Abstract
Background:
Focal therapy (FT) and partial gland ablation (PGA) are quickly adopted by urologists and radiologists as an option for the management of localized prostate cancer.
Objective:
To find consensus on a standardized nomenclature and to define a follow-up guideline after FT and PGA for localized prostate cancer in clinical practice.
Design, setting, and participants:
A review of the literature identified controversial topics in the field of FT. Online questionnaires were distributed to experts during three rounds, with the goal to achieve consensus on debated topics. The consensus project was concluded with a face-to-face meeting in which final conclusions were formulated.
Outcome measurements and statistical analysis:
Controlled feedback of responses of previous rounds were summarized and returned to the participants allowing them to re-evaluate their decisions. The level of agreement to achieve consensus on a topic was set at 80%.
Results and limitations:
Sixty-five experts participated in this interdisciplinary consensus study (72% urologists; 28% radiologists). The experts propose the use of the herein standardized nomenclature for ablative procedures. After FT/PGA, the following tests should be performed to assess treatment outcomes: prostate-specific antigen (PSA), imaging, biopsies, and functional outcome assessment. Although not a reliable marker for treatment failure, PSA should be measured every 3 mo in the 1st year and every 6 mo thereafter. Magnetic resonance imaging is the preferred image modality and should be performed at 6 and 18 mo after treatment. A systematic 12-core transrectal ultrasound-guided biopsy combined with a targeted biopsy of the treated area should be performed 6–12 mo after treatment. Functional outcomes should be obtained 3–6 mo after treatment for the first time and until stability is attained.
Conclusions:
The panel recommends the use of the proposed nomenclature and follow-up protocols to generate reliable data supporting a broader implementation of FT as a standard of care for select patients with localized prostate cancer.
Keywords: Focal therapy, Partial gland ablation, Nomenclature, Surveillance
Patient summary:
In this report, we present expert opinion on the use of a standardized nomenclature, and surveillance methodologies after focal therapy and partial gland ablation for localized prostate cancer.
1. Introduction
With an estimated incidence of 1 276 106 new patients, in 2018, prostate cancer (PCa) was the most commonly diagnosed cancer in men worldwide [1]. Despite the high incidence of this disease, its mortality remains low and most men die from unrelated causes. While whole-gland treatments such as radiation and radical prostatectomy represent the gold standard for the definitive management of PCa, both treatment modalities are associated with considerable side effects including urinary incontinence and erectile dysfunction [2–5].
Partial gland ablation (PGA) therapies, including image-targeted focal treatment (FT), have an emerging role in the management of localized PCa [6–12], as early results from trials and prospective studies have supported these approaches as promising standard of care options based on encouraging short- and intermediate-term outcomes providing evidence for regulatory approvals [10,13,14]. However, it is important to emphasize that FT/PGA is not included in current treatment guidelines due to the paucity of high-quality data and lack of randomized trials supporting these novel treatment strategies. As longer-term results are awaited, the availability of multiple ablative technologies and treatment templates, as well as varied postprocedural follow-up, has the potential to complicate the comparison of patient outcome data.
In order to advance the field of FT, standardized evaluation of treatment outcomes and a uniform nomenclature for treatment templates are urgently needed to support FT/PGA as an alternative to whole-gland treatment options [15]. The Focal Therapy Society feels obligated to spearhead all efforts to outline guidance and recommendations for clinicians offering this treatment modality to their patients.
Hence, an international multidisciplinary consensus panel was convened to reach consensus on standardized nomenclature for image-guided, organ-preserving PGA procedures for localized PCa and to establish uniform postprocedural surveillance after FT/PGA. The project was executed using the modified Delphi method, a well-accepted research tool to reach consensus on complex topics with opposing stances [16]. Implementation of the recommended terminology as well as postprocedural surveillance standards in future clinical studies will facilitate the generation of uniformly comparable results and data, expediting earlier ground truth for validated roles and indications.
