Abstract
Background
Peak systolic volume (PSV), the essential parameter of penile Doppler ultrasonography (PDU), can reflect the penile artery blood supply. The present study was conducted to explore the correlations between PDU parameters and shear wave elastography (SWE), a feasible technology to measure penile stiffness.
Material/Methods
A total of 78 erectile dysfunction (ED) patients and 32 healthy controls were enrolled in our study. The PDU and SWE were performed to each participant simultaneously by a blinded radiologist. The penoscrotal junction was used to measure the PDU parameters and the SWE values. The PDU and SWE measurements were conducted formally at flaccid state and 5, 10, 15, 20, and 25 min after intra-cavernous injection (ICI) of vasoactive agents.
Results
The significant correlation between PSV and SWE value was found in both ED patients (r=−0.748, P<0.001) and healthy controls (r=−0.815, P<0.001). SWE values of corpus cavernosum penis (CCP) decreased significantly with the increase of PSV during penile erection in both the ED patients and healthy controls. When the SWE value of CCP was less than 11.57 kPa, it showed that the penile artery blood supply was sufficient to finish satisfactory sexual intercourse. The sensitivity and specificity were 0.838 and 0.872, respectively.
Conclusions
Quantitative measurement of SWE values in CCP can reflect the penile arterial blood supply during PDU examination. The SWE technique could be used for evaluating the penile artery blood supply combined with the ICI test, with the advantages of noninvasiveness, simple operation, and excellent repeatability.
Keywords: Erectile Dysfunction, Penile Erection, Ultrasonography
Background
Erectile dysfunction (ED), defined as the persistent inability to attain and/or maintain erection sufficient for satisfactory sexual performance [1], can be divided into 3 categories according to the etiology: psychological, organic, and mixed [2]. Among ED patients with organic etiology, approximately 80% of cases can be attributed to vascular factors owing to the particular vascular network of the penis [3], including penile artery blood supply (referred to as arterial ED), venous occlusive dysfunction (referred to as venous ED), and mixed dysfunction of artery and venous occlusive dysfunction (referred to as mixed vascular ED) [4]. Moreover, arterial ED has been considered a sentinel symptom of subclinical cardiovascular disease (CVD) [5]. Thus, it is essential to diagnose arterial ED precisely to prevent CVD in the future. Several methods are used to evaluate penile hemodynamic in ED patients: penile Doppler ultrasonography (PDU) [6], nocturnal penile tumescence RigiScan (NPTR) [7], RigiScan combined with intracavernosal injection (ICI) [8], and arterial angiography [9]. Among these methods, the PDU is considered the criterion standard method for evaluating penile hemodynamic to diagnose vascular ED since it was introduced by Lue et al in 1985 [10], and it has been used widely, with more availability and less invasiveness than other penile hemodynamics methods. The PDU test can quantitatively assess the penile hemodynamic parameters in greater detail, which should be combined with the ICI of vasoactive agents to induce penile erection [11]. The PDU test is also recommended by the American Urological Association for ED patients with complex histories [12].
The hemodynamic parameters measured by PDU included peak systolic velocity (PSV), which evaluates the penile artery blood supply directly, and end-diastolic velocity (EDV) and resistive index (RI), which evaluates the veno-occlusive mechanism indirectly [13]. Among these parameters, PSV is the most important parameter to be associated with CVD risk, and can be used to identify men at risk for CVD in the future [14,15]. Of course, the PSV was also the most important parameter of PDU to diagnose arterial ED [16]. If ED patients have PSV <30 cm/s, arterial ED is diagnosed, meaning that the ED was mainly caused by insufficient penile artery blood supply when patients engage in sexual intercourse [17]. However, several shortcomings limited the PDU application for ED patients, including checking time, optimal cut-off values, and the variability among different radiologists [18]. With the development of the technology, more and more ultrasound methods are applied for clinical diagnoses. Shear wave elastography (SWE), a novel ultrasonography method, is increasingly used for diagnosing the ED [19]; it can estimate tissue stiffness by using shear waves and display it in a quantitative manner using the SWE values [20]. During SWE examination, the transducer sends ultrasonic waves to the tissue and quickly detects shear waves created in the tissue. The wave speed detected by the transducer is used to calculate the SWE values, named Young’s modulus (YM) [21], which are directly related to the stiffness of the measured tissue [21]. Higher SWE values indicate greater stiffness of the assessed tissue [22].
