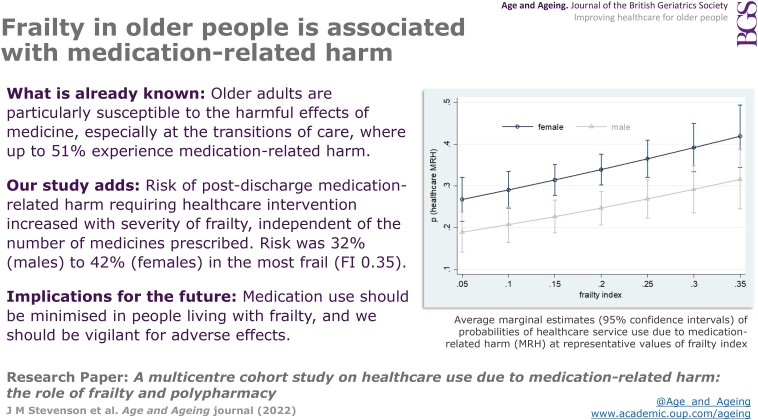
Abstract
Objectives
To determine the association between frailty and medication-related harm requiring healthcare utilisation.
Design
Prospective observational cohort study.
Setting
Six primary and five secondary care sites across South East England, September 2013–November 2015.
Participants
One thousand and two hundred and eighty participants, ≥65 years old, who were due for discharge from general medicine and older persons’ wards following an acute episode of care. Exclusion criteria were limited life expectancy, transfer to another hospital and consent not gained.
Main outcome measures
Medication-related harm requiring healthcare utilisation (including primary, secondary or tertiary care consultations related to MRH), including adverse drug reactions, non-adherence and medication error determined via the review of data from three sources: patient/carer reports gathered through a structured telephone interview; primary care medical record review; and prospective consultant-led review of readmission to recruiting hospital. Frailty was measured using a Frailty Index, developed using a standardised approach. Marginal estimates were obtained from logistic regression models to examine how probabilities of healthcare service use due to medication-related harm were associated with increasing number of medicines and frailty.
Results
Healthcare utilisation due to medication-related harm was significantly associated with frailty (OR = 10.06, 95% CI 2.06–49.26, P = 0.004), independent of age, gender, and number of medicines. With increasing frailty, the need for healthcare use as a result of MRH increases from a probability of around 0.2–0.4. This is also the case for the number of medicines.
Conclusions
Frailty is associated with MRH, independent of polypharmacy. Reducing the burden of frailty through an integrated health and social care approach, alongside strategies to reduce inappropriate polypharmacy, may reduce MRH related healthcare utilisation.
Keywords: adverse drug reactions, non-adherence, aged, frailty, risk stratification, older people
Graphical Abstract
Graphical Abstract.
Key Points
Frailty increases susceptibility to adverse outcomes, yet its influence on medication-related harm is under-investigated.
Frailty is associated with post-discharge medication-related harm requiring healthcare intervention, independent of polypharmacy.
Future strategies to reduce harm from medicines in older adults must consider frailty and avoid focusing on polypharmacy alone.
Introduction
Frailty and medication-related harm (MRH) are two major challenges that increase the risk of poor outcomes such as hospital admission and death in older adults [1]. MRH includes harm arising from adverse drug reactions (ADRs), non-adherence and medication errors and affects one in three older adults following an acute hospital episode, costing approximately £400 million to the National Health Service (NHS) [2]. The World Health Organisation (WHO) has challenged member states to reduce avoidable MRH by 50% by 2022 [3].
Frailty is defined as a state of increased vulnerability to suboptimal restoration of homoeostasis after a stressor event, and increases the risk of adverse outcomes [4]. Multiple models of frailty have been developed to measure frailty however, the phenotype model [5] and cumulative deficits (Frailty Index) [6] dominate the literature. Frailty affects 14% of adults >50 years old in the UK and the prevalence increases exponentially with age [7]. In the USA it is reported to be increasing across younger adults. Whilst the increased risk of frailty associated mortality remains stable; the proportion of adults living with frailty is rising [8].
