Abstract
Objectives:
To determine the skeletal relationships in patients with hypodontia and analyze the effects of severity and pattern.
Materials and Methods:
Pretreatment lateral cephalograms from 277 patients with hypodontia, categorized by the number of missing teeth as mild (1–2), moderate (3–5), or severe (≥6), were digitized recording angular measurements and ratios and compared with published norms matched for age and gender. Pattern was determined as mandibular, maxillary, bimaxillary, bilateral, anterior, posterior, and anteroposterior. Linear regression models assessed relationships between number of missing teeth and cephalometric parameters, controlling for the pattern of hypodontia.
Results:
For every additional missing tooth, SNA, SNB, and ANB decreased 0.3°, 0.1°, and 0.2°, respectively; this was clinically significant for >4, >10, and >5 missing teeth, respectively. Mandibular to cranial base ratio decreased 0.3% for every additional missing tooth; this was clinically significant for >10 missing teeth. The MMPA decreased 0.3° for every additional missing tooth; this was clinically significant for >7 missing teeth. Percentage LAFH decreased 0.2% for every additional missing tooth; this was significant for >7 missing teeth. Jarabak ratio increased 0.2% for each additional missing tooth; this was clinically significant for >10 missing teeth. Anterior hypodontia significantly decreased most cephalometric parameters.
Conclusions:
Patients with hypodontia demonstrated a tendency toward a Class III relationship, caused by decreased maxillary and mandibular angular prognathism and MnCB ratio, though the effect was greater on the maxilla than the mandible. Clinical significance was only associated with severe hypodontia. Vertically, there was a tendency toward decreased MMPA and %LAFH; this was clinically relevant only with severe hypodontia. Anterior hypodontia had a significant effect on skeletal relationship.
Keywords: Hypodontia, Skeletal, Cephalometric analysis, Pattern
INTRODUCTION
Hypodontia is the developmental absence of one or more teeth, excluding third molars,1 and it is classified according to the number of missing teeth: mild for 1 or 2 missing teeth,2 moderate for 3 to 5 missing teeth,2 and severe where 6 or more permanent teeth are missing.3,4 A meta-analysis of white population surveys showed a mean gender prevalence of 5.5% in Europe, 6.6% in Australia, and 3.9% in North America; 82.9% of affected individuals have mild hypodontia.5 Females are 1.37 times more likely to have dental agenesis than males.5
The management of hypodontia often involves orthodontic repositioning of the remaining teeth to allow for strategic prosthetic replacements, and it is best undertaken within a multidisciplinary team.1,3 Orthodontic treatment must be carried out within the anatomic constraints of an individual's three-dimensional dentoalveolar structure, and the ultimate aim is to create a harmonious facial form through dental and skeletal changes. Devising a suitable long-term treatment plan requires knowledge of the likely pattern of skeletal development in individuals with hypodontia.
The aims of this study were to investigate the vertical and antero-posterior facial skeletal relationships in patients with increasing severity of hypodontia and to determine whether the pattern of hypodontia affects any specific component of facial form.
MATERIALS AND METHODS
Ethical approval was sought and granted by the University College London Hospitals Research and Ethics Committee. The sample consisted of patients with hypodontia of their permanent dentition who were treated in the Orthodontic Department at the Eastman Dental Hospital. Subjects were classified into the following categories: mild (1 or 2 teeth missing), moderate (3 to 5 teeth missing), and severe hypodontia (≥6 teeth missing). The severity and distribution of hypodontia were identified from dental panoramic tomograms and supplemented with clinical notes.
A sample-size calculation was performed using Altman's nomogram and the standard deviation of angular measurements from a UK sample of patients with hypodontia.6 Clinically meaningful differences for ANB and MMPA were calculated as 1° greater than their standard deviations.7 For horizontal measurements, 70 patients in each severity group provided 96% power (α = .05). For vertical measurements, 70 patients in each group provided 97% power (α = .05).