The SWE technique has been used extensively in the prostate [23], breast [24], and liver [25], with the advantages of non-invasiveness, quantitation, simple operation, and perfect repeatability. Recently, more and more studies have verified the role of SWE in the diagnosis of ED, particular vascular ED [26,27]. Turkay et al conducted a case-control study to explore the application of SWE in the diagnosis of ED [19]. Recently, a well-designed study was conducted to explore the role of SWE in the rigidity assessment of penile corpus cavernosum for venogenic ED [26]. Furthermore, our published study also verified the role of SWE in the diagnosis of arterial ED [27]. However, no studies were conducted to explore the relationship between PDU parameters and SWE values of corpus cavernosum penis (CCP). When conducting the PDU test, the hemodynamic parameters and penile rigidity cannot be measured, limiting the clinical value of PDU. If the positive correlations between PDU parameters and SWE values were discovered, we could measure the SWE to evaluate the penile rigidity and hemodynamic parameters simultaneously, expanding the clinical value of SWE in the diagnosis of vascular ED.
Therefore, the present study aimed to explore the relationship between PDU parameters and SWE values in ED patients and healthy controls, and to analyze the change trend between the PSV and SWE. We also sought to determine the most appropriate cut-off value of SWE for related PSV, to verify the feasibility of SWE.
Material and Methods
Study Population
Our study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and received approval from the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Quick-PJ2021-15-39). The informed consents were signed by all patients volunteering to participate in our study before the study, which contained the advantages and disadvantages for the enrolled patients. We screened 100 ED patients and 32 healthy controls from January 2020 to September 2021 in the Department of Urology and Andrology and Healthy Physical Examination Center of our hospital, respectively.
Inclusion and Exclusion Criteria
The inclusion criteria of our study were: 1) aged 20 years old or older with regular heterosexual activity (at least once a week); 2) with a history of ED for at least 6 months. The patients were excluded if they met any of the following exclusion criteria: 1) a history of pelvic or penile trauma or operation, 2) diagnosis of penile curvature or Peyronie’s disease, 3) penile congenital deformity, 4) diabetes mellitus or hypertension. Ultimately, a total of 78 ED patients were enrolled in our study, with 10 patients excluded due to diagnosis of Peyronie’s disease, 8 patients excluded due to a history of pelvic trauma, and 4 patients excluded due to lack of regular heterosexual activity. All patients were asked to complete a brief International Index of Erectile Function (IIEF-5) form [28] and were diagnosed with ED when they scored 21 or less based on the IIEF-5 questionnaire [29]. Each patient underwent history taking and physical examination by a senior andrologist. Blood samples from all patients were collected in 8: 00 AM to check serum fasting glucose, testosterone, triglyceride, and total cholesterol after 12 h of fasting.
PDU Measurement and SWE Imaging
The PDU and SWE were performed for every patient simultaneously by the same senior radiologist utilizing the Aixplorer™ ultrasound system (Supersonic Imagine S.A., Aix-en-Provence, France) with a SuperLinear™ SL15-4 probe (frequency 4–15 MHz). The radiologist was blind to the clinical characteristics of all patients to avoid influencing the examination results. We performed the scans in a quiet, dim, and isolated room to reduce patients’ discomfort and anxiety and prevent disturb from the external environment. The penile ventral surface was scanned using longitudinal and transverse views with patients lying in supine position. We guide every patient to hold the glans penis to keep the dorsal penis tightly contacting the abdominal wall. To prevent the penis from moving out of position during the examination, an experimental examination was performed for each patient to teach them to control the position.
First of all, we scanned the penis thoroughly in the flaccid state to evaluate the anatomical structure to exclude fibrosis, calcification, and Peyronie’s disease. Then, we performed PDU and SWE measurements formally in the flaccid state and 5, 10, 15, 20, and 25 min after intra-cavernous injection (ICI) of vasoactive agents (alprostadil, Caverject®; Pfizer, New York, NY, USA). The penoscrotal junction was used to measure the PDU parameters and the SWE values. The PDU parameters included PSV, EDV, and RI. The RI was calculated by the formula PSV-EDV/PSV. The PSV and EDV were recorded when 3 consecutive similar spectra displayed. Considering the difference of the PSV measured at the left and right CCP, the mean PSV of the bilateral CCP were used to compare in our study, as in a previously published study [30]. The SWE mode was used every time to measure the SWE values of the corpus cavernosum penis (CCP) just at the end of PDU measurement. After switching to SWE mode, a target region of interest (ROI) with a 4.0 mm diameter was activated when color images filled the frames to more than 90%. Four ROI positions of both sides of the cavernosum were used to measure the SWE value of the penis, and 2 valid measurements of each ROI position were obtained. The average value of 8 sets of data was calculated to be the final SWE values of the penis cavernosum, as measured in kilopascals (kPa).