2. Patients and methods
2.1. Consensus process
We performed a multistage modified Delphi consensus project (Supplementary material) to achieve consensus among a panel of invited experts in the field of focal image-guided therapy. Expert panelists were selected based on a dedicated literature search or peer recommendations. The literature search was further used to identify controversial topics in the field of surveillance after FT. The initial survey was created by a focus group (A.H.L., P.A.P., O.U., B.G.M., A.K.G., and A.R.R.) and pilot tested prior to distribution to the experts. In accordance with a modified Delphi survey technique, online questionnaires were distributed to experts during three rounds, between October 26, 2018 and February 5, 2019, using an online questionnaire platform (www.surveymonkey.com; San Mateo, CA, USA), with the goal to achieve consensus on debated topics [16,17]. Questionnaires were modified based on the panelists’ feedback and electronic responses after each round. Controlled feedback of responses of prior rounds were summarized and returned to the participants allowing them to re-evaluate their decisions, with enhanced consensus feedback. The level of agreement to achieve consensus was set at 80%. The modified Delphi consensus project was finalized, fine-tuned, ratified, and subjectively verified with a meeting at the 11th International Symposium on Focal Therapy and Imaging in Prostate and Kidney Cancer on February 9, 2019, in Kyoto, Japan. The final results of the first three rounds of the Delphi consensus project were presented during the face-to-face meeting, and topics with low levels of consensus were discussed.
2.2. Standardized nomenclature for image-guided, organ-preserving ablative procedures
The consensus panel proposes the utilization of a standardized nomenclature for image-guided organ-preserving ablative procedures, since there are various minimally invasive tissue ablation strategies generically labeled as “FT” of the prostate [18]. Prior consensus statements have made a distinction between PGA strategies based on regional tumor localization (either by biopsy or by imaging) and FT, which is an image-targeted, biopsy-confirmed treatment modality [19]. The panelists agreed on the presented standardized nomenclature for targeted, parenchyma-preserving ablative procedures during the final meeting.
3. Results
Ninety-seven experts were invited to participate, and 65 experts filled out the initial survey. Questionnaires were submitted in three rounds, and the response rate declined for the second (56/65; 86.2%) and third (48/65; 74%) rounds but remained robust. In the panel, 72% were urologists and 28% were radiologists. Tables 1 and 2 summarize the demographics and FT practice of the experts, respectively.
Table 1 –
Characteristics of focal therapy experts
| Specialty (%) | |
| Urology | 72 |
| Radiology | 28 |
| Gender (%) | |
| Male | 95 |
| Female | 4.6 |
| Location of practice (%) | |
| North and South America | 49 |
| Europe | 31 |
| Asia | 20 |
| Age of expert (%) | |
| <40 yr | 23 |
| 40–50 yr | 42 |
| 50–60 yr | 28 |
| >60 yr | 7.7 |
| Major professional associations | |
| American Urological Association | 57 |
| European Association of Urology | 37 |
| Society of Urological Oncology | 27 |
| Société Internationale d’Urologie | 24 |
| Endourological Society | 18 |
| Radiological Society of North America | 14 |
Table 2 –
Focal therapy practice
| Primarily used focal therapy modality (%) a | ||
| HIFU | 41 | |
| Cryotherapy | 36 | |
| Laser | 27 | |
| IRE | 13 | |
| Radiotherapy | 11 | |
| PDT | 6.3 | |
| Practice of focal therapy experts b | ||
| Number of patients followed on active surveillance on a yearly basis (%) | ||
| <10 | 9.4 | |
| 10–50 | 36 | |
| 50–100 | 20 | |
| >100 | 34 | |
| Number of patients treated with focal therapy on a yearly basis (%) | ||
| <10 | 25 | |
| 10–50 | 59 | |
| 50–100 | 13 | |
| >100 | 3.1 | |
| Number of patients treated with surgery on a yearly basis (%) | ||
| <10 | 9.7 | |
| 10–50 | 18 | |
| 50–100 | 32 | |
| >100 | 40 | |
HIFU = high-intensity focused ultrasound; IRE = irreversible electroporation; PDT = photodynamic therapy.
Primarily used focal therapy modality of surveyed participants.
Practice of focal therapy experts surveyed: number of patients they follow on active surveillance, and treat with focal therapy or surgery.
