Abstract
Objective
The aim of this study was to compare the prevalence of heart failure (HF) in relation to age, multimorbidity and socioeconomic status of primary healthcare centres in southern Sweden.
Design
A cross-sectional study.
Setting
The data were collected concerning diagnoses at each consultation in all primary healthcare centres and secondary healthcare in the southernmost county of Sweden at the end of 2015.
Participants
The individuals living in southern Sweden in 2015 aged 20 years and older. The study population of 981 383 inhabitants was divided into different categories including HF, multimorbidity, different levels of multimorbidity and into 10 CNI (Care Need Index) groups depending on the socioeconomic status of their listed primary healthcare centre.
Outcomes
Prevalence of HF was presented according to age, multimorbidity level and socioeconomic status. Logistic regression was used to further analyse the associations between HF, age, multimorbidity level and socioeconomic status in more complex models.
Results
The total prevalence of HF in the study population was 2.06%. The prevalence of HF increased with advancing age and the multimorbidity level. 99.07% of the patients with HF fulfilled the criteria for multimorbidity. The total prevalence of HF among the multimorbid patients was only 5.30%. HF had a strong correlation with the socioeconomic status of the primary healthcare centres with the most significant disparity between 40 and 80 years of age: the prevalence of HF in primary healthcare centres with the most deprived CNI percentile was approximately twice as high as in the most affluent CNI percentile.
Conclusion
The patients with HF were strongly associated with having multimorbidity. HF patients was a small group of the multimorbid population associated with socioeconomic deprivation that challenges efficient preventive strategies and health policies.
Keywords: Heart failure, EPIDEMIOLOGY, PRIMARY CARE, PUBLIC HEALTH, Adult cardiology
Strengths and limitations of this study.
Our large cohort with almost 1 million inhabitants included 20 193 patients with heart failure and 377 161 patients with multimorbidity in southern Sweden, which increases the validity of our results.
The data were based on clinical diagnoses registered by physicians, rather than self-reported data, which eliminated any recall bias.
Many patients have diagnoses that are usually neglected by the patients and staff in the healthcare, because these do not impair their quality of life or prognosis, which constitutes a consistent error source to our statistics.
As heart failure has none-specific symptoms at the onset, we suspect that many people were underdiagnosed regarding this condition.
We had no data on the quality of healthcare in the neighbourhood.
Introduction
Heart failure (HF) and multimorbidity (MM) are leading causes of morbidity, hospitalisations, disability and death in Western countries.1 2 The prevalence of HF and MM increases with age and the cost of care and treatment constitutes a considerable burden on primary healthcare and on healthcare as a whole.1 In high-income countries, HF is the most common diagnosis in hospitalised elderly patients aged >65 years.2 In Sweden, 31% of medical expenditures were spent for HF patients with reduced ejection fraction (HFrEF) in primary healthcare, 29% for primary cardiac hospitalisations and 40% were for non-cardiac hospitalisations.3
HF is classified into three major groups: HF with reduced EF (HFrEF), HF with midrange EF (HFmrEF), and HF with preserved ejection fraction (HFpEF).4 All subtypes of HF have the same clinical phenotype,5 but different pathophysiology and prognosis.6 The systolic failure or HFrEF (or systolic dysfunction) is established when the left ventricle loses its ability to contract normally, resulting in EF <40%. The heart cannot pump with enough force to push enough blood into the circulation. HFrEF develops usually in response to larger-scale myocyte loss/dysfunction, with the most common aetiologies including acute myocardial infarction, genetic abnormalities, myocarditis or toxin effects (eg, alcohol or chemotherapy).7 Diagnosis of systolic dysfunction is easier than the diagnosis of diastolic dysfunction due to the objective finding of reduced ejection fraction. HFmrEF shares features with both HFrEF and HFpEF, including the aetiology, symptomatology, age of the patients and comorbidities.8 Four diagnostic criteria are simultaneously required for HFmrEF: symptoms with or without signs of HF, LVEF of 40%–49%. Elevated natriuretic peptides, and relevant structural heart disease: left ventricle hypertrophy or left atrial enlargement or diastolic dysfunction.9 HFpEF or diastolic HF (or diastolic dysfunction) is established when the left ventricle loses its ability to relax normally, because the muscle has become stiff. The heart cannot properly fill with blood during the resting period between each beat. The pathophysiological derangements in HFpEF include concentric remodelling, ventricular-vascular stiffening and loss of ventricular-vascular reserve function are resulted from chronic pressure overload due to arterial hypertension.10 Diastolic HFpEF with LVEF ≥50%, and is preferably found among elderly, women, and patients with diabetes mellitus and hypertension.11–14
Beside the risk factors like physical inactivity, obesity, chemotherapy, heritability and hyperlipidaemia, which increases the incidence of HF, the incidence also varies with the patient’s socioeconomic status (SES).15–20 Higher income has previously been associated with a lower risk of developing HF.21 Moreover, the risk factors for HF, such as hypertension and coronary heart disease, also vary with SES.22 HF is often a chronic complication of other cardiovascular comorbidities, particularly ischaemic heart disease, atrial fibrillation and valve dysfunctions.23 Due to improved medical management, the age-adjusted incidence and prevalence of HF are decreasing, and the HF patients have got prolonged life expectancy.1 Consequently, the absolute number of patients with HF has drastically increased, secondary to global ageing, as well as general population growth.24 Although reliable estimates for middle-income and low-income nations are lacking, evidence from the current literature suggests that HF is the fastest growing cardiovascular condition globally.25 26
The aetiology of HF is diverse and varies geographically worldwide: High-income countries are disproportionally affected by ischaemic heart disease and COPD (chronic obstructive pulmonary disease) compared with low-income countries, which in turn are primarily affected by hypertensive heart disease, rheumatic heart disease, cardiomyopathy and myocarditis.27 More than two-thirds of all cases of HF can be attributed to four underlying conditions: ischaemic heart disease, COPD, hypertensive heart disease and rheumatic heart disease.1
HF is often a chronic condition with insidious symptoms at the onset, which could make early and accurate diagnosis difficult. The diagnosis of HF requires three criteria to be fulfilled: typical clinical symptoms, such as dyspnoea, fatigue, exertional intolerance and oedema of the lower body, elevated BNP value and objective findings of impaired cardiac function on echocardiography, myocardial scintigraphy, magnet resonance tomography or other imaging.13
The aim of this study was to compare the prevalence of HF in relation to age, MM level and SES of primary healthcare centres in southern Sweden.