To obtain accurate measurements, it is necessary to prevent excessive sympathetic discharge owing to the pain of ICI and anxiety about the measurement process. We applied audio-visual sexual stimulation (AVSS) to all patients using a glasses-type video player. In addition, prostaglandin E-1 was used for inducing penis erection with the dosage of 10 ug, which also is the second-line therapy drug for ED patients.
Statistical Analysis
Statistical analyses were performed with SPSS version 25.0 (SPSS, Inc., Chicago, IL, USA). Quantitative variables with normal distribution are expressed as the mean±standard deviation (SD), and the distribution was tested by the Kolmogorov-Smirnov test. For clinical characteristics, an additional t test was used to compare the difference in variables following normal distribution. Otherwise, the Mann-Whitney test was used for nonparametric variables. The Spearman correlation test was used to explore the correlation between PDU parameters and SWE values. One-way analysis of variance (ANOVA) was used to test differences among 4 groups of different PSV degrees. Because the PSV >30 cm/s was considered sufficient for penile function, we conducted receiver operating characteristic (ROC) curve analysis to calculate the area under the curve (AUC) to assess the efficacy of SWE value for predicting the PSV values. In addition, the cut-off value of SWE was calculated by the ROC curve. The Youden criterion, which consists of identifying the value of SWE that maximizes the sum of sensitivity and specificity, was used to identify the optimal SWE value for ED patients. Statistical significance was set at P value under 0.05, and all tests were two-sided.
Results
Clinical Characteristics of Study Population
A total of 78 ED patients and 32 healthy controls were recruited in our study. The clinical characteristics of all patients are described in Table 1, including fasting blood glucose, testosterone, triglycerides, and total cholesterol. The mean IIEF-5 value of total patients was 14.88±4.14. For each patient, the PDU parameters and SWE values were measured 6 times with an interval of 5 min between each measurement, meaning that each patient had 6 values each of PSV, EDV, and SWE (0 min [flaccid state], 5 min, 10 min, 15 min, 20 min, and 25 min [erectile state after the ICI]). Therefore, 468 values of PSV, EDV, and SWE in ED patients and 192 values of PSV, EDV, and SWE in healthy controls were used to test our hypothesis.
Table 1.
Clinical characteristics of the samples including erectile dysfunction (ED) patients and healthy controls.
| ED patients (n=78) | Healthy controls (n=32) | P value | |
|---|---|---|---|
| Age | 36.77±10.93 | 34.31±8.75 | 0.261 |
| IIEF-5 | 14.88±4.14 | 23.87±1.24 | <0.001 |
| Fasting sugar, mmol/L | 4.65±0.58 | 4.43±0.51 | 0.059 |
| Testosterone, nmol/L | 15.67±4.57 | 16.90±3.76 | 0.151 |
| Triglycerides, mmol/L | 1.30±0.47 | 1.44±0.45 | 0.154 |
| Total cholesterol, mmol/L | 4.18±0.96 | 3.87±1.04 | 0.139 |
| Parameters | |||
| PSV max, cm/s | 30.66±10.34 | 42.33±7.65 | <0.001 |
| EDV, cm/s | 2.11±2.25 | −1.45±1.91 | <0.001 |
| SWE min, kPa | 11.66±4.98 | 8.08±1.58 | <0.001 |
ED – erectile dysfunction; IIEF-5 – the five-item version of the International Index of Erectile Function; PSV – peak systolic velocity; EDV – end-diastolic velocity; SWE – shear wave elastography.
Correlation Between PDU Parameters and SWE Values
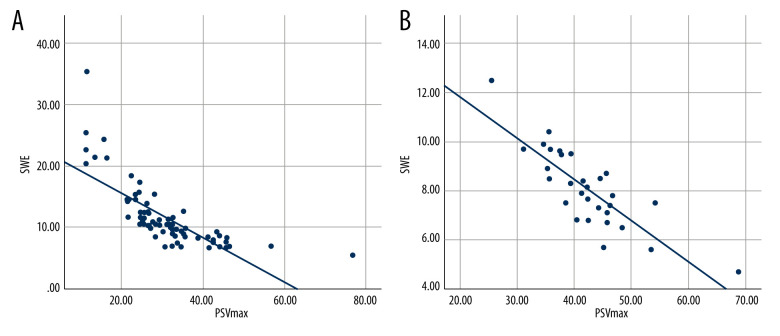
To explore the correlation between PDU parameters and SWE values, we calculated the correlation coefficients among PSV, EDV, and SWE. Notably, the highest PSV, corresponding EDV, and corresponding SWE values of 78 ED patients and 32 healthy controls were obtained to explore the correlations among them. The results are shown in Table 2 and Figure 1. After the calculation, a significant correlation between PSV and SWE value was found in both ED patients (r=−0.748, p<0.001) and healthy controls (r=−0.815, P<0.001), but there was no significant correlation between EDV and SWE values.
