye took longer than 18 days for the epithelium to heal. Five control eyes had a final visual acuity of less than 0.50. In contrast, no participant in the AMT treatment group had a final visual acuity of less than 0.30. While a treatment effect cannot be excluded, moderately burned eyes typically have a good prognosis. This raises the possibility that some injuries in the control group were misclassified at presentation (high).
There was a significantly longer follow‐up for treated eyes (MD 14.5 months, range 10 to 24 versus MD 12 months, range 6 to 24; Mann‐Whitney U = 94, z = ‐2.31, P = 0.02). Since there was no common time‐point for reporting visual acuity, this constitutes a potential source of bias. In the severe group, length of follow‐up (12 months) was not significantly different between the groups (unclear).
The methodology of measurement of epithelial healing may be a source of bias. The study authors state that in some AMT cases, the margins of the epithelial defect could not be made out beneath the amniotic membrane. There is no indication that any data were missing or excluded, yet it is not explained in the report how the problem was circumvented. The unit of measurement of the healing rate (mm2/day) implies that the eyes were seen every day until complete healing, which was not the case. This suggests that the precise timing of epithelial closure was a best guess between appointments or the appointment day itself. This may have led to imputation of data (Higgins 2011) (unclear).
Effects of interventions
AMT and medical therapy versus medical therapy alone for acute ocular burns
Moderate burns
Epithelialisation by day 21 post‐injury
Thirteen out of 20 control eyes and 14 out of 16 treatment eyes epithelialised completely within 21 days. This represents a risk ratio (RR) of failure of epithelialisation by day 21 of 0.18 in the treatment group (95% confidence interval (CI) 0.02 to 1.31; P = 0.09).
This gives the NNTB for one patient with AMT to achieve complete epithelialisation at day 21 as 3.5 (95% CI 2 to 104.5).
Visual acuity
Mean visual acuity (LogMAR) at final follow‐up was 0.06 in the AMT treatment group (SD 0.10). In the control group, LogMAR visual acuity was 0.38 (SD 0.52). The mean difference (MD) was ‐0.32 (95% CI ‐0.09 to ‐0.55; P = 0.007).
Corneal neovascularisation
The RR of developing 1 to 2 quadrants of corneal new vessels in the AMT treatment group was 0.63 (4/16 eyes in the treatment group and 8/20 eyes in the control group) (95% CI 0.23 to 1.71; P = 0.36).
Symblepharon
The RR of developing symblepharon among the AMT participants in the moderate group was 0.41 (0/16 treatment and 1/20 control eyes) (95% CI 0.02 to 9.48; P = 0.58).
Time to epithelialisation
Survival curves representing the time‐to‐epithelialisation for the treatment and control arms of the moderate burns group are shown in Figure 4. The lines cross, showing that the proportional hazards assumption is not valid with these data. A single hazard ratio is also not appropriate.
4.
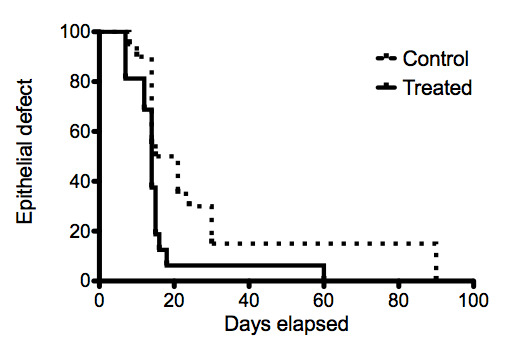
Survival curves of epithelial defects of moderate ocular burns treated with AMT.
Other
No information is available about the effects on pain or the presence of fibrovascular pannus. The study reported that no complications were encountered during the study period.
Severe burns
Epithelialisation by day 21 post‐injury
Only one eye in each group (17 eyes in treatment group and 15 controls) had complete epithelial recovery at 21 days; a RR of 1.01 (95% CI 0.84 to 1.21; P = 0.93).
This gives the NNTH for one patient with AMT to fail to achieve complete epithelialisation at day 21 as 127.5 (95% CI NNTH 4 to ∞ to NNTB 5).
Visual acuity
Mean LogMAR visual acuities at final follow‐up for AMT groups and controls respectively were 1.77 (SD 1.31) and 1.64 (SD 1.48) (MD 0.13; 95% CI ‐0.84 to 1.10) (P = 0.79).
Corneal neovascularisation
The RR of developing 3 to 4 quadrants of new vessels was 1.06 in the AMT treatment group (12/17 treatment eyes and 10/15 control eyes) (95% CI 0.66 to 1.70; P = 0.81).
Symblepharon
The RR of developing symblepharon among the AMT participants was 0.98 (10/17 treatment and 9/15 control eyes) (95% CI 0.55 to 1.74; P = 0.95).
Time to epithelialisation
Survival curves representing the time‐to‐epithelialisation for the treatment and control arms of the severe burns group are shown in Figure 5. The proportional hazards assumption and a single hazard ratio are not valid with these data.
5.
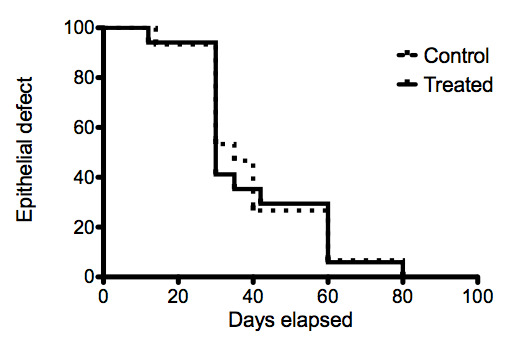
Survival curves of epithelial defects of severe ocular burns treated with AMT.
Other
No adverse events reported.
Discussion
The putative mechanisms of action of amniotic membrane (AM) on the ocular surface broadly fall into two categories: those that can be explained by directly observable physical interactions between the membrane and the eye, and those that are inferred by presumed biological properties of AM. In the first of these categories, AM functions as a biocompatible membrane with empirically observable benefits, such as sealing a perforation (Duchesne 2001) or preventing a leak from a surgical procedure (Budenz 2000). In the second category, a biological property is attributed to AM over and above its self‐evident value as a biomaterial, such as promotion of epithelial healing and suppression of inflammation (Tseng 2004). For the second category, high quality evidence is lacking. In the treatment of acute burns, these two categories may become conflated, obscuring the true value of amniotic membrane transplantation (AMT). Since the promotion of AMT may be driven by commercial interests, it is particularly important to clarify its true potential.
