Abstract
Congenital anomalies of the kidney and urinary tract (CAKUTs) represent the leading cause of chronic kidney disease and end-stage kidney disease in children. Increasing evidence points to critical roles for the urothelium in the developing urinary tract and in the genesis of CAKUTs. The involvement of the urothelium in patterning the urinary tract is supported by evidence that CAKUTs can arise as a result of abnormal urothelial development. Emerging evidence indicates that congenital urinary tract obstruction triggers urothelial remodelling that stabilizes the obstructed kidney and limits renal injury. Finally, the diagnostic potential of radiological findings and urinary biomarkers derived from the urothelium of patients with CAKUTs might aid their contribution to clinical care.
Introduction
Congenital anomalies of the kidney and urinary tract (CAKUTs) constitute a heterogeneous group of clinical disorders that vary in aetiology, severity and need for urological intervention. The diverse spectrum of phenotypes encompassed by CAKUTs includes renal agenesis, renal hypodysplasia, multicystic dysplastic kidney, renal duplication, horseshoe kidney, ureteropelvic junction obstruction (UPJO), megaureter, ureterovesical junction obstruction, ureterocoele, vesicoureteral reflux (VUR), prune belly syndrome, urethral atresia, anterior urethral valve and posterior urethral valve (PUV)1. Collectively, CAKUTs occur in >1% of live births and account for up to 23% of birth defects2-6. In the past decade, the estimated incidence of CAKUTs has been 0.4–4.0 cases per 1,000 births7,8. In the majority of individuals, CAKUTs are detected during fetal ultrasonography. Early detection of CAKUTs facilitates early clinical care, subspecialty consultation, antenatal counselling and — depending on the nature of the CAKUT and the treating centre — in utero interventions such as vesico-amniotic shunting and fetal cystoscopy.
As a consequence of earlier detection and improvements in surgical and medical management, more children born with CAKUTs are surviving infancy, but these individuals develop progressive chronic kidney disease as they age. In paediatric populations, CAKUTs account for 48–59% of chronic kidney disease diagnoses and 34–43% of end-stage kidney disease (ESKD) diagnoses9. Registry data highlight a continued risk of ESKD progression among adults with a history of CAKUT, which has been attributed to aberrant nephron development and reduced nephron endowment that primes the kidney for subsequent functional deterioration in adulthood10,11. In a large study of over 1.5 million Israeli adolescents, a history of CAKUT was associated with a fivefold increase in the risk of ESKD during adulthood, even in individuals who had normal renal function at the time of study entry12. Overall, CAKUTs account for 40% of ESKD cases occurring in the first three decades of life13.
The clinical presentation of CAKUTs is highly heterogeneous. This variability is due in part to the wide anatomical spectrum of CAKUTs themselves, the patient’s age and circumstances at the time of their detection, and the occurrence of CAKUTs within syndromes that include prominent extrarenal malformations. Indeed, over 200 genomic disorders have been attributed to syndromic CAKUT, including renal coloboma syndrome, renal cysts and diabetes syndrome, branchio-oto-renal syndrome, Alagille syndrome and Townes–Brocks syndrome14. Even so, extrarenal manifestations are absent in the vast majority of individuals with CAKUTs, who are considered to have non-syndromic or isolated CAKUTs.
Genes associated with syndromic CAKUTs, such as PAX2 and HNF1B, are also associated with isolated CAKUTs. Although over 40 genes have been causally implicated in isolated CAKUTs to date, the vast majority of CAKUT phenotypes have not yet been linked to a single gene defect. In part, the difficulty of genetic characterization is attributable to the highly variable phenotypic manifestations of CAKUTs, as well as incomplete penetrance and variable expressivity among siblings who inherit the same defective allele. Furthermore, epidemiological studies point to potential aetiological roles for environmental factors in the pathogenesis of CAKUTs, most notably maternal pregestational diabetes mellitus8,15-17. Vitamin A serves a critical role in urinary tract morphogenesis, and either deficiency or excess of vitamin A can lead to CAKUT. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers have teratogenic effects in the developing fetus, particularly during the second and third trimesters; exposure to these drugs can result in maldevelopment of multiple organs, including renal dysplasia18.
The diversity of malformations observed across CAKUTs has been attributed to the complexity of temporally and spatially coordinated reciprocal interactions between the metanephric mesenchyme and ureteric bud2,14. These carefully coordinated interactions are absolutely essential for metanephric kidney development. Given the fundamental role of these interactions in patterning the urinary tract, it is not surprising that genes encoding mediators of these interactions (including extracellular matrix components, ligands, receptors, mediators of intracellular signalling cascades, transcription factors and chromatin remodelling proteins2,14) are disrupted in human patients with CAKUTs and have been shown to confer CAKUTs when such disrupted native genes or transgenes are introduced into model organisms such as the mouse and zebrafish. Just as they serve vital roles in metanephric kidney development, epithelial–mesenchymal interactions are also absolutely essential for normal organogenesis throughout the urinary tract. In particular, the urothelium serves critical roles in patterning the developing ureter and bladder through its reciprocal interactions with the underlying mesenchyme.
This Review describes the role of the urothelium in normal and aberrant patterning of the urinary tract from a scientific and clinical perspective. We discuss the importance of specific urothelial proteins and signalling pathways in urinary tract development, drawing insight from relevant findings in animal models and human embryogenesis. The evidence that specific CAKUTs arise as a direct consequence of aberrant urothelial development is presented, highlighting the influence of disrupted plaque synthesis on CAKUTs. Emerging evidence that the urothelium (and specifically urothelial membrane plaque) undergoes extensive remodelling during congenital obstructive uropathy and that this remodelling serves a critical role in limiting renal injury are discussed. Finally, we consider the utility of potential radiological and urinary urothelial biomarkers in the management of patients with CAKUTs.
The urothelium
The urothelium is a stratified epithelial layer that lines the urinary tract from the proximal urethra to the renal pelvis. Appreciation of its structural properties is essential for understanding the role of the urothelium in both urinary tract development and CAKUTs (FIG. 1). The urothelium consists of distinct basal, intermediate and superficial cells. The superficial cells, also known as umbrella cells, are large, binucleated and highly specialized cells that elaborate an apical membrane plaque composed of uroplakins19. In mammals, four major uroplakins are synthesized by the superficial cells: UPK1A, UPK1B, UPK2 and UPK3A. These proteins initially form heterodimers (UPK1A–UPK2 and UPK1B–UPK3A). The UPK2 and UPK3A moieties of these heterodimers then interact, to form heterotetramers. Six heterotetramers make up each urothelial plaque, which is packed into a hexagonal lattice and delivered to the apical membrane19. Urothelial plaque covers ~90% of the surface of superficial cells and is constantly being recycled. This highly specialized plaque confers transcellular resistance, minimizing water and solute absorption from the urine. Tight junctions and adherens junctions between umbrella cells form the paracellular urine permeability barrier20-22. Together, the urothelial plaque and junctional complexes establish exquisitely high electrical resistance and provide highly effective control of the passage of water and ions from urine and underlying tissue20-23. Indeed, the urothelium is the tightest barrier of all the epithelia.
