Abstract
Background: The aim was to explore a novel risk score to predict mortality in hospitalized patients with COVID-19 pneumonia. Methods: This was a retrospective, multicenter study. Results: A total of 1013 patients with COVID-19 were included. The mean age was 60.5 ± 14.4 years, and 581 (57.4%) patients were male. In-hospital death occurred in 124 (12.2%) patients. Multivariate analysis revealed peripheral capillary oxygen saturation (SpO2), albumin, D-dimer and age as independent predictors. The mortality score model was given the acronym SAD-60, representing SpO2, Albumin, D-dimer, age ≥60 years. The SAD-60 score (0.776) had the highest area under the curve compared with CURB-65 (0.753), NEWS2 (0.686) and qSOFA (0.628) scores. Conclusion: The SAD-60 score has a promising predictive capacity for mortality in hospitalized patients with COVID-19.
Keywords: : albumin, COVID-19, D-dimer, mortality, SAD-60 score
The pandemic of COVID-19 continues to be a significant public health issue [1]. COVID-19 has caused hospitalization and intensive care unit (ICU) admission, and results in mortality especially in older adults with comorbid diseases [2]. Previous studies have identified the epidemiological features, clinical characteristics and outcomes of COVID-19 [2–8]. Wu et al. reported that 14% of the 72,314 patients had a severe illness, and the mortality rate among 44,672 critical patients with confirmed COVID-19 was 49.0% [2]. Mortality rates in critically ill patients were varied and reported to be as high as 61.5% [2,4,9].
Estimating the risk of poor prognosis and early recognition of critical disease in patients with COVID-19 is important for the care planning process in reducing morbidity and mortality, as immediate intensive care treatment is a crucial step to recovery [9]. Patient outcomes would be improved by prioritizing patient care resources especially in limited-resource settings [10]. Several studies have determined predictors for clinical deterioration and mortality [11–13]. Moreover, recent studies have focused on the discriminative ability of previously known risk scores for community-acquired pneumonia in patients with COVID-19 [14–17]. Further, prediction models for early identification of COVID-19 progression have been reported [18–22]. However, although recent studies have created various novel scores, there is no certain and specific risk score to predict poor prognosis in patients with COVID-19.
In this study, the aim was to explore a novel risk score to predict mortality in hospitalized patients with COVID-19 pneumonia. In addition, the accuracy of the novel risk score was compared with CURB-65, qSOFA and NEWS2 scores.
Patients & methods
This retrospective multicenter study was conducted in all hospitalized adult patients (≥18 years old) with laboratory and radiologically confirmed COVID-19 pneumonia who were diagnosed by the Department of Infectious Diseases and Clinical Microbiology between 1 November 2020 and 30 November 2020 from six public/governmental hospitals in Istanbul, Turkey. Demographic features, clinical findings, laboratory results and patient outcomes from medical records via a datasheet form were retrospectively collected. Peripheral capillary oxygen saturation (SpO2) was measured on room air. All data was recorded at admission or within 24 h after hospitalization. SARS-CoV-2 real-time reverse transcription PCR (RT-PCR) was performed in samples collected by nasopharyngeal and/or oropharyngeal swabs. Chest computed tomography confirmed patients with COVID-19 pneumonia requiring hospitalization were included, whereas asymptomatic patients, outpatients and radiologically unconfirmed patients were excluded.
A confirmed COVID-19 pneumonia was defined as a case diagnosed with a molecularly and radiologically confirmed COVID-19 pneumonia by both SARS-CoV-2 RT-PCR and chest computed tomography among suspected patients. Underlying diseases including hypertension, diabetes mellitus, congestive heart failure, chronic artery disease, chronic renal failure and cerebrovascular disease, which were significantly more frequent in deceased patients, were defined as a new variable named ‘any comorbidity’. Mortality was defined as all-cause in-hospital death.
The primary outcome of this study was in-hospital death. Secondary outcome was ICU admission or in-hospital death. Once the novel score was developed, the predictive ability of the novel score for both outcomes was compared with CURB-65, NEWS2 and qSOFA.
Continuous variables were described as median ± interquartile range, whereas categorical variables were described as numbers and percentages. Chi-square and Fisher’s exact tests were performed to compare categorical variables. The independent sample t-test was performed for variables with normal distribution, whereas the Mann–Whitney U test was performed for variables without normal distribution. The optimal cut-off values of the independent variables were calculated by Youden’s index of the receiver operating characteristic (ROC) curve. ROC analyses defined cut-off values for candidate variables were performed before univariate and multivariate analyses regarding survival. Univariate analysis was performed, and all significant variables except parameters with high percentages (>20%) of missing values were included in multivariable logistic regressions. Odds ratio (OR) values with 95% CI were calculated. Independent predictors were identified using multivariate logistic regression analysis. When there was a strong correlation, only the variable with a high contribution to the model was included. OR values of the variables in a nomogram were used to assign score points. Score points were rounded to the nearest 0.5 or whole number, whichever was closer, to make the calculation easier. In addition, ROC analysis with area under the curve (AUC) was used to evaluate the performance of the novel score. Statistical Package for Social Sciences (SPSS) version 20.0 was used for statistical analyses. A p-value <0.05 was considered statistically significant.
All procedures were performed in accordance with the ethical standards of the Declaration of Helsinki and National Research Committee. This study was approved by the Ethics Committee of Haseki Training and Research Hospital (approval number: 2020–239; date: 23 December 2020). Written informed consent was waived given the retrospective nature of this study.