Table 2.
Correlation coefficients between peak systolic velocity (PSV), end-diastolic velocity (EDV), and shear wave elastography (SWE) in erectile dysfunction (ED) patients and healthy controls.
| ED patients (n=78) | Healthy controls (n=32) | |
|---|---|---|
| PSV max-SWE min | r=−0.748, P<0.001 | r=−0.815, P<0.001 |
| EDV-SWE min | r=0.012, P=0.917 | r=0.319, P=0.08 |
ED – erectile dysfunction; PSV max – maximum peak systolic velocity; EDV – end-diastolic velocity; SWE min – minimum shear wave elastography.
Figure 1.
Correlation between peak systolic velocity (PSV) and shear wave elastography (SWE) values in erectile dysfunction (ED) patients and healthy controls. (A) Correlation between peak systolic velocity (PSV) and shear wave elastography (SWE) values in erectile dysfunction (ED) patients. (B) Correlation between peak systolic velocity (PSV) and shear wave elastography (SWE) values in healthy controls. The figure was created using SPSS software (version 25.0).
Changes Trends Between PSV and SWE Values
We divided all data into 5 groups based on the PSV values: Group 1 with PSV value ranging from 0 to 10 cm/s, Group 2 with PSV value ranging from 10 to 20 cm/s, Group 3 with PSV value ranging from 20 to 30 cm/s, Group 4 with PSV value ranging from 30 to 40 cm/s, and Group 5 with PSV value larger than 40 cm/s. We also recorded corresponding SWE values of the different PSV values. The results are shown in Table 3. Moreover, there were significant differences in the SWE values and PSV values among the 5 groups in ED patients and healthy controls. The PSV values of ED patients were as follows: Group 1: 7.48±1.38 cm/s; Group 2: 13.42±2.43 cm/s; Group 3: 24.34±2.66 cm/s; Group 4: 33.54±2.69 cm/s; Group 5: 44.52±7.41 cm/s. The color of CCP decreased significantly as the PSV improved, as follows: Group 1: 18.82±3.37 kPa; Group 2: 16.35±3.19 kPa; Group 3: 13.52±3.61 kPa; Group 4: 10.55±2.13 kPa; Group 5: 7.62±1.19 kPa. The detailed data are shown in Table 3. As shown in Table 3, the PSV values of healthy controls were as follows: Group 1: 9.54±0.51 cm/s; Group 2: 18.12±1.35 cm/s; Group 3: 27.03±2.77 cm/s; Group 4: 37.81±1.72 cm/s; Group 5: 51.12±7.21 cm/s. The color of CCP decreased significantly as the PSV improved, as follows: Group 1: 15.74±1.47 kPa; Group 2: 14.59±2.67 kPa; Group 3: 12.11±1.96 kPa; Group 4: 9.21±1.49 kPa; Group 5: 7.23±1.68 kPa.
Table 3.
Shear wave elastography values of the corpus cavernosum at different agrees of peak systolic velocity (PSV).
| ED patients (n=78) | Healthy controls (n=32) | |||||
|---|---|---|---|---|---|---|
| N | PSV, cm/s (mean±SD) | SWE, kPa (mean±SD) | N | PSV, cm/s (mean±SD) | SWE, kPa (mean±SD) | |
| PSV [0–10 cm/s] | 105 | 7.48±1.38 | 18.82±3.37 | 8 | 9.54±0.51 | 15.74±1.47 |
| PSV [10–20 cm/s] | 118 | 13.42±2.43 | 16.35±3.19 | 32 | 18.12±1.35 | 14.59±2.67 |
| PSV [20–30 cm/s] | 136 | 24.34±2.66 | 13.52±3.61 | 51 | 27.03±2.77 | 12.11±1.96 |
| PSV [30–40 cm/s] | 79 | 33.54±2.69 | 10.55±2.13 | 51 | 37.81±1.72 | 9.21±1.49 |
| PSV [40-cm/s] | 30 | 44.52±7.41 | 7.62±1.19 | 50 | 51.12±7.21 | 7.23±1.68 |
| P value | – | <0.001 | <0.001 | – | <0.001 | <0.001 |
ED – erectile dysfunction; PSV – peak systolic velocity; SWE – shear wave elastography; SD – standard deviation.