The physical functions of AM reflect its versatility as a thin and pliable membrane. From its initial use as a conjunctival substitute, AM evolved into a dressing for the burnt ocular surface to improve comfort (Lavery 1946; Sorsby 1946; Sorsby 1947). Subsequently, AM was used to prevent symblepharon, by acting as a spacer between inflamed tissues (Shafto 1950). Multiple layers of AM were later applied to prevent the impending perforation of bacterial corneal ulcers (Shukla 1962). More recently, AM has been used as a physical substrate for epithelial cell growth by placing it flat inside the boundary of persistent epithelial defects (Lee 1997). As such, it can be used to grow sheets of limbal epithelial cells (Tsai 2000) and mucosal epithelial cells (Kinoshita 2004) for ocular surface reconstruction. AM may also be used as a physical barrier against conjunctival ingrowth, protecting strips of transplanted limbal tissue on the stem cell deficient cornea (Tsubota 1996). These physical functions have directly observable benefits.
In contrast, the inferred ability of therapeutic AM to influence the biological healing response is unproven. AM is known to express biological factors, such as anti‐inflammatory and anti‐angiogenic proteins (Hao 2000), and soluble AM‐derived factors appear to be capable of suppressing the immune response in vitro (Li 2005). However, the concept that biological factors found in therapeutic AM have a beneficial clinical effect is speculative. The presumed mediators may become degraded during preservation and may be present in insufficient quantities to have an effect. Some of the factors have opposing effects, not all of which can be expected to be positive (Dua 2004). For example, regulatory proteins retained in therapeutic AM include transforming growth factor β1 (TGF‐β1) (Hopkinson 2006), which may cause scarring. Similarly, anti‐angiogenic factors such as tissue inhibitor of metalloproteinase 1 (TIMP‐1) (Hao 2000) may promote ischaemia. Furthermore, the concentration of biological factors in therapeutic AM is variable, both due to its heterogeneity (Gicquel 2009) and as a result of processing (Hopkinson 2006). This suggests an important need for clinical research into this treatment to continue.
The lack of evidence surrounding the 'pharmaceutical' use of AM is compounded by a lack of clarity in defining the purpose and objectives of AM treatment and in identifying treatment successes and failures (Maharajan 2007). This is in evidence in studies of the treatment of burns, which fall into the following four main groups.
Non‐randomised prospective studies of AMT for moderate burns (Table 5): these either lacked controls (Arora 2005; Meller 2000) or reported outcomes that are not directly clinically relevant, such as histological findings (López‐García 2006) and impression cytology (López‐García 2007). All of the studies claim success for AMT.
Retrospective case series studies of AMT for moderate burns (Table 6): most had fewer than 10 participants (Chen 2000; Kheirkah 2008; Kobayashi 2003; Uçakhan 2002). Two retrospective, uncontrolled studies of more than 20 participants have been reported, both claiming that AMT was beneficial for acute burns (Tejwani 2007; Zhou 2004). A study from Thailand compared the treatment group to a set of matched controls, finding faster epithelial healing in the treatment group (Prabhasawat 2007). All of these studies claimed that AMT was beneficial.
Non‐comparative case series studies of severe ocular burns (Table 7): in this group, burns were treated with AMT during the early and late reparative periods, rather than the acute phase. The reported assessments of the treatment are guarded in all four studies (da Silva Ricardo 2009; Joseph 2001; Muraine 2001; Sridhar 2000).
Randomised controlled trials (RCTs) (Table 8).
3. Prospective studies.
| Reference | Grade of burn | No. of eyes with AMT | No. of control eyes | Timing of AMT (days) | Outcome |
| Meller 2000 | II‐IV (R‐H) | 13 | ‐ | Mean 9.4 (SD 4.5) | “AMT alone rapidly restores both corneal and conjunctival surfaces” |
| Arora 2005 | II‐IV (R‐H) | 15 | ‐ | Mean 9.9 (SD 3.8) | “AMT increases patient comfort and reduces inflammation” |
| Tamhane 2005 | II‐IV | 24 | 24 | Range 1 to 14 | “Reduces pain and promotes epithelialisation" |
| López‐García 2006 | Moderate | 12 | 12 | 4 to 6 | “Corneal epithelialisation occurred earlier in patients treated with AMT” |
| López‐García 2007 | II‐IV (Dua) | 5 | 4 | 4 to 6 | “AMT improved limbal stromal and epithelial regeneration” |
AMT: amniotic membrane transplantation; R‐H: Roper‐Hall classification; SD: standard deviation
4. Retrospective studies.
| Study | Grade of burn | No. of eyes with AMT | No. of control eyes | Timing of AMT (days) | Outcome (moderate burns) |
| Chen 2000 | ‐ | 6 | 0 | ‐ | “...can effectively reduce neovascularisation, fibrosis and inflammation” |
| Uçakhan 2002 | II‐IV (R‐H) | 5 | 0 | Mean 14.2 (SD 12.3) | “...decreased the extent and severity of vascularisation” |
| Kobayashi 2003 | II‐III (R‐H) | 5 | 0 | Median 4 (0 to 6) | “...facilitating rapid epithelialisation and pain relief” |
| Zhou 2004 | III | 20 | 0 | ‐ | “...can prevent corneal ulcer” |
| Tejwani 2007 | II‐IV (Dua) | 24 | 0 | Median 2 (1 to 20) | “...partially restores limbal stem cell function” |
| Prabhasawat 2007 | II‐IV (R‐H) | 13 | 8 | Range 1 to 10 days | “...promoted rapid epithelial healing and reduced corneal complication” |
| Kheirkah 2008 | I‐III (R‐H) | 5 | 0 | Mean 3.7 (SD 3.1) | “...may help preserve remaining limbal stem cells” |
AMT: amniotic membrane transplantation; R‐H: Roper‐Hall classification; SD: standard deviation
5. Severe burns.
| Study | No. of eyes with AMT | Timing of AMT | Outcome |
| Sridhar 2000 | 2 | "Within weeks" | “Long‐term studies are warranted” |
| Muraine 2001 | 9 | 1 month to 5 years | "preferable to conjunctival advancement" |
| Joseph 2001 | 4 | 14 to 21 days | “..AMT did not help to restore ocular surface” |
| da Silva Ricardo 2009 | 5 | 10 to 30 days | “...not possible to avoid the limbic deficiency” |
AMT: amniotic membrane transplantation
6. Randomised controlled trials.
| Reference | Grade of burn | No. of eyes with AMT | No. of control eyes | Timing of AMT (days) | Outcome |
| Tamhane 2005 | II‐IV (R‐H) | 24 | 24 | Range 1 to 14 | “Reduces pain and promotes epithelialisation" |
| Tandon 2011 | II‐VI (Dua) | 50 | 50 | Range 0 to 15 | “promotes faster healing of epithelial defect” |
AMT: amniotic membrane transplantation; R‐H: Roper‐Hall classification
Studies from the first two groups include ocular burns of different causes and severity. This makes it difficult to identify any benefit of AMT, since different grades of burn carry very different prognoses. The variability in timing of the AM treatment after injury, ranging from a few hours (Kobayashi 2003) to 20 days (Tejwani 2007), and the duration of treatment, from two weeks (Kobayashi 2003) to indefinite (Meller 2000), further obfuscate any treatment benefits.