Methods
Setting and study population
Most residents in Sweden are listed at a primary healthcare centre, either a public or private healthcare centre. Scania is the southernmost county of Sweden with around 1.3 million inhabitants during 2015.28 Approximately one-quarter of the study population were born abroad.29 The biggest city in Scania is Malmö with about 320 000 inhabitants during 2015, ranked as the third largest city in Sweden.28 About one-third of the residents in Malmö were born abroad representing most countries in the world.30 Almost half of the residents in Malmö (48.40%) were under 35 years during 2015.31 The study population comprised individuals aged 20 years and older living in Scania during the last week of 2015. This age cut-off was chosen because the types of HF affecting children and younger people are pathologically distinct from those found in older adults.
The study population was divided into age groups: 20, 30, 40, 50, 60, 70, 80+. The age group 20 included inhabitants aged 20 to 29 years, the age group 30 included inhabitants aged 30–39 years and so on. The age group 80+ included all inhabitants from 80 years and over.
Data source and measurements
The data used in this study was retrieved from the County Council healthcare register in Scania that contains anonymised registry information from the study population, including age, gender, SES and diagnostic data in the last week of 2015.
The data were collected concerning diagnoses at each consultation in all primary healthcare centres and secondary healthcare. Diagnoses were recorded according the International Statistical Classification of Diseases and Related Health Problems version 10 (ICD 10). HF was diagnosed following the diagnosis criteria for HF according to ESC (European Society of Cardiology) guidelines and recorded as I50, which comprised all subtypes of HF. Totally 152 primary healthcare centres were operating during 2015 in Scania, with on average 8587 listed patients (95% CI 7971.49 to 9292.88) including 133 patients with HF (95% CL 122.60 to 143.80) at each primary healthcare centre.
Multimorbidity
MM was defined as coexistence of two or more chronic conditions in the same person, independently if cardiovascular or not. To measure MM, we used a method to identify chronic conditions developed by Calderón-Larrañaga et al at the Ageing Research Centre in Stockholm.32 They analysed the full list of ICD-10 codes on a four-digit level to define if a diagnosis is chronic or not in an elderly population. To determine if a condition is chronic or not the following key features were identified and discussed concerning their pertinence and suitability in older populations: duration, course, reversibility, treatment and consequences. They were then grouped into 60 groups of chronic conditions if their duration exceeded 3 months. We applied their definition and list of chronic conditions to estimate the MM in our study population. All information about diagnoses was obtained from electronic medical record database in the county council in Scania. MM was then estimated by counting the number of chronic conditions in each patient. To study the MM level in relation to the prevalence of HF, the patients were further divided into groups MM0 (less than 2 chronic conditions), MM1 (2–4 chronic conditions), MM2 (5–9 chronic conditions) and MM3 (10 chronic conditions or more).
Socioeconomics
We used the term Care Need Index (CNI)33 to divide the primary healthcare centres into 10 groups depending on their SES. CNI is based on different measures of a group, in this case the patients listed to different primary healthcare centres in Scania. CNI 1 was assigned to those patients listed at primary healthcare centres who belonged to the most socioeconomically affluent percentile, and CNI 10 was assigned to those patients listed at primary healthcare centres who belonged to the most socioeconomically deprived percentile.33
Statistical analyses
We analysed data from 981 383 (about a tenth of the Swedish population) inhabitants aged 20 years and older living in Scania during the last week of 2015. Associations between the variables were studied using univariate and multivariate statistics.
We used frequencies, percentages and cross tabulations for descriptive analysis. Logistic regression was used to analyse the associations between the univariate and multivariate models. Only the linear predications of the fully adjusted models were shown in the figures.
A p <0.05 was considered statistically significant. The predicted mean probability of HF was calculated as average marginal effects using the Delta-method.
We used STATA V.16.0 and V.17.0 (Stata) for statistical analyses.
Patient and public involvement
Data in this study are based on anonymised information provided by the County Council of Scania.
The study participants were not involved in the recruitment to the study by themselves. Due to the requirement of anonymised data, each individual could not be asked for consent to participate; active refusal of participation was instead applied. This was done by publishing information about the planned study in the Swedish local newspaper ‘Sydsvenskan’. The advertisement outlined the study and contained information on how to contact the research manager (first author) to opt out of the study. The study results are published anonymised in group level, and cannot be disseminated to every study participant.