3.1. Standardized nomenclature for the application of FT
3.1.1. Definition of the term “focal therapy” of the prostate
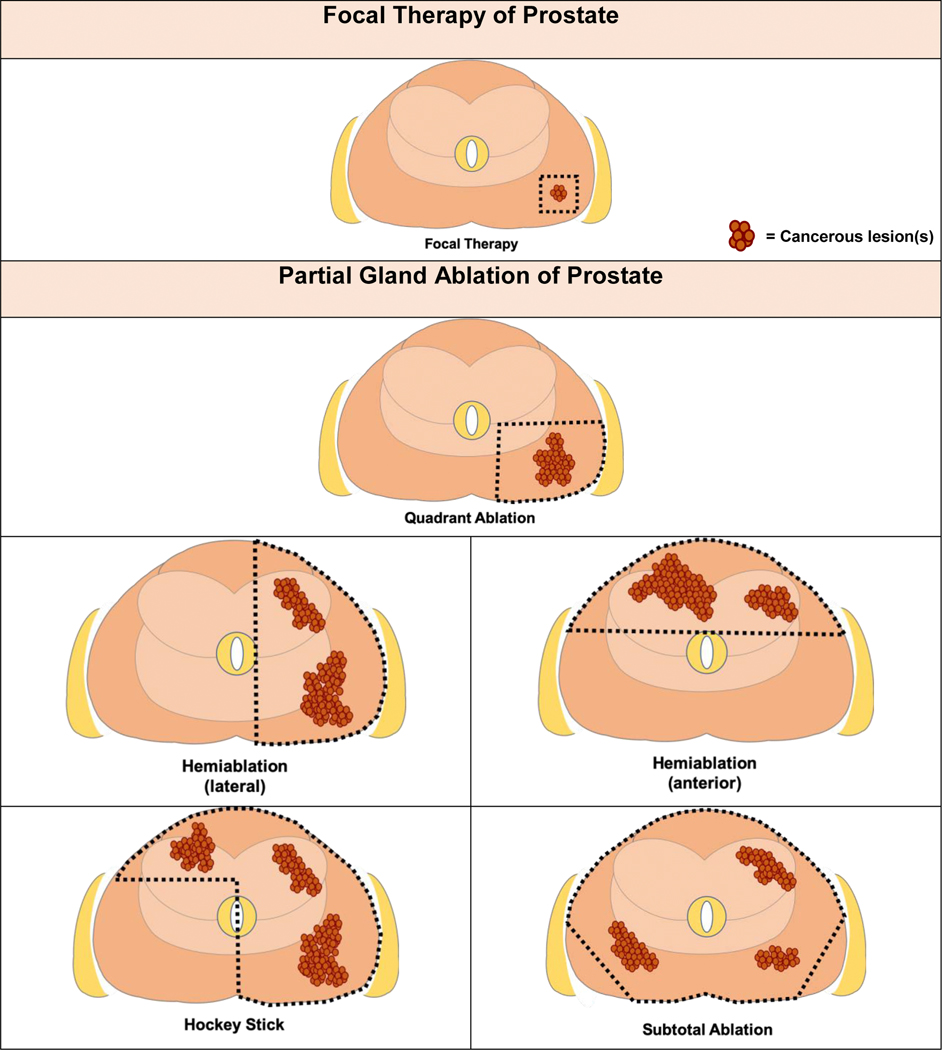
In distinction to regional PGA therapies, the panel reached agreement that the term “focal therapy” is meant to describe guided ablation of an image-defined, biopsy-confirmed, cancerous lesion with a safety margin surrounding the targeted lesion (94%). The panel acknowledges that although multiple image modalities may be used (including multiparametric magnetic resonance imaging [mpMRI], computed tomography, positron emission tomography, and ultrasound), the current standard of care and the most widely utilized imaging modality is mpMRI (Fig. 1).
Fig. 1 –
Focal therapy versus partial gland ablative procedures. Graphics demonstrating distinction between focal therapy and templated organ-preserving partial gland ablations. Focal therapy: image-guided focused ablation of image-visible, biopsy-confirmed malignant lesion(s) plus an adequate safety margin. Quadrant ablation: destruction of all prostate tissue within a quadrant of the prostate. Hemiablation: destruction of all prostate tissue within a lateralized hemisphere of the prostate or the anterior half of the prostate. Hockey stick: destruction of all prostate tissue within a lateralized hemisphere plus anterior contralateral region. Subtotal ablation: destruction of most of the prostate tissue with preservation of a posterior lateral region (unilaterally or bilaterally). The goal intended is to preserve at least one neurovascular bundle during ablation.