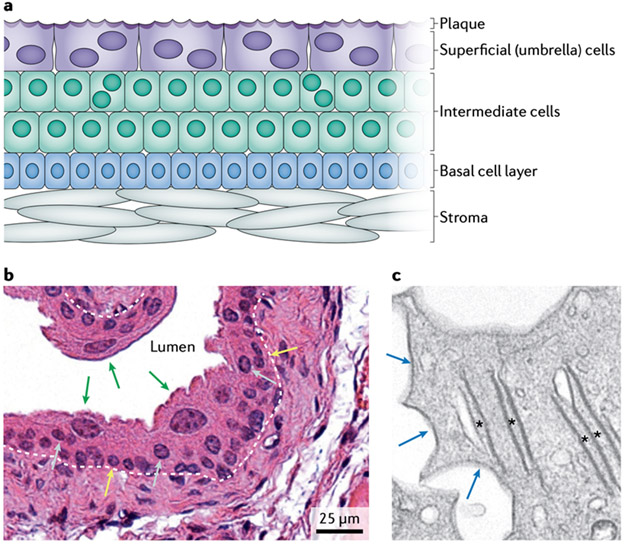
Figure 1. Structural properties of urothelium.
a Urothelium is composed of distinct basal, intermediate and superficial (umbrella) cell populations. Superficial cells secrete an apical membrane plaque composed of uroplakin proteins at the luminal surface. b Haematoxylin and eosin staining demonstrates large, binucleated superficial cells (green arrows), as well as intermediate cells (grey arrows), some of which are binucleated, and basal cells (yellow arrows); ×40 magnification. The urothelial basement membrane is indicated by a white dashed line. c Transmission electron microscopy of superficial cells reveals their unique ultrastructural characteristics, including uroplakin-rich fusiform vesicles (asterisks) and scallop-shaped apical urothelial plaque (blue arrows); ×3,000 magnification.
The urothelium also serves other physiological functions in the urinary tract. The urothelial plaque modifies the umbrella cell surface area and confers properties of compliance on this cell layer, which are needed as the bladder deforms during filling and voiding24,25. Whether the presence of plaques or the absence of a subapical F-actin network in superficial cells accounts for this compliance is unclear. The roles of specific uroplakins in the barrier function of the urothelium have been established by gene-deletion studies and analyses of the resulting developmental phenotypes. In addition to its barrier function, each urothelial cell type expresses a broad repertoire of receptors that enable the urothelium to sense a variety of physical, chemical and biological stimuli and to communicate with underlying stromal cells, neurons and immune cells26. Thus, the urothelium is well situated to serve a pivotal role in coordinating urinary tract development.
Urothelial development.
Development of the urothelium is only briefly outlined here. Urothelial development has been traditionally studied mainly in the bladder, but studies during the past 5 years have increasingly focused on ureteral and renal urothelium27,28. The anatomical site studied is an important consideration, as the ontogeny of the urothelium varies: the proximal urethral and bladder urothelium derive from the endoderm, whereas the urothelial lining of the ureters and renal pelvicalyceal system derives from the Wolffian duct, which in turn derives from the intermediate mesoderm29. Regardless of the germ layer of origin, the primordial urothelium starts off as a single-cell layer of immature, cuboidal epithelial cells. Under the direction of ligands produced by the underlying stroma, these cells undergo cell division and ultimately differentiate into three defined strata of basal, intermediate and superficial cells30-32.
Mounting evidence attests to the presence of dedicated progenitor cell populations in the developing and adult urothelium that govern development of the urothelium and I renewal during homeostasis and following injury. In mice, a rare subset of basal cells has been identified in the embryonic bladder that expresses Krt14 (encoding keratin type I cytoskeletal 14) and has progenitor properties, based on genetic fate-mapping studies in vivo and their superior self-renewal capacity in vitro33. Another mouse study identified a transient urothelial progenitor cell population during embryogenesis but reported that a population of uroplakin-positive intermediate cells were the source of both intermediate and superficial cells in juvenile and adult bladders30. In one study of the developing mouse ureter, the authors similarly concluded that the superficial and basal cell layers of the primordial urothelium chiefly derive from an intermediate layer of uroplakin-positive progenitor cells27. Our genetic fate-mapping study of intrarenal urothelial development revealed that progenitor cells expressing Krt5 (encoding keratin, type II cytoskeletal 5) can give rise to uroplakin-expressing cells in adult mice. Although the majority of late embryonic and neonatal KRT5+ cells acquired uroplakin expression, the differentiation of KRT5+ cells into uroplakin-expressing cells was largely restricted to these early time periods, as juvenile and adult KRT5+ cells exhibited lineage restriction28. Our study illustrates the benefit of evaluating candidate progenitor populations at multiple time frames throughout embryonic and postnatal development. Whether the results of studies in intrarenal urothelium can be generalized to other urothelial locations remains to be seen. Moreover, as these progenitor studies were performed in rodent models, their relevance to human urothelial development remains unknown.
Urothelium in urinary tract development.
Epithelial– stromal interactions serve broad, pivotal roles during embryonic development, including in the urinary tract. During kidney formation, waves of reciprocal interactions between the ureteric bud and metanephric mesenchyme account for the genesis and maturation of nephrons and the renal collecting system34,35. Similarly, interactions between the immature urothelium and stromal cells have crucial roles in patterning the developing urinary tract. Tissue recombination experiments in embryonic rat bladders provided the first evidence that the primordial urothelium provides an essential signal that promotes both the survival of the underlying mesenchyme and its subsequent differentiation into smooth muscle36: embryonic bladder mesenchyme grafts placed beneath the renal capsule fail to undergo smooth-muscle differentiation, unlike grafts containing both bladder mesenchyme and urothelium36. Experiments in mouse embryos have similarly demonstrated an essential role for the primordial urothelium in the patterning of bladder smooth muscle, supporting the hypothesis that primordial urothelial cells secrete a diffusible factor that inhibits smooth muscle at high concentrations while inducing smooth-muscle differentiation at low concentrations37.
Subsequent studies identified the underlying signal transduction pathways initiated by these epithelial– stromal interactions during bladder development (FIG. 2). Sonic hedgehog (SHH) was identified as a key epithelial morphogen that induces smooth-muscle differentiation within the bladder mesenchyme. During mouse bladder development, SHH expression occurs within the developing urothelium on embryonic day (E) 12.5, 1 day before the bladder mesenchyme begins to express smooth-muscle genes38. Studies in human embryos have confirmed that SHH is expressed at the onset of urothelial differentiation39. Smooth-muscle-specific gene expression is considerably decreased in explanted embryonic mouse bladders treated with the SHH inhibitor cyclopamine38. Moreover, isolated mouse bladder mesenchyme cultured with recombinant SHH showed cell proliferation and smooth-muscle differentiation40. Thus, urothelial SHH is responsible for orchestrating the formation of smooth muscle in the fetal bladder.
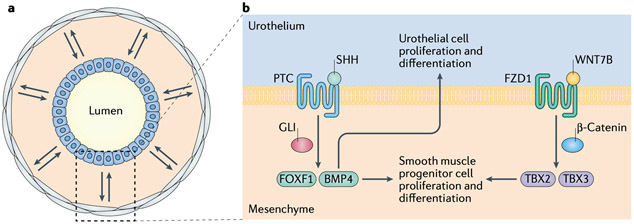
Figure 2. Urothelial–stromal crosstalk serves essential roles in urinary tract development.
a Bidirectional crosstalk (double arrows) between urothelial cells (blue) and stromal cells (grey) within the mesenchyme is essential for the normal differentiation of urothelium and smooth muscle in the developing bladder and ureter. b Urothelial–mesenchyme interactions during ureteral development. Urothelial sonic hedgehog protein (SHH) binds to its receptor patched (PTC), leading to the activation of GLI transcription factors, which drive transcription of bone morphogenetic protein 4 (BMP4) and forkhead box protein F1 (FOXF1), leading to the proliferation and differentiation of smooth-muscle progenitor cells. BMP4 also promotes urothelial proliferation and differentiation44,45. In parallel, urothelial WNT7B and WNT9B activate frizzled 1 (FZD1) receptors, leading to nuclear translocation of β-catenin and the transcription of target genes such as Tbx2 and Tbx3 (encoding the T-box transcription factors TBX2 and TBX3, respectively), thereby promoting the proliferation and differentiation of smooth-muscle progenitor cells51,52.