Results
The total prevalence of HF in the study population was found to be 2.06% (20193 patients) in 2015. HF was a rare disease under 40 years of age in the whole study population, but the prevalence increased at least twofold in all age groups and CNI percentiles from 30 years of age onwards and reached 17.31% in the age group 80+ (table 1). The individuals listed at primary healthcare centres with deprived CNI percentiles were more likely to have a higher proportion of individuals younger than 40 years and the opposite was true for primary healthcare centres with affluent CNI percentiles. The primary healthcare centres with the most deprived CNI percentile had the lowest proportion of population from middle age, only 33.25% were 50 years and older, whereas the affluent CNI percentiles were likely to be dominated by individuals from 50 years and over (table 1).
Table 1.
Prevalence of heart failure (HF) and multimorbidity (MM) in all age groups and CNI percentiles
| CNI percentiles | HF | |||||||||
| No MM |
Yes MM |
MM (%) | HF (%) | HF with MM (%) | MM with HF (%) | |||||
| Age | N | No | Yes | No | Yes | |||||
| CNI 1 | 20 | 12 866 | 10 842 | 2020 | 1 | 3 | 15.72 | 0.03 | 75.00 | 0.15 |
| 30 | 17 890 | 14 347 | 3533 | 2 | 8 | 19.79 | 0.06 | 80.00 | 0.23 | |
| 40 | 24 753 | 18 672 | 6047 | 3 | 31 | 24.55 | 0.14 | 91.18 | 0.51 | |
| 50 | 17 806 | 11 062 | 6656 | 5 | 83 | 37.85 | 0.49 | 94.32 | 1.23 | |
| 60 | 19 358 | 7857 | 11 190 | 5 | 306 | 59.39 | 1.61 | 98.39 | 2.66 | |
| 70 | 13 345 | 2894 | 9769 | 5 | 677 | 78.28 | 5.11 | 99.27 | 6.48 | |
| 80 | 5 614 | 610 | 4055 | 1 | 948 | 89.12 | 16.90 | 99.89 | 18.95 | |
| CNI 2 | 20 | 16 173 | 13 755 | 2411 | 1 | 6 | 14.94 | 0.04 | 85.71 | 0.25 |
| 30 | 16 095 | 12 861 | 3230 | 0 | 4 | 20.09 | 0.02 | 100.00 | 0.12 | |
| 40 | 20 750 | 15 497 | 5220 | 0 | 33 | 25.32 | 0.16 | 100.00 | 0.63 | |
| 50 | 18 892 | 11 602 | 7196 | 2 | 92 | 38.58 | 0.50 | 97.87 | 1.26 | |
| 60 | 19 729 | 8378 | 10 990 | 6 | 355 | 57.50 | 1.83 | 98.34 | 3.13 | |
| 70 | 12 752 | 3090 | 9024 | 5 | 633 | 75.73 | 5.00 | 99.22 | 6.55 | |
| 80 | 6 278 | 833 | 4468 | 2 | 975 | 86.70 | 15.56 | 99.80 | 17.91 | |
| CNI 3 | 20 | 16 970 | 14 424 | 2540 | 1 | 5 | 15.00 | 0.04 | 83.33 | 0.20 |
| 30 | 15 252 | 12 212 | 3030 | 0 | 10 | 19.93 | 0.07 | 100.00 | 0.33 | |
| 40 | 16 596 | 12 045 | 4520 | 1 | 30 | 27.42 | 0.19 | 96.77 | 0.66 | |
| 50 | 14 638 | 8843 | 5693 | 2 | 100 | 39.58 | 0.70 | 98.04 | 1.73 | |
| 60 | 15 383 | 6310 | 8760 | 4 | 309 | 58.95 | 2.03 | 98.72 | 3.41 | |
| 70 | 10 056 | 2269 | 7163 | 4 | 620 | 77.40 | 6.21 | 99.36 | 7.97 | |
| 80 | 5 553 | 649 | 3903 | 8 | 993 | 88.17 | 18.03 | 99.20 | 20.28 | |
| CNI 4 | 20 | 14 112 | 11 835 | 2271 | 3 | 3 | 16.11 | 0.04 | 50.00 | 0.13 |
| 30 | 13 429 | 10 665 | 2753 | 1 | 10 | 20.57 | 0.08 | 90.91 | 0.36 | |
| 40 | 15 769 | 11 417 | 4309 | 1 | 42 | 27.59 | 0.27 | 97.67 | 0.97 | |
| 50 | 14 658 | 8622 | 5915 | 3 | 118 | 41.16 | 0.83 | 97.52 | 1.96 | |
| 60 | 14 826 | 6017 | 8459 | 7 | 343 | 59.37 | 2.36 | 98.00 | 3.90 | |
| 70 | 9 409 | 2221 | 6558 | 0 | 630 | 76.39 | 6.70 | 100.00 | 8.76 | |
| 80 | 5 122 | 646 | 3493 | 6 | 977 | 87.27 | 19.19 | 99.39 | 21.86 | |
| CNI 5 | 20 | 12 796 | 10 794 | 2000 | 1 | 1 | 15.64 | 0.02 | 50.00 | 0.05 |
| 30 | 13 168 | 10 455 | 2706 | 0 | 7 | 20.60 | 0.05 | 100.00 | 0.26 | |
| 40 | 13 879 | 10 028 | 3816 | 2 | 33 | 27.73 | 0.25 | 94.29 | 0.86 | |
| 50 | 12 142 | 7171 | 4897 | 2 | 72 | 40.92 | 0.61 | 97.30 | 1.45 | |