The PSV value >30 cm/s was considered sufficient artery blood supply to complete sexual intercourse; therefore, we conducted ROC analysis to find the cut-off value of SWE in CCP to evaluate the penile artery blood supply. SWE values of CCP less than 11.57 kPa show that the penile artery blood supply was sufficient to finish satisfied sexual intercourse. The sensitivity and specificity were 0.838 and 0.872, respectively, and the ROC figure is exhibited in Figure 2.
Figure 2.
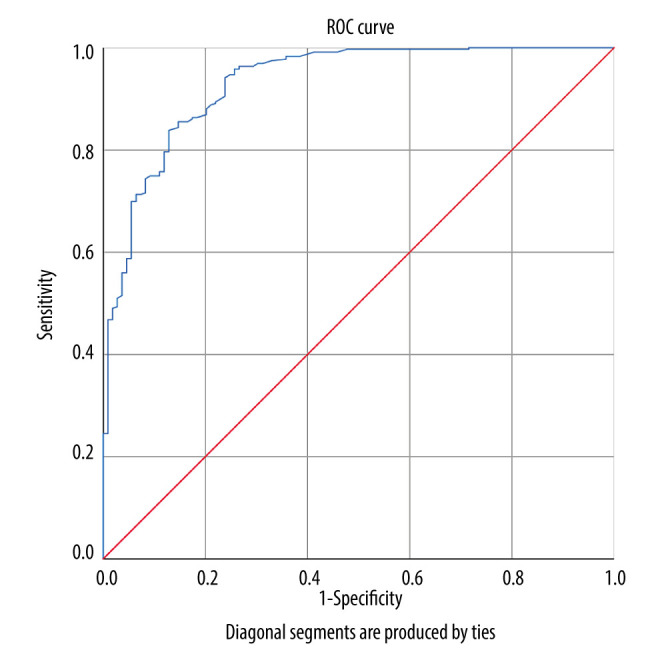
Receiver operating characteristics curve of shear wave elastography (SWE) for penile corpus cavernosum. The area under the ROC curve (AUC)=0.932, 95% CI (confidential interval)=0.904–0.959). The figure was created using SPSS software (version 25.0).
Discussion
We demonstrated that the SWE value of CCP was negatively related to the PSV value, and the SWE value decreased significantly with rising PSV. The SWE value in CCP can reflect the penile artery blood supply during the PDU test. With a cut-off value of 10.05 kPa in SWE for CCP, the SWE value of CCP can evaluate whether the penile artery blood supply exceeds 30 cm/s, which is a critical threshold for assessing penile artery function.
The penile artery, also referred to as the cavernous artery, was more vulnerable than the cardiac vessel, which is explained by the artery size hypothesis of Montorsi [31]. When narrowed by other risk factors, a smaller penile artery will be destroyed earlier than larger cardiac vessels. Currently, PSV is the only index used to reflect the penile artery blood supply directly, and it has been shown to be strongly associated with future CVD [32]. However, precise measurement of PSV in the PDU examination requires a more technical operation for radiologists. To date, no other methods can reflect the penile artery blood supply and compensate for the limitations of PSV measurement.
SWE is a novel sonography method for evaluating tissue stiffness, which includes 2 main types: strain elastography and shear wave elastography [21]. Strain elastography has been used rarely as a semi-quantitative method, which requires applying external force manually [33]. The accuracy of strain elastography highly depends on the user’s ability. Shear wave elastography measures the tissue stiffness quantitatively, which reflects the stiffness by calculating the Young’s modulus (YM) [34]; the calculation formula is E=Pc2, in which E is YM, c is the propagation velocity of shear wave, and P is the tissue density [35]. Therefore, higher SWE values indicate greater stiffness. Tissue stiffness measured by the SWE technique was not equal to the hardness. “Stiffness” assessed by SWE is a reflection of tissue structures [21]. The structure of the CCP mainly consists of approximately 50% smooth muscle cells (SMC) and extracellular matrix [20]. An experimental study [36] demonstrated that the penis SWE values differed between pre-sexual maturity rats and sexual decline rats (10.18±1.09 kPa vs 8.02±1.34 kPa). Further analysis of immunochemical staining indicates that the decline of collagen fibers accounted for the decrease of SWE values. Another experimental study, conducted in rabbits, also verified the role of SWE in the detection of the pathological changes of penile lesions induced by hyperlipidemia [37].