Two RCTs from the same centre have addressed AM patching for acute burns (Tamhane 2005; Tandon 2011). In the first RCT (Tamhane 2005), 20 eyes with ocular burns were treated with AM, with 24 controls. Participants with grades II‐IV (Roper‐Hall classification) were included, and treatment was carried out between 1 and 14 days after the injury. The study found that AMT improved pain and promoted early epithelialisation. This was determined by comparing the logarithm of the mean percentage reduction of the epithelial defect size at various time points. Although the study found some statistically significant difference in the healing rates, the treatment and control groups did not differ in clinical parameters such as final visual outcome. There was also a high participant dropout rate, further weakening the study. Moreover, there was significant baseline imbalance, with the treatment group having more severe injuries, and this was attributed to the small sample size and the randomisation strategy. This was reported as a "fallacy" in a subsequent RCT (Tandon 2011). The second RCT was conducted to redress this bias by increasing the sample size and improving the randomisation process. A total of 100 participants were recruited. The authors found epithelial healing to be significantly faster in the treatment arm of the moderate burns group. While the later study addressed some of the limitations of the former, including insufficient randomisation and a small sample size, it has been considered to be underpowered (Chew 2011). Both RCTs considered the epithelial healing rate as a primary outcome measure. While the speed of epithelial healing is important, it is not considered a 'patient‐centred' outcome, since it cannot be used to prognosticate the extent of recovery after a burn. Moreover, the rate of epithelial healing may reflect variables other than a treatment effect, including timing of presentation, size of epithelial defect, severity of injury and patient factors. Besides being technically difficult to achieve, accurate and precise measurements of the rate of corneal epithelial healing following burns are limited by the number of time‐points of patient attendance and by treatment with semi‐opaque AM patches. For these reasons, the first of two primary outcome measures addressed in the review was not the epithelial healing rate, but the proportion of eyes with a persistent epithelial defect at the end of the early reparative phase, as designated by McCulley 1987. A further primary outcome measure was final visual acuity. In patients with moderate burns treated with AMT within the first seven days of injury in the Tandon 2011 study, there was no significant difference in the proportion of eyes with a persistent epithelial defect between the treatment and control groups. Although visual outcomes were better, there was a high risk of bias from a baseline imbalance. The value of this systematic review is therefore to highlight the lack of high quality evidence supporting the use of AMT in acute ocular burns and to provide a protocol for future RCTs (Clare 2011).
Summary of main results
There is currently not enough evidence to recommend the treatment of ocular burns with amniotic membrane transplantation (AMT) in the first seven days following injury. In one randomised controlled trial (RCT) of acutely burned eyes treated with AMT, there was no statistically significant increase in the proportion of eyes with complete epithelial healing by day 21 or in mean visual acuity.
Overall completeness and applicability of evidence
We could only include one RCT in this systematic review, indicating a need for further studies. There is a high risk of bias in the included RCT (Tandon 2011) in three domains (performance, detection, and baseline imbalance) and a low risk in four (selection, allocation concealment, attrition, and selective reporting). The variations in the timings of presentation and treatment, as well as the grade of burn and causative agent, all contribute to the high level of outcome variability, pointing to a need for more specificity in further studies.
Quality of the evidence
The GRADEprofiler software (GRADEpro) allowed us to make an assessment of the quality of the evidence based on the risk of bias and any inconsistencies, indirectness and imprecision, identified as absent, serious or very serious. This was done for the moderate burns group (Table 1) and the severe burns group (Table 2).
There was a risk of bias from the inability to mask personnel and outcome assessors from the treatment. The quality of the evidence was further downgraded because of possible imputation of data, resulting in imprecision (measured in days despite no daily reviews). The calculation of mean visual outcomes measured at final follow‐up rather than a fixed interval is a further limitation of the study quality.
In the moderate burns group, the risk of bias in the assessment of failure of epithelialisation was classified as serious. The risk of bias in the assessment of visual outcomes was classified as very serious as there was a baseline imbalance in visual acuities between the treatment and control groups, and there are very poor outcomes in the control group, suggesting misclassification. This gave an assessment of the quality of the evidence as low and very low for these respective outcomes.
In the severe burns group, the risk of bias and imprecision were assessed to be serious, resulting in an assessment of quality of evidence as low for both outcomes.
The small sample size was small, which resulted in an underpowered study and wide confidence intervals (CIs).
Potential biases in the review process
It is likely that all relevant studies have been included in this review. The decision to exclude one RCT (Tamhane 2005) was based on the difficulty in obtaining all the necessary data. The clinical trial that was identified (NCT00370812) does not appear to have produced results, and efforts to contact the trialists have so far failed. It has been classified as 'ongoing'.
Agreements and disagreements with other studies or reviews
The vast majority of reports on AMT for acute ocular burns support its use, frequently citing its pro‐epithelial, anti‐fibrotic and anti‐inflammatory properties. We are not affirming our disagreement with this position, but highlighting the lack of clinical evidence to support it.
Authors' conclusions
Implications for practice.
The value of this systematic review is perhaps to highlight the lack of evidence supporting the use of amniotic membrane transplantation (AMT) in acute ocular burns. At one end of the scale, AMT is not indicated for an uncomplicated mild burn, which has an excellent prognosis. At the other end of the scale, AMT is not sufficient to mitigate the sequelae of a very severe burn. For moderate burns, more evidence is required to justify the application of AMT to treat pain or to prevent inflammation, scarring and visual loss.
Implications for research.
Although the lack of suitable randomised controlled trials (RCTs) precludes a meta‐analysis, some important conclusions may be drawn for future RCTs, both to minimise biases and to make them more clinically meaningful. We propose the following recommendations to increase the quality of further studies.
Well‐designed RCTs are preferable to uncontrolled case series studies.
For greater precision, the Dua classification should be adopted in future studies.
The inclusion criteria should specify a single category of causative agent.
Treatment should be performed in the acute phase (by day seven).
Treatment should be temporary (e.g. patch removed at 21 days post‐injury) in order to facilitate a masked assessment of epithelial recovery. Temporary patching may also reduce the risk of adverse outcomes, such as fungal keratitis, which often take several weeks to develop. Moreover, it is unlikely that an AM patch will continue to have any effect after this period.