The following inclusion criteria were used:
Pretreatment dental panoramic tomogram and lateral cephalogram of good quality, taken as part of routine orthodontic treatment
Age 8–20 years old at time of cephalogram being taken
White
The following exclusion criteria were used:
Craniofacial syndromes, including ectodermal dysplasia
History of trauma to anterior teeth
Digit-sucking habits
Previous orthodontic treatment
The following additional data were captured:
Date of birth
Age at time of cephalogram (rounded down to the nearest whole year)
Gender
Charting of missing teeth
Total number of missing teeth
The pattern of hypodontia was classified as follows:
Bilateral
Mandibular
Maxillary
Bimaxillary
Anterior (incisors only)
Posterior (canines, premolars, molars)
Anteroposterior
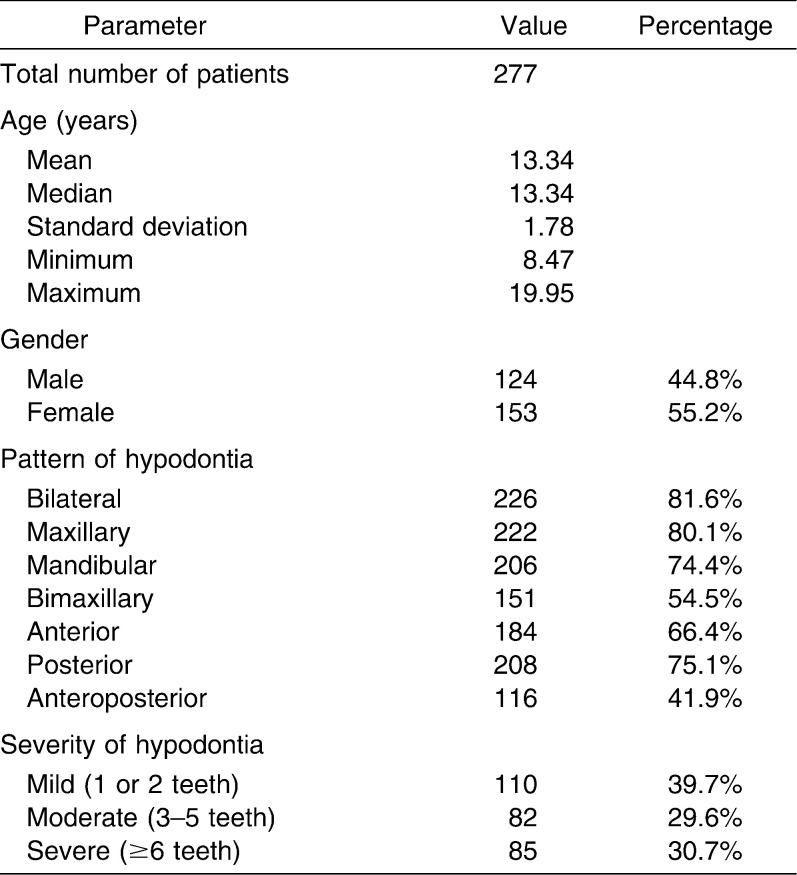
A total of 277 patients were included in the study (Table 1). Pretreatment cephalograms were analyzed by direct digitization using a digitizer (Numonics Model IPS/BLG, Numonics Corp, 101 Commerce Drive, Montgomeryville, Pa) linked to a computer installed with a customized geometric digitizing program (GELA Version 1.5, British Orthodontic Society, London, UK). The program prompted sequential identification of landmarks, calculating linear and angular measurements. Each radiograph was secured to a flat light box with surrounding light blocked out using a black cardboard frame and digitized in dark ambient conditions. For bilateral landmarks, the midpoint was determined by hand tracing.8 No more than 10 radiographs were digitized in any session, to reduce operator fatigue.
Table 1.
Demographic Overview of the Hypodontia Sample
To eliminate the effect of magnification from the cephalograms, ratios and angular cephalometric measurements were used to determine the vertical and A-P skeletal relationships. The results were compared to UK published norms for equivalent control groups, matched for age and gender.9
Intraoperator error and repeatability were measured by digitizing each cephalogram twice, at least 1 week apart, to avoid landmark memorization.10 The mean of both measurements was used in the final statistical analysis. Systematic and random errors were assessed using STATA 10.0 (Stata Corp, Intercooled STATA 10.0, 1984–2007 for Windows, College Station, Tex) to calculate the paired t-test and the repeatability coefficient, respectively, and the Bland and Altman method was used to calculate the combined error. Linear regression was used to calculate the effect of increase in the number of missing teeth on each cephalometric parameter. Any parameters showing statistical significance were subsequently subjected to multiple linear regressions using the number and pattern of missing teeth as the independent variables.