Results
A total of 1013 patients with COVID-19 were included. The median age was 61 ± 21 years, and 581 (57.4%) patients were male. A total of 672 patients (66.3%) had at least one comorbidity. Increased age was significantly associated with mortality (p < 0.001). The most common comorbidities were hypertension (n = 428, 42.3%) and diabetes mellitus (n = 323, 31.9%). Diabetes mellitus (41.1% vs 30.6%, p = 0.02), hypertension (58.1% vs 40.0%, p < 0.001), congestive heart failure (16.1% vs 4.3%, p = 0.04), chronic artery disease (21.8% vs 13.0%, p = 0.01), chronic renal failure (16.1% vs 4.3%, p < 0.001) and cerebrovascular disease (6.5% vs 2.3%, p = 0.01) were more frequent in deceased patients compared with survived patients. The median length of hospital stay was 9 ± 7 days. The median duration from hospitalization to death was 13 ± 11 days. A total of 331patients (32.7%) had severe or critical COVID-19 on admission defined by WHO criteria. A total of 175 (17.3%) patients were admitted to the ICU. In-hospital death occurred in 124 (12.2%) patients. A total of 18 patients (14.5%) died during the first week of hospitalization. Demographic characteristics of survived and deceased patients with COVID-19 are available in Table 1.
Table 1. Demographic characteristics and clinical outcomes of patients with COVID-19.
| Parameters | Mortality | |||
|---|---|---|---|---|
| No |
Yes |
Total |
p-value | |
| n (%) | n (%) | n (%) | ||
| Age (years) | <0.001 | |||
| – IQR | 21 | 17 | 21 | |
| – Median | 59 | 71 | 61 | |
| Age group (years) | ||||
| – 18–64 | 574 (64.6) | 42 (33.9) | 616 (60.8) | <0.001 |
| – 65–74 | 191 (21.5) | 37 (29.8) | 228 (22.5) | |
| – 75–84 | 98 (11) | 27 (21.8) | 125 (12.3) | |
| – >84 | 26 (2.9) | 18 (14.5) | 44 (4.3) | |
| Gender | ||||
| – Male | 516 (58) | 65 (52.4) | 581 (57.4) | 0.24 |
| – Female | 373 (42) | 59 (47.6) | 432 (42.6) | |
| Underlying diseases | 569 (64) | 103 (83) | 672 (66.3) | <0.001 |
| – COPD | 20 (2.3) | 4 (3.2) | 24 (2.4) | 0.52 |
| – Diabetes mellitus | 272 (30.6) | 51 (41.1) | 323 (31.9) | 0.02 |
| – Hypertension | 356 (40.0) | 72 (58.1) | 428 (42.3) | <0.001 |
| – Congestive heart failure | 36 (4.0) | 10 (8.1) | 46 (4.5) | 0.04 |
| – Chronic artery disease | 116 (13.0) | 27 (21.8) | 143 (14.1) | 0.01 |
| – Chronic renal failure | 38 (4.3) | 20 (16.1) | 58 (5.7) | <0.001 |
| – Malignancy | 21 (2.4) | 6 (4.8) | 27 (2.7) | 0.11 |
| – Cerebrovascular disease | 20 (2.3) | 8 (6.5) | 28 (2.8) | 0.01 |
| – Rheumatological disease | 18 (2) | 5 (4) | 23 (2.3) | 0.16 |
| – Neurological disorder | 16 (1.8) | 5 (4) | 21 (2) | 0.10 |
| Clinical outcomes | ||||
| – Invasive ventilation | 19 (2.1) | 116 (93.6) | 135 (13.3) | <0.001 |
| – ICU admission | 57 (6.4) | 118 (95.2) | 175 (17.3) | <0.001 |
| – Dialysis | 1 (0.1) | 10 (8.1) | 11 (1.1) | <0.001 |
| – Noninvasive ventilation | 57 (6.4) | 115 (92.7) | 172 (17) | <0.001 |
COPD: Chronic obstructive pulmonary disease; ICU: Intensive care unit; IQR: Interquartile range.
The most common symptoms in the cohort were dyspnea (n = 651, 64.3%), cough (n = 581, 57.4%), fatigue (n = 505, 49.9%) and fever (n = 274, 27.0%). Dyspnea (72.6% vs 63.1%, p = 0.04), vomiting (16.1% vs 8.2%, p = 0.01), diarrhea (9.7% vs 5.3%, p = 0.05) and confusion (5.3% vs 0.7%, p < 0.001) were more frequent in deceased patients compared with survived patients. Median respiratory rate (24 ± 8 vs 20 ± 4, p < 0.001) and SpO2 on room air (88 ± 10 vs 92 ± 6, p < 0.001) were higher in deceased patients than survived patients. Comparison of clinical presentations of survived and deceased patients with COVID-19 are represented in Table 2.
Table 2. Clinical presentations of patients with COVID-19.
| Symptoms | Mortality | |||
|---|---|---|---|---|
| No |
Yes |
Total |
p-value | |
| n (%) | n (%) | n (%) | ||
| Fever | 249 (28) | 25 (20.2) | 274 (27) | 0.07 |
| Cough | 514 (57.8) | 67 (54) | 581 (57.4) | 0.43 |
| Dyspnea | 561 (63.1) | 90 (72.6) | 651 (64.3) | 0.04 |
| Chest pain | 44 (5) | 3 (2.4) | 47 (4.6) | 0.21 |
| Myalgia | 164 (18.5) | 24 (19.4) | 188 (18.6) | 0.81 |
| Arthralgia | 114 (12.8) | 20 (16.1) | 134 (13.2) | 0.31 |
| Fatigue | 435 (48.9) | 70 (56.5) | 505 (49.9) | 0.12 |
| Sore throat | 26 (2.9) | 7 (5.7) | 33 (3.3) | 0.11 |
| Abdominal pain | 32 (3.6) | 8 (6.5) | 40 (4) | 0.13 |
| Nausea | 133 (15) | 23 (18.6) | 156 (15.4) | 0.30 |
| Vomiting | 73 (8.2) | 20 (16.1) | 93 (9.2) | 0.01 |
| Diarrhea | 47 (5.3) | 12 (9.7) | 59 (5.8) | 0.05 |
| Anosmia | 46 (5.2) | 8 (6.5) | 54 (5.3) | 0.55 |
| Ageusia | 45 (5.1) | 7 (5.7) | 52 (5.1) | 0.78 |
| Confusion | 6 (0.7) | 5 (4.3) | 11 (1.1) | 0.001 |
| Vital signs | Median (IQR) | Median (IQR) | Median (IQR) | p-value |
|---|---|---|---|---|
| Heart rate/min | 86 (18) | 86 (22) | 86 (18) | 0.83 |
| SpO2 (%) | 92 (6) | 88 (10) | 92 (7) | <0.001 |
| Body temperature (°C) | 36.6 (0.8) | 36.6 (0.9) | 36.6 (0.8) | 0.66 |
| Systolic blood pressure (mmHg) | 120 (20) | 120 (24) | 120 (20) | 0.60 |
| Diastolic blood pressure (mmHg) | 70 (10) | 70 (20) | 70 (10) | 0.44 |
| Respiratory rate/min | 20 (4) | 24 (8) | 20 (6) | <0.001 |
IQR: Interquartile range; SpO2: Peripheral capillary oxygen saturation.