Investigations of SHH signalling during mouse and human bladder development have demonstrated that urothelial SHH serves as the ligand for patched homologue 1 protein (PTC1), a receptor on the surface of inner mesenchymal cells of the bladder39,41. Upon binding to SHH, PTC1 undergoes a conformational change that releases its inhibition of the transmembrane protein smoothened homologue (SMO). This change activates GLI transcription factors, which translocate to the nucleus and activate gene transcription. The gene targets of GLI transcription factors encode structural proteins with integral roles in smooth-muscle formation and secreted factors such as bone morphogenetic protein 4 (BMP4)42. The distribution of PTC1 and GLI transcription factor gene expression is most dense in the inner mesenchyme abutting the urothelium, where muscle differentiation is suppressed, and least dense in the outer mesenchyme, where muscle differentiation occurs. The mechanisms that restrict muscle differentiation to the outer mesenchyme are unclear. A signal originating from the developing urothelium might repel (that is, induce outward migration of) GLI1+ mesenchymal cells or the bladder adventitia might emit a signal that attracts these cells. Alternatively, a cell-autonomous property of the outer mesenchyme might specify muscle differentiation.
Urothelial SHH also serves essential, complex roles in coordinating the differentiation of smooth muscle in the developing ureter (FIG. 2). Specifically, SHH promotes the proliferation of smooth-muscle progenitor cells and the induction of smooth-muscle differentiation within the ureteral mesenchyme43. Evidence points to a critical role for forkhead box protein F1 (FOXF1) as a downstream mediator of this SHH signalling. This transcription factor both induces expression of BMP4 and synergizes with BMP4 to promote ureteral smooth-muscle differentiation44. In addition, ureteral SHH signalling also promotes ureteral urothelial proliferation and differentiation in an FOXF1-dependent manner44: exogenous BMP4 rescues urothelial proliferation and differentiation when either SHH or FOXF1 is inhibited44. Pharmacological studies demonstrate that BMP4 promotes ureteral urothelial proliferation and differentiation in a manner that depends on RACα serine–threonine protein kinase (AKT)45. Thus, a SHH–FOXF1–BMP4 signalling pathway promotes growth and differentiation of both the urothelium and the mesenchyme during ureteral development (FIG. 2).
Urothelial SHH also has an essential role in the development of renal pacemaker cells in the mouse46. These neural-crest-derived cells are located in close proximity to the ureteropelvic junction47,48 and coordinate ureteral peristalsis after birth. Defects in pacemaker-cell development and function lead to non-obstructive hydronephrosis48,49. In mice, urothelial SHH promotes pacemaker development by suppressing production of the GLI3 transcriptional repressor46. Thus, urothelial SHH engages multiple cellular populations in the developing ureter to regulate its morphogenesis and peristaltic function. Interestingly, constitutive activation of SHH signalling in the mouse ureteral mesenchyme leads to bilateral UPJO, which suggests that the fine-tuning of SHH signalling is essential for normal kidney development50. Additional studies are needed to determine whether this SHH fine-tuning normally occurs at the level of the urothelium or the underlying mesenchyme and to define its effect on the development and maintenance of ureteral smooth muscle and pacemaker function.
Other studies in the developing mouse ureter point to an important role for two urothelium-derived wingless/ integrated 1 (WNT) proteins, WNT7B and WNT9B, in regulating smooth-muscle development. These proteins bind to frizzled 1 (FZD1) receptors on mesenchymal cells, which results in nuclear translocation of β-catenin and the transcription of genes involved in promoting cell proliferation and smooth-muscle differentiation51. In 2018, the T-box transcription factors TBX2 and TBX3 were shown to participate in this pathway downstream of WNT–β-catenin signalling52 (FIG. 2).
A major limitation of these studies lies in their near-exclusive reliance on the laboratory mouse as the experimental system. Although mouse experiments have greatly advanced our understanding of urinary tract development, further studies are required to determine the extent to which these findings are broadly generalizable to the human urinary tract. This limitation is especially important in light of important anatomical differences between the human and mouse urothelium: the increased stratification of human urothelium; the restriction of uroplakin expression to the most superficial cells in human urothelium, compared with their widespread expression throughout intermediate and superficial urothelial cells in the mouse53,54; and the discontinuous nature of uroplakin expression in human ureteral and renal pelvic urothelium55, which contrasts with the continuous expression of uroplakins in the mouse urothelium. These species differences must be borne in mind when seeking to translate experimental observations derived in mice to human urinary-tract development and vice versa.
Roles for urothelium in CAKUTs
A growing convergence of data from mouse gene-deletion models and human patients with CAKUTs attest to the primacy of interactions between the developing urothelium and its underlying mesenchyme that are conserved across species and, when disrupted, account for CAKUTs. Mouse studies have demonstrated that genetic manipulation of the WNT–β-catenin and SHH–FOXF1–BMP4 signalling pathways results in a spectrum of CAKUTs, highlighting the essential role of urothelial–mesenchymal interactions in the developmental patterning of the urinary tract. Specifically, mice with conditional deletion of Cnntb1 (encoding β-catenin) in the ureteral mesenchyme develop congenital hydroureteronephrosis owing to impaired smooth-muscle differentiation51. Deletion of Shh results in a spectrum of urinary tract anomalies, including hydroureter, hydronephrosis and hypoplastic bladder43,56. These phenotypes have been attributed to the prominent role of early SHH signalling in directing mesenchymal proliferation and differentiation56. In mice with selective deletion of Shh in Wolffian-derived tissues, attenuated proliferation of ureteral mesenchyme and delayed smooth-muscle differentiation results in hydroureter, which confirms a central role for urothelial SHH in mesenchymal patterning43. A similar phenotype occurs when FOXF1 transcriptional activity is inhibited in the developing ureteral mesenchyme44. Likewise, mice with deletion of the BMP4 receptor Bmpr1a in SHH-responsive cells develop impaired smooth-muscle differentiation and hydroureteronephrosis57.
Mutations in components of these pathways might also account for human CAKUTs, as suggested by the increased incidence of BMP4 mutations in patients with congenital UPJO and renal hypodysplasia58-60. In addition, SHH and FOXF1 mutations have occasionally been identified in human patients with CAKUTs61,62. Altogether, these studies attest to essential roles for WNT–β-catenin and SHH–FOXF1–BMP4 signalling in normal urinary tract development. With further application of whole-genome sequencing in human patients with CAKUTs, additional examples of convergence between clinical and experimental CAKUTs are likely to emerge63.
Disrupted urothelial differentiation.
Mounting evidence indicates that genetic defects in urothelial differentiation pathways also give rise to CAKUTs (TABLE 1). In human kindreds with autosomal dominant bladder outlet obstruction, mutations in BNC2 (encoding basonuclin 2) have been associated with a constellation of urethral obstruction phenotypes in male carriers (female carriers are unaffected)64, including urethral stenosis, urethral agenesis and PUV64. Further studies are required to determine whether BNC2 variants also account for sporadic PUV. The function of basonuclin 2 is poorly understood. This zinc finger protein localizes to the urogenital sinus and outflow tract in 7-week-old human embryos, where BNC2 is expressed most strongly in the primitive urothelium and to a lesser extent in the mesenchyme64. In zebrafish, bnc2 has an instrumental role in maintaining a patent bladder outlet, as its genetic disruption results in obstruction of the pronephros and a dilated cloaca64. This study illustrates the power of the zebrafish pronephros as a model system to test evolutionarily conserved developmental pathways. However, unlike in humans, both female and male Bnc2-knockout mice exhibit distal urethral defects65. Further studies are required to resolve these interspecies differences and to address the mechanistic contribution of basonuclin 2 to urethral development.
Table 1 ∣.
