Abstract
A man in his 70s with a complex medical history, including cadaveric renal transplant, presented with recurrent urinary tract infections. Investigation revealed recurrent urinary pathogens, including Enterobacter cloacae and persistent BK viruria. Cystoscopy revealed a pedunculated mass in the right posterior–lateral wall, inferior to the transplant urethral orifice, and biopsy of this mass showed invasive small cell carcinoma with a prominent adenocarcinoma component. The tumour was treated with complete transurethral resection followed by carboplatin, etoposide and radiation. Laboratory analysis of biopsied samples showed immunostaining and molecular evidence of BK virus DNA in the cancer cells. Follow-up cystoscopies have shown no recurrence of the cancer.
Keywords: Malignant disease and immunosuppression, Infections, Urological cancer, Carcinogenesis
Background
BK virus is a common polyomavirus that disproportionately reactivates in transplant recipients and can cause nephropathy, allograft dysfunction or rejection, and rarely haemorrhagic cystitis. There is a growing body of cases reported in the literature showing an association between persistent BK viruria in transplant recipients and urothelial carcinoma. Our clinical report is unique and provides the first description of small cell carcinoma with foci of adenocarcinoma of the bladder in the setting of persistent BK virus infection after solid organ transplantation.
Beyond offering this new association, we investigated for a possible mechanism of oncogenesis related to BK virus. We present immunohistological and molecular evidence from our patient’s tumour that aligns with this oncogenic mechanism. This represents the first compelling evidence for a role for BK virus in the pathogenesis of our patient’s unusual malignancy.
We wish to offer our experience to alert providers of patients with renal transplants of the potential neoplastic consequences of BK virus reactivation, that may become more prevalent with increasing utilisation of solid organ transplantation.
Case presentation
A man in his 70s with hypertension, hyperlipidaemia, diabetes mellitus, diabetic neuropathy and end-stage diabetic renal disease initially received haemodialysis. He eventually underwent a cadaveric renal transplant and was placed on immunosuppressive therapy of mycophenolate 750 mg two times per day, tacrolimus 1 mg two times per day and leflunomide 10 mg once daily.
Post transplant, he suffered from recurrent bacterial urinary tract infections (UTIs) with a variety of pathogens, predominantly Enterobacter cloacae. Urinary and plasma BK virus loads were as high as 5.0×108 copies/mL and 3.26×104 copies/mL respectively.
Investigations
His recurrent UTIs were investigated by cystoscopy that revealed a pedunculated mass in the right posterior–lateral wall, inferior to the transplant urethral orifice. Biopsy of the resected tumour revealed invasive small cell carcinoma with a prominent adenocarcinoma component (figure 1).
Figure 1.
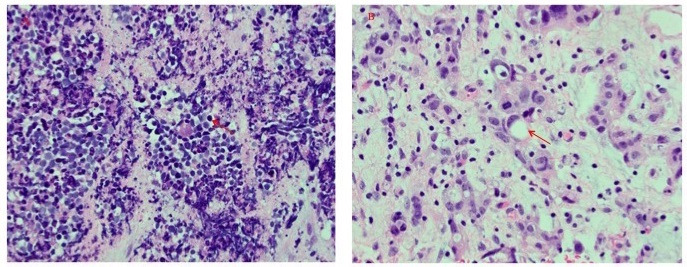
H&E stains of bladder neoplasm (400×). Histologic features of small cell carcinoma (panel (A)) and adenocarcinoma (panel (B)) were both detected. Representative neoplastic cells are labelled (arrows).
In the course of tumour staging, CT scans of the chest and abdomen and positron emission tomography detected no evidence of malignant foci elsewhere. However, the CT scan of the chest revealed a right upper lobe cavitary lesion. Bronchoscopic biopsy confirmed a non-invasive aspergilloma and no evidence of malignancy.
The bladder biopsy sample was fixed in 10% formalin, routinely processed and embedded, and stained by H&E.
Immunohistochemistry
An immunohistochemical (IHC) assay was performed by procedures that we have previously detailed,1 using a monoclonal antibody targeting the large T antigen (LTA) of SV40 polyomavirus (Calbiochem/EMD Milipore, https://www.emdmillipore.com, clone PAb416). IHC was performed on 4 µm sections of the formalin-fixed, paraffin-embedded (FFPE) biopsy tissue. The positive control consisted of FFPE cells infected with SV40 polyomavirus.