Median neutrophil count (5725 ± 4410 vs 4870 ± 3560, p < 0.001), neutrophil to lymphocyte ratio (4.31 ± 5.97 vs 3.89 ± 4.15, p < 0.001), C-reactive protein (CRP) (104 ± 102 vs 81 ± 87, p < 0.001), procalcitonin (0.3 ± 0.5 vs 0.1 ± 0.1, p < 0.001), D-dimer (1.3 ± 2.1 vs 0.8 ± 0.9, p < 0.001), creatinine (1.2 ± 0.8 vs 0.9 ± 0.4, p < 0.001), urea (51 ± 46 vs 32 ± 19, p < 0.001), lactate dehyrogenase (393 ± 226 vs 338 ± 167, p < 0.001) and urea to albumin ratio (1.47 ± 1.90 vs 0.87 ± 0.59, p < 0.001) were significantly higher in patients who died than those who survived. Median lymphocyte count (920 ± 680 vs 1170 ± 750, p < 0.001), platelet count (179 ± 100 vs 206 ± 109, p = 0.01), glomerular filtration rate (56 ± 49 vs 85 ± 34, p < 0.001) and albumin (34 ± 7 vs 37 ± 5, p < 0.001) were significantly lower in deceased patients than survived patients. Comparison of the laboratory parameters with the median and interquartile range values are represented in Table 3.
Table 3. Baseline laboratory parameters on admission in patients with COVID-19.
| Parameters |
Mortality | ||
|---|---|---|---|
| Leukocyte count (/μl) | No | Yes | Total |
| IQR | 3800 | 4620 | 3960 |
| Median | 6750 | 7020 | 6800 |
| p-value | 0.100 | ||
| Lymphocyte count (/μl) | No | Yes | Total |
|---|---|---|---|
| IQR | 750 | 680 | 750 |
| Median | 1170 | 920 | 1140 |
| p-value | <0.001 | ||
| Neutrophil count (/μl) | No | Yes | Total |
|---|---|---|---|
| IQR | 3560 | 4410 | 3710 |
| Median | 4870 | 5725 | 4935 |
| p-value | <0.001 | ||
| Platelet count × 103 | No | Yes | Total |
|---|---|---|---|
| IQR | 109 | 100 | 108 |
| Median | 206 | 179 | 204 |
| p-value | <0.010 | ||
| Neutrophil/lymphocyte ratio | No | Yes | Total |
|---|---|---|---|
| IQR | 4.15 | 5.97 | 4.38 |
| Median | 3.89 | 4.31 | 4.15 |
| p-value | <0.001 | ||
| Platelet/lymphocyte ratio | No | Yes | Total |
|---|---|---|---|
| IQR | 0.127 | 0.157 | 0.129 |
| Median | 01171 | 0.194 | 0.173 |
| p-value | 0.04 | ||
| C-reactive protein (mg/l) | No | Yes | Total |
|---|---|---|---|
| IQR | 87 | 102 | 89 |
| Median | 81 | 104 | 83 |
| p-value | <0.001 | ||
| Procalcitonin (ng/ml) | No | Yes | Total |
|---|---|---|---|
| IQR | 0.1 | 0.5 | 0.1 |
| Median | 0.1 | 0.3 | 0.1 |
| p-value | <0.001 | ||
| D-dimer (μg/l) | No | Yes | Total |
|---|---|---|---|
| IQR | 0.9 | 2.1 | 1 |
| Median | 0.8 | 1.3 | 0.8 |
| p-value | <0.001 | ||
| Ferritin (ng/ml) | No | Yes | Total |
|---|---|---|---|
| IQR | 500 | 900 | 533 |
| Median | 385 | 422 | 394 |
| p-value | 0.11 | ||
| Creatinine (mg/dl) | No | Yes | Total |
|---|---|---|---|
| IQR | 0.4 | 0.8 | 0.4 |
| Median | 0.9 | 1.2 | 0.9 |
| p-value | <0.001 | ||
| GFR | No | Yes | Total |
|---|---|---|---|
| IQR | 34 | 49 | 39 |
| Median | 85 | 56 | 84 |
| p-value | <0.001 | ||
| Urea (mg/dl) | No | Yes | Total |
|---|---|---|---|
| IQR | 19 | 46 | 22 |
| Median | 32 | 51 | 33 |
| p-value | <0.001 | ||
| Lactate dehydrogenase (U/l) | No | Yes | Total |
|---|---|---|---|
| IQR | 167 | 226 | 174 |
| Median | 338 | 393 | 345 |
| p-value | <0.001 | ||
| Fibrinogen (mg/dl) | No | Yes | Total |
|---|---|---|---|
| IQR | 206 | 188 | 206 |
| Median | 572 | 561 | 571 |
| p-value | 0.51 | ||
| Albumin (g/dl) | No | Yes | Total |
|---|---|---|---|
| IQR | 5 | 7 | 6 |
| Median | 37 | 34 | 36 |
| p-value | <0.001 | ||
| Urea/albumin ratio | No | Yes | Total |
|---|---|---|---|
| IQR | 0.59 | 1.9 | 0.69 |
| Median | 0.87 | 1.47 | 0.92 |
| p-value | <0.001 | ||
| Troponin (pg/ml) | No | Yes | Total |
|---|---|---|---|
| IQR | 4 | 9 | 5 |
| Median | 0 | 0 | 0 |
| p-value | <0.001 | ||
GFR: Glomerular filtration rate; IQR: Interquartile range.