| 60 | 11 723 | 4870 | 6597 | 3 | 253 | 58.43 | 2.18 | 98.83 | 3.69 | |
| 70 | 7 333 | 1704 | 5162 | 0 | 467 | 76.76 | 6.37 | 100.00 | 8.30 | |
| 80 | 4 178 | 489 | 2884 | 3 | 802 | 88.22 | 19.27 | 99.63 | 21.76 | |
| CNI 6 | 20 | 18 134 | 15 365 | 2766 | 0 | 3 | 15.27 | 0.02 | 100.00 | 0.11 |
| 30 | 15 745 | 12 638 | 3099 | 2 | 6 | 19.72 | 0.05 | 75.00 | 0.19 | |
| 40 | 18 285 | 13 316 | 4928 | 2 | 39 | 27.16 | 0.22 | 95.12 | 0.79 | |
| 50 | 16 530 | 9833 | 6588 | 2 | 107 | 40.50 | 0.66 | 98.17 | 1.60 | |
| 60 | 16 438 | 6943 | 9163 | 5 | 327 | 57.73 | 2.02 | 98.49 | 3.45 | |
| 70 | 11 457 | 2667 | 8171 | 4 | 615 | 76.69 | 5.40 | 99.35 | 7.00 | |
| 80 | 6 894 | 940 | 4845 | 6 | 1103 | 86.28 | 16.09 | 99.46 | 18.54 | |
| CNI 7 | 20 | 18 045 | 15 624 | 2411 | 1 | 9 | 13.41 | 0.06 | 90.00 | 0.37 |
| 30 | 14 656 | 11 977 | 2669 | 1 | 9 | 18.27 | 0.07 | 90.00 | 0.34 | |
| 40 | 14 400 | 10 590 | 3777 | 2 | 31 | 26.44 | 0.23 | 93.94 | 0.81 | |
| 50 | 12 597 | 7597 | 4907 | 4 | 89 | 39.66 | 0.74 | 95.70 | 1.78 | |
| 60 | 13 119 | 5696 | 7147 | 5 | 271 | 56.54 | 2.10 | 98.19 | 3.65 | |
| 70 | 8 930 | 2194 | 6193 | 1 | 542 | 75.42 | 6.08 | 99.82 | 8.05 | |
| 80 | 5 569 | 788 | 3788 | 5 | 988 | 85.76 | 17.83 | 99.50 | 20.69 | |
| CNI 8 | 20 | 22 405 | 18 803 | 3597 | 1 | 4 | 16.07 | 0.02 | 80.00 | 0.11 |
| 30 | 21 019 | 16 659 | 4341 | 0 | 19 | 20.74 | 0.09 | 100.00 | 0.44 | |
| 40 | 19 268 | 13 828 | 5395 | 2 | 43 | 28.22 | 0.23 | 95.56 | 0.79 | |
| 50 | 17 755 | 10 435 | 7175 | 7 | 138 | 41.19 | 0.82 | 95.17 | 1.89 | |
| 60 | 17 014 | 7233 | 9435 | 3 | 343 | 57.47 | 2.03 | 99.13 | 3.51 | |
| 70 | 10 651 | 2616 | 7388 | 4 | 643 | 75.40 | 6.07 | 99.38 | 8.01 | |
| 80 | 6 039 | 838 | 4189 | 7 | 1005 | 86.01 | 16.76 | 99.31 | 19.35 | |
| CNI 9 | 20 | 23 116 | 19 785 | 3328 | 1 | 2 | 14.41 | 0.01 | 66.67 | 0.06 |
| 30 | 21 531 | 17 553 | 3967 | 2 | 9 | 18.47 | 0.05 | 81.82 | 0.23 | |
| 40 | 16 388 | 12 072 | 4277 | 1 | 38 | 26.33 | 0.24 | 97.44 | 0.88 | |
| 50 | 14 812 | 8881 | 5828 | 2 | 101 | 40.03 | 0.70 | 98.06 | 1.70 | |
| 60 | 12 646 | 5696 | 6616 | 2 | 332 | 54.94 | 2.64 | 99.40 | 4.78 | |
| 70 | 8 915 | 2342 | 6013 | 4 | 556 | 73.68 | 6.28 | 99.29 | 8.46 | |
| 80 | 6 064 | 1042 | 4043 | 8 | 971 | 82.68 | 16.14 | 99.18 | 19.37 | |
| CNI 10 | 20 | 26 259 | 22 707 | 3542 | 2 | 8 | 13.52 | 0.04 | 80.00 | 0.23 |
| 30 | 21 295 | 17 348 | 3931 | 1 | 15 | 18.53 | 0.08 | 93.75 | 0.38 | |
| 40 | 15 007 | 10 531 | 4428 | 4 | 44 | 29.80 | 0.32 | 91.67 | 0.98 | |
| 50 | 12 602 | 7145 | 5338 | 0 | 119 | 43.30 | 0.94 | 100.00 | 2.18 | |
| 60 | 9 304 | 4061 | 4938 | 3 | 302 | 56.32 | 3.28 | 99.02 | 5.76 | |
| 70 | 5 751 | 1643 | 3580 | 2 | 526 | 71.40 | 9.18 | 99.62 | 12.81 | |
| 80 | 3 450 | 662 | 2117 | 2 | 669 | 80.75 | 19.45 | 99.70 | 24.01 | |
| All CNI percentiles | 20 | 180 876 | 153 934 | 26 886 | 12 | 44 | 14.89 | 0.03 | 78.57 | 0.16 |
| 30 | 170 080 | 136 715 | 33 259 | 9 | 97 | 19.61 | 0.06 | 91.51 | 0.29 | |
| 40 | 175 095 | 127 996 | 46 717 | 18 | 364 | 26.89 | 0.22 | 95.29 | 0.77 | |
| 50 | 152 432 | 91 191 | 60 193 | 29 | 1019 | 40.16 | 0.69 | 97.23 | 1.66 | |
| 60 | 149 540 | 63 061 | 83 295 | 43 | 3141 | 57.80 | 2.13 | 98.65 | 3.63 | |
| 70 | 98 599 | 23 640 | 69 021 | 29 | 5909 | 75.99 | 6.02 | 99.51 | 7.89 | |
| 80 | 54 761 | 7497 | 37 785 | 48 | 9431 | 86.22 | 17.31 | 99.49 | 19.97 | |
| Total | 981 383 | 604 034 | 357 156 | 188 | 20 005 | 38.43 | 2.06 | 99.07 | 5.30 | |
MM (%)=total prevalence of MM.