Molecular analysis
DNA was extracted from the FFPE bladder biopsy tissue section using the QIAamp DNA Mini Kit (Qiagen, Valencia, California, USA) following the tissue extraction protocol. DNA sample was evaluated by a human polyomavirus-specific conventional PCR assay targeting the VP2 gene using the High Fidelity PCR Kit (Roche Diagnostics, Indianapolis, Indiana, USA) and previously published primers.2
PCR amplicons were visualised by running on a 2% agarose gel containing ethidium bromide and the PCR amplicons were directly sequenced on a GenomeLab GeXP Genetic Analysis System (AB SCIEX, LLC, Redwood City, California, USA). The search for homologies to known sequences was done using the Basic Local Alignment Search Tool at http://wwwncbinlmnihgov/BLAST.
To evaluate the quality of DNA extracts and assess the level of fragmentation, we also tested the sample by a duplex human house-keeping genes PCR assay targeting the glyceraldehyde-3-phosphate dehydrogenase and β-globin genes.3 4
| Primer sequences for human polyomavirus PCR2: | |
| BJS-FP | GGG GAC CTA RTT GCY AST GT |
| BJS-RP | GCA ASR GAT GCA AKT TSMAC |
| IUPAC ambiguity codes: R=AG; Y=CT; K=GT; S=CG; W=AT; M=AC | |
Treatment
He underwent complete transurethral resection of the bladder tumour followed by 2 cycles, at 3-week intervals, of carboplatin (170 mg), etoposide (3 consecutive days per cycle; 130 mg for 1st cycle and 100 mg for 2nd cycle) and radiation. Treatment was limited to two cycles because of instability in renal function and severe neutropenia. Both were transient. Subsequent cystoscopies detected no recurrence of the tumour.
A wedge resection of the lung aspergilloma, discovered on staging, was performed due to haemoptysis.
To control the BK viruria, tacrolimus was switched to cyclosporine 50 mg two times per day followed by further dosage adjustment. A decrease in viral load resulted with repeat concentrations of <500 copies/mL in the plasma. A timeline of events and associated BK viruria/viraemia is shown in figure 2.
Figure 2.
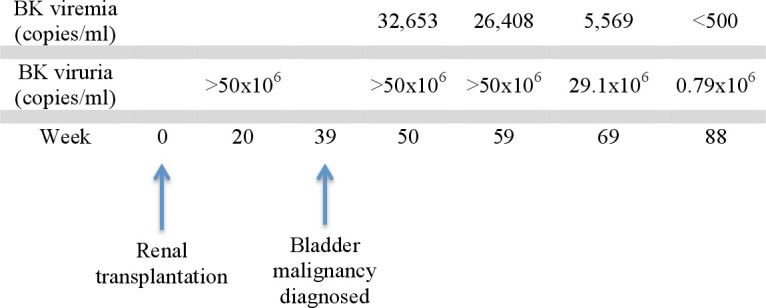
Timeline of events. Eventual decline in BK viruria occurred with modification of our patient’s immunosuppressive regimen.
Outcome and follow-up
Immunohistochemistry
Immunostaining was performed to investigate for the presence of BK virus in the bladder tumour. Although the underlying cells were distorted by crush artefact, IHC staining detected strong focal nuclear positivity of the LTA in the epithelium of the biopsied mass (figure 3).
Figure 3.
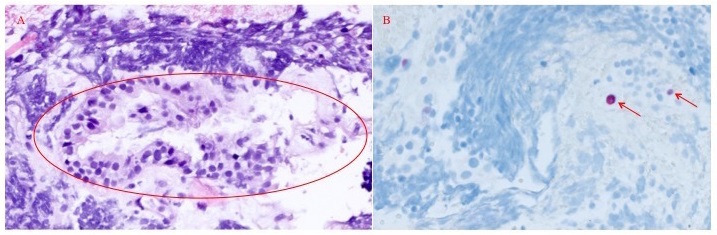
H&E stain (100×) of bladder mass biopsy from the area of polyoma immunostaining (panel (A)). Circled area corresponds to the area of immunohistochemical staining for polyoma virus (panel (B), 100×). Positive staining of nuclei is shown with red arrows.
Molecular analysis
DNA extracted from the FFPE bladder biopsy tissue specimen was positive by the human polyomavirus PCR assay. The sequence analysis of the 113 bp positive amplicon showed highest (99%) nucleotide sequence identities with BK polyomavirus (BKpV).
Clinical follow-up
Repeat surveillance cystoscopies detected no recurrence of his bladder malignancy over the next 3 years. Our patient remains well with greatly improved quality of life.