3.1.2. Partial gland ablation
The consensus panel proposes the utilization of a standardized nomenclature for image-guided regional ablation procedures based on biopsy localization of tumors. PGA templates do not rely on image identification of tumors but instead utilize anatomic boundaries of ablation intended to preserve organ function while achieving complete tumor treatment. Zonal ablations include previously described approaches such as quadrant ablation, hemiablation, hockey-stick ablation, and subtotal ablation (Fig. 1).
3.1.3. Index lesion as a target for ablation
The expert panel reached agreement that all biopsy-confirmed MRI-visible lesions with clinically significant cancer defined as Gleason grade group (GGG) ≥2 (Gleason 3 + 4 ≥ 7) should be used as a target for FT (83%). At the same time, the panelists could not achieve consensus that an index lesion can be defined solely by being the largest lesion. Furthermore, there was no consensus that GGG1 tumors could be defined as index lesions.
3.2. Surveillance after FT/PGA for PCa
3.2.1. Prostate-specific antigen
Serum prostate-specific antigen (PSA) was accepted as the preferred “nonimaging” biomarker in the follow-up and should be included in postprocedural surveillance (98%). While experts see a role in monitoring PSA after ablation, they acknowledged that PSA alone is insufficient to determine oncological success. The panel recommends obtaining first post-treatment PSA measurement within 3 mo after treatment (89%) and subsequently every 3 mo during the 1st year (91%). After the 1st year, PSA should be measured every 6 mo (80%).
3.2.2. Imaging in surveillance after FT
While adequate state-of-the-art imaging is a cornerstone for FT, its role to evaluate treatment response remains under investigation. Experts agreed that at this time mpMRI is the preferred image modality (97%) to evaluate treatment response, as it is the most established imaging modality. However, the panel acknowledges that this is an evolving field and alternative image modalities may prove beneficial.
3.2.2.1. MRI findings suggestive of failure
The panel reached agreement that early contrast enhancement in the treated lesion is suggestive of a failure after FT (81%). While there was no consensus, the panel considers following features suggestive of treatment failure and hence recommends acquiring the following information: hypointense T2 signal, or the combination of hypointense signal on apparent diffusion coefficient map with hyperintense signal on high b values(b ≥ 1400) diffusion-weighted MRI in the treated region.
3.2.2.2. Imaging schedule
Initial postoperative imaging should be obtained within 6 mo after FT (80%) and subsequent mpMRI should be scheduled 12 mo after the first postprocedural mpMRI (85%). Consensus was not reached as to whether further scheduled imaging is mandatory beyond these two postprocedural mpMRI scans if the results were negative. However, consensus recommendation was for further imaging for triggering factors (eg, clinical suspicion, young age, genetic predisposition rise in PSA, and digital rectal examination [DRE] abnormality) or as according to institutional active surveillance protocols.
3.2.3. Role of biopsy in surveillance after FT
The panel reached agreement that the preferred biopsy in the post-FT setting is an MRI-targeted biopsy, either in gantry or MRI fusion, to evaluate the treated lesion and a systematic extended sextant biopsy to evaluate the untreated area (86%). Other whole-gland biopsy techniques based on the clinician’s expertise (eg, equivalent transperineal based sampling) are also accepted, but should aim to sample the treated and untreated regions to timely identify patients with persistent in-field disease and/or out-of-field tumors. Subsequent biopsies should follow a risk-adapted approach depending on the clinical scenario as endorsed by existing guidelines for active surveillance and adopted based on institutional standards of care. The panel acknowledged that those undergoing focal radiation therapy techniques (brachytherapy and external beam radiation) may experience continued treatment response several years after intervention. The timing of biopsy in this setting may need to be deferred for up to 2–3 yr following focal radiation in the setting of favorable PSA kinetics.
3.2.3.1. Image-guided targeted biopsy of treated region (assessment of in-field persistence; treatment failure)
There was consensus that the first image-guided targeted biopsy should be obtained within the 1st year after FT to assess for in-field persistence, that is, treatment failure (83%). Furthermore, consensus was achieved that subsequent biopsies after a completely negative post-treatment image-guided targeted biopsy might be obtained only in the setting of triggering factors such as a rise in PSA, imaging abnormalities, or an abnormal DRE (87%). The panel recommends a risk-adapted approach that allows for the discretion of the treating clinician to perform more frequent biopsies from the treated region if there are clinical concerns. Furthermore, a targeted biopsy should be always utilized when surveillance imaging demonstrates new lesions.