When penis erection is stimulated by sexual stimulation, the penile artery relaxes, and a continuously increasing blood supply results in penile erection [38]. From flaccid state to erectile state, the PSV improved significantly along with the erection hardness, which changed from grade 1 to grade 4 in different erection phases [39]. When comparing the SWE values of different erection hardness, Zheng et al demonstrated that the shear wave velocity significantly decreased with the increase of erectile hardness [40]. In another study, the SWE values of CCP in erectile state were significantly lower than that in flaccid state [26]. The increases of blood supply in the CCP mainly accounted for the phenomenon. When the CCP is filled with blood, the CCP tissue density decreases gradually. So, when the penis becomes erect, the SWE for CCP will decrease with the decreased CCP density. Based on this analysis, the conclusion of our study is consistent with those of previous studies. A prospective study conducted in venogenic ED patients showed that the SWE values changed from 21.75±4.25 kPa to 8.10±3.38 kPa when the penis was stimulated to achieve optimal erection [26], as the blood flow in the penis decreased the penile SWE values, but the EDV were unrelated to the penile artery blood supply, which could reflect the venous return from the penis and thus would not change the SWE values of CCP.
A study by Altinbas et al had also found the difference of SWE values between normal controls and arterial ED by strain elastography. Nowadays, more andrologists preferred to the ultrasonography methods for evaluating ED. Translational PDU only focused on the artery supply of penis, neglecting the evaluation of the penile structures. Novel SWE could assess the penile artery supply and structures as a whole [41]. So, the SWE technique would display more superiority in the territory of ED. In the published study by our group also found that the SWE could be used for diagnosis of arterial ED, and the results showed that the penile SWE values would decreased significantly when the penis were induced to erection [27]. More importantly, the SWE value in flaccid state were related inversely with it in erectile state [41]. In 2021, Lee et al conducted a study with larger sample size to assess the role of SWE in the penile erectile rigidity evaluation in ED patients with different vascular subtype. They found that a cut-off value of 8.05 kPa would predicting the vascular ED with a specificity of 41.5%, a sensitivity of 84.6% [42]. Combined with our results, the SWE measurements in ED patients would assess the penile rigidity and penile hemodynamic parameters simultaneously, which could expand the clinical value and practical application in vascular ED.
However, several limitations of our study should be considered. First of all, the sample enrolled for our study was small; thus, our data were limited. Second, we did not further analyze the correlation between SWE values of CCP and the criterion standard of penile rigidity. However, our study results have clinical utility for the precise prediction of vascular ED. SWE measurement needs a more precise ultrasound machine and more skilled radiologist to conduct it, limiting the clinical application in basic medical institutions. In subsequent research, we plan to link the SWE values to the NPTR results in ED patients in a study with a larger sample size. We believe that the clinical value of the SWE technique in ED will be extensively verified by further studies.