Discussion
BKpV infects as much as 90% of the world’s population by adulthood.5 After initial infection, the virus typically persists asymptomatically in the kidney or urothelium of the bladder unless reactivated.6 With common immunosuppressive regimens, transplant recipients are particularly vulnerable to reactivation, which can cause haemorrhagic cystitis in bone marrow transplant recipients and ureteral stenosis and interstitial nephritis in kidney transplant recipients, thus jeopardising the viability of the transplanted kidney.7 8 Less well-known associations of BKpV reactivation are the development of urothelial cancers.9
Even in the absence of BKpV, bladder cancer is threefold–fourfold more common and more severe in patients with renal transplants, with rapid progression and poorer response to treatment.5 7 However, this increased susceptibility to bladder cancer is even greater in the presence of polyomavirus. Liu et al showed that bladder carcinoma in patients with transplants with polyomavirus replication had a relative risk of 11.9 compared with transplant recipients without active virus (p=0.0014).10 Further study of six bladder tumours determined that the integration of BKpV generates heterogeneous tumour cell populations, possibly adding to metastatic potential.11
Compelling evidence supports the idea that BKpV has tumorigenic potential. BK virus expresses an LTA, which inactivates two key tumour suppressor proteins, p53 and pRB.10 In the unique setting of immunosuppression, with viral reactivation and loss of immune checkpoints, the virus can potentially initiate unchecked cellular proliferation, promoting neoplasia.12 Furthermore, LTA from polyoma viruses closely related to BKpV has induced aggressive transitional cell carcinoma in transgenic mice, as well as extended proliferation of human fibroblasts in vitro.10 13 In fact, LTA expression and p53 inactivation are associated with high-grade aggressive urothelial carcinoma subtypes.14
A comparable oncogenic process is seen in the closely related Merkel cell polyomavirus (MCpV), which causes Merkel cell carcinoma. In this rare form of skin cancer, MCpV expresses LTA, which, combined with integration into the host cell genome, is able to induce unchecked cellular replication.9
Despite a plausible mechanism of oncogenesis, the presence of a causal relationship between BKpV infection and renourinary carcinoma is still controversial. Perhaps, the strongest data to support causation in organ transplant recipients lies in 20 well-documented renourinary cancers showing IHC evidence of BKpV with or without DNA evidence.9 However, these reports differ from the distinct histology of the patient’s tumour presented here.
Interestingly, several of these case reports documented positive IHC staining for LTA specifically in the tumours and not in the surrounding benign bladder.15 16 Crush artefact in our biopsied specimen limited our ability to clearly delineate the area expressing LTA by IHC and BKpV DNA by PCR (figure 2).
Most literature surrounding this topic has focused on transitional cell cancer of the bladder, with a few case reports of BKpV-associated renal cell carcinoma and collecting duct carcinoma.9 While transitional cell cancer is by far the most common cause of bladder cancer, 0.5%–0.7% of primary bladder tumours are small cell carcinomas that are high grade and often aggressive.17
We have not found any reports of small cell carcinoma in the setting of BK virus reactivation. However, it is intriguing that extrapulmonary small cell carcinoma has occurred with MCpV infection, which bears pathogenic similarities to BKpV.18 19
This is the first documented case of small cell carcinoma with foci of adenocarcinoma of the bladder in the setting of persistent BKpV infection after solid organ transplants and also provides strong evidence implicating BK virus in the pathogenesis of the patient’s tumour. Although our patient had relatively high-grade BK viruria and had diagnosis of his malignancy within a year of his transplant (figure 2), it is unclear if the magnitude or duration of BK viruria are predictive variables for the time of onset of neoplasia. Our investigation not only detected BK virus antigens within the tumour by immunohistochemistry, but also most notably detected viral DNA from the tumour sample, providing convincing evidence for a role for BK virus in the pathogenesis of bladder cancers in patients with transplants. We offer our experience to alert providers of patients with renal transplants to potentially serious, unforeseen neoplastic consequences of BK virus reactivation.
Patient’s perspective.
Related to us by our patient: ‘I felt real good knowing that finding this out in me might help other people’.
Learning points.
BK viruria is increasingly linked to cases of bladder cancer in recipients with renal transplant.
Although associated with urothelial carcinoma, our patient’s course exemplifies association of BK viruria with small cell carcinoma and adenocarcinoma of the bladder.
Our experience highlights the potential neoplastic consequences of BK virus reactivation in the setting of solid organ transplantation.
Providers for patients with solid organ transplants with persistent BK viruria should maintain a low threshold for investigation of bladder malignancies.