In a secondary analysis of inpatient hospital admission data (n = 737) collected between 2012–13, the interaction between ADRs and frailty showed that frailty was more predictive of ADRs than the number of medicines alone [9]. Frailty was identified using a frailty index, consisting of 34 items across a range of domains (including polypharmacy). A higher frailty index was associated with a greater likelihood of potentially inappropriate prescriptions and of experiencing at least one ADR during hospital admission. Patients taking more than six medicines were three times more likely to have at least one instance of potentially inappropriate prescriptions, but ADR occurrence was not associated with the number of medicines [9].
Investigating the interaction between frailty and the broader context of MRH, beyond merely focusing on ADRs, might influence the way in which healthcare professionals approach mitigating MRH. Approaches to reducing MRH in older adults, have traditionally focused on polypharmacy, medicines appropriateness and medicines reconciliation, but have thus far demonstrated limited impact on quality of life, clinical or economic outcomes [10, 11]. The influence of frailty on MRH has been little explored, and crucially needs to be investigated independent of polypharmacy.
This study, therefore, aimed to determine whether frailty is independently associated with MRH requiring healthcare utilisation within a UK multi-centre prospective cohort study (The Predicting RIsk of Medication-related harm in Elderly (PRIME) study) [12]. The PRIME Study developed a risk-prediction model to identify older adults at risk of post-discharge MRH that was superior to routine clinical judgement [2, 12–14].
Methods
Study participants
The data presented here relate to a sub-study of the PRIME study, the methods for which are described in detail in the published protocol [12]. The PRIME study was approved by the National Research Ethics Service, East of England (REC Reference 13/EE/0075), and was funded by NIHR Research for Patient Benefit and Guy’s and St. Thomas’ Charity.
In summary, PRIME was a multi-centre prospective cohort study of patients over the age of 65 years old. Patients from medical wards of five teaching hospitals in South England were invited to participate, immediately prior to hospital discharge. Those transferred to another acute healthcare setting (excluding transfer to intermediate care facilities), had a short life expectancy and were unlikely to survive to the end of the study 8 week follow-up period, or lacked capacity with no nominated consultee were excluded.
Baseline data included demographic, clinical, functional, psychological and social data. MRH and associated healthcare utilisation (including primary, secondary or tertiary consultations relating to MRH) within 8-week post-discharge were identified by senior pharmacists using three sources: (i) primary care records; (ii) patient telephone interviews and (iii) prospective review of hospital readmission in conjunction with the admitting medical consultant. MRH included ADRs, non-adherence and medication errors.
Measures
Medication-related harm
MRH was classified as ‘doubtful’, ‘possible’, ‘probable’ or ‘definite’ and verified by an independent endpoint committee, consisting of two consultant geriatricians and a professor of clinical pharmacy. Events classified as ‘possible, probable and, definite’ were considered MRH.
Frailty
A post-hoc measurement of frailty in the PRIME Study cohort was conducted through the development of a Frailty Index using data collected as part of the original PRIME Study. The Frailty index (FI) was developed using the standardised approach described by Searle and colleagues [15]. Development of a FI was necessary because existing frailty measures, e.g. Fried frailty phenotype [5] and the Clinical Frailty Scale [6] were not routinely recorded, and the electronic FI was developed for primary care datasets [16] and therefore not appropriate for this hospital cohort. The approach adopted was based on the premise that the more health-related deficits a person has, the more likely they are to be frail [17]. The number of deficits were counted and a ratio of the total number of deficits accumulated to the number of deficits considered calculated. Consistency has been demonstrated in this approach, even when the number and type of deficits differ [17–24]; the frailer the person (that is, the higher the number of deficits), the more susceptible they are to adverse outcomes, including death [17].
Five criteria should be considered when developing a FI [15], four of which were appropriate for this study: (i) Variables must be deficits associated with health status; (ii) The prevalence of the deficit must increase with age; (iii) Deficits must not saturate too early; (iv) Deficits must cover a range of systems. A fifth criteria was suggested (serial use of a single FI in the same population should include the same items each time) but was not applicable to our study where the FI could only be applied during the one episode of inpatient admission.
Finally, in the development of a FI there is no set number of deficits to be included however at least 30–40 deficits is recommended, and generally the more variables included the more precise is the index [15].