Conclusions
In conclusion, we demonstrated that quantitative measurement of SWE values in CCP could reflect the penile artery blood supply during PDU examination. The SWE technique could be used for evaluating the penile artery blood supply combining the ICI test with the advantages of noninvasiveness, simple operation, and excellent repeatability.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Hatzimouratidis K, Amar E, Eardley I, et al. Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation. Eur Urol. 2010;57(5):804–14. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Ma Z, Zhang XL, et al. Significance of blood lipid parameters as effective markers for arteriogenic erectile dysfunction. Andrology. 2020;8(5):1086–94. doi: 10.1111/andr.12776. [DOI] [PubMed] [Google Scholar]
- 3.Guo LQ, Liu YQ, Sun WD, et al. Significance of platelet distribution width as a severity marker of erectile dysfunction. Andrologia. 2017;49(3):12628. doi: 10.1111/and.12628. [DOI] [PubMed] [Google Scholar]
- 4.Tal R, Voelzke BB, Land S, et al. Vasculogenic erectile dysfunction in teenagers: A 5-year multi-institutional experience. BJU international. 2009;103(5):646650. doi: 10.1111/j.1464-410X.2008.08037.x. [DOI] [PubMed] [Google Scholar]
- 5.Uddin SMI, Mirbolouk M, Dardari Z, et al. Erectile dysfunction as an independent predictor of future cardiovascular events. Circulation. 2018;138(5):540–42. doi: 10.1161/CIRCULATIONAHA.118.033990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carneiro F, Nascimento B, Miranda EP, et al. Audiovisual sexual stimulation improves diagnostic accuracy of penile doppler ultrasound in patients with erectile dysfunction. J Sex Med. 2020;17(2):249–56. doi: 10.1016/j.jsxm.2019.11.263. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhang Z, Zhang N. Role of RigiScan parameters in differentiation of vascular erectile dysfunction. Andrologia. 2020;52(10):e13620. doi: 10.1111/and.13620. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Zhuan L, Liu Z, et al. Audiovisual sexual stimulation and RigiScan test for the diagnosis of erectile dysfunction. Chin Med J. 2018;131(12):1465–71. doi: 10.4103/0366-6999.233945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C-C, Ruan X-Z, Tang Y-F, et al. Diagnostic value of four-dimensional CT angiography in arterial erectile dysfunction using 320-detector row dynamic volume CT. Biosci Rep. 2017;37 doi: 10.1042/BSR20170200. BSR20170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lue TFHH, Marich K, et al. Vasculogenic impotence evaluated by high-resolution ultrasonography and pulsed Doppler spectrum analysis. Radiology. 1985;155:777–81. doi: 10.1148/radiology.155.3.3890009. [DOI] [PubMed] [Google Scholar]
- 11.Sikka SC, Hellstrom WJ, Brock G, et al. Standardization of vascular assessment of erectile dysfunction: Standard operating procedures for duplex ultrasound. J Sex Med. 2013;10(1):120–29. doi: 10.1111/j.1743-6109.2012.02825.x. [DOI] [PubMed] [Google Scholar]
- 12.Burnett AL, Nehra A, Breau RH, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200(3):633–41. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Aversa A, Sarteschi LM. The role of penile color-duplex ultrasound for the evaluation of erectile dysfunction. J Sex Med. 2007;4(5):1437–47. doi: 10.1111/j.1743-6109.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Herati A, Gilbert BR. Penile Doppler ultrasound predicting cardiovascular disease in men with erectile dysfunction. Curr Urol Rep. 2015;16(3):16. doi: 10.1007/s11934-015-0482-1. [DOI] [PubMed] [Google Scholar]
- 15.Ioakeimidis N, Vlachopoulos C, Rokkas K, et al. Dynamic penile peak systolic velocity predicts major adverse cardiovascular events in hypertensive patients with erectile dysfunction. J Hypertens. 2016;34(5):860–68. doi: 10.1097/HJH.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 16.Varela CG, Yeguas LAM, Rodriguez IC, et al. Penile Doppler ultrasound for erectile dysfunction: Technique and interpretation. Am J Roentgenol. 2020;214(5):1112–21. doi: 10.2214/AJR.19.22141. [DOI] [PubMed] [Google Scholar]
- 17.Aversa A, Crafa A, Greco EA, et al. The penile duplex ultrasound: How and when to perform it? Andrology. 2021;9(5):1457–66. doi: 10.1111/andr.13029. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento B, Miranda EP, Terrier JE, et al. A critical analysis of methodology pitfalls in duplex Doppler ultrasound in the evaluation of patients with erectile dysfunction: Technical and interpretation deficiencies. J Sex Med. 2020;17(8):1416–22. doi: 10.1016/j.jsxm.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkay R, Inci E, Yenice MG, et al. Shear wave elastography: Can it be a new radiologic approach for the diagnosis of erectile dysfunction? Ultrasound. 2017;25(3):150–55. doi: 10.1177/1742271X17697512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inci E, Turkay R, Nalbant MO, et al. The value of shear wave elastography in the quantification of corpus cavernosum penis rigidity and its alteration with age. Eur J Radiol. 2017;89:106–10. doi: 10.1016/j.ejrad.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Nowicki A, Dobruch-Sobczak K. Introduction to ultrasound elastography. J Ultrason. 2016;16(65):113–24. doi: 10.15557/JoU.2016.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng H, Niu Z, Xin F, et al. A new method to quantify penile erection hardness: Real-time ultrasonic shear wave elastography. Transl Androl Urol. 2020;9(4):1735–42. doi: 10.21037/tau-20-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correas J-M, Tissier A-M, Khairoune A, et al. Prostate cancer: Diagnostic performance of real-time shear-wave elastography. Radiology. 2015;275:280–89. doi: 10.1148/radiol.14140567. [DOI] [PubMed] [Google Scholar]