RESULTS
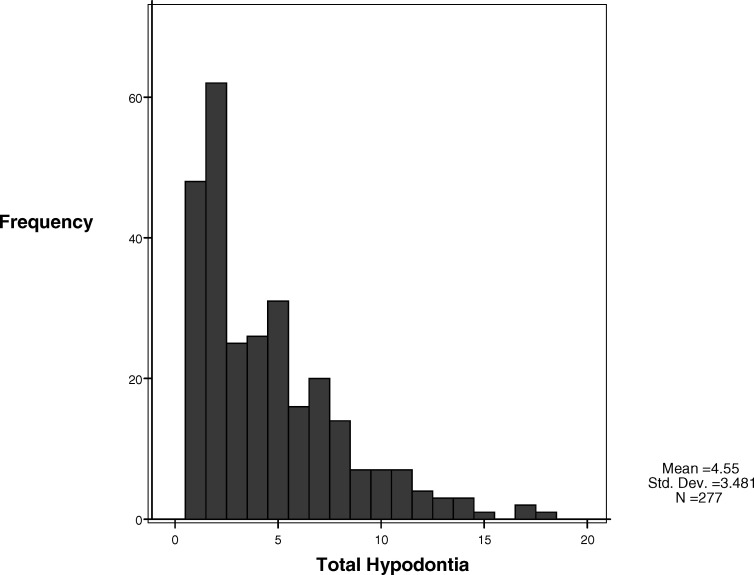
The sample consisted of 124 male and 153 female subjects with an age range of 8.47–19.95 years (mean age 13.34 years). Just over 41% of subjects had anteroposterior hypodontia, more than 54% had hypodontia affecting both jaws, 66% had anterior hypodontia, approximately 75% had mandibular hypodontia or posterior hypodontia, and more than 80% had maxillary or bilateral hypodontia (Table 1). A histogram demonstrated that most patients had 1 or 2 teeth missing; the most severely affected patient in the sample had 18 missing teeth (Figure 1).
Figure 1.
Histogram of the number of missing teeth.
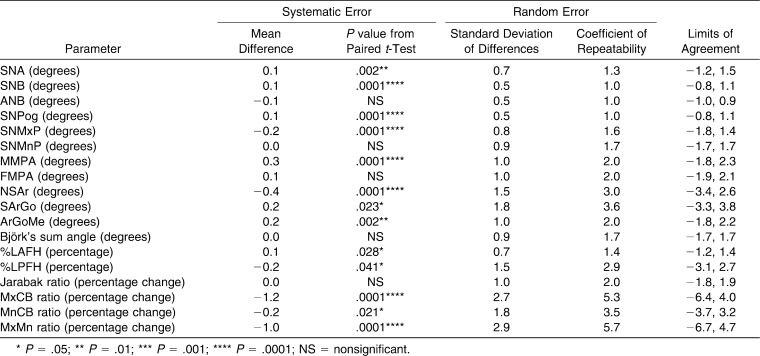
Systematic error was very small (0.1–0.2°) for SNA, SNB, SNPog, SNMxP, FMPA, S-Ar-Go, and Ar-Go-Me, whereas N-S-Ar and MMPA showed systematic errors of 0.4° and 0.3°, respectively. The percentage face height and skeletal base ratios showed systematic errors from 0.1%–1.2%, although the A-P ratios were more substantially affected (Table 2).
Table 2.