A narrow grade of burn should be specified for each outcome to help equalise the baseline characteristics between treatment and control arms. For epithelial healing studies, the grade of burn should be severe enough to warrant treatment, but not so severe as to carry a high likelihood of secondary procedures or dismal prognosis. A series of grade III alkaline burns (prognosis good to guarded, Dua classification) may be sufficient to clearly demonstrate a treatment effect.
Separate studies could address the prevention of symblepharon, helping to direct treatment with AMT in increasingly case‐specific ways. For symblepharon prevention studies, AMT would be reserved for cases in which there was forniceal involvement. These would tend to be more severe.
A multicentre RCT could be organised to ensure sufficient numbers of cases.
Each outcome should be categorised as continuous, ordinal, dichotomous or time‐to‐event.
-
Outcome measurement and reporting are of great importance in these studies. Outcome measures should be meaningful and simple to understand.
Primary outcome measures should answer clinically meaningful questions. For example, does AMT in the acute phase of a Dua grade IV alkaline burn result in a higher likelihood of complete epithelial healing by the end of the early reparative phase, compared to an untreated control? Does it reduce pain? Does it prevent scarring?
Patient‐important outcomes should be considered in future research as well as the objective measures described. These can be defined by interviewing patients.
The complexity of comparing epithelial healing rates can be reduced by restricting measurement of the epithelial defect to two time‐points (pre‐ and post‐AMT). Epithelial healing rates are highly dependent on case‐by‐case factors, such as timing of presentation and depth of burn. The more uniform the treatment timing, the more accurate and precise the outcome measurement will be.
Where possible, outcome reporting should be kept simple for the study message to be made clear. Outcome measures such as the log mean percentage reduction in size of epithelial defect lack clarity. Conversely, a statistically significant difference in the size of an epithelial defect between treated and untreated groups at a specified time‐point would constitute a clear outcome report.
An assessment of visual outcomes after a fixed interval is also important for any future RCTs. It would help to specify whether any participants had previously identified visual loss and whether visual acuities were measured with or without correction.
Simple grading systems for pain and ocular surface inflammation are better than none at all. An assessment of pain relief could include visual analogue pain rating scales before and after treatment. Convincing evidence of improved comfort alone could justify treatment of acute burns with AMT, but even this is currently lacking.
The remaining outcomes of acute burns, such as symblepharon, fibrovascular pannus and corneal neovascularisation will be harder to assess because they are less common outcomes and are associated with more severity. These can be dealt with separately in larger studies of severe injuries.
In the absence of more RCTs, evidence for this kind of treatment will be difficult to obtain. The authors of the included RCT (Tandon 2011) have suggested that a stepwise sequential treatment trial with stratified randomisation would be a better model to determine best practice (personal communication from Dr Radhika Tandon). An RCT may not be the best model to study a relatively uncommon and complex disease, and an intervention that is influenced by multiple clinical parameters, including the timing of presentation. Animal models may also help to clarify the role of AMT in acute burns.
What's new
| Date | Event | Description |
|---|---|---|
| 9 April 2015 | Amended | Contact details updated. |
History
Protocol first published: Issue 10, 2011 Review first published: Issue 9, 2012
| Date | Event | Description |
|---|---|---|
| 17 October 2013 | Amended | Contact details updated. |
Acknowledgements
The Cochrane Eyes and Vision Group (CEVG) created and executed the search strategies for the electronic databases. We thank Steve Tuft, Ann‐Margret Ervin and Michael Marrone for their comments on the protocol. We thank Anupa Shah, Managing Editor for CEVG for her assistance throughout the review process.
The authors would like to thank Dr Radhika Tandon of the Cornea and Refractive Surgery Services at the Dr Rajendra Prasad Centre for Ophthalmic Sciences and the All India Institute of Medical Sciences for her help with this systematic review.
Richard Wormald (Co‐ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health (UK) through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Eye Burns #2 (eye* or ocular) near/6 (burn*) #3 (#1 OR #2) #4 MeSH descriptor Amnion #5 amniotic near/3 membrane* #6 AMT #7 (#4 OR #5 OR #6) #8 (#3 AND #7)
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. Eye burns/ 14. ((eye$ or ocular) adj6 burn$).tw. 15. or/13‐14 16. exp Amnion/ 17. (amniotic adj3 membrane$).tw. 18. AMT.tw. 19. or/16‐18 20. 15 and 19 21. 12 and 20
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. Eye burn/ 34. Cornea burn/ 35. ((eye$ or ocular) adj6 burn$).tw. 36. or/33‐35 37. exp Amnion/ 38. (amniotic adj3 membrane$).tw. 39. AMT.tw. 40. or/37‐39 41. 36 and 40 42. 32 and 41
Appendix 4. LILACS search strategy
amniotic and eye or ocular burn$
Appendix 5. metaRegister of Controlled Trials search strategy
amniotic and ocular burn
Appendix 6. ClinicalTrials.gov search strategy
amniotic AND ocular burn
Appendix 7. ICTRP search strategy
amniotic AND ocular burn
Data and analyses
Comparison 1. AMT and medical therapy versus medical therapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of epithelialisation (21 days) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Moderate burns | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Severe burns | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Visual acuity at final follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Moderate burns | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Severe burns | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Corneal neovascularisation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Moderate burns (1 to 2 quadrants) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Severe burns (3 to 4 quadrants) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Symblepharon | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Moderate burns | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Severe burns | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
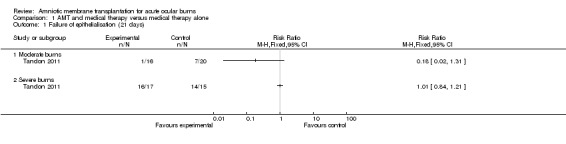
Comparison 1 AMT and medical therapy versus medical therapy alone, Outcome 1 Failure of epithelialisation (21 days).
1.2. Analysis.
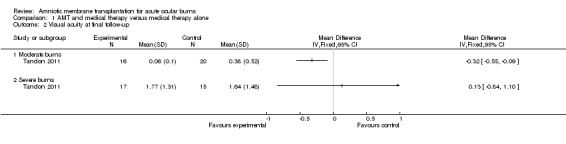
Comparison 1 AMT and medical therapy versus medical therapy alone, Outcome 2 Visual acuity at final follow‐up.
1.3. Analysis.

Comparison 1 AMT and medical therapy versus medical therapy alone, Outcome 3 Corneal neovascularisation.