- 24.Athanasiou A, Tardivon A, Tanter M, et al. Breast lesions: Quantitative elastography with supersonic shear imaging – preliminary results. Radiology. 2010;256:297–303. doi: 10.1148/radiol.10090385. [DOI] [PubMed] [Google Scholar]
- 25.Barr RG. Shear wave liver elastography. Abdom Radiol (NY) 2018;43(4):800–7. doi: 10.1007/s00261-017-1375-1. [DOI] [PubMed] [Google Scholar]
- 26.Cui A, Xu L, Mu J, et al. The role of shear wave elastography on evaluation of the rigidity changes of corpus cavernosum penis in venogenic erectile dysfunction. Eur J Radiol. 2018;103:1–5. doi: 10.1016/j.ejrad.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Zhou W, Zhang Y, Li L, et al. Evaluation of arterial erectile dysfunction using shear wave elastography: A feasibility study. J Ultrasound Med. 2021;40(6):1209–16. doi: 10.1002/jum.15502. [DOI] [PubMed] [Google Scholar]
- 28.Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 29.Salonia A, Bettocchi C, Boeri L, et al. European Association of Urology Guidelines on Sexual and Reproductive Health – 2021 update: Male sexual dysfunction. Eur Urol. 2021;80(3):333–57. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Kuo Y-C, Liu S-P, Chen J-H, et al. Feasability of a novel audio-video sexual stimulation system: An adjunct to the use of penile duplex Doppler ultrasonography for the investigation of erectile dysfunction. J Sex Med. 2010;7(12):3979–83. doi: 10.1111/j.1743-6109.2009.01583.x. [DOI] [PubMed] [Google Scholar]
- 31.Montorsi P, Ravagnani PM, Galli S, et al. Association between erectile dysfunction and coronary artery disease: Matching the right target with the right test in the right patient. Eur Urol. 2006;50(4):721–31. doi: 10.1016/j.eururo.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Corona G, Fagioli G, Mannucci E, et al. Penile Doppler ultrasound in patients with erectile dysfunction (ED): Role of peak systolic velocity measured in the flaccid state in predicting arteriogenic ED and silent coronary artery disease. J Sex Med. 2008;5(11):2623–34. doi: 10.1111/j.1743-6109.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 33.Garra BS. Elastography: History, principles, and technique comparison. Abdom Imaging. 2015;40(4):680–97. doi: 10.1007/s00261-014-0305-8. [DOI] [PubMed] [Google Scholar]
- 34.Arda K, Ciledag N, Aktas E, et al. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am J Roentgenol. 2011;197(3):532–36. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- 35.Sarvazyan A, Rudenko O, Swanson S, et al. Shear wave elasticity imaging: A new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24(9):1419–35. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 36.Qiao XH, Zhang JJ, Gao F, et al. An experimental study: Quantitatively evaluating the change of the content of collagen fibres in penis with two-dimensional ShearWave™ Elastography. Andrologia. 2017;49(5):12653. doi: 10.1111/and.12653. [DOI] [PubMed] [Google Scholar]
- 37.Hu JL, Chen HX, Chen HR, et al. Novel noninvasive quantification of penile corpus cavernosum lesions in hyperlipidemia-induced erectile dysfunction in rabbits by two-dimensional shear-wave elastography. Asian J Androl. 2019;21(2):143–49. doi: 10.4103/aja.aja_78_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381(9861):153–65. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 39.Jung DC, Park SY, Lee JY. Penile Doppler ultrasonography revisited. Ultrasonography. 2018;37(1):16–24. doi: 10.14366/usg.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng X, Ji P, Mao H, et al. Evaluation of penile erection rigidity in healthy men using virtual touch tissue quantification. Radiol Oncol. 2012;46(2):114–18. doi: 10.2478/v10019-012-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Zhou W, Wu X, et al. Role of shear wave elastography measured in the flaccid state in predicting arteriogenic erectile dysfunction. Andrologia. 2021;53(4):e13996. doi: 10.1111/and.13996. [DOI] [PubMed] [Google Scholar]
- 42.Lee JY, Jung DC, Lee S, et al. Stiffness of the central corpus cavernosum on Shear-Wave Elastography is inversely correlated with the penile rigidity score in patients with erectile dysfunction. World J Mens Health. 2021;39(1):123–30. doi: 10.5534/wjmh.190094. [DOI] [PMC free article] [PubMed] [Google Scholar]