HF (%)=total prevalence of HF.
HF with MM (%)=prevalence of HF with MM.
MM with HF (%)=prevalence of MM with HF.
CNI, Care Need Index.;
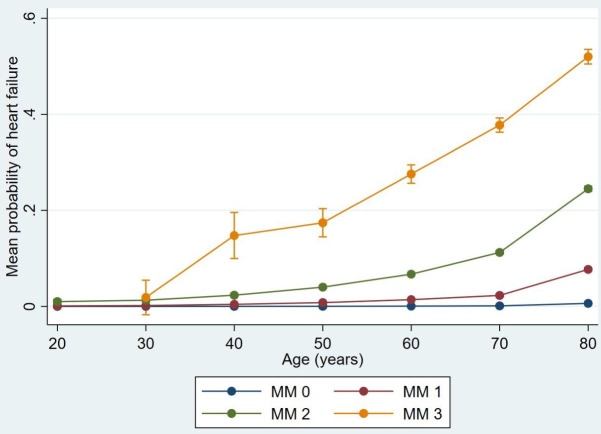
MM was present in 38.40% (377161 patients) of the study population and followed different patterns according to age groups and CNI percentiles of the primary healthcare centres (table 1). HF was strongly correlated to MM: 99.07% of the patients with HF fulfilled the criteria for MM, independently of the age at their diagnosis. The prevalence of MM increased steadily with advancing age, from 14.89% in the age group 20 to 86.22% in the age group 80+ (table 1). The prevalence of HF increased consistently with the MM level: the MM1 (2–4 chronic conditions) group had 1.49% patients with HF, the MM2 (5–9 chronic conditions) group had 11.16% patients with HF and the MM3 (>10 chronic conditions) group had 39.28% patients with HF. The total prevalence of HF among the multimorbid patients was only 5.30% (20005 patients) (table 1). The predicted mean probability of HF adjusted for age and MM level is shown in figure 1.
Figure 1.
The predicted mean probability of heart failure adjusted for different age groups and multimorbidity levels with 95% CIs using delta methods. MM0, less than 2 chronic conditions (not multimorbid); MM1, 2–4 chronic conditions; MM2, 5–9 chronic conditions; MM3, 10 or more chronic conditions.
If we consider the prevalence of HF in different MM levels: 19.19% (3875 patients) of all patients with HF belonged to the MM1 group, 58.18% (11748 patients) belonged to the MM2 group and 21.70% (4382 patients) belonged to the MM3 group. The MM2 group as a whole was more than nine times larger than the MM3 group (105 241 vs 11 156 patients).
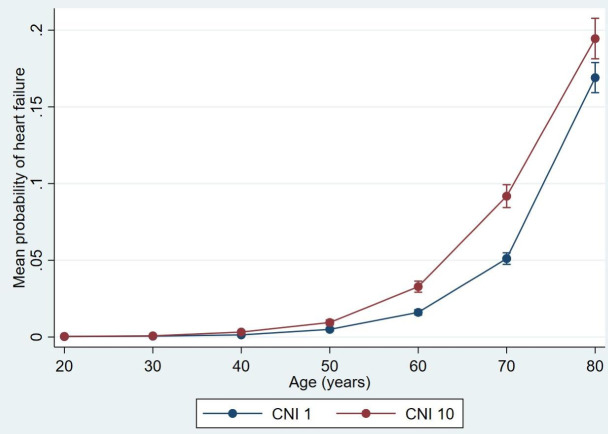
The prevalence of HF had a strong correlation with the SES of the primary healthcare centres (figure 2). The most significant disparity was between 40 and 80 years of age: the prevalence of HF in primary healthcare centres with the most deprived CNI percentile was significantly increased and approximately twice as high as in the most affluent CNI percentile (table 1). Although at much lower levels, significant disparities in prevalence of HF could be observed when comparing the most deprived CNI percentile with other CNI percentiles of the primary healthcare centres. The primary healthcare centres with the most deprived CNI percentile had the highest prevalence of HF from 40 years of age, although their prevalence of MM was lowest from 70 years of age. In contrast, the prevalence of HF in the most affluent CNI percentile remained relatively low in most age groups, even from 60 years of age as their prevalence of MM became highest (table 1). Only 4.58% of the multimorbid individuals belonging to this CNI percentile had HF, which was lowest compared with the more deprived CNI percentiles. The association between the prevalence of HF and CNI percentiles followed different patterns compared with MM as shown in table 1.