1.4. Analysis.
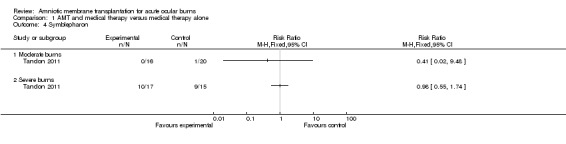
Comparison 1 AMT and medical therapy versus medical therapy alone, Outcome 4 Symblepharon.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Tandon 2011.
| Methods | Single centre, single surgeon masked RCT conducted in New Delhi, India | |
| Participants | One hundred patients with chemical (acid or alkali) or thermal ocular burns, of which 50 were moderate and 50 severe. Of these 100, 68 fulfilled the inclusion criteria for this review | |
| Interventions | Amniotic membrane patch sutured to entire ocular surface, within 7 days of injury with conventional medical therapy versus medical therapy alone | |
| Outcomes | Number of eyes with completely re‐epithelialised ocular surface at 21 days; visual acuity at final follow‐up, number of quadrants of corneal vascularisation, presence of symblepharon | |
| Notes | Authors provided database. The results presented here constitute a secondary analysis which differs from the published version | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were randomised using a treatment assignment list prepared with the help of a table of random numbers" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Serial numbers were given to the cases, and concealed randomisation using sealed envelopes was followed to decide whether a subject would receive either AMT combined with conventional medical therapy (study group) or conventional medical therapy alone (control group)" Comment: Probably done, although no explicit mention on when the envelopes were opened or if they were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment obvious to participants and clinicians. No way of masking treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Digital photographs at each visit were obtained and stored for independent comparative assessment by masked observers" Comment: Digital photographs were taken of the eyes at each visit for assessment by observers, yet AMT is obvious, at least for a few weeks |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reports of incomplete follow‐up |
| Selective reporting (reporting bias) | Low risk | Not detected |
| Other bias | High risk | There is baseline imbalance in visual acuity between control and treatment arms of moderate burns group The control group contains a high proportion of eyes with very poor outcomes, suggesting possible misclassification This could have skewed findings in favour of treatment |
AMT: amniotic membrane transplantation GC corresponded with Dr Radhika Tandon by email to obtain the required data
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arora 2005 | Prospective non‐randomised study |
| López‐García 2006 | Prospective non‐randomised study |
| López‐García 2007 | Prospective non‐randomised study |
| Meller 2000 | Prospective non‐randomised study |
| Muraine 2001 | Prospective non‐randomised study; non‐acute injuries |
| Tamhane 2005 | Treatment not stratified into acute and early phase groups. Insufficient data on number of eyes with intact epithelium at 21 days and on visual acuity |
Characteristics of ongoing studies [ordered by study ID]
NCT00370812.
| Trial name or title | The role of amniotic membrane transplantation in ocular chemical burns |
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | Amniotic membrane transplantation |
| Outcomes | |
| Starting date | August 31, 2006 |
| Contact information | Shaheed Beheshti Medical University; labbafi@hotmail.com |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently |
Differences between protocol and review
As we only included one randomised controlled trial (RCT), we did not prepare a funnel plot comparing different treatment effects. We could not conduct sensitivity analyses for the main findings. Calculations of statistical heterogeneity and inconsistency were not necessary.
The search strategies published in the protocol did not include a RCT filter as we were considering searching for all study designs. However, after further discussion we decided to incorporate a RCT filter as the review will only use data from RCTs.
Contributions of authors
Conceiving the review: Gerry Clare (GC)
Designing the review: GC
Co‐ordinating the review: GC
-
Data collection for the review:
Designing electronic search strategies: Cochrane Eyes and Vision Group editorial base
Undertaking manual searches: GC
Screening search results: GC, Hanif Suleman (HS)
Organising retrieval of papers: GC
Screening retrieved papers against inclusion criteria: GC, HS
Appraising quality of papers: GC, HS
Extracting data from papers: GC, HS
Writing to authors of papers for additional information: GC
Providing additional data about papers: GC
Obtaining and screening data on unpublished studies: GC
-
Data management for the review:
Entering data into Review Manager: GC
Checking that data entered into Review Manager is correct: HS
Analysis of data: GC and Catey Bunce (CB)
-
Interpretation of data: GC
Providing a methodological perspective: GC, CB, Harminder Dua (HD)
Providing a clinical perspective: GC, HD
Providing a policy perspective: GC, HD
Writing the review: GC
Providing general advice on the review: CB, HD
Securing funding for the review: GC
Performing previous work that was the foundation of the current study: GC, HS, CB, HD
Sources of support
Internal sources
No sources of support supplied
External sources
-
Ministry of Defence, UK.
This study was conducted in conjunction with a project to use dried amniotic membrane as a battlefield dressing for the ocular surface.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Tandon 2011 {published and unpublished data}
- Tandon R, Gupta N, Kalaivani M, Sharma N, Titiyal JS, Vajpayee RB. Amniotic membrane transplantation as an adjunct to medical therapy in acute ocular burns. British Journal of Ophthalmology 2011;95(2):199‐204. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arora 2005 {published data only}
- Arora R, Mehta D, Jain V. Amniotic membrane transplantation in acute chemical burns. Eye 2005;19(3):273‐8. [DOI] [PubMed] [Google Scholar]
López‐García 2006 {published data only}
- López‐García JS, Rivas L, García‐Lozano I, Murube J. Analysis of corneal surface evolution after moderate alkaline burns by using impression cytology. Cornea 2006;25(8):908‐13. [DOI] [PubMed] [Google Scholar]
López‐García 2007 {published data only}
- López‐García JS, Rivas Jara L, García‐Lozano I, Murube J. Histopathologic limbus evolution after alkaline burns. Cornea 2007;26(9):1043‐8. [DOI] [PubMed] [Google Scholar]
Meller 2000 {published data only}
- Meller D, Pires RT, Mack RJ, Figueiredo F, Heiligenhaus A, Park WC, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology 2000;107(5):980‐9. [DOI] [PubMed] [Google Scholar]
Muraine 2001 {published data only}
- Muraine M, Descargues G, Franck O, Villeroy F, Toubeau D, Menguy E, et al. Amniotic membrane graft in ocular surface disease. Prospective study with 31 cases [La greffe de membrane amniotique dans les pathologies oculaires de surface. Etude prospective a partir de 31 cas]. Journal Francais d' Opthalmologie 2001;24(8):798‐12. [PubMed] [Google Scholar]
Tamhane 2005 {published data only (unpublished sought but not used)}
- Tamhane A, Vajpayee RB, Biswas NR, Pandey RM, Sharma N, Titiyal JS, et al. Evaluation of amniotic membrane transplantation as an adjunct to medical therapy as compared with medical therapy alone in acute ocular burns. Ophthalmology 2005;112(11):1963‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00370812 {published data only (unpublished sought but not used)}
- NCT00370812. The role of amniotic membrane transplantation in ocular chemical burns. clinicaltrials.gov/ct2/show/NCT00370812 (accessed 20 July 12).