Bland and Altman Table of Repeatability Testing
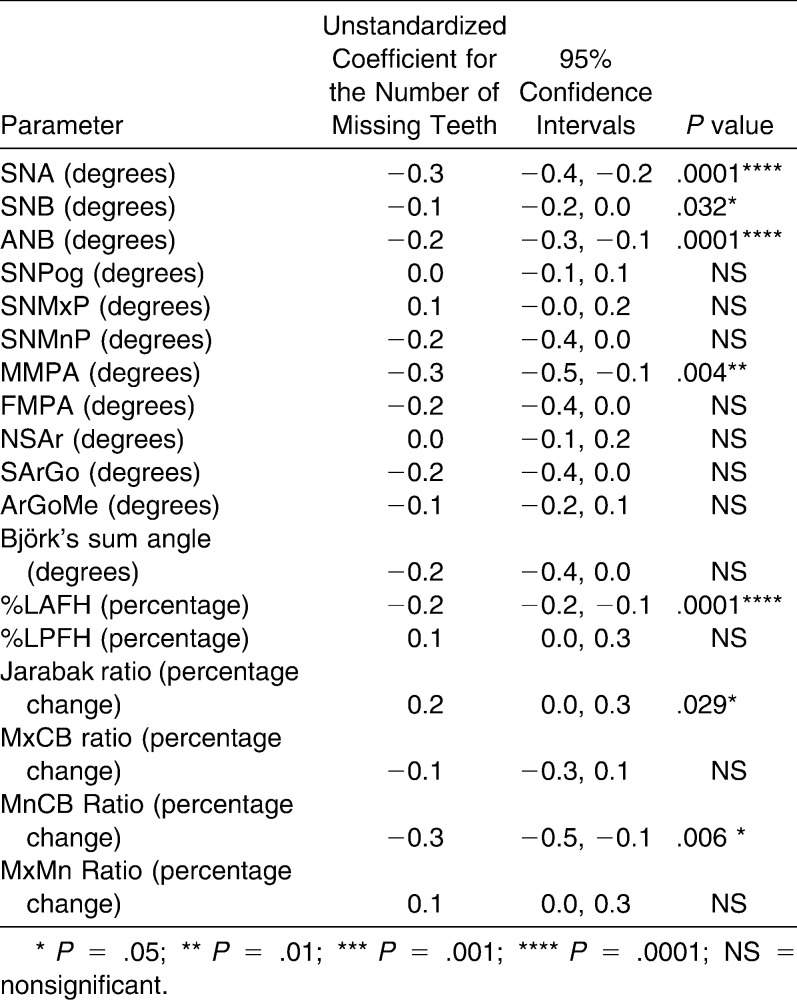
Linear regression testing revealed that SNA, SNB, ANB, and MMPA were significantly affected by the number of missing teeth; the effect for SNA and ANB was highly statistically significant (P = .0001). SNA decreased by 0.3°, SNB decreased by 0.1°, ANB decreased by 0.2°, and MMPA decreased by 0.3° for every additional tooth missing. The results for the %LAFH, Jarabak ratio, and MnCB ratio were also significant, and %LAFH was highly statistically significant (P = .0001). The %LAFH decreased by 0.2%, Jarabak ratio increased by 0.2%, and MnCB ratio decreased by 0.3% for every additional tooth missing. All other measurements were unaffected by the severity of hypodontia (Table 3).
Table 3.
Univariate Effect of the Number of Missing Teeth on Cephalometric Parameters Measured (Compared with Age- and Gender-matched Controls)
Multiple linear regressions for seven parameters that had a statistically significant association with the number of missing teeth were further examined with respect to the pattern of hypodontia (Table 4). A pattern of anterior hypodontia was associated with a statistically significant reduction in all cephalometric parameters, excluding the Jarabak ratio, and there were highly statistically significant reductions of 0.3° for SNA, 0.2° for ANB, and 0.2% for %LAFH, respectively (P = .0001).
Table 4.
Bivariate Effect of Pattern of Hypodontia Adjusted for the Number of Missing Teeth on Cephalometric Parameters Measured (Compared with Age- and Gender-matched Controls)
For the other patterns of hypodontia, only six parameters were affected by the pattern of hypodontia after adjusting for the number of missing teeth. The Jarabak ratio decreased by 1.5% with mandibular hypodontia; MMPA increased by 2.3° with bimaxillary hypodontia; the Jarabak ratio and MnCB ratio decreased by 1.6% and 2.6%, respectively, with bimaxillary hypodontia; MMPA increased by 1.9° with posterior hypodontia; and the Jarabak ratio decreased by 1.7% with posterior hypodontia . Excluding anterior hypodontia, there was no consistent underlying relationship between skeletal morphology and the pattern of hypodontia.