Figure 2.
Disparities in the predicted mean probability of heart failure adjusted for age between the most affluent (CNI 1) and deprived (CNI 10) CNI percentile with 95% CIs using delta methods. CNI, Care Need Index.
Discussion
The total prevalence of HF was about 2% in Scania during 2015, which was the same as the prevalence in Sweden and other Western countries.34 35 HF was a rare disease under 40 years of age and increased substantially with advancing age. 99.07% of the patients with HF in our study population had MM, which could be explained by the diagnosis HF mostly constitutes a complication of other cardiovascular conditions.23 36 MM was present in 38.40% of the study population, but included only 5.30% patients with HF. The high prevalence of MM could be explained by the socioeconomic difference within the study population and the considerable part of elderly with high prevalence of MM. With increasing MM level, the prevalence of HF increased from 1.49% in the MM1 (2–4 chronic conditions) group to 39.28% in the MM3 (more than 10 chronic conditions) group. The MM3 group had fewer patients, but a higher prevalence of HF than the MM2 group, which makes us to believe that the MM3 group had a higher mortality in general.
Most primary healthcare centres are public and organised similarly irrespective of CNI. The socioeconomic boundaries are quite sharp and agree with uptake areas of the different primary healthcare centres. The CNI category was an average socioeconomic level of the patients listed at the primary healthcare centres.
The prevalence of HF also had a strong association with the SES of primary healthcare centres with the most significant disparity between 40 and 80 years of age: the prevalence of HF in primary healthcare centres with the most deprived CNI percentile was approximately twice as high as in the most affluent CNI percentile. The fact that the prevalence of HF was highest from 40 years of age in the most deprived CNI percentile of primary healthcare centres indicates that HF is a disease associated with socioeconomic deprivation. The correlation was assessed visually as the difference in prevalence of HF was obvious between the most affluent and deprived CNI percentiles.
The individuals listed at primary healthcare centres with deprived CNI percentiles were more likely to have high proportion of inhabitants younger than 40 years, and the opposite wastrue for primary healthcare centres with affluent CNI percentiles. The primary healthcare centres with the most deprived CNI percentile had the lowest proportion of population (33.25%) from 50 years and the highest prevalence of HF from 40 years of age compared with the more affluent population, which makes us to suspect that they suffered from SES related MM with worse prognosis, including HF.
HF is common in multimorbid patients with COPD,37 with prevalence in 33.2% of women and 35.7% of men over 80 years of age.38 In most countries, low SES is associated with higher prevalence of COPD and mortality.39 The estimated mortality in patients with COPD and coexisting HF was seven times higher than in patients with COPD alone, thus the patients with these two conditions were reported with the highest mortality among patients hospitalised with COPD exacerbation.40 Other conditions with high impact on mortality in patients with HF including stroke, renal disease and diabetes mellitus,41 are strongly associated with low SES as well.42–44
With respect to the global burden of ischaemic heart disease, the incidence of acute myocardial infarction worldwide is highest in Eastern Europe and Central Asia.45 Compared with the Swedish population, the first-generation immigrants from Iraq and Bosnia had the highest incidence of HF, probably due to a higher incidence of coronary heart disease.4 When this incidence of HF was further adjusted for SES, marital status and educational level, the HR for HF raised significantly compared with the immigrants from other countries. As many of these immigrants are socioeconomically highly disadvantaged in Sweden, these results support our findings. Interestingly, the HF risk pattern among the second-generation immigrants in most cases differed only marginally compared with their Swedish counterparts, indicating that their risk factor is not purely genetic, rather responsive to other factors.4
A similar study in Scotland revealed that older people typically have more morbidities with lower functional status, whereas younger people are more often affected by combinations physical and mental health disorders. Except that the most affluent population being on average 2–5 years older at onset of morbidity (dependent on the disorder), conditions like coronary heart disease, diabetes mellitus, COPD, depression, painful disorders or cancer were more common in people living in deprived areas.46 This could explain that people in the affluent areas suffered from MM with less disability and had better prognosis.
We do not know if MM causes socioeconomic deprivation or if low SES causes MM. There is presumably an impact in both directions. Many people with MM do retire earlier, and have more socioeconomic consequences than the working population. Statistically, this group degrades in the SES, which even may influence their family members. On the other hand, many people in the deprived areas have to accept a job which is more health challenging, and become multimorbid many years earlier than the affluent population.
Strengths and limitations
Our study has a number of strengths. Our large cohort with almost 1 million inhabitants included all patients with HF and MM in Scania during the study period, which increases the validity of our results. The outcome data were based on clinical diagnoses registered by physicians, rather than self-reported data, which eliminated any recall bias. Our findings have similarities with correlative studies in other countries,21 23 which increases the credibility of our results.