3.2.3.2. Systematic biopsy of untreated regions (assessment of out-of-field -tumors; selection failure)
The experts agreed that a careful re-evaluation of the entire prostate is required in patients undergoing FT. This is commonly performed by a systematic extended sextant biopsy and should be obtained between 6 and 12 mo after treatment to assess for disease outside of the treated area (out-of-field progression/selection failure; 87%). Since it is possible that these cancerous lesions already existed at the time of treatment, these failures are classified as selection failures if clinically significant disease is present. While a 12-core systematic biopsy is the most commonly used biopsy technique to screen areas not found to be suspicious on MRI, the panel acknowledges that clinicians may apply alternative sampling strategies (eg, transperineal mapping biopsy and transrectal saturation biopsies).
The panel recommends that after a negative initial systematic biopsy, further systematic biopsies are not mandated, but may be obtained at the physician’s discretion or if there are triggering factors (rise in PSA, imaging abnormalities, and an abnormal DRE; 81%).
3.3. Assessment of quality of life and functional outcomes after FT/PGA
The desire to avoid treatment-related toxicities associated with prostatectomy and radiation is the key driver for developing organ-sparing treatment strategies prompting strong agreement that erectile function, continence, and urinary symptoms should be assessed in the post-FT setting (95%). Consensus was not attained on the value of mental, physical, bowel, and hormonal assessments in routine post-treatment evaluation. The recommendation was to assess functional outcomes for the first time between 3 and 6 mo after treatment (93%). Satisfactory urinary control was defined as requiring “no pads” after treatment (96%), although no agreement could be reached on the definition of success for erectile function. While 72% of experts considered no change of erectile function from baseline as satisfactory, it did not meet the consensus threshold of 80%. While the specific instruments to evaluate patient-reported outcomes was not a subject of this investigation, the panel acknowledged that there are multiple validated measures and these decisions are best made at an institutional/practice level.
3.4. Management of failures after FT (in-field persistence and out-of-field tumors)
Similar to other forms of organ-sparing therapies, a low but acceptable rate of post-treatment tumor recurrence is expected. The management of failure after FT/PGA is important to achieve oncological success. One feature of FT/PGA is that it can be repeated, particularly if the reason for FT failure can be identified and surmounted [20]. Biopsy strategies described above allow for assessment of in-field and out-of-field new or residual sites of disease. Success of FT/PGA for PCa was defined by consensus as eradication of all identified GGG ≥2 cancerous lesions based on targeted biopsy or systematic biopsy (86%). The experts agreed that no further treatment is indicated in the setting of treatment success, including the presence of GGG1 disease. The panel recommended continued monitoring of the entire prostate for de novo disease or grade reclassification of untreated GGG1 lesions. Repeated radiological or histological assessments as per institutional active surveillance pathways are appropriate to monitor long-term outcomes.
3.4.1. Management of in-field failure
A variety of causes may contribute to in-field failure, defined as residual tumor identified within the targeted lobe of the prostate. The panel agreed that patients with in-field failures may be offered repeat FT/PGA (with the same or different modality) based on clinical judgment and expectation of success (89%). Radical prostatectomy and radiation therapy are options that can also be appropriate for patients with in-field failure, particularly if the patient would benefit from whole-gland treatment. Low-grade GGG1 tumors in field can be followed according to institutional active surveillance protocols.
3.4.2. Management of out-of-field failure
These failures, sometimes referred to as “selection failures,” have likely existed prior to initial ablation but missed by initial evaluation. They were likely missed or not sampled by conventional biopsy, and represented MRI-invisible tumors. Other out-of-field tumors may represent de novo lesions that became clinically apparent or represent progression of known tumors monitored on active surveillance protocols.
Depending on the suitability for treatment, these patients may be offered additional FT/PGA (with same or different modality; 85%). However, since these foci are sometimes MRI invisible, they could represent a challenging target for FT, and are more suitable for PGA or definitive treatment options including radiation and radical prostatectomy.