Urothelial genes and proteins with roles in CAKUTs
| Gene | Protein | Phenotype | Refs |
|---|---|---|---|
| Human | |||
| BMP4 | Bone morphogenetic protein 4 | Congenital UPJO, renal hypodysplasia | 58-60 |
| BNC2 | Zinc finger protein basonuclin 2 | Bladder outlet obstruction, distal hypospadias, PUV | 64,65 |
| FOXF1 | Forkhead box protein F1 | Renal agenesis, hypospadias | 62 |
| SHH | Sonic hedgehog protein | Renal hypodysplasia, urogenital formations | 61 |
| TP63 | Tumour protein 63 | Bladder exstrophy | 69 |
| UPK2 | Uroplakin 2 | VUR | 85 |
| UPK3A | Uroplakin 3a | Renal aplasia, dysplasia, multicystic dysplastic kidney and VUR | 54,82 |
| Mouse | |||
| Bnc2 | Zinc finger protein basonuclin 2 | Distal urethral defects | 65 |
| Cldn4 | Claudin 4 | Urothelial hyperplasia resulting in ureteral atresia and hydroureteronephrosis | 86 |
| Exoc5 | Exocyst complex component 5 | Impaired ureteral urothelial differentiation, triggering fibroproliferative response and bilateral congenital UPJO | 87 |
| Shh | Sonic hedgehog protein | Bladder hypoplasia and hydroureteronephrosis | 43,56 |
| Tp63 | Tumour protein 63 | Bladder exstrophy | 67 |
| Upk1b | Uroplakin 1b | Urothelial hyperplasia without ureteral atresia, duplex kidneys, non-obstructive hydronephrosis with onset in adulthood | 71 |
| Upk2 | Uroplakin 2 | VUR, urothelial hyperplasia resulting in ureteral atresia and hydroureteronephrosis | 70 |
| Upk3a | Uroplakin 3a | Small urothelial plaque size and VUR | 73 |
CAKUTs, congenital anomalies of the kidney and urinary tract; PUV, posterior urethral valve; UPJO, ureteropelvic junction obstruction; VUR, vesicoureteral reflux.
In the bladder, evidence points to a role for the urothelium in specifying bladder wall development and formation of the bladder outlet. Tumour protein 63 (TP63) is a p53-like protein with key roles in cellular differentiation that are regulated in part through alternative splicing66. In mice, TP63 is expressed by the developing urothelium and its absence leads to apoptosis of the ventral urothelium and impaired ventral smooth-muscle development, which result in bladder exstrophy67. The suggestion from these experiments is that additional, as yet unidentified, factors specify dorsal smooth-muscle development and that TP63 links urothelial differentiation and bladder-wall differentiation, although the mechanism is unclear. Patients with bladder exstrophy exhibit dysregulated expression of alternatively spliced TP63 isoforms68, and insertions or deletions in the TP63 promoter have been identified as risk factors for bladder exstrophy69. However, further studies are needed to determine the precise contribution of TP63 to bladder exstrophy in humans.
Additional data that support a direct role for the urothelium in CAKUTs stem from studies in uroplakin-knockout mice and human patients with CAKUTs. Uroplakin-knockout mice have been extensively characterized, and the spectrum of CAKUT phenotypes in these mice ranges widely in severity and anatomical basis (TABLE 1). In Upk2−/− mice, excessive urothelial hyperplasia results in variable obliteration of the ureteral lumen, leading to azotaemia and death by around postnatal days 8–10 in some but not all strains70. The fact that this severe phenotype is not universally displayed by all Upk2−/− mice illustrates the concept that unknown genetic modifiers can influence the severity or penetrance of phenotypes arising from monogenic CAKUTs — an important consideration that might account for the heterogeneity of CAKUT phenotypes in human carriers of common causative mutations. Upk1b-knockout mice also have urothelial hyperplasia but to a lesser extent. These mice have no evidence of urinary tract obstruction or VUR but do develop hydronephrosis in early adulthood that progresses as they age. Approximately 17% of Upk1b−/− mice have duplex kidneys, suggesting a potential role for UPK1B in ureteric bud branching morphogenesis71,72. In mice, deletion of the Upk3a gene results in increased urothelial permeability to water and solutes, including urea23. These mice develop urothelial hyperplasia, small urothelial plaques, urothelial leakage and VUR73. UPK3B is a 35-kDa protein originally identified as upregulated in the bladders of Upk3a-knockout mice74. Although UPK3B is structurally related to UPK3A and capable of binding to UPK1B74, deletion of Upk3b does not impair urothelial plaque synthesis. UPK3B is considered a minor uroplakin owing to its functional redundancy with UPK3A75.
Urothelial hyperplasia occurs almost universally in uroplakin-mutant mice, which raises the question of whether an intact urothelial plaque transmits a physiological signal that suppresses urothelial hyperplasia or whether hyperplasia occurs as a result of a compromised urine permeability barrier. With regard to the potential for signalling from the urothelial plaque, multiple lines of evidence obtained in various species demonstrate that uroplakins undergo phosphorylation and can participate in intracellular signalling cascades. In mouse and frog oocytes, UPK3A transduces an extracellular signal that is required for fertilization and depends on recruitment of a tyrosine kinase to its cytoplasmic tail76-79. In zebrafish, Upk1b-like protein (an orthologue of UPK3A) is expressed by tubules in the pronephros, where it has an essential role in apical–basolateral polarization that is contingent on tyrosine phosphorylation80. When cultured human urothelial cells are exposed to the bacterial protein FimH, UPK3A undergoes serine/threonine phosphorylation of its cytoplasmic tail, an event that triggers apoptosis81. Clearly, more work is required to determine whether uroplakin heterodimers or the assembled urothelial plaque transduces a specific signal within developing urothelial cells (via phosphorylation or otherwise), but the convergence of experimental data in disparate model systems support this hypothesis. Alternatively, the absence of urothelial plaque might expose underlying quiescent urothelial cells to mitogenic proteins in the urine, leading to hyperplasia70.
The identification of mutations in uroplakin-related genes in mice with CAKUTs prompted efforts to screen patients with CAKUT for similar mutations. De novo heterozygous mutations in UPK3A were detected in 4 of 17 unrelated patients with kidney failure due to renal adysplasia54. In 2 of these individuals, a missense mutation resulted in a Pro–Leu amino acid substitution in the cytoplasmic tail of the UPK3A protein54. The other 2 patients had single-nucleotide substitutions in the 3' untranslated region of UPK3A mRNA54,82. In a follow-up study conducted in 170 patients with CAKUTs, 1 patient with a unilateral multicystic dysplastic kidney had a de novo missense mutation in UPK3A consistent with a pathogenic effect82. By contrast, genomic sequencing of 76 children with primary (non-syndromic) VUR did not identify nonsense or frameshift mutations in the four major uroplakin genes, although a single-nucleotide polymorphism in UPK3A resulting in a Pro–Ala amino acid substitution was weakly associated with VUR in this cohort83. Another study of 126 sibling pairs found no associations between VUR and markers at the UPK3A locus84. In a cohort of 42 children with a diverse spectrum of CAKUTs, a single patient who had VUR, bilateral nephropathy and renal failure was heterozygous for a frameshift mutation in UPK2, which, if expressed, would generate a truncated protein85. Taken together, these studies establish that mutations in uroplakin genes are associated with CAKUTs in rare individuals, but the pathogenic role of these mutations in CAKUT remains unclear. This lack of clarity is in part due to the paucity of knowledge regarding the prevalence of mutations in uroplakin genes in the general population.