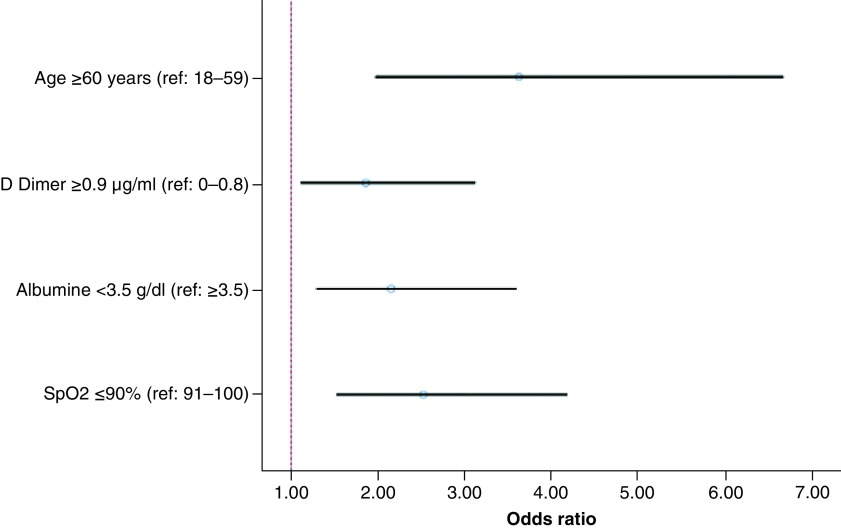
In univariate analysis, increased age (OR = 4.63; CI = 2.93–7.32; p < 0.001), presence of dyspnea (OR = 1.55; CI = 1.02–2.35; p = 0.040) and confusion (OR = 6.18; CI = 1.89–20.57; p = 0.003), increased respiratory rate (OR = 4.06; CI = 2.42–6.84; p < 0.001), decreased SpO2 (OR = 3.50; CI = 2.38–5.15; p < 0.001), presence of any comorbidity (OR = 2.39; CI = 1.56–3.66; p < 0.001), high levels of platelet to lymphocyte ratio (OR = 1.54; CI = 1.06–2.24; p = 0.025) and D-dimer (OR = 2.66; CI = 1.73–4.09; p < 0.001), and a low level of albumin (OR = 3.63; CI = 2.32–5.69; p < 0.001) were associated with increased mortality. Multivariate analysis revealed that SpO2 (OR = 2.52; CI = 1.52–4.18; p < 0.001), albumin (OR = 2.15; CI = 1.29–3.59; p = 0.003), D-dimer (OR = 1.86; CI = 1.11–3.12; p = 0.019) and age (OR = 3.62; CI = 1.97–6.66; p < 0.001) were independent predictors for mortality (Table 4). OR and CI (95%) values for each predictor are demonstrated in the Forest plot diagram (Figure 1).
Table 4. Univariate and multivariate analysis of factors predicting mortality.
| Logistic regression | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | CI | p-value | OR | CI | p-value | |
| Age ≥60 years | 4.63 | 2.93–7.32 | <0.001 | 3.62 | 1.97–6.66 | <0.001 |
| Dyspnea | 1.55 | 1.02–2.35 | 0.040 | 1.00 | 0.56–1.79 | 0.992 |
| Confusion | 6.18 | 1.89–20.57 | 0.003 | 8.05 | 0.72–89.84 | 0.090 |
| Respiratory rate ≥28/min | 4.06 | 2.42–6.84 | <0.001 | 1.74 | 0.82–3.73 | 0.151 |
| SpO2 ≤90% | 3.50 | 2.38–5.15 | <0.001 | 2.52 | 1.52–4.18 | <0.001 |
| Any comorbidity† | 2.39 | 1.56–3.66 | <0.001 | 1.31 | 0.72–2.37 | 0.373 |
| Platelet/lymphocyte ratio ≥190 | 1.54 | 1.06–2.24 | 0.025 | 1.07 | 0.63–1.80 | 0.801 |
| Albumin <3.5 g/dl | 3.63 | 2.32–5.69 | <0.001 | 2.15 | 1.29–3.59 | 0.003 |
| D-dimer ≥0.9 μg/ml | 2.66 | 1.73–4.09 | <0.001 | 1.86 | 1.11–3.12 | 0.019 |
Includes diabetes mellitus, hypertension, congestive heart failure, chronic artery disease, chronic renal failure and cerebrovascular disease.
OR: Odds ratio; SpO2: Peripheral capillary oxygen saturation.
Figure 1. Forest plot diagram based on multivariate analysis of mortality predictors in patients with COVID-19 pneumonia.