4. Discussion
While FT and PGA continue to gain increasing interest in the urology and radiology communities as an attractive, less invasive alternative to whole-gland treatment options, clinicians and patients contemplating these approaches should be mindful of the limitations of available supporting data including long-term outcomes. A variety of modalities have been approved or are under investigation, which creates challenges in optimizing treatment strategies since treatment effects may vary based on technique, technology, and tumor location. The efficacy for clinical use in the treatment of PCa is often extrapolated from experimental, preclinical studies or use in other organs. Another concern and complicating factor for producing reliable data is the lack of a uniform nomenclature. Clinicians use numerous definitions for organ-preserving image-guided ablative prostate procedures. While larger ablation templates or whole-gland ablations are potentially associated with superior oncological success, they are inherently associated with more side effects. The expert panel strongly recommends the use of a standardized nomenclature that reserves the term “focal therapy” for the targeted ablation of a prostate focus with a safety margin and for distinguishing it from all other image-guided organ-preserving PGA. Once agreed on the implementation of a standardized nomenclature, clinicians should also adhere to a standardized follow-up to generate robust and reliable data that support its broader implementation as a standard of care for select patients with localized PCa (Table 3).
Table 3 –
Summary of recommendation for follow-up after focal therapy of the prostate
| First time | Years after treatment | ||||||
|---|---|---|---|---|---|---|---|
| 0–1 | 1–2 | 2–3 | 3–4 | 4–5 | |||
| PSA | 3 mo | Every 3 mo | Every 6 mo | Every 6 mo | Every 6 mo | Every 6 mo | |
| Imaging | 6 mo | Once (at 6 mo) | Once (12 mo after initial) | Every 12 mo or as per institutional active surveillance protocol or triggering factor/clinical suspicion | |||
| Biopsy | |||||||
| Systematic | 6–12 mo | Once (at 6–12 mo) | If negative: as per institutional active surveillance protocol or triggering factor/clinical suspicion | ||||
| MRI guided | 6–12 mo | Once (at 6–12 mo) | If negative: as per institutional active surveillance protocol or triggering factor/clinical suspicion | ||||
| Assessment of functional outcomes | 3–6 mo | Once (at 3–6 mo) | Assessment until stability/baseline attained | ||||
MRI = magnetic resonance imaging; PSA = prostate-specific antigen.
Our study has several limitations. While the modified Delphi method is a well-established research tool to reach agreement among experts, it is subject to possible biases as it probes participants with a vested interest in this treatment modality. Second, while the study represents an interdisciplinary consensus, the lack of participation of radiation oncologist represents a weakness of our survey. Although urological oncologists with significant experience in focal brachytherapy were included and pertinent aspects of surveillance after radiation were discussed in the consensus meeting, the panel would have been further supported by the inclusion of radiation oncologists. Third, the Delphi study design prevents a retrospective analysis of surveyed topics to explore more granular details. Our study confirmed the importance of mpMRI in the post-treatment setting but did not investigate mpMRI details further, as these were the subject of a previous consensus [21]. Fourth, the anonymous nature of the study design also prevents discernment if participants responded based on institutional or individual practices, although questions specifically enquired about individual recommendations. Another inherent problem of anonymous surveys is that demographics of the participants in the subsequent consensus round remain unknown. Finally, our survey focused on nomenclature and surveillance after FT, and did not explore patient selection, as this was previously reported by our group [22]. Tay et al [22] showed that consensus was achieved in patient selection for FT, namely, men with clinically localized low- or intermediate-risk PCa, PSA ≤10, and any cancer foci <1.5 cc or lesions <3 cc confined to a single lobe would be appropriate candidates contingent on the ability of the energy source to completely ablate the lesion with an appropriate margin.
5. Conclusions
FT and PGA are quickly adopted by urologists and radiologists as an option for the management of localized PCa. The panel recommends the use of standardized nomenclature and follow-up protocols to generate reliable data, supporting a broader implementation of this treatment strategy as a standard of care for select patients with localized PCa.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: None.