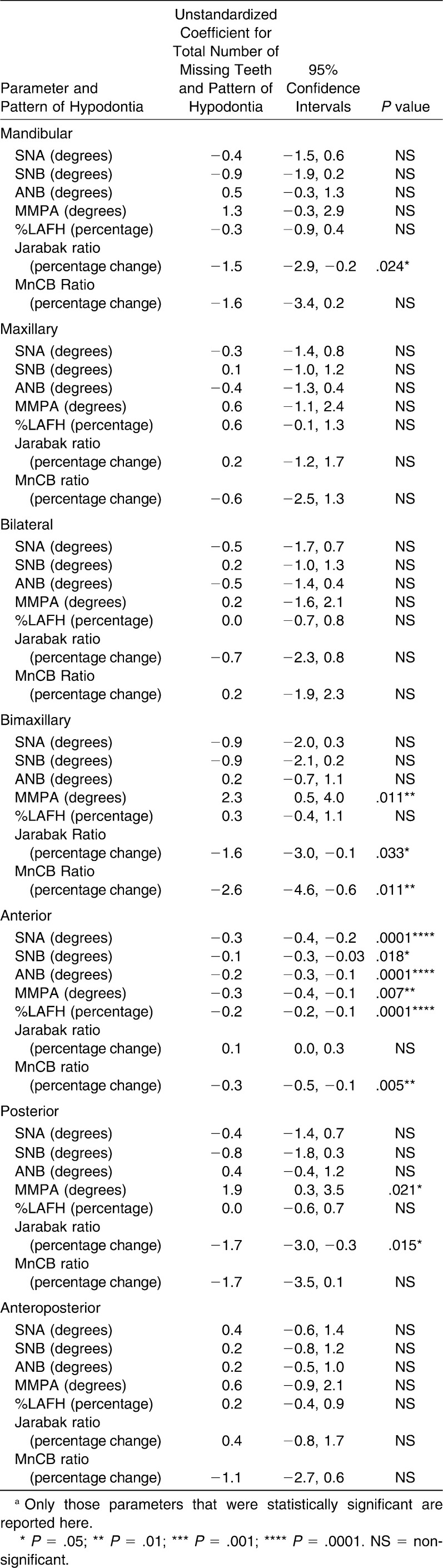
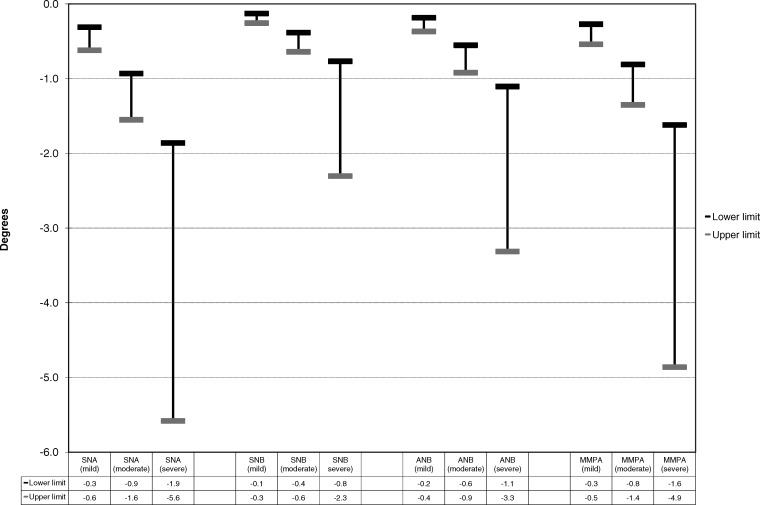
Regression analyses provide an indication of the unit effect of hypodontia severity on various clinical parameters in terms of missing teeth. Clinicians more usually consider severity of hypodontia as a series of categories (mild, moderate, and severe). To aid clinical interpretation of the results, those cephalometric measurements that showed a statistically significant linear association with the total number of missing teeth (Table 3) were recategorized into the clinical grades of severity. The anticipated typical ranges of average effects on the clinical parameters for each severity category are displayed in Figures 2 and 3.
Figure 2.
Plots for angular measurements showing a statistically significant linear association with the total number of missing teeth, categorized as mild, moderate and severe hypodontia.
Figure 3.
Plots for ratio measurements showing a statistically significant linear association with the total number of missing teeth, categorized as mild, moderate and severe hypodontia.
DISCUSSION
The effect of hypodontia on mandibular and maxillary growth has been subject to relatively few research publications, perhaps because of the difficulties of obtaining a sufficiently large sample size, variation in age groups and a range of cephalometric analyses used. Differences in the classification of the severity of hypodontia make comparisons difficult.