Footnotes
Contributors: SJL and NNS: primary research and authorship of the manuscript. JMG: conception, design and interpretation of immunohistochemical and molecular studies. JB, BCB and BA: performance and interpretation of histopathological studies. CSB: conception, design, acquisition, analysis and interpretation of data.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Gary JM, Welch SR, Ritter JM, et al. Lassa virus targeting of anterior uvea and endothelium of cornea and conjunctiva in eye of guinea pig model. Emerg Infect Dis 2019;25:865–74. 10.3201/eid2505.181254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elfaitouri A, Hammarin A-L, Blomberg J. Quantitative real-time PCR assay for detection of human polyomavirus infection. J Virol Methods 2006;135:207–13. 10.1016/j.jviromet.2006.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Murdoch DR, Anderson TP, Beynon KA, et al. Evaluation of a PCR assay for detection of Streptococcus pneumoniae in respiratory and nonrespiratory samples from adults with community-acquired pneumonia. J Clin Microbiol 2003;41:63–6. 10.1128/JCM.41.1.63-66.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Beers EH, Joosse SA, Ligtenberg MJ, et al. A multiplex PCR predictor for aCGH success of FFPE samples. Br J Cancer 2006;94:333–7. 10.1038/sj.bjc.6602889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts ISD, Besarani D, Mason P, et al. Polyoma virus infection and urothelial carcinoma of the bladder following renal transplantation. Br J Cancer 2008;99:1383–6. 10.1038/sj.bjc.6604711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaldo CH, Tylden GD, Sharma BN. The human polyomavirus BK (BKPyV): virological background and clinical implications. APMIS 2013;121:728–45. 10.1111/apm.12134 [DOI] [PubMed] [Google Scholar]
- 7.Geetha D, Tong BC, Racusen L, et al. Bladder carcinoma in a transplant recipient: evidence to implicate the BK human polyomavirus as a causal transforming agent. Transplantation 2002;73:1933–6. 10.1097/00007890-200206270-00015 [DOI] [PubMed] [Google Scholar]
- 8.Kuppachi S, Holanda D, Eberlein M, et al. An unexpected surge in plasma BKPyV viral load heralds the development of BKPyV-associated metastatic bladder cancer in a lung transplant recipient with BKPyV nephropathy. Am J Transplant 2017;17:813–8. 10.1111/ajt.14057 [DOI] [PubMed] [Google Scholar]
- 9.Papadimitriou JC, Randhawa P, Rinaldo CH, et al. Bk polyomavirus infection and Renourinary tumorigenesis. Am J Transplant 2016;16:398–406. 10.1111/ajt.13550 [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Chaudhry MR, Berrebi AA, et al. Polyomavirus replication and smoking are independent risk factors for bladder cancer after renal transplantation. Transplantation 2017;101:1488–94. 10.1097/TP.0000000000001260 [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Liu Y, Deng W, et al. Viral integration in BK polyomavirus-associated urothelial carcinoma in renal transplant recipients: multistage carcinogenesis revealed by next-generation virome capture sequencing. Oncogene 2020;39:5734–42. 10.1038/s41388-020-01398-6 [DOI] [PubMed] [Google Scholar]
- 12.Alexiev BA, Randhawa P, Vazquez Martul E, et al. Bk virus-associated urinary bladder carcinoma in transplant recipients: report of 2 cases, review of the literature, and proposed pathogenetic model. Hum Pathol 2013;44:908–17. 10.1016/j.humpath.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 13.Ahuja D, Sáenz-Robles MT, Pipas JM. Sv40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 2005;24:7729–45. 10.1038/sj.onc.1209046 [DOI] [PubMed] [Google Scholar]
- 14.Bertz S, Ensser A, Stoehr R, et al. Variant morphology and random chromosomal integration of BK polyomavirus in posttransplant urothelial carcinomas. Mod Pathol 2020;33:1433–42. 10.1038/s41379-020-0489-0 [DOI] [PubMed] [Google Scholar]
- 15.Alexiev BA, Papadimitriou JC, Chai TC, et al. Polyomavirus (BK)-associated pleomorphic giant cell carcinoma of the urinary bladder: a case report. Pathol Res Pract 2013;209:255–9. 10.1016/j.prp.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 16.Alexiev BA, Papadimitriou JC, Drachenberg CB. Bk polyomavirus-infected nephrogenic adenoma of the urinary bladder in a renal transplant recipient: a case report. Pathol Res Pract 2015;211:697–701. 10.1016/j.prp.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 17.Sved P, Gomez P, Manoharan M, et al. Small cell carcinoma of the bladder. BJU Int 2004;94:12–17. 10.1111/j.1464-410X.2003.04893.x [DOI] [PubMed] [Google Scholar]
- 18.Fisher CA, Harms PW, McHugh JB, et al. Small cell carcinoma in the parotid harboring Merkel cell polyomavirus. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;118:703–12. 10.1016/j.oooo.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 19.Hourdequin KC, Lefferts JA, Brennick JB, et al. Merkel cell polyomavirus and extrapulmonary small cell carcinoma. Oncol Lett 2013;6:1049–52. 10.3892/ol.2013.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]