Additional genetic studies have implicated a dysfunctional urothelium in CAKUTs. In mice, deletion of Cldn4 (which encodes claudin 4, a tight-junction protein expressed throughout the urothelium) leads to urinary-tract obstruction as a result of ureteral urothelial hyperplasia, a phenotype resembling that observed in Upk2-knockout mice70,86. However, Cldn4-knockout mice do not exhibit the defects in urothelial ultrastructure or barrier function observed in Upk2-knockout mice86. In mice, too, conditional deletion of Exoc5 (also known as Sec10) in the ureteral urothelium leads to 100% penetrance of severe, bilateral hydronephrosis and anuria caused by congenital bilateral UPJO87. Exoc5 encodes exocyst complex component 5, which serves an essential role in trafficking secretory vesicles to the plasma cell membrane88. Inactivation of Exoc5 in developing ureteral urothelial cells results in impaired umbrella-cell differentiation, urothelial-cell apoptosis and compromised urothelial barrier function. These defects in epithelial integrity drive a local wound-healing response characterized by increased production of transforming growth factor-β, which drives myofibroblast activation. In turn, myofibroblast activation leads to an uncontrolled fibroproliferative response that ultimately obliterates the ureteral lumen and causes bilateral UPJO89. Altogether, these mouse studies indicate that defects in urothelial proteins are sufficient to cause congenital urinary-tract obstruction. In some patients with congenital UPJO, urothelial hyperplasia is a prominent finding, highlighting its potential role in the pathogenesis of UPJO in children90-92. Further studies are required to determine whether abnormal urothelial differentiation accounts for UPJO in such individuals and whether mutations in CLDN4 or EXOC5 occur in humans with CAKUTs.
Intrarenal urothelium in CAKUTs
The intrarenal urothelium lining the renal inner medulla and papilla is morphologically distinct from that lining the renal pelvis and ureter, in that it exhibits limited expression of uroplakin genes and lacks ultrastructural evidence of urothelial plaque93. In the megabladder mouse model of congenital urinary tract obstruction (these mice are homozygous for a gene translocation in which a fragment of chromosome 16 is inserted into chromosome 11), the intrarenal urothelium undergoes initial proliferation followed by stratification and differentiation, such that apical cells acquire expression of uroplakin genes and begin to produce urothelial plaque94,95 (FIG. 3). These morphological changes are associated with the induction of genes expressed uniquely by terminally differentiated umbrella cells, encoding adherens junction proteins, proteins involved in urothelial plaque synthesis and urothelium-specific transcription factors. Similar intrarenal changes occur in adult mice following unilateral ureteral obstruction95-97. When urothelial plaque synthesis is inhibited, megabladder mice develop an accelerated onset of severe, bilateral hydronephrosis and die during adolescence with biochemical evidence of renal failure95. These findings suggest that morphological alterations in the intrarenal urothelium, and changes in urothelial plaque production in particular, serve essential roles in maintaining renal structure and function during congenital urinary-tract obstruction. Further studies are needed to identify the cellular and molecular mechanisms that drive intrarenal urothelial remodelling and to devise pharmacological strategies to promote this process, with the aim of attenuating congenital obstructive nephropathy.
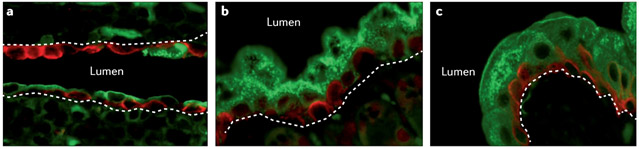
Figure 3. Intrarenal urothelial remodelling in the setting of congenital urinary tract obstruction.
Intrarenal urothelium in the normal adult mouse consists of one to two layers of interspersed cells expressing uroplakin 3A (green) and keratin 5 (red) (part a)93,95. In the megabladder mouse model of congenital urinary tract obstruction, a marked uniform apical redistribution of uroplakin-expressing cells to the intrarenal urothelium occurs (part b) to the extent that it resembles bladder urothelium (part c)94,95. The position of the urothelial basement membrane is indicated by white dashed lines.
Urothelium-specific biomarkers
Given the emerging evidence of the importance of the urothelium during CAKUTs, researchers have focused on the development of urothelium-specific radiological and biochemical markers as a non-invasive means of CAKUT detection and diagnosis. These biomarkers might also aid in predicting prognosis and in directing appropriate selection of medical and/or surgical interventions. For all potential biomarkers considered to date, further studies are required to independently validate their utility before they can be used in the clinic.
Radiological markers.
Renal pelvic uroepithelial thickening (UET) is a common ultrasound finding that has been detected in patients with urinary tract infection (UTI), inflammation, urolithiasis and renal transplant rejection98-102. Scrutiny of its clinical meaning in children with possible CAKUTs has identified an association between UET and dilating VUR in two groups of patients: in those with prenatal hydronephrosis, a renal ultrasound finding of UET improved the sensitivity and specificity of this technique for identifying high-grade VUR (when present in combination with other previously defined renal ultrasound abnormalities such as hydroureter and renal dysmorphia)103. In children with a first febrile UTI, a renal ultrasound finding of UET similarly improved the sensitivity of this technique when used as a screening investigation to determine which children needed to undergo the more invasive investigation voiding cystourethrography to diagnose dilating VUR104. The value of detecting UET was evident even in children with a febrile UTI under 2 months of age, among whom UET was strongly associated with detection of grade 4–5 VUR105.
The anatomical basis for a radiological finding of UET is unclear in the setting of high-grade VUR, as high grades of VUR are clinically more commonly associated with an underlying renal parenchymal abnormality than with UET. Extensive urothelial injury and inflammation are seen in mouse models of reflux-associated pyelonephritis106, and a similar pathological response in infants with dilating VUR and febrile UTI could lead to local oedema and radiological UET. To our knowledge, no studies have evaluated the association between UET and renal scarring to date. A major limitation of clinical studies of UET lies in their retrospective design103,104. Future, prospective, multicentre studies are required to determine the predictive value of UET in CAKUTs.
Urinary biomarkers.
The search for possible urothelial markers of CAKUTs has considered various urine biomarkers. A study of urothelium from children with congenital UPJO identified increased infiltration of eosinophils and degranulation of mast cells, accompanied by increased expression of IL5 and CCL11 (encoding eotaxin). Urine from the obstructed kidney contained increased levels of IL-5 and eotaxin. In addition, elevated IL5 expression and increased cytoplasmic IL-5 levels in all urothelial cell types was associated with impaired function on diuretic renography91. Patients with UPJO (but not healthy controls) also showed increased IL-5 levels in smooth-muscle and inflammatory cells.
Our group has similarly evaluated urothelial markers such as antimicrobial peptides in children undergoing surgical intervention for UPJO. Urinary levels of two urothelium-specific antimicrobial peptides, namely regenerating islet-derived protein 3α (also known as hepato-intestinal pancreatic protein) and cathelicidin antimicrobial peptide (also known as LL-37), were markedly elevated in patients with clinically significant UPJO compared with their levels in healthy controls. Area under the curve plots suggested that the sensitivity and specificity of LL-37 for obstruction were in excess of 70% at an optimized threshold value of 3.3 ng/mg creatinine107. However, further prospective studies are required to validate these findings. In addition, the predictive power of these urothelial peptides might be confounded by their broad induction in response to urothelial injury in the setting of infection and inflammation108-110.
Conclusions
The urothelium is instrumental in patterning the development of the normal urinary tract, and mounting evidence suggests that urothelial abnormalities have a causal relationship with CAKUTs. As the search for additional genes associated with CAKUTs continues, we predict that some genes specifically expressed in the urothelium will have causal associations with CAKUTs and serve as genetic modifiers of CAKUT phenotypes. Additionally, alterations in urothelial composition associated with congenital urinary-tract obstruction are now known to promote structural stability and preserve renal function. Strategies to promote these stabilizing features of the urothelium could have therapeutic value in mitigating parenchymal injury in patients with urinary tract obstruction. Last, prospective studies are warranted to evaluate the roles of urothelial biomarkers in assisting with accurate diagnosis of CAKUTs and predicting the prognosis of affected patients.
Key points.
Interactions between the developing urothelium and its underlying mesenchyme serve critical roles in developmental patterning of the bladder and ureter.
Genetic disruption of signaling pathways that mediate terminal differentiation of urothelium and urothelial-mesenchymal interactions results in CAKUT phenotypes in humans and mice.
The urothelial plaque serves a critical protective role in response to congenital urinary tract obstruction.