To recruit a sufficient sample size, hypodontia was initially classified by clinical severity. No consistent pattern of classification exists, except for severe hypodontia with ≥6 teeth missing.3,4 In this study, moderate hypodontia was classified as 3–5 missing teeth and mild hypodontia as 1–2 missing teeth.2 The pattern of hypodontia in previous studies used the tooth type missing6 or the affected jaw.11 The simple classification of mandibular, maxillary, bilateral, bimaxillary, anterior, posterior, and anteroposterior was considered appropriate for this study. We used published UK norms to permit direct comparison of the hypodontia sample with controls matched for age, ethnicity, and gender.9
Systematic error was very small (0.1° and 0.1%–0.2%) for all significant measurements (SNA, SNB, ANB, %LAFH, MnCB ratio) except the angle MMPA (0.3°). Although Björk's sum angle was not prone to systematic error, its three component angles (N-S-Ar, S-Ar-Go, and Ar-Go-Me) were.
The present study found a linear correlation between a reduction in SNA, SNB, and ANB and the number of missing teeth. The SNA angle decreased more than the SNB angle, resulting in a decreased ANB angle. Previous studies have reported SNA angle reduction12–15 and maxillary retrusion.16 Maxillary base shortening is possibly attributed to the lack of bony apposition in the tuberosity and anterior alveolar process, caused by molar and incisor agenesis.17 The present study found no direct relationship between hypodontia and maxillary base length, in keeping with previous studies,11,13 although one study found a biassociation of Nasion and A point retrusion, resulting in a normal SNA angle.17
Severe hypodontia has been associated with a decreased ANB13–15 angle and Class III skeletal relationship,6,14,18–20 although previous studies have highlighted the problems associated with drawing conclusions from ANB changes.18,21 Published results on the mandibular effects of hypodontia vary. Some report an increased mandibular corpus length and SNB angle,16,19 whereas others have found no changes in SNB,13 even in patients with ≥10 missing teeth. Interestingly, although SNB decreased in the present study, SNPog did not. In direct contrast, one study22 reported increased mandibular skeletal prognathism as assessed by the SNPog angle but decreased mandibular alveolar prognathism. Overall, it seems that hypodontia affects relative growth of the maxilla more than the mandible,11,16 but the effects are only evident with severe hypodontia.6,13 MMPA decreased as the severity of hypodontia increased, which concurs with previous studies.6,18 Some authors relate these changes to dental and functional compensation due to the lack of posterior dental support.13,23 Although the present study found no statistically significant association between hypodontia and the SNMnP angle, others have.13,22,24,25 Similarly, the present study found no association between FMPA and the number of missing teeth, which is in keeping with one previous study20 but not others.14,15,26
No association was found between hypodontia and changes in the saddle angle (N-S-Ar), joint angle (S-Ar-Go), gonial angle (Ar-Go-Me), or sum of the three (Björk's sum angle). In contrast, a previous study found a reduction in Björk's sum angle25 and lower gonial angle (nasion-gonion-gnathion),25 which was not measured in this study.
The %LAFH decreased, which was in keeping with several previous studies.13,25 The Jarabak ratio increased to a clinically significant level when >10 teeth were missing, indicating that in the presence of severe hypodontia the total posterior face height increases, the total anterior face height decreases, or a combination of both occurs. This demonstrates a closing growth rotation in individuals with severe hypodontia, as previously reported.13,24
Given the magnitude of vertical and A-P skeletal differences seen, the clinical relevance of the effect of hypodontia on each of the seven statistically significant cephalometric parameters is likely to manifest only in patients with severe hypodontia. Underlying relationships were found between the pattern of hypodontia and each cephalometric parameter, and there was a statistically significant association to the number of missing teeth. Of the 49 possible bivariate associations tested, 12 showed statistical significance. Interestingly, half of these were associated with a pattern of anterior hypodontia, and the results of the present study seem to suggest that the effects of anterior hypodontia are entirely responsible for the reduction in the ANB angle. Also, three of four bivariable associations involved bimaxillary hypodontia. This probably reflects the severity of hypodontia, as seen with the univariate effects of total hypodontia on the A-P and vertical skeletal relationship. However, it should be noted that none of the parameters showed a significant correlation to anteroposterior hypodontia.