This study has certain limitations. We had no data on several risk factors for HF, such as smoking, obesity or physical inactivity. However, some prior works on SES and HF had adjusted for smoking and physical inactivity and still found an independent association.21 We had no results of echocardiography, and thus could not analyse the subtypes of HF in our study population. As HF has none-specific symptoms at the onset, we suspect that many people were underdiagnosed regarding this condition. Those patients with HF belonging to the MM0 group were probably underdiagnosed as well, because HF usually constitutes a complication of other diseases or treatments. Many patients have diagnoses that are usually neglected by the patients and staff in the healthcare, because these do not impair their quality of life or prognosis, which constitutes a consistent error source to our statistics. We had no data on the severity of HF and other conditions, which have high impact on the mortality. We had no data on the quality of healthcare in the neighbourhood. Our results could be more accurate if the age group 80+ were divided into age group 80 and 90+, and analysed separately.
Conclusion
The prevalence of HF was strongly associated with MM, with increasing prevalence of HF with MM level. The patients listed at primary healthcare centres with the most socioeconomic deprived CNI percentile had a significantly elevated risk of developing HF and probably MM with worse prognosis, which resulted in the lowest proportion of population from 50 years compared with the more affluent population in our study. HF patients was a small group of the multimorbid population associated with socioeconomic deprivation that challenges efficient preventive strategies and health policies.
Supplementary Material
Acknowledgments
We thank the County Council of Scania for providing the patient data enabling this study. We are indebted to Patrick Reilly for his expertise and invaluable advice in proofreading the manuscript.
Footnotes
Contributors: In accordance with the Vancouver Protocol, AH was involved in data collection, design of the study, data analysis, editing the manuscript and student supervision. MS contributed with data collection, data analysis, writing and editing the manuscript. PM provided critical comments and feedback on the manuscript. AH acts as a gaurantor for this study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository. Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Scania County council provided anonymised data of the study population, which is confidential and not available for open access according to the Swedish law.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The regional Ethical Review Board at Lund University (application no. 2018/778) approved the study.
References
- 1.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016;13:368–78. 10.1038/nrcardio.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015;385:812–24. 10.1016/S0140-6736(14)61889-4 [DOI] [PubMed] [Google Scholar]
- 3.Mejhert M, Lindgren P, Schill O, et al. Long term health care consumption and cost expenditure in systolic heart failure. Eur J Intern Med 2013;24:260–5. 10.1016/j.ejim.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 4.Wändell P, Carlsson AC, Li X, et al. Heart failure in immigrant groups: a cohort study of adults aged 45 years and over in Sweden. Scand Cardiovasc J 2018;52:292–300. 10.1080/14017431.2018.1546892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 2011;123:2006–14. 10.1161/CIRCULATIONAHA.110.954388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aziz F, Tk L-A, Enweluzo C, et al. Diastolic heart failure: a Concise review. J Clin Med Res 2013;5:327–34. 10.4021/jocmr1532w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines: developed in collaboration with the International Society for heart and lung transplantation. Circulation 2009;119:e391–479. 10.1161/CIRCULATIONAHA.109.192065 [DOI] [PubMed] [Google Scholar]
- 8.Savarese G, Stolfo D, Sinagra G, et al. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol 2022;19:100–16. 10.1038/s41569-021-00605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of cardiology (ESC). developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 10.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 1993;73:413–67. 10.1152/physrev.1993.73.2.413 [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, et al. , WRITING COMMITTEE MEMBERS . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines. Circulation 2013;128:e240–327. 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 12.Andersson C, Vasan RS. Epidemiology of heart failure with preserved ejection fraction. Heart Fail Clin 2014;10:377–88. 10.1016/j.hfc.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 14.Garcia M, Mulvagh SL, Merz CNB, et al. Cardiovascular disease in women: clinical perspectives. Circ Res 2016;118:1273–93. 10.1161/CIRCRESAHA.116.307547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agunbiade TA, Zaghlol RY, Barac A. Heart failure in relation to anthracyclines and other chemotherapies. Methodist Debakey Cardiovasc J 2019;15:243–9. 10.14797/mdcj-15-4-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halldin A-K, Schaufelberger M, Lernfelt B, et al. Obesity in middle age increases risk of later heart failure in Women-Results from the prospective population study of women and H70 studies in Gothenburg, Sweden. J Card Fail 2017;23:363–9. 10.1016/j.cardfail.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 17.Lindgren MP, PirouziFard M, Smith JG, et al. A Swedish nationwide adoption study of the heritability of heart failure. JAMA Cardiol 2018;3:703–10. 10.1001/jamacardio.2018.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins NM, Jhund PS, McMurray JJV, et al. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur J Heart Fail 2012;14:138–46. 10.1093/eurjhf/hfr168 [DOI] [PubMed] [Google Scholar]
- 19.Ramsay SE, Whincup PH, Papacosta O, et al. Inequalities in heart failure in older men: prospective associations between socioeconomic measures and heart failure incidence in a 10-year follow-up study. Eur Heart J 2014;35:442–7. 10.1093/eurheartj/eht449 [DOI] [PubMed] [Google Scholar]