Urothelium-specific radiological and biochemical markers represent potential means to detect CAKUT, but prospective studies are warranted to evaluate their diagnostic accuracy.
Acknowledgements
The authors’ research work is supported by National Institutes of Health grants F32DK115085 (A.R.J.), K08-DK122119 (C.B.C.), R01DK085242 (K.M.M.) and K08-DK102594 (B.B.). B.B. is also supported by a Norman Siegel Research Scholar Grant from the American Society of Nephrology Foundation for Kidney Research.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Murugapoopathy V & Gupta IR A Primer on Congenital Anomalies of the Kidneys and Urinary Tracts (CAKUT). Clinical journal of the American Society of Nephrology : CJASN 15, 723–731, doi: 10.2215/CJN.12581019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanna-Cherchi S, Westland R, Ghiggeri GM & Gharavi AG Genetic basis of human congenital anomalies of the kidney and urinary tract. The Journal of clinical investigation 128, 4–15, doi: 10.1172/JCI95300 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birth Defects Monitoring Program (BDMP)/Commission on Professional and Hospital Activities (CPHA) surveillance data, 1988-1991. Teratology 48, 658–675, doi: 10.1002/tera.1420480608 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Garne E, Dolk H, Loane M, Boyd PA & Eurocat. EUROCAT website data on prenatal detection rates of congenital anomalies. J Med Screen 17, 97–98, doi: 10.1258/jms.2010.010050 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Metropolitan Atlanta Congenital Defects Program surveillance data, 1988-1991. Teratology 48, 695–709, doi: 10.1002/tera.1420480610 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Schulman J, Edmonds LD, McClearn AB, Jensvold N & Shaw GM Surveillance for and comparison of birth defect prevalences in two geographic areas--United States, 1983-88. MMWR CDC Surveill Summ 42, 1–7 (1993). [PubMed] [Google Scholar]
- 7.Postoev VA et al. Congenital anomalies of the kidney and the urinary tract: A murmansk county birth registry study. Birth defects research. Part A, Clinical and molecular teratology 106, 185–193, doi: 10.1002/bdra.23475 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Tain YL, Luh H, Lin CY & Hsu CN Incidence and Risks of Congenital Anomalies of Kidney and Urinary Tract in Newborns: A Population-Based Case-Control Study in Taiwan. Medicine (Baltimore) 95, e2659, doi: 10.1097/MD.0000000000002659 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harambat J, van Stralen KJ, Kim JJ & Tizard EJ Epidemiology of chronic kidney disease in children. Pediatric nephrology 27, 363–373, doi: 10.1007/s00467-011-1939-1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanna-Cherchi S et al. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int 76, 528–533, doi: 10.1038/ki.2009.220 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Wuhl E et al. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clinical journal of the American Society of Nephrology : CJASN 8, 67–74, doi: 10.2215/CJN.03310412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderon-Margalit R et al. History of Childhood Kidney Disease and Risk of Adult End-Stage Renal Disease. The New England journal of medicine 378, 428–438, doi: 10.1056/NEJMoa1700993 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Chesnaye N et al. Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERA-EDTA registry. Pediatric nephrology 29, 2403–2410, doi: 10.1007/s00467-014-2884-6 (2014). [DOI] [PubMed] [Google Scholar]
- 14.van der Ven AT, Vivante A & Hildebrandt F Novel Insights into the Pathogenesis of Monogenic Congenital Anomalies of the Kidney and Urinary Tract. Journal of the American Society of Nephrology : JASN 29, 36–50, doi: 10.1681/ASN.2017050561 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu CW, Yamamoto KT, Henry RK, De Roos AJ & Flynn JT Prenatal risk factors for childhood CKD. Journal of the American Society of Nephrology : JASN 25, 2105–2111, doi: 10.1681/ASN.2013060582 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dart AB, Ruth CA, Sellers EA, Au W & Dean HJ Maternal diabetes mellitus and congenital anomalies of the kidney and urinary tract (CAKUT) in the child. American journal of kidney diseases : the official journal of the National Kidney Foundation 65, 684–691, doi: 10.1053/j.ajkd.2014.11.017 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Parikh CR, McCall D, Engelman C & Schrier RW Congenital renal agenesis: case-control analysis of birth characteristics. American journal of kidney diseases : the official journal of the National Kidney Foundation 39, 689–694, doi: 10.1053/ajkd.2002.31982 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Pryde PG, Sedman AB, Nugent CE & Barr M Jr. Angiotensin-converting enzyme inhibitor fetopathy. Journal of the American Society of Nephrology : JASN 3, 1575–1582 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Wu XR, Kong XP, Pellicer A, Kreibich G & Sun TT Uroplakins in urothelial biology, function, and disease. Kidney Int 75, 1153–1165, doi: 10.1038/ki.2009.73 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acharya P et al. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, −8, and −12 in bladder epithelium. American journal of physiology. Renal physiology 287, F305–318, doi: 10.1152/ajprenal.00341.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Lavelle J et al. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. American journal of physiology. Renal physiology 283, F242–253, doi: 10.1152/ajprenal.00307.2001 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Smith NJ et al. The human urothelial tight junction: claudin 3 and the ZO-1alpha(+) switch. Bladder (San Franc) 2, e9, doi: 10.14440/bladder.2015.33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu P et al. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283, F1200–1207, doi: 10.1152/ajprenal.00043.2002 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Mathai JC et al. Hypercompliant apical membranes of bladder umbrella cells. Biophys J 107, 1273–1279, doi: 10.1016/j.bpj.2014.07.047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truschel ST et al. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Molecular biology of the cell 13, 830–846, doi: 10.1091/mbc.01-09-0435 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill L, Gonzalez EJ, Girard BM & Vizzard MA Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol 13, 193–204, doi: 10.1038/nrurol.2016.13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohnenpoll T et al. Diversification of Cell Lineages in Ureter Development. J Am Soc Nephrol 28, 1792–1801, doi: 10.1681/ASN.2016080849 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson AR et al. Krt5(+) urothelial cells are developmental and tissue repair progenitors in the kidney. Am J Physiol Renal Physiol 317, F757–F766, doi: 10.1152/ajprenal.00171.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenwolf GC, Bleyl SB, Brauer PR & Francis-West PH Larsen's human embryology. Fifth edition. edn, (Elsevier/Churchill Livingstone, 2015). [Google Scholar]
- 30.Gandhi D et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell 26, 469–482, doi: 10.1016/j.devcel.2013.07.017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC & Hultgren SJ Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe 5, 463–475, doi: 10.1016/j.chom.2009.04.005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tash JA, David SG, Vaughan EE & Herzlinger DA Fibroblast growth factor-7 regulates stratification of the bladder urothelium. J Urol 166, 2536–2541 (2001). [PubMed] [Google Scholar]
- 33.Papafotiou G et al. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun 7, 11914, doi: 10.1038/ncomms11914 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faa G et al. Morphogenesis and molecular mechanisms involved in human kidney development. Journal of cellular physiology 227, 1257–1268, doi: 10.1002/jcp.22985 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Michos O Kidney development: from ureteric bud formation to branching morphogenesis. Curr Opin Genet Dev 19, 484–490, doi: 10.1016/j.gde.2009.09.003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baskin LS, Hayward SW, Young P & Cunha GR Role of mesenchymal-epithelial interactions in normal bladder development. The Journal of urology 156, 1820–1827 (1996). [PubMed] [Google Scholar]