One other previous study12 categorized the pattern of hypodontia in a similar fashion but limited it to hypodontia affecting the mandible only or maxilla only, and there was no gender differentiation. Individuals with maxillary hypodontia had significantly smaller SNA and ANB angles and increased N-A-Pog angles. Mandibular hypodontia was associated with significant reduction of ANB. The authors published linear measurements of vertical face height, which cannot be directly related to the present findings. The authors concluded that patients with hypodontia had a slightly increased face height and that hypodontia affects A-P maxillary growth most, irrespective of gender or pattern. A later study reported no significant cephalometric changes associated with hypodontia but found a significant decrease in maxillary jaw size associated with maxillary hypodontia. No significant associations were found for mandibular tooth agenesis. Bolton growth study templates were used for comparison.11 A more recently published study27 categorized pattern as anterior, posterior, and anteroposterior and tested for their effect on three cephalometric parameters that were also measured in the present study: SNA, SNB, and ANB. The authors of that study also concluded that an anterior hypodontia pattern has a predominant effect on the dentoskeletal pattern.
CONCLUSIONS
With increasing severity of hypodontia, the A-P skeletal relationship demonstrated a tendency toward a Class III pattern. This was caused by a combination of a decrease in maxillary and mandibular angular prognathism and mandibular length in ratio to the anterior cranial base length.
Hypodontia affected the A-P size of the maxilla more than the mandible, although these effects were clinically significant only in the presence of severe hypodontia.
The vertical skeletal pattern with increasing severity of hypodontia demonstrated a decreased MMPA and %LAFH. This was only clinically relevant in severe hypodontia and may be attributed to a lack of posterior occlusal support.
A pattern of anterior hypodontia had a significant effect on the A-P and vertical skeletal relationships with increasing severity of hypodontia.
Acknowledgments
The GELA program was kindly written by Mr Adrian Hart, Consultant Orthodontist, Raigmore Hospital, Inverness, UK.
REFERENCES
- 1.Goodman J. R, Jones S. P, Hobkirk J. A, King P. A. Hypodontia: 1. Clinical features and the management of mild to moderate hypodontia. Dent Update. 1994;21:381–384. [Google Scholar]
- 2.Dhanrajani P. J. Hypodontia: etiology, clinical features, and management. Quintessence Int. 2002;33:294–302. [PubMed] [Google Scholar]
- 3.Hobkirk J. A, Brook A. H. The management of patients with severe hypodontia. J Oral Rehabil. 1980;7:289–298. doi: 10.1111/j.1365-2842.1980.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 4.Hobkirk J. A, King P. A, Goodman J. R, Jones S. P. Hypodontia: 2. The management of severe hypodontia. Dent Update. 1995;22:8–11. [PubMed] [Google Scholar]
- 5.Polder B. J, Van't Hof M. A, Van der Linden F. P. G. M, Kuijpers-Jagtman A. M. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent Oral Epidemiol. 2004;32:217–226. doi: 10.1111/j.1600-0528.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung L. K. L, Hobson R. S, Nunn J. H, Gordon P. H, Carter N. E. An analysis of the skeletal relationships in a group of young people with hypodontia. J Orthod. 2000;27:315–318. doi: 10.1093/ortho/27.4.315. [DOI] [PubMed] [Google Scholar]
- 7.Mills J. R. E. Principles and Practice of Orthodontics 2nd ed. Edinburgh, UK: Churchill Livingstone; 1987. p. 80. [Google Scholar]
- 8.Sandler P. J. Reproducibility of cephalometric measurements. Br J Orthod. 1988;15:105–110. doi: 10.1179/bjo.15.2.105. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia S. N, Leighton B. C. A Manual of Facial Growth A Computer Analysis of Longitudinal Cephalometric Growth Data. Oxford, UK: Oxford University Press / Oxford Medical Publications; 1993. [Google Scholar]