Additional references
Alberth 1971
- Alberth B, Kettesy A. Surgical treatment of caustic eye injuries [Die chirurgische behandlung der atzverletzungen des auges]. Bucherei des Augenarztes 1971;57:1‐119. [PubMed] [Google Scholar]
Altman 1998
- Altman DG. Confidence intervals for the number needed to treat. BMJ 1998;317(7168):1309‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batmanov 1990
- Batmanov I, Egorova KS, Kolesnikova LN. Use of fresh amnion in the treatment of corneal diseases. Vestnik Oftalmologii 1990;106(5):17‐9. [PubMed] [Google Scholar]
Battle 1993
- Battle JF, Perdomo FJ. Placental membranes as a conjunctival substitute. Ophthalmology 1993; Vol. 100:107 Abstract 9A.
Baum 2002
- Baum J. Thygeson lecture. Amniotic membrane transplantation: why is it effective?. Cornea 2002;21(4):339‐41. [DOI] [PubMed] [Google Scholar]
Bouchard 2004
- Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: indications and outcomes. Ocular Surface 2004;2(3):201‐11. [DOI] [PubMed] [Google Scholar]
Brown 1941
- Brown AL. Lime burns of the eye: Use of rabbit peritoneum to prevent severe delayed effects. Archives of Ophthalmology 1941;26:754‐69. [Google Scholar]
Budenz 2000
- Budenz DL, Barton K, Tseng SC. Amniotic membrane transplantation for repair of leaking glaucoma filtering blebs. American Journal of Ophthalmology 2000;130(5):581‐8. [DOI] [PubMed] [Google Scholar]
Chao 1940
- Chao YC, Humphreys S, Penfield W. A new method of preventing adhesions. The use of amnioplastin after craniotomy. British Medical Journal 1940;1:517‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2000
- Chen J, Zhou S, Huang T, Liu Z, Chen L, Lin Y. A clinical study on fresh amniotic membrane transplantation for treatment of severe ocular surface disorders at acute inflammatory and cicatricial stage. Zhonghua Yan Ke Za Zhi 2000;36(1):13‐7. [PubMed] [Google Scholar]
Chew 2011
- Chew HF. Amniotic membrane transplantation as an adjunct to medical therapy in acute ocular burns. Evidence‐Based Ophthalmology 2011;12(3):152‐3. [DOI] [PubMed] [Google Scholar]
Clare 2011
- Clare G, Suleman H, Bunce C, Dua H. Amniotic membrane transplantation for acute ocular burns. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD009379] [DOI] [PMC free article] [PubMed] [Google Scholar]
Connon 2007
- Connon CJ, Nakamura T, Hopkinson A, Quantock A, Yagi N, Doutch J, et al. The biomechanics of amnion rupture: an X‐ray diffraction study. PLoS One 2007;2(11):e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connon 2010
- Connon CJ, Doutch J, Chen B, Hopkinson A, Mehta JS, Nakamura T, et al. The variation in transparency of amniotic membrane used in ocular surface regeneration. British Journal of Ophthalmology 2010;94(8):1057‐61. [DOI] [PubMed] [Google Scholar]
da Silva Ricardo 2009
- Silva Ricardo JR, Barros SL, dos Santos MS, Souza LB, Gomes JA. Amniotic membrane transplantation for severe acute cases of chemical ocular burn and Stevens‐Johnson syndrome. Arquivos Brasileiros de Oftalmologia 2009;72(2):215‐20. [DOI] [PubMed] [Google Scholar]
Das 2009
- Das S, Ramamurthy B, Sangwan VS. Fungal keratitis following amniotic membrane transplantation. International Ophthalmology 2009;29(1):49‐51. [DOI] [PubMed] [Google Scholar]
De Rötth 1940
- Rötth A. Plastic repair of conjunctival defects with fetal membranes. Archives of Ophthalmology 1940;23(3):522‐5. [Google Scholar]
Denig 1918
- Denig R. Transplantation of mucous membrane of mouth for various diseases and burns of the cornea. New York Medical Journal 1918;107:1074‐5. [Google Scholar]
Dua 2001
- Dua HS, King AJ, Joseph A. A new classification of ocular surface burns. British Journal of Ophthalmology 2001;85(11):1379‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dua 2004
- Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Survey of Ophthalmology 2004;49(1):51‐77. [DOI] [PubMed] [Google Scholar]
Dua 2005
- Dua HS, Shanmuganathan VA, Powell‐Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. British Journal of Ophthalmology 2005;89(5):529–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dua 2010
- Dua HS, Rahman I, Miri A, Said DG. Variations in amniotic membrane: relevance for clinical applications. British Journal of Ophthalmology 2010;94(8):963‐4. [DOI] [PubMed] [Google Scholar]
Duchesne 2001
- Duchesne B, Tahi H, Galand A. Use of human fibrin glue and amniotic membrane transplant in corneal perforation. Cornea 2001;20(2):230‐2. [DOI] [PubMed] [Google Scholar]
Fini 1998
- Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Archives of Dermatological Research 1998;290:S12‐23. [DOI] [PubMed] [Google Scholar]
Gabler 2000
- Gabler B, Lohmann CP. Hypopyon after repeated transplantation of human amniotic membrane onto the corneal surface. Ophthalmology 2000;107(7):1344–6. [DOI] [PubMed] [Google Scholar]
Gicquel 2009
- Gicquel JJ, Dua HS, Brodie A, Mohammed I, Suleman H, Lazutina E, et al. Epidermal growth factor (EGF) variations in amniotic membrane used for ex vivo tissue constructs. Tissue Engineering. Part A 2009;15(8):1919‐27. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows 2008.