- 37.Cao M, Liu B, Cunha G & Baskin L Urothelium patterns bladder smooth muscle location. Pediatric research 64, 352–357, doi: 10.1203/PDR.0b013e318180e4c9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiroyanagi Y et al. Urothelial sonic hedgehog signaling plays an important role in bladder smooth muscle formation. Differentiation; research in biological diversity 75, 968–977, doi: 10.1111/j.1432-0436.2007.00187.x (2007). [DOI] [PubMed] [Google Scholar]
- 39.Jenkins D, Winyard PJ & Woolf AS Immunohistochemical analysis of Sonic hedgehog signalling in normal human urinary tract development. Journal of anatomy 211, 620–629, doi: 10.1111/j.1469-7580.2007.00808.x (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao M et al. Urothelium-derived Sonic hedgehog promotes mesenchymal proliferation and induces bladder smooth muscle differentiation. Differentiation; research in biological diversity 79, 244–250, doi: 10.1016/j.diff.2010.02.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeSouza KR, Saha M, Carpenter AR, Scott M & McHugh KM Analysis of the Sonic Hedgehog signaling pathway in normal and abnormal bladder development. PLoS One 8, e53675, doi: 10.1371/journal.pone.0053675 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng W et al. Sonic Hedgehog mediator Gli2 regulates bladder mesenchymal patterning. The Journal of urology 180, 1543–1550, doi: 10.1016/j.juro.2008.06.003 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Carroll TJ & McMahon AP Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129, 5301–5312 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Bohnenpoll T et al. A SHH-FOXF1-BMP4 signaling axis regulating growth and differentiation of epithelial and mesenchymal tissues in ureter development. PLoS Genet 13, e1006951, doi: 10.1371/journal.pgen.1006951 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mamo TM et al. BMP4 uses several different effector pathways to regulate proliferation and differentiation in the epithelial and mesenchymal tissue compartments of the developing mouse ureter. Hum Mol Genet 26, 3553–3563, doi: 10.1093/hmg/ddx242 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Cain JE, Islam E, Haxho F, Blake J & Rosenblum ND GLI3 repressor controls functional development of the mouse ureter. The Journal of clinical investigation 121, 1199–1206, doi: 10.1172/JCI45523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.David SG, Cebrian C, Vaughan ED Jr. & Herzlinger D c-kit and ureteral peristalsis. J Urol 173, 292–295, doi: 10.1097/01.ju.0000141594.99139.3d (2005). [DOI] [PubMed] [Google Scholar]
- 48.Iskander SM, Feeney MM, Yee K & Rosenblum ND Protein Kinase 2beta Is Expressed in Neural Crest-Derived Urinary Pacemaker Cells and Required for Pyeloureteric Contraction. Journal of the American Society of Nephrology : JASN 29, 1198–1209, doi: 10.1681/ASN.2017090951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feeney MM & Rosenblum ND Urinary tract pacemaker cells: current knowledge and insights from nonrenal pacemaker cells provide a basis for future discovery. Pediatric nephrology 29, 629–635, doi: 10.1007/s00467-013-2631-4 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Sheybani-Deloui S et al. Activated Hedgehog-GLI Signaling Causes Congenital Ureteropelvic Junction Obstruction. Journal of the American Society of Nephrology : JASN 29, 532–544, doi: 10.1681/ASN.2017050482 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trowe MO et al. Canonical Wnt signaling regulates smooth muscle precursor development in the mouse ureter. Development 139, 3099–3108, doi: 10.1242/dev.077388 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Aydogdu N et al. TBX2 and TBX3 act downstream of canonical WNT signaling in patterning and differentiation of the mouse ureteric mesenchyme. Development 145, doi: 10.1242/dev.171827 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Zupancic D & Romih R Heterogeneity of uroplakin localization in human normal urothelium, papilloma and papillary carcinoma. Radiol Oncol 47, 338–345, doi: 10.2478/raon-2013-0052 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenkins D et al. De novo Uroplakin IIIa heterozygous mutations cause human renal adysplasia leading to severe kidney failure. J Am Soc Nephrol 16, 2141–2149, doi: 10.1681/ASN.2004090776 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Riedel I et al. Urothelial umbrella cells of human ureter are heterogeneous with respect to their uroplakin composition: different degrees of urothelial maturity in ureter and bladder? Eur J Cell Biol 84, 393–405, doi: 10.1016/j.ejcb.2004.12.011 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Haraguchi R et al. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development 134, 525–533, doi: 10.1242/dev.02736 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Haraguchi R et al. The hedgehog signal induced modulation of bone morphogenetic protein signaling: an essential signaling relay for urinary tract morphogenesis. PLoS One 7, e42245, doi: 10.1371/journal.pone.0042245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He JL et al. Mutation screening of BMP4 and Id2 genes in Chinese patients with congenital ureteropelvic junction obstruction. Eur J Pediatr 171, 451–456, doi: 10.1007/s00431-011-1561-z (2012). [DOI] [PubMed] [Google Scholar]
- 59.Reis GS et al. Study of the association between the BMP4 gene and congenital anomalies of the kidney and urinary tract. J Pediatr (Rio J) 90, 58–64, doi: 10.1016/j.jped.2013.06.004 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Weber S et al. SIX2 and BMP4 mutations associate with anomalous kidney development. Journal of the American Society of Nephrology : JASN 19, 891–903, doi: 10.1681/ASN.2006111282 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubourg C et al. Molecular screening of SHH, ZIC2, SIX3, and TGIF genes in patients with features of holoprosencephaly spectrum: Mutation review and genotype-phenotype correlations. Hum Mutat 24, 43–51, doi: 10.1002/humu.20056 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Hilger AC et al. Targeted Resequencing of 29 Candidate Genes and Mouse Expression Studies Implicate ZIC3 and FOXF1 in Human VATER/VACTERL Association. Hum Mutat 36, 1150–1154, doi: 10.1002/humu.22859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Ven AT et al. Whole-Exome Sequencing Identifies Causative Mutations in Families with Congenital Anomalies of the Kidney and Urinary Tract. Journal of the American Society of Nephrology : JASN 29, 2348–2361, doi: 10.1681/ASN.2017121265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolvenbach CM et al. Rare Variants in BNC2 Are Implicated in Autosomal-Dominant Congenital Lower Urinary-Tract Obstruction. Am J Hum Genet 104, 994–1006, doi: 10.1016/j.ajhg.2019.03.023 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhoj EJ et al. Human balanced translocation and mouse gene inactivation implicate Basonuclin 2 in distal urethral development. Eur J Hum Genet 19, 540–546, doi: 10.1038/ejhg.2010.245 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang A et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Molecular cell 2, 305–316, doi: 10.1016/s1097-2765(00)80275-0 (1998). [DOI] [PubMed] [Google Scholar]
- 67.Cheng W et al. DeltaNp63 plays an anti-apoptotic role in ventral bladder development. Development 133, 4783–4792, doi: 10.1242/dev.02621 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Ching BJ et al. p63 (TP73L) a key player in embryonic urogenital development with significant dysregulation in human bladder exstrophy tissue. Int J Mol Med 26, 861–867, doi: 10.3892/ijmm_00000535 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Wilkins S et al. Insertion/deletion polymorphisms in the DeltaNp63 promoter are a risk factor for bladder exstrophy epispadias complex. PLoS Genet 8, e1003070, doi: 10.1371/journal.pgen.1003070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kong XT et al. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol 167, 1195–1204, doi: 10.1083/jcb.200406025 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carpenter AR et al. Uroplakin 1b is critical in urinary tract development and urothelial differentiation and homeostasis. Kidney Int 89, 612–624, doi: 10.1016/j.kint.2015.11.017 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carpenter AR & McHugh KM Role of renal urothelium in the development and progression of kidney disease. Pediatric nephrology 32, 557–564, doi: 10.1007/s00467-016-3385-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu P et al. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol 151, 961–972 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng FM et al. Uroplakin IIIb, a urothelial differentiation marker, dimerizes with uroplakin Ib as an early step of urothelial plaque assembly. J Cell Biol 159, 685–694, doi: 10.1083/jcb.200204102 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rudat C et al. Upk3b is dispensable for development and integrity of urothelium and mesothelium. PloS one 9, e112112, doi: 10.1371/journal.pone.0112112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liao Y et al. Uroplakins play conserved roles in egg fertilization and acquired additional urothelial functions during mammalian divergence. Mol Biol Cell, mbcE18080496, doi: 10.1091/mbc.E18-08-0496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahbub Hasan AK et al. The egg membrane microdomain-associated uroplakin III-Src system becomes functional during oocyte maturation and is required for bidirectional gamete signaling at fertilization in Xenopus laevis. Development 141, 1705–1714, doi: 10.1242/dev.105510 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Mahbub Hasan AK et al. Uroplakin III, a novel Src substrate in Xenopus egg rafts, is a target for sperm protease essential for fertilization. Dev Biol 286, 483–492, doi: 10.1016/j.ydbio.2005.08.020 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Sakakibara K et al. Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization. J Biol Chem 280, 15029–15037, doi: 10.1074/jbc.M410538200 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Mitra S et al. Requirement for a uroplakin 3a-like protein in the development of zebrafish pronephric tubule epithelial cell function, morphogenesis, and polarity. PLoS One 7, e41816, doi: 10.1371/journal.pone.0041816 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thumbikat P et al. Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog 5, e1000415, doi: 10.1371/journal.ppat.1000415 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schonfelder EM et al. Mutations in Uroplakin IIIA are a rare cause of renal hypodysplasia in humans. American journal of kidney diseases : the official journal of the National Kidney Foundation 47, 1004–1012, doi: 10.1053/j.ajkd.2006.02.177 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Jiang S et al. Lack of major involvement of human uroplakin genes in vesicoureteral reflux: implications for disease heterogeneity. Kidney Int 66, 10–19, doi: 10.1111/j.1523-1755.2004.00703.x (2004). [DOI] [PubMed] [Google Scholar]
- 84.Kelly H et al. Uroplakin III is not a major candidate gene for primary vesicoureteral reflux. Eur J Hum Genet 13, 500–502, doi: 10.1038/sj.ejhg.5201322 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Jenkins D et al. Mutation analyses of Uroplakin II in children with renal tract malformations. Nephrol Dial Transplant 21, 3415–3421, doi: 10.1093/ndt/gfl465 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Fujita H, Hamazaki Y, Noda Y, Oshima M & Minato N Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PLoS One 7, e52272, doi: 10.1371/journal.pone.0052272 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fogelgren B et al. Urothelial Defects from Targeted Inactivation of Exocyst Sec10 in Mice Cause Ureteropelvic Junction Obstructions. PloS one 10, e0129346, doi: 10.1371/journal.pone.0129346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin-Urdiroz M, Deeks MJ, Horton CG, Dawe HR & Jourdain I The Exocyst Complex in Health and Disease. Front Cell Dev Biol 4, 24, doi: 10.3389/fcell.2016.00024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee AJ et al. Fibroproliferative response to urothelial failure obliterates the ureter lumen in a mouse model of prenatal congenital obstructive nephropathy. Sci Rep 6, 31137, doi: 10.1038/srep31137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hou T et al. Aberrant differentiation of urothelial cells in patients with ureteropelvic junction obstruction. Int J Clin Exp Pathol 7, 5837–5845 (2014). [PMC free article] [PubMed] [Google Scholar]
- 91.Chiou YY, Shieh CC, Cheng HL & Tang MJ Intrinsic expression of Th2 cytokines in urothelium of congenital ureteropelvic junction obstruction. Kidney Int 67, 638–646, doi: 10.1111/j.1523-1755.2005.67120.x (2005). [DOI] [PubMed] [Google Scholar]
- 92.Huang WY, Olumi AF & Rosen S Urothelial mucosal malformation: a rare cause for ureteropelvic junction obstruction. Pediatr Dev Pathol 9, 72–74, doi: 10.2350/06-05-0071.1 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Romih R, Korosec P, de Mello W Jr. & Jezernik K Differentiation of epithelial cells in the urinary tract. Cell Tissue Res 320, 259–268, doi: 10.1007/s00441-004-1005-4 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Becknell B et al. Molecular basis of renal adaptation in a murine model of congenital obstructive nephropathy. PLoS One 8, e72762, doi: 10.1371/journal.pone.0072762 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jackson AR et al. The uroplakin plaque promotes renal structural integrity during congenital and acquired urinary tract obstruction. Am J Physiol Renal Physiol 315, F1019–F1031, doi: 10.1152/ajprenal.00173.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Girshovich A et al. Ureteral obstruction promotes proliferation and differentiation of the renal urothelium into a bladder-like phenotype. Kidney Int 82, 428–435, doi: 10.1038/ki.2012.110 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Chen WY et al. IL-33/ST2 axis mediates hyperplasia of intrarenal urothelium in obstructive renal injury. Exp Mol Med 50, 36, doi: 10.1038/s12276-018-0047-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sorantin E, Fotter R, Aigner R, Ring E & Riccabona M The sonographically thickened wall of the upper urinary tract system: correlation with other imaging methods. Pediatric radiology 27, 667–671, doi: 10.1007/s002470050208 (1997). [DOI] [PubMed] [Google Scholar]
- 99.Tain YL Renal pelvic wall thickening in childhood urinary tract infections--evidence of acute pyelitis or vesicoureteral reflux? Scand J Urol Nephrol 37, 28–30, doi: 10.1080/00365590310008640 (2003). [DOI] [PubMed] [Google Scholar]
- 100.Nicolet V et al. Thickening of the renal collecting system: a nonspecific finding at US. Radiology 168, 411–413, doi: 10.1148/radiology.168.2.3293110 (1988). [DOI] [PubMed] [Google Scholar]
- 101.Birnholz JC & Merkel FK Submucosal edema of the collecting system: a new ultrasonic sign of severe, acute renal allograft rejection. A clinical note. Radiology 154, 190, doi: 10.1148/radiology.154.1.3880604 (1985). [DOI] [PubMed] [Google Scholar]
- 102.Avni EF et al. US demonstration of pyelitis and ureteritis in children. Pediatric radiology 18, 134–139 (1988). [DOI] [PubMed] [Google Scholar]
- 103.Gordon ZN et al. Uroepithelial thickening improves detection of vesicoureteral reflux in infants with prenatal hydronephrosis. J Pediatr Urol 12, 257 e251–257, doi: 10.1016/j.jpurol.2016.04.018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gordon ZN, McLeod DJ, Becknell B, Bates DG & Alpert SA Uroepithelial Thickening on Sonography Improves Detection of Vesicoureteral Reflux in Children with First Febrile Urinary Tract Infection. J Urol 194, 1074–1079, doi: 10.1016/j.juro.2015.05.001 (2015). [DOI] [PubMed] [Google Scholar]
- 105.Wallace SS et al. Renal Ultrasound for Infants Younger Than 2 Months With a Febrile Urinary Tract Infection. AJR Am J Roentgenol 205, 894–898, doi: 10.2214/AJR.15.14424 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Li B et al. Inflammation drives renal scarring in experimental pyelonephritis. Am J Physiol Renal Physiol 312, F43–F53, doi: 10.1152/ajprenal.00471.2016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gupta S et al. Urinary antimicrobial peptides: Potential novel biomarkers of obstructive uropathy. J Pediatr Urol 14, 238 e231–238 e236, doi: 10.1016/j.jpurol.2018.03.006 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Chromek M et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 12, 636–641, doi: 10.1038/nm1407 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Makino T, Kawashima H, Konishi H, Nakatani T & Kiyama H Elevated urinary levels and urothelial expression of hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein in patients with interstitial cystitis. Urology 75, 933–937, doi: 10.1016/j.urology.2009.05.044 (2010). [DOI] [PubMed] [Google Scholar]
- 110.Spencer JD et al. Expression and Significance of the HIP/PAP and RegIIIgamma Antimicrobial Peptides during Mammalian Urinary Tract Infection. PLoS One 10, e0144024, doi: 10.1371/journal.pone.0144024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]