- 10.You Q. L, Hägg U. A comparison of three superimposition methods. Eur J Orthod. 1999;21:717–725. doi: 10.1093/ejo/21.6.717. [DOI] [PubMed] [Google Scholar]
- 11.Tavajohi-Kermani H, Kapur R, Sciote J. J. Tooth agenesis and craniofacial morphology in an orthodontic population. Am J Orthod Dentofacial Orthop. 2002;122:39–47. doi: 10.1067/mod.2002.123948. [DOI] [PubMed] [Google Scholar]
- 12.Wisth P. J, Thunold K, Böe O. E. The craniofacial morphology of individuals with hypodontia. Acta Odontol Scand. 1974;32:281–290. doi: 10.3109/00016357409026344. [DOI] [PubMed] [Google Scholar]
- 13.Øgaard B, Krogstad O. Craniofacial structure and soft tissue profile in patients with severe hypodontia. Am J Orthod Dentofacial Orthop. 1995;108:472–477. doi: 10.1016/s0889-5406(95)70047-1. [DOI] [PubMed] [Google Scholar]
- 14.Chan D. W. S, Samman N, McMillan A. S. Craniofacial profile in southern Chinese with hypodontia. Eur J Orthod. 2009;31:300–305. doi: 10.1093/ejo/cjn111. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Bassat Y, Brin I. Skeletal and dental patterns in patients with severe congenital absence of teeth. Am J Orthod Dentofacial Orthop. 2009;135:349–356. doi: 10.1016/j.ajodo.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Roald K. L, Wisth P. J, Bøe O. E. Changes in cranio-facial morphology of individuals with hypodontia between the ages of 9 and 16. Acta Odontol Scand. 1982;40:65–74. doi: 10.3109/00016358209041117. [DOI] [PubMed] [Google Scholar]
- 17.Endo T, Yoshino S, Ozoe R, Kojima K, Shimooka S. Association of advanced hypodontia and craniofacial morphology in Japanese orthodontic patients. Odontology. 2004;92:48–53. doi: 10.1007/s10266-004-0034-5. [DOI] [PubMed] [Google Scholar]
- 18.Woodworth D. A, Sinclair P. M, Alexander R. G. Bilateral congenital absence of maxillary lateral incisors: a craniofacial and dental cast analysis. Am J Orthod Dentofacial Orthop. 1985;87:280–293. doi: 10.1016/0002-9416(85)90003-x. [DOI] [PubMed] [Google Scholar]
- 19.Bondarets N, Jones R. M, McDonald F. Analysis of facial growth in subjects with syndromic ectodermal dysplasia: a longitudinal analysis. Orthod Craniofac Res. 2002;5:71–84. doi: 10.1034/j.1600-0544.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- 20.Chung C. J, Han J. H, Kim K. H. The pattern and prevalence of hypodontia in Koreans. Oral Dis. 2008;14:620–625. doi: 10.1111/j.1601-0825.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 21.Hussels W, Nanda R. S. Analysis of factors affecting angle ANB. Am J Orthod Dentofacial Orthop. 1984;85:411–423. doi: 10.1016/0002-9416(84)90162-3. [DOI] [PubMed] [Google Scholar]
- 22.Nodal M, Kjær I, Solow B. Craniofacial morphology in patients with multiple congenitally missing permanent teeth. Eur J Orthod. 1994;16:104–109. doi: 10.1093/ejo/16.2.104. [DOI] [PubMed] [Google Scholar]
- 23.Dermaut L. R, Goeffers K. R, De Smit A. A. Prevalence of tooth agenesis correlated with jaw relationship and dental crowding. Am J Orthod Dentofacial Orthop. 1986;90:204–210. doi: 10.1016/0889-5406(86)90067-3. [DOI] [PubMed] [Google Scholar]
- 24.Sarnäs K. V, Rune B. The facial profile in advanced hypodontia: a mixed longitudinal study of 141 children. Eur J Orthod. 1983;5:133–143. doi: 10.1093/ejo/5.2.133. [DOI] [PubMed] [Google Scholar]
- 25.Bondarets N, McDonald F. Analysis of the vertical facial form in patients with severe hypodontia. Am J Phys Anthropol. 2000;111:177–184. doi: 10.1002/(SICI)1096-8644(200002)111:2<177::AID-AJPA4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Endo T, Ozoe R, Yoshino S, Shimooka S. Hypodontia patterns and variations in craniofacial morphology in Japanese orthodontic patients. Angle Orthod. 2006;76:996–1003. doi: 10.2319/082905-303. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Bassat Y, Brin I. Skeletodental patterns in patients with multiple congenitally missing teeth. Am J Orthod Dentofacial Orthop. 2003;124:521–525. doi: 10.1016/s0889-5406(03)00620-6. [DOI] [PubMed] [Google Scholar]