Following these standards, a FI was developed for the PRIME study cohort including 55 deficits from multiple domains (morbidity, cognition, mood, strength and mobility, nutrition, daily function; Table 1). Furthermore, in accordance with the standardised approach, the relationship between frailty and mortality was determined with the aim of validating the FI developed in this study [15]. Kaplan–Meier plots were generated comparing survival in frail and non-frail patients using the established cutoff of 0.2, reflective of an individual approaching a frail state [5, 15, 20, 21]. Validity of our 55-item FI was demonstrated; frail patients had significantly reduced 18-month survival. Median survival of frail patients was 14.2 months (95% CI 13.8–14.8) compared to non-frail patients 16.6 months (95% CI 16.3–16.9) (Log-Rank test P < 0.001; Figure 1).
Table 1.
Domains and deficits included in Frailty Index
| Domains | Variable | Deficit coding |
|---|---|---|
| Morbidity | Charlson co-morbidities | |
| HIV/AIDS | Yes = 1, No = 0 | |
| Connective tissue disorder | Yes = 1, No = 0 | |
| Any dementia | Yes = 1, No = 0 | |
| Any malignancy | Yes = 1, No = 0 | |
| GI haematological | Yes = 1, No = 0 | |
| Any liver disease | Yes = 1, No = 0 | |
| CKD | Yes = 1, No = 0 | |
| Diabetes | Yes = 1, No = 0 | |
| Asthma/COPD | Yes = 1, No = 0 | |
| Hemiplegia | Yes = 1, No = 0 | |
| Previous stroke/TIA | Yes = 1, No = 0 | |
| Peripheral vascular disease | Yes = 1, No = 0 | |
| CHF | Yes = 1, No = 0 | |
| Previous MI | Yes = 1, No = 0 | |
| Other diagnosis | ||
| Glaucoma | Yes = 1, No = 0 | |
| Hypertension | Yes = 1, No = 0 | |
| IHD | Yes = 1, No = 0 | |
| Osteoporosis | Yes = 1, No = 0 | |
| Parkinsons | Yes = 1, No = 0 | |
| Seizures | Yes = 1, No = 0 | |
| Thyroid dysfunction | Yes = 1, No = 0 | |
| Peripheral neuropathy | Yes = 1, No = 0 | |
| Other cardiac disease | Yes = 1, No = 0 | |
| Anaemia | Yes = 1, No = 0 | |
| Hiatus hernia | Yes = 1, No = 0 | |
| Gout | Yes = 1, No = 0 | |
| TB | Yes = 1, No = 0 | |
| VTE | Yes = 1, No = 0 | |
| Atrial fibrillation | Yes = 1, No = 0 | |
| Depression | Yes = 1, No = 0 | |
| Hyperlipidaemia | Yes = 1, No = 0 | |
| Sensory | ||
| Impaired hearing (and not corrected with hearing aid) | Yes = 1, No = 0 | |
| Retinopathy | Yes = 1, No = 0 | |
| Eyesight impaired (and not corrected with glasses) | Yes = 1, No = 0 | |
| Falls | ||
| ≥2 falls in 12 months | Yes = 1, No = 0 | |
| Laboratory | ||
| Hyponatraemia (Na < 135 mmol/l) | Yes = 1, No = 0 | |
| Abnormal Hb: Female <121 g/, Male <138 g/l | Yes = 1, No = 0 | |
| Cognition | AMTS < 8 | Yes = 1, No = 0 |
| Anxiety screen GAD-2 ≥ 3 | Yes = 1, No = 0 | |
| Depression screen PHQ-2 ≥ 3 | Yes = 1, No = 0 | |
| Strength | Grip strength: female <14 kg, male <24 kg# | Yes = 1, No = 0 |
| Function* | Help with stairs | Yes = 1, No = 0 |
| Help with mobility on level surfaces | Yes =1, No = 0 | |
| Help with transfers bed to chair and back | Yes =1, No = 0 | |
| Help with toilet use | Yes =1, No = 0 | |
| Help with bladder | Yes =1, No = 0 | |
| Help with bowels | Yes =1, No = 0 | |
| Help with dressing | Yes =1, No = 0 | |
| Help with grooming | Yes =1, No = 0 | |
| Help with bathing | Yes =1, No = 0 | |
| Help with feeding | Yes =1, No = 0 | |
| Nutrition | MUST ≥ 1 | Yes =1, No = 0 |
| Albumin < 35 g/l | Yes =1, No = 0 | |
| Social | Has social care package (any frequency) | Yes =1