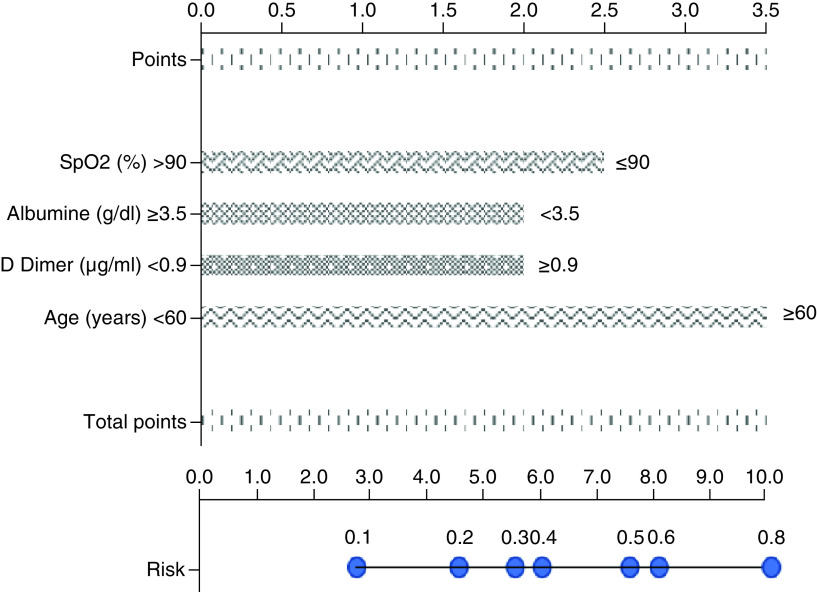
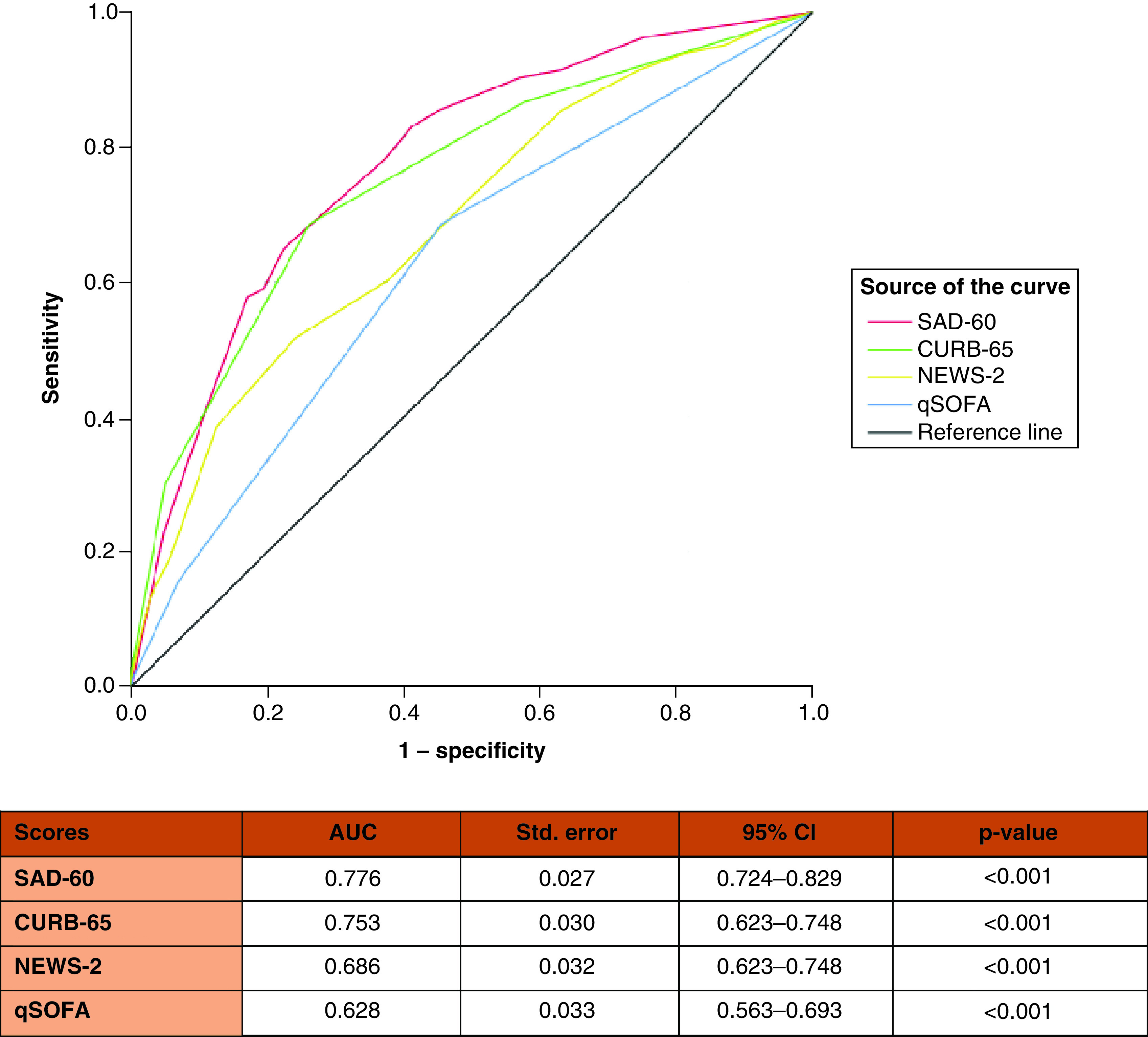
A nomogram was developed based on four independent predictors in multivariable analysis. The score point of each parameter was determined according to its OR values. The total point, which varied from 0 to 10, was calculated by summing the points obtained from each parameter. The risk of death by total points is demonstrated in the nomogram in Figure 2. The mortality score model was given the acronym SAD-60, representing SpO2, Albumin, D-dimer, age ≥60 years. The SAD-60 score (0.776) had the highest AUC compared with CURB-65 (0.753), NEWS2 (0.686) and qSOFA (0.628) scores (Figure 3). The risk of death was higher than 75% in patients with more than 8 points and lower than 25% in patients with fewer than 5.5 points.
Figure 2. Nomogram predicting in-hospital mortality in patients with COVID-19 pneumonia.
Figure 3. Comparison of CURB-65, qSOFA, NEWS-2 and SAD-60 for predicting mortality in hospitalized patients with COVID-19 pneumonia by receiver operating characteristic analysis.