Gupta 2011
- Gupta N, Kalaivani M, Tandon R. Comparison of prognostic value of Roper Hall and Dua classification systems in acute ocular burns. British Journal of Ophthalmology 2011;95(2):194‐8. [DOI] [PubMed] [Google Scholar]
Hackett 2011
- Hackett JM, Lagali NS, Merrett K, Edelhauser HF, Sun Y, Gan L, et al. Biosynthetic corneal implants for replacement of pathologic corneal tissue: performance in a controlled rabbit alkali burn model. Investigative Ophthalmology and Visual Science 2011;52(2):651‐7. [DOI] [PubMed] [Google Scholar]
Hao 2000
- Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of anti‐angiogenic and anti‐inflammatory proteins in human amniotic membrane. Cornea 2000;19(3):348‐52. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopkinson 2006
- Hopkinson A, McIntosh RS, Tighe PJ, James DK, Dua HS. Amniotic membrane for ocular surface reconstruction: donor variations and the effect of handling on TGF‐β content. Investigative Ophthalmology and Visual Science 2006;47(10):4316‐22. [DOI] [PubMed] [Google Scholar]
Hori 2006
- Hori J, Wang M, Kamiya K, Takahashi H, Sakuragawa N. Immunological characteristics of amniotic epithelium. Cornea 2006;25(10 Suppl 1):S53‐8. [DOI] [PubMed] [Google Scholar]
Joseph 2001
- Joseph A, Dua HS, King AJ. Failure of amniotic membrane transplantation in the treatment of acute ocular burns. British Journal of Ophthalmology 2001;85(9):1065‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2001
- Jϋni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kheirkah 2008
- Kheirkhah A, Johnson DA, Paranjpe DR, Raju VK, Casas V, Tseng SC. Temporary sutureless amniotic membrane patch for acute alkaline burns. Archives of Ophthalmology 2008;126(8):1059‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 1995a
- Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 1995;14(5):473‐84. [PubMed] [Google Scholar]
Kim 1995b
- Kim JC, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean Journal of Ophthalmology 1995;9(1):32‐46. [DOI] [PubMed] [Google Scholar]
Kim 2000
- Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Experimental Eye Research 2000;70(3):329‐37. [DOI] [PubMed] [Google Scholar]
Kinoshita 2004
- Kinoshita S, Nakamura T. Development of cultivated mucosal epithelial sheet transplantation for ocular surface reconstruction. Artificial Organs 2004;28(1):22‐7. [DOI] [PubMed] [Google Scholar]
Kobayashi 2003
- Kobayashi A, Shirao Y, Yoshita T, Yagami K, Segawa Y, Kawasaki K, et al. Temporary amniotic membrane patching for acute chemical burns. Eye 2003;17(2):149‐58. [DOI] [PubMed] [Google Scholar]
Koizumi 2000a
- Koizumi N, Inatomi T, Quantock AJ, Fullwood NJ, Dota A, Kinoshita S. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea 2000;19(1):65‐71. [DOI] [PubMed] [Google Scholar]
Koizumi 2000b
- Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Current Eye Research 2000;20(3):173‐7. [PubMed] [Google Scholar]
Koizumi 2001
- Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 2001;108(9):1569‐74. [DOI] [PubMed] [Google Scholar]
Kuckelkorn 1995
- Kuckelkorn R, Schrage N, Reim M. Treatment of severe eye burns by Tenon‐plasty. Lancet 1995;345(8950):657‐8. [DOI] [PubMed] [Google Scholar]
Lavery 1946
- Lavery FS. Lime burn of conjunctiva and cornea treated with amnioplastin graft. Transactions of the Ophthalmological Society of the United Kingdom 1946;66:668. [Google Scholar]
Lee 1997
- Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. American Journal of Ophthalmology 1997;123(3):303‐12. [DOI] [PubMed] [Google Scholar]
Levinson 1976
- Levinson RA, Paterson CA, Pfister RR. Ascorbic acid prevents corneal ulceration and perforation following experimental alkali burns. Investigative Ophthalmology and Visual Science 1976;15(12):986–93. [PubMed] [Google Scholar]
Li 2005
- Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Investigative Ophthalmology and Visual Science 2005;46(3):900‐7. [DOI] [PubMed] [Google Scholar]
Loon 2009
- Loon SC, Tay WT, Saw SM, Wang JJ, Wong TY. Prevalence and risk factors of ocular trauma in an urban south‐east Asian population: the Singapore Malay Eye Study. Clinical and Experimental Ophthalmology 2009;37(4):362‐7. [DOI] [PubMed] [Google Scholar]
Ma 2009
- Ma DH, Kuo MT, Tsai YJ, Chen HC, Chen XL, Wang SF, et al. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye 2009;23(6):1442‐50. [DOI] [PubMed] [Google Scholar]
Maharajan 2007
- Maharajan VS, Shanmuganathan V, Currie A, Hopkinson A, Powell‐Richards A, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction: indications and outcomes. Clinical and Experimental Ophthalmology 2007;35(2):140‐7. [DOI] [PubMed] [Google Scholar]
Malhotra 2009
- Malhotra R, Sheikh I, Dheansa B. The management of eyelid burns. Survey of Ophthalmology 2009;54(3):356‐71. [DOI] [PubMed] [Google Scholar]
McCulley 1987
- McCulley JP. The Cornea: Scientific Foundation and Clinical Practice. 2nd Edition. Boston: Little, Brown and Co, 1987. [Google Scholar]
McCulley 1990
- McCulley JP. Ocular hydrofluoric acid burns: animal model, mechanism of injury and therapy. Transactions of the American Ophthalmological Society 1990;88:649‐84. [PMC free article] [PubMed] [Google Scholar]
Murdoch 1998
- Murdoch IE, Morris SS, Cousens SN. People and eyes: statistical approaches in ophthalmology. British Journal of Ophthalmology 1998;82(8):971‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakamura 2004
- Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. British Journal of Ophthalmology 2004;88(10):1280‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newcombe 1998
- Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Statistics in Medicine 1998;17(8):873‐90. [DOI] [PubMed] [Google Scholar]
Négrel 1998
- Négrel AD, Thylefors B. The global impact of eye injuries. Ophthalmic Epidemiology 1998;5(3):143‐69. [DOI] [PubMed] [Google Scholar]
Panda 2002
- Panda A, Nainiwal SK, Sudan R. Failure of amniotic membrane transplantation in the treatment of acute ocular burns. British Journal of Ophthalmology 2002;86(7):831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfister 1984
- Pfister RR, Haddox JL, Dodson RW, Deshazo WF. Polymorphonuclear leukocytic inhibition by citrate, other metal chelators, and trifluoperazine. Evidence to support calcium binding protein involvement. Investigative Ophthalmology and Visual Science 1984;25(8):955–70. [PubMed] [Google Scholar]
Prabhasawat 2007
- Prabhasawat P, Tesavibul N, Prakairungthong N, Booranapong W. Efficacy of amniotic membrane patching for acute chemical and thermal ocular burns. Journal of the Medical Association of Thailand 2007;90(2):319‐26. [PubMed] [Google Scholar]