Financial disclosures: Peter A. Pinto certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Footnotes
Credit Author Statement
Amir H. Lebastchi: Conceptualizing, Methodology, Formal analysis, Investigation, Data curation, Writing- original draft, Writing- review and editing, Visualization
Arvin K. George: Conceptualizing, Methodology, Formal analysis, Writing- review and editing
Thomas J. Polascik: Conceptualizing, Methodology, Formal analysis, Writing- review and editing
Jonathan Coleman: Writing- review and editing, Formal analysis
Jean de la Rosette: Writing- review and editing, Formal analysis
Baris Turkbey: Writing- review and editing, Formal analysis
Bradford J. Wood: Writing- review and editing, Formal analysis
Michael A. Gorin: Writing- review and editing, Formal analysis
Abhinav Sidana: Writing- review and editing, Formal analysis
Sangeet Ghai: Writing- review and editing, Formal analysis
Kae Jack Tay: Writing- review and editing, Formal analysis
John F. Ward: Writing- review and editing, Formal analysis
Rafael Sanchez-Salas: Writing- review and editing, Formal analysis
Berrend G. Muller: Conceptualizing, Methodology, Formal analysis, Writing- review and editing
Bernard Malavaud: Writing- review and editing, Formal analysis
Pierre Mozer: Writing- review and editing, Formal analysis
Sebastien Crouzet: Writing- review and editing, Formal analysis
Peter L. Choyke: Writing- review and editing
Osamu Ukimura: Conceptualizing, Methodology, Formal analysis, Writing- review and editing
Ardeshir R. Rastinehad: Conceptualizing, Formal analysis, Writing- review and editing
Peter A. Pinto: Conceptualizing, Resources, Funding acquisition, Methodology, Formal analysis, Writing- review and editing Supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Culp MB, et al. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol 2020;77:38–52. [DOI] [PubMed] [Google Scholar]
- [2].Donovan JL, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hamdy FC, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–24. [DOI] [PubMed] [Google Scholar]
- [4].Lardas M, et al. Quality of life outcomes after primary treatment for clinically localised prostate cancer: a systematic review. Eur Urol 2017;72:869–85. [DOI] [PubMed] [Google Scholar]
- [5].Resnick MJ, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 2013;368:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Valerio M, et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol 2014;66:732–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ahdoot M, et al. Contemporary treatments in prostate cancer focal therapy. Curr Opin Oncol 2019;31:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Eggener SE, et al. Phase II evaluation of magnetic resonance imaging guided focal laser ablation of prostate cancer. J Urol 2016;196:1670–5. [DOI] [PubMed] [Google Scholar]
- [9].Tourinho-Barbosa RR, et al. Focal therapy for localized prostate cancer with either HIFU or cryoablation: a single institution experience. J Urol 2020;203:320–30. [DOI] [PubMed] [Google Scholar]
- [10].Rastinehad AR, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci U S A 2019;116:18590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Scheltema MJ, et al. Pair-matched patient-reported quality of life and early oncological control following focal irreversible electroporation versus robot-assisted radical prostatectomy. World J Urol 2018;36:1383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zheng X, et al. Focal laser ablation versus radical prostatectomy for localized prostate cancer: survival outcomes from a matched cohort. Clin Genitourin Cancer 2019;17:464–9.e3. [DOI] [PubMed] [Google Scholar]
- [13].Johnston MJ, et al. Focal high-intensity focussed ultrasound partial gland ablation for the treatment of localised prostate cancer: a report of medium-term outcomes from a single-center in the United Kingdom. Urology 2019;133:175–81. [DOI] [PubMed] [Google Scholar]
- [14].Bahn D, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol 2012;62:55–63. [DOI] [PubMed] [Google Scholar]
- [15].Muller BG, et al. Follow-up modalities in focal therapy for prostate cancer: results from a Delphi consensus project. World J Urol 2015;33:1503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Williams PL, Webb C. The Delphi technique: a methodological discussion. J Adv Nurs 1994;19:180–6. [DOI] [PubMed] [Google Scholar]
- [17].Stewart J, et al. Identifying appropriate tasks for the preregistration year: modified Delphi technique. BMJ 1999;319:224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ward JF, Jones JS. Classification system: organ preserving treatment for prostate cancer. Urology 2010;75:1258–60. [DOI] [PubMed] [Google Scholar]
- [19].Postema AW, et al. Standardization of definitions in focal therapy of prostate cancer: report from a Delphi consensus project. World J Urol 2016;34:1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tay KJ, et al. Surveillance after prostate focal therapy. World J Urol 2019;37:397–407. [DOI] [PubMed] [Google Scholar]
- [21].Muller BG, et al. Role of multiparametric magnetic resonance imaging (MRI) in focal therapy for prostate cancer: a Delphi consensus project. BJU Int 2014;114:698–707. [DOI] [PubMed] [Google Scholar]
- [22].Tay KJ, et al. Patient selection for prostate focal therapy in the era of active surveillance: an international Delphi consensus project. Prostate Cancer Prostatic Dis 2017;20:294–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.