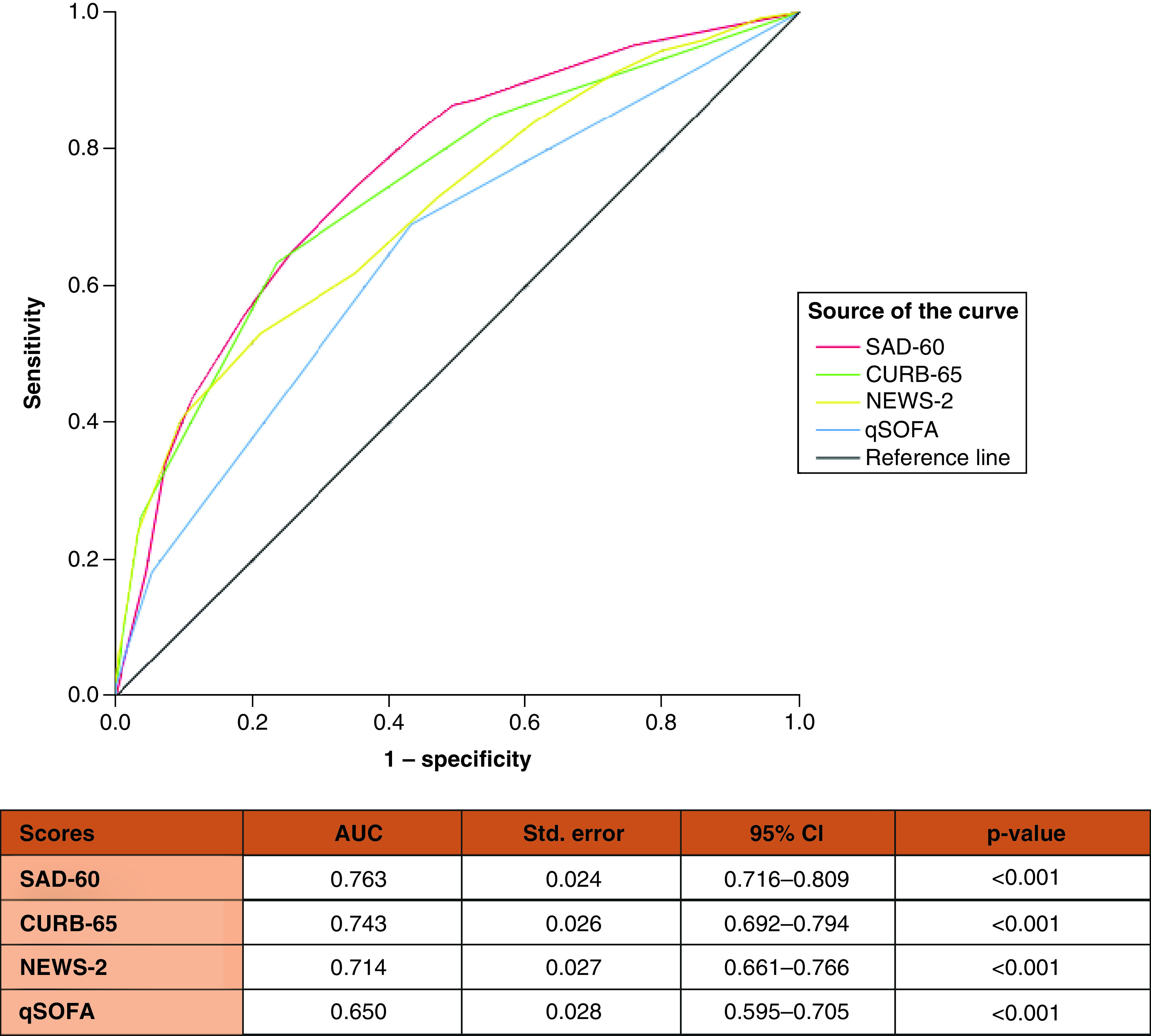
Additionally, the predictive ability of the SAD-60 score for ICU admission or in-hospital death was assessed and compared with CURB-65, NEWS2 and qSOFA. The results are demonstrated in Figure 4.
Figure 4. Comparison of CURB-65, qSOFA, NEWS-2 and SAD-60 for predicting intensive care admission or in-hospital death in patients with COVID-19 pneumonia by receiver operating characteristic analysis.
Discussion
In this study, we presented a detailed analysis of 1013 patients with COVID-19 pneumonia in a multicenter retrospective cohort study and created a simple prediction model based on two biomarkers of albumin and D-dimer, as well as the clinical features of age and SpO2. The SAD-60 score that was derived from the model in the present study had a promising predictive capacity for mortality in hospitalized patients with COVID-19. Age [23], SpO2 [11], albumin [24] and D-dimer [25,26] were demonstrated as independent predictors for mortality in patients with COVID-19 in different previous studies. However, to our knowledge, this is the first study to combine these four parameters to predict mortality in hospitalized patients with COVID-19 pneumonia. In multivariate analysis, increased age, SpO2, albumin and D-dimer were associated with about 3.5-fold, 2.5-fold, twofold and twofold increased risk for mortality, respectively.
Previous studies have identified that underlying diseases are one of the risk factors for mortality [27,28]. In the present study, although underlying diseases were associated with in-hospital death, they were not detected as independent predictors in multivariate analysis.
There is an increased number of studies investigating clinical deterioration and mortality predictors of COVID-19 [27–31]. Acar et al. reported that 11% of COVID-19 patients (n = 75/709) died; in their study, the independent predictors of mortality were specific comorbidities, dyspnea, SpO2, hematocrit, CRP, aspartate aminotransferase and ferritin. They developed a novel score with the combination of these seven predictors in addition to age. In the study of Guner et al., 15.2% of COVID-19 patients (n = 104/686) transferred to the ICU [29]. In their final model, the independent predictors of the need for ICU transfer were SpO2, CRP, procalcitonin, lactate dehidyrogenase and troponin. Guner et al. reported a good predictive value in the ROC analysis (AUC = 0.93; CI = 0.90–0.95) [29]. Bayram et al. [30] developed a novel score named CAPA, which allows for the prediction of mortality and ICU admission in patients with COVID-19. They reported that the AUC values of the CAPA score in predicting mortality and ICU admission were 0.67 and 0.66, respectively. In our study, the AUC values of the SAD-60 score in predicting mortality and ICU admission were 0.776 and 0.763, respectively. However, these studies were conducted in single tertiary care centers. Liang et al. [31] established the COVID-GRAM score with a cohort of 1590 COVID-19 patients from 575 hospitals in China and demonstrated that the mean AUC was 0.88 (CI = 0.85–0.91) in the development cohort.
Although most patients have mild or moderate disease, COVID-19 can progress to severe disease and result in acute respiratory distress syndrome, multiorgan failure, septic shock and death [2]. Therefore, early stratifying of COVID-19 patients based on disease severity is vital. CURB-65 has been the widely used scoring system for severity classification, outcome and mortality prediction of community-acquired pneumonia. NEWS2 has been recommended by the National Institute of Clinical Excellence (NICE) for the prediction of clinical deterioration in patients with COVID-19 [32]. However, although there are studies to identify risk factors for disease progression and to develop scoring models in patients with COVID-19, no concensus has been reached [18,33–35]. In addition, previous reports do not identify cut-off values of continues variables [36] or have not used combined parameters similar to our previous report (blinded). Even if studies categorize continuous variables, they do not stratify patients according to mortality risk by scoring [37,38]. These issues cause difficulties in calculation of the risk by physicians. In the present study, a simplified nomogram was used to assess the risk of mortality in patients with COVID-19 pneumonia.
The SAD-60 score, which was derived from the model in the present study, could be helpful for detecting patients at high risk of clinical deterioration and mortality. This might improve patient outcomes by enhancing physicians clinical decision making. Indicators of inflammation could be useful for predicting prognosis in patients with COVID-19. Our biomarker-based model incorporated albumin and D-dimer. Recent studies have confirmed that these biomarkers provide substantive information about clinical deterioration and the risk of mortality [25,26,38–40]. In addition, some studies revealed the role of albumin in COVID-19 prognostication reflecting both possible liver damage, inflammation and the nutritional status of patients [41,42].
This study has several strengths. First, this was a multicenter study conducted in six hospitals with 1013 patients with COVID-19. Second, different types of variables, such as multiple comorbidities, symptoms, vital signs and laboratory parameters, were included in the multivariate regression analysis. Moreover, as laboratory parameters were routinely tested in all six hospitals, we could collect biomarkers probably associated with disease severity. Third, we had a relatively a large sample size.
This study has several limitations. First, our study was retrospectively conducted. Second, external validation was not performed. The generalizability of the results might be limited even with this being a multicenter study. Therefore, we need new large-scale studies to further improve the robustness of this model. Last, we did not perform longitudinal evaluation of vital signs and laboratory parameters.
A part of this study was presented at IDWeek-2021. The abstract, Table 4 & Figure 3 were published in the journal Open Forum Infectious Diseases [43].
Conclusion
We created a simple prediction model based on two biomarkers, as well as the clinical features of age and SpO2, and demonstrated that the SAD-60 score has promising predictive capacity for mortality in hospitalized patients with COVID-19. Thus, patients with high risk scores at admission should be carefully monitored and preventive strategies should be implemented to reduce mortality.
Summary points.
The pandemic of COVID-19 continues to be a significant public health issue.
In this retrospective multicenter study, the aim was to explore a novel risk score to predict mortality in hospitalized patients with COVID-19 pneumonia. In addition, the accuracy of the novel risk score with CURB-65, qSOFA and NEWS2 scores was compared.
A total of 1013 patients with COVID-19 were included. In-hospital death occurred in 124 (12.2%) patients.
Multivariate analysis revealed that peripheral capillary oxygen saturation, albumin, D-dimer and age were independent predictors for mortality.
The mortality score model was given the acronym SAD-60, representing SpO2, Albumin, D-dimer, age ≥60 years.
The SAD-60 score (0.776) had the highest area under the curve compared with CURB-65 (0.753), NEWS2 (0.686) and qSOFA (0.628) scores.
The SAD-60 score has a promising predictive capacity for mortality in hospitalized patients with COVID-19.
Author contributions
S Surme, G Tuncer and OF Bayramlar proposed the concept, designed the study, wrote the protocol and managed the study. S Surme, G Tuncer, B Copur, OF Bayramlar and YE Ozdemir performed the statistics, interpreted the data and wrote the manuscript. S Surme, G Tuncer, B Copur, E Zerdali, IY Nakir, M Alkan, A Buyukyazgan, ARK Cinar, Y Kurekci and YE Ozdemir were involved in collecting the data. G Tuncer, B Copur, YE Ozdemir, G Sengoz and F Pehlivanoglu performed a critical review of the manuscript. All authors provided inputs for revision of the manuscript. S Surme communicated with the journal and addressed comments from reviewers. All authors contributed to data acquisition, data analysis or data interpretation, and reviewed and approved the final version.
Acknowledgments
The authors acknowledge all healthcare professionals who contribute to the care of patients.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All procedures were performed in accordance with the ethical standards of the Declaration of Helsinki and National Research Committee. This study was approved by the Ethics Committee of Haseki Training and Research Hospital (approval number: 2020–239; date: 23 December 2020). Written informed consent was waived given the retrospective nature of this study.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.WHO. Weekly Epidemiological Update on COVID-19 - 30 November 2021 (Accessed 3 December 2021). https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---30-november-2021 ; •• Highlights weekly epidemiological reports on COVID-19.
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72–314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323(13), 1239–1242 (2020). [DOI] [PubMed] [Google Scholar]; •• Reports clinical characteristics of COVID-19 cases by a large-scale surveillance.
- 3.Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl. J. Med. 382, 1708–1720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8, 475–481 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 395, 689–697 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu XW, Wu XX, Jiang XG et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 368, m606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen T, Wu D, Chen H et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368, m1091 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang M, Li C, Zheng L et al. A biomarker-based age, biomarkers, clinical history, sex (ABCS)-mortality risk score for patients with coronavirus disease 2019. Ann. Transl. Med. 9(3), 230 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Li L, Song K et al. A nomogram for predicting mortality in patients with COVID-19 and solid tumors: a multicenter retrospective cohort study. J. Immunother. Cancer 8(2), e001314 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surme S, Buyukyazgan A, Bayramlar OF et al. Predictors of intensive care unit admission or mortality in patients with coronavirus disease 2019 pneumonia in Istanbul, Turkey. Jpn J. Infect. Dis. 74(5), 458–464 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Suleyman G, Fadel RA, Malette KM et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw. Open 3(6), e2012270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G, Greco M, Zanella A, COVID-19 Lombardy ICU Network et al. Risk Factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 181(7), 1021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satici C, Demirkol MA, Sargin Altunok E et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int. J. Infect. Dis. 98, 84–89 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Hu ZW, Hu Y et al. Comparison of severity classification of Chinese protocol, pneumonia severity index and CURB-65 in risk stratification and prognostic assessment of coronavirus disease 2019. Zhonghua Jie. He. He Hu. Xi. Za. Zhi. 43(10), 834–838 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Zhou B, Zhu M et al. CURB-65 may serve as a useful prognostic marker in COVID-19 patients within Wuhan, China: a retrospective cohort study. Epidemiol. Infect. 148, e241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang JG, Hur J, Hong KS, Lee W, Ahn JH. Prognostic accuracy of the SIRS, qSOFA, and NEWS for early detection of clinical deterioration in SARS-CoV-2 infected patients. J. Korean Med. Sci. 35(25), e234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji D, Zhang D, Xu J et al. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin. Infect. Dis. 71(6), 1393–1399 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Establishes a new scoring system named the CALL score.
- 19.Varol Y, Hakoglu B, Kadri CA et al. The impact of Charlson comorbidity index on mortality from SARS-CoV-2 virus infection and A novel COVID-19 mortality index: coLACD. Int. J. Clin. Pract. 75, e13858 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancilla-Galindo J, Vera-Zertuche JM, Navarro-Cruz AR et al. Development and validation of the patient history COVID-19 (PH Covid19) scoring system: a multivariable prediction model of death in Mexican patients with COVID-19. Epidemiol. Infect. 148, e286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imran MM, Ahmad U, Usman U, Ali M, Shaukat A, Gul N. Neutrophil/lymphocyte ratio – a marker of COVID-19 pneumonia severity. Int. J. Clin. Pract. 75, e13698 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Zhang S, Hu H, Liu T, Huang J. The role of neutrophil–lymphocyte ratio and lymphocyte–monocyte ratio in the prognosis of type 2 diabetics with COVID-19. Scott. Med. J. 65, 154–160 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821), 430–436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuncer G, Surme S, Bayramlar OF et al. National Early Warning Score 2 and laboratory predictors correlate with clinical deterioration in hospitalized patients with COVID-19. Biomark. Med. 15(11), 807–820 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reveals that National Early Warning Score 2, procalcitonin, neutrophil to lymphocyte ratio and albumin are the best predictors for clinical deterioration.
- 25.Zhang L, Yan X, Fan Q et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 18(6), 1324–1329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao D, Zhou F, Luo L et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 7(9), 671–678 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acar HC, Can G, Karaali R et al. An easy-to-use nomogram for predicting in-hospital mortality risk in COVID-19: a retrospective cohort study in a university hospital. BMC Infect Dis. 21(1), 148 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu R, Ai S, Cai J et al. Predictive model and risk factors for case fatality of COVID-19: a cohort of 21,392 cases in Hubei, China. Innovation (NY). 1(2), 100022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guner R, Kayaaslan B, Hasanoglu I et al. Development and validation of nomogram to predict severe illness requiring intensive care follow up in hospitalized COVID-19 cases. BMC Infect. Dis. 21(1), 1004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayram M, Yildirim O, Ozmen R S et al. Prognostic Nutritional Index and CRP, age, platelet count, albumin level score in predicting mortality and intensive care unit admission for COVID-19. Biomark. Med. 17, 1733–1740 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is the first report to identify the Prognostic Nutritional Index as a predictor for mortality and intensive care admission in patients with COVID-19.
- 31.Liang W, Liang H, Ou L, China Medical Treatment Expert Group for COVID-19 et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 180(8), 1081–1089 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenhalgh T, Treadwell J, Burrow R. NEWS (or NEWS2) score when assessing possible COVID-19 patients in primary care? Centre for Evidence-Based Medicine (2020). www.cebm.net/covid-19/should-we-use-the-news-or-news2-score-when-assessing-patients-with-possible-covid-19-in-primary-care [Google Scholar]
- 33.Chu K, Alharahsheh B, Garg N, Guha P. Evaluating risk stratification scoring systems to predict mortality in patients with COVID-19. BMJ Health. Care Inform. 28(1), e100389 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguirre-García GM, Ramonfaur D, Torre-Amione G et al. Stratifying risk outcomes among adult COVID-19 inpatients with high flow oxygen: the R4 score. Pulmonology. (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sze S, Pan D, Williams CML et al. Letter to the Editor: variability but not admission or trends in NEWS2 score predicts clinical outcome in elderly hospitalised patients with COVID-19. J. Infect. 82(1), 159–198 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagadinou M, Salomou EE, Zareifopoulos N, Marangos M, Gogos C, Velissaris D. Prognosis of COVID-19: changes in laboratory parameters. Infez. Med. 28(Suppl. 1), 89–95 (2020). [PubMed] [Google Scholar]; •• Reveals prognostic indicators for COVID-19 including some laboratory parameters but does not detect cut-off values.
- 37.Xu JB, Xu C, Zhang RB et al. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci. Rep. 10(1), 15058 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang W, Li Y, Li H et al. Correlation between laboratory parameters on admission and outcome of COVID-19 in maintenance hemodialysis patients. Int. Urol. Nephrol. 53(1), 165–169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W, Tao ZW, Wang L et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. (Engl.) 133(9), 1032–1038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit. Care 24(1), 255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucijanić M, Stojić J, Atić A et al. Clinical and prognostic significance of C-reactive protein to albumin ratio in hospitalized coronavirus disease 2019 (COVID-19) patients: data on 2309 patients from a tertiary center and validation in an independent cohort. Wien Klin Wochenschr. (2022) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paštrovic F, Lucijanic M, Atic A et al. Prevalence and prognostic impact of deranged liver blood tests in COVID-19: experience from the regional COVID-19 center over the cohort of 3812 hospitalized patients. J. Clin. Med. 10(18), 4222 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surme S, Karanalbant HK, Tuncer G, Bayramlar O, Copur B, Zerdali E et al. Development a novel score (SAD-60) for predicting mortality in hospitalised patients with COVID-19 pneumonia: a multicenter retrospective study of 1013 patients. Open Forum Infect. Dis. 8(Suppl. 1), 21–22 (2021). [Google Scholar]