Pratoomsoot 2008
- Pratoomsoot C, Tanioka H, Hori K, Kawasaki S, Kinoshita S, Tighe PJ, et al. A thermoreversible hydrogel as a biosynthetic bandage for corneal wound repair. Biomaterials 2008;29(3):272‐81. [DOI] [PubMed] [Google Scholar]
Rahman 2010
- Rahman I, Said DG, Maharajan SV, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye 2009;23(10):1954‐61. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roper‐Hall 1965
- Roper‐Hall MJ. Thermal and chemical burns. Transactions of the Ophthalmological Society of the United Kingdom 1965;85:631‐53. [PubMed] [Google Scholar]
Saw 2007
- Saw VP, Minassian D, Dart JK, Ramsay A, Henderson H, Poniatowski S, et al. Amniotic membrane transplantation for ocular disease: a review of the first 233 cases from the UK user group. British Journal of Ophthalmology 2007;91(8):1042‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schechter 2005
- Schechter BA, Rand WJ, Nagler RS, Estrin I, Arnold SS, Villate N, et al. Corneal melt after amniotic membrane transplant. Cornea 2005;24(1):106‐7. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sekiyama 2007
- Sekiyama E, Nakamura T, Kurihara E, Cooper LJ, Fullwood NJ, Takaoka M, et al. Novel sutureless transplantation of bioadhesive‐coated, freeze‐dried amniotic membrane for ocular surface reconstruction. Investigative Ophthalmology and Visual Science 2007;48(4):1528‐34. [DOI] [PubMed] [Google Scholar]
Shafto 1950
- Shafto CM. A simple method of inserting amniotic membrane grafts into the conjunctival sac. British Journal of Ophthalmology 1950;34(7):445‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shimazaki 1997
- Shimazaki K, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology 1997;104(12):2068‐76. [DOI] [PubMed] [Google Scholar]
Shimazaki 2006
- Shimazaki J, Konomi K, Shimmura S, Tsubota K. Ocular surface reconstruction for thermal burns caused by fireworks. Cornea 2006;25(2):139‐45. [DOI] [PubMed] [Google Scholar]
Shimmura 2001
- Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Anti‐inflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea 2001;20(4):408‐13. [DOI] [PubMed] [Google Scholar]
Shimmura 2008
- Shimmura S, Tsubota K. Surgical treatment of limbal stem cell deficiency: are we really transplanting stem cells?. American Journal of Ophthalmology 2008;146(2):154‐5. [DOI] [PubMed] [Google Scholar]
Shukla 1962
- Shukla IM. Amniotic membrane grafts in corneal ulcer. Indian Journal of Ophthalmology 1962;10(3):55‐60. [PubMed] [Google Scholar]
Smith 2004
- Smith VA, Cook SD. Doxycycline ‐ a role in ocular surface repair. British Journal of Ophthalmology 2004;88(5):619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorsby 1946
- Sorsby A, Symons HM. Amniotic membrane grafts in caustic burns of the eye (burns of the second degree). British Journal of Ophthalmology 1946;30:337‐45. [PubMed] [Google Scholar]
Sorsby 1947
- Sorsby A, Haythorne J, Reed H. Further experience with amniotic membrane grafts in caustic burns of the eye. British Journal of Ophthalmology 1947;31:409‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sridhar 2000
- Sridhar MS, Bansal AK, Sangwan VS, Rao GN. Amniotic membrane transplantation in acute chemical and thermal injury. American Journal of Ophthalmology 2000;130(1):134‐7. [DOI] [PubMed] [Google Scholar]
Srinivasan 2007
- Srinivasan R, T SS, Gupta A, Kaliaperumal S. Hypopyon iritis after primary fresh amniotic membrane transplantation. Cornea 2007;26(10):1275–6. [DOI] [PubMed] [Google Scholar]
Tejwani 2007
- Tejwani S, Kolari RS, Sangwan VS, Rao GN. Role of amniotic membrane graft for ocular chemical and thermal injuries. Cornea 2007;26(1):21‐6. [DOI] [PubMed] [Google Scholar]
Thoft 1977
- Thoft R. Conjunctival transplantation. Archives of Ophthalmology 1977;95(8):1425‐7. [DOI] [PubMed] [Google Scholar]
Tsai 2000
- Tsai JF, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. New England Journal of Medicine 2000;343(2):86‐93. [DOI] [PubMed] [Google Scholar]
Tseng 2004
- Tseng SC, Espana EM, Kawakita T, Pascuale MA, Li W, He H, et al. How does amniotic membrane work?. Ocular Surface 2004;2(3):177‐87. [DOI] [PubMed] [Google Scholar]
Tsubota 1996
- Tsubota K, Satake Y, Ohyama M, Toda I, Takano Y, Ono M, et al. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens‐Johnson syndrome. American Journal of Ophthalmology 1996;122(1):38‐52. [DOI] [PubMed] [Google Scholar]
Tuft 2009
- Tuft SJ, Shortt AJ. Surgical rehabilitation following severe ocular burns. Eye 2009;23(10):1966‐71. [DOI] [PubMed] [Google Scholar]
Ueta 2002
- Ueta M, Kweon MN, Sano Y, Sotozono C, Yamada J, Koizumi N, et al. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clinical and Experimental Immunology 2002;129(3):464‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uglova 1957
- Uglova T, Goleminova R. Amnion in caustic injuries of the eye [Prilozhenie na amnion pri izgariane na okoto]. Khirurgiia 1957;10(5):444‐51. [PubMed] [Google Scholar]
Uçakhan 2002
- Uçakhan OO, Köklü G, Firat E. Nonpreserved human amniotic membrane transplantation in acute and chronic chemical eye injuries. Cornea 2002;21(2):169‐72. [DOI] [PubMed] [Google Scholar]
von Versen‐Hoeynck 2008
- Versen‐Hoeynck F, Steinfeld AP, Becker J, Hermel M, Rath W, Hesselbarth U. Sterilization and preservation influence the biophysical properties of human amnion grafts. Biologicals 2008;36(4):248‐55. [DOI] [PubMed] [Google Scholar]
von Versen‐Höynck 2004
- Versen‐Höynck F, Syring C, Bachmann S, Möller DE. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts‐light and scanning electron microscopic studies. Cell Tissue Bank 2004;5(1):45‐56. [DOI] [PubMed] [Google Scholar]
Wagoner 1997
- Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Survey of Ophthalmology 1997;41(4):275‐13. [DOI] [PubMed] [Google Scholar]
Whitcher 2001
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bulletin of the World Health Organization 2001;79(3):214‐21. [PMC free article] [PubMed] [Google Scholar]
Wong 2000
- Wong TY, Klein BE, Klein R. The prevalence and 5‐year incidence of ocular trauma. The Beaver Dam Eye Study. Ophthalmology 2000;107(12):2196‐202. [DOI] [PubMed] [Google Scholar]
Ye 2006
- Ye J, Yao K, Kim JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing. Eye 2006;20(4):482‐90. [DOI] [PubMed] [Google Scholar]
Zhou 2004
- Zhou S, Chen J, Liu Z, Huang T, Chen L. A clinical study of amniotic membrane transplantation for severe eye burns at the acute stage. Zhonghua Yan Ke Za Zhi 2004;40(2):97‐100. [PubMed] [Google Scholar]