- 20.Halldin A-K, Lissner L, Lernfelt B, et al. Impact of changes in physical activity or BMI on risk of heart failure in women - the prospective population study of women in Gothenburg. Scand J Prim Health Care 2020;38:56–65. 10.1080/02813432.2020.1717083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akwo EA, Kabagambe EK, Harrell FE, et al. Neighborhood deprivation predicts heart failure risk in a low-income population of blacks and whites in the southeastern United States. Circ Cardiovasc Qual Outcomes 2018;11:e004052. 10.1161/CIRCOUTCOMES.117.004052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlsson AC, Li X, Holzmann MJ, et al. Neighbourhood socioeconomic status and coronary heart disease in individuals between 40 and 50 years. Heart 2016;102:775–82. 10.1136/heartjnl-2015-308784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor CJ, Ryan R, Nichols L, et al. Survival following a diagnosis of heart failure in primary care. Fam Pract 2017;34:161–8. 10.1093/fampra/cmw145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333–41. 10.1056/NEJMoa1406656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett DA, Eliasz TK, Forbes A, et al. Study protocol: systematic review of the burden of heart failure in low- and middle-income countries. Syst Rev 2012;1:59. 10.1186/2046-4053-1-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee A, Mendis S. Heart failure: the need for global health perspective. Curr Cardiol Rev 2013;9:97–8. 10.2174/1573403X11309020001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2163–96. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Statistics Sweden y . Population by region, marital status, sex and year [internet]. Statistics Sweden. [cited 2021 Mar 30]. Available: http://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__BE__BE0101__BE0101A/BefolkningNy/table/tableViewLayout1/
- 29.Statistics Sweden y . Population by region, age, sex, region of birth and year [internet]. Statistics Sweden. [cited 2021 Mar 30]. Available: http://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__BE__BE0101__BE0101E/InrUtrFoddaRegAlKon/table/tableViewLayout1/
- 30.Statistics Sweden y . Population by region, sex, region of birth and year [internet]. Statistics Sweden; [cited 2021 Mar 29]. Available: http://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__BE__BE0101__BE0101E/InrUtrFoddaRegAlKon/table/tableViewLayout1/
- 31.Statistics Sweden y . Population by region, marital status, age, sex and year [internet]. Statistics Sweden; [cited 2021 Mar 28]. Available: http://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__BE__BE0101__BE0101A/BefolkningNy/table/tableViewLayout1/
- 32.Calderón-Larrañaga A, Vetrano DL, Onder G, et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its Operationalization. J Gerontol A Biol Sci Med Sci 2017;72:1417–23. 10.1093/gerona/glw233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundquist K, Malmström M, Johansson S-E, et al. Care need index, a useful tool for the distribution of primary health care resources. J Epidemiol Community Health 2003;57:347–52. 10.1136/jech.57.5.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarrinkoub R, Wettermark B, Wändell P, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 2013;15:995–1002. 10.1093/eurjhf/hft064 [DOI] [PubMed] [Google Scholar]
- 35.Savarese G, D'Amario D. Sex differences in heart failure. Adv Exp Med Biol 2018;1065:529–44. 10.1007/978-3-319-77932-4_32 [DOI] [PubMed] [Google Scholar]
- 36.Gimeno-Miguel A, Gracia Gutiérrez A, Poblador-Plou B, et al. Multimorbidity patterns in patients with heart failure: an observational Spanish study based on electronic health records. BMJ Open 2019;9:e033174. 10.1136/bmjopen-2019-033174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutten FH, Cramer M-JM, Grobbee DE, et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J 2005;26:1887–94. 10.1093/eurheartj/ehi291 [DOI] [PubMed] [Google Scholar]
- 38.Almagro P, Calbo E, Ochoa de Echagüen A, et al. Mortality after hospitalization for COPD. Chest 2002;121:1441–8. 10.1378/chest.121.5.1441 [DOI] [PubMed] [Google Scholar]
- 39.Pleasants RA, Riley IL, Mannino DM. Defining and targeting health disparities in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2016;11:2475–96. 10.2147/COPD.S79077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaszuba E, Odeberg H, Råstam L, et al. Heart failure and levels of other comorbidities in patients with chronic obstructive pulmonary disease in a Swedish population: a register-based study. BMC Res Notes 2016;9:215. 10.1186/s13104-016-2008-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joffe SW, Webster K, McManus DD, et al. Improved survival after heart failure: a community-based perspective. J Am Heart Assoc 2013;2:e000053. 10.1161/JAHA.113.000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vart P, Grams ME, Ballew SH, et al. Socioeconomic status and risk of kidney dysfunction: the Atherosclerosis risk in Communities study. Nephrol Dial Transplant 2019;34:1361–8. 10.1093/ndt/gfy142 [DOI] [PubMed] [Google Scholar]
- 43.Marshall IJ, Wang Y, Crichton S, et al. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol 2015;14:1206–18. 10.1016/S1474-4422(15)00200-8 [DOI] [PubMed] [Google Scholar]
- 44.Wändell P, Carlsson AC, Gasevic D, et al. Neighbourhood socio-economic status and all-cause mortality in adults with atrial fibrillation: a cohort study of patients treated in primary care in Sweden. Int J Cardiol 2016;202:776–81. 10.1016/j.ijcard.2015.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moran AE, Forouzanfar MH, Roth GA, et al. The global burden of ischemic heart disease in 1990 and 2010: the global burden of disease 2010 study. Circulation 2014;129:1493–501. 10.1161/CIRCULATIONAHA.113.004046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Scania County council provided anonymised data of the study population, which is confidential and not available for open access according to the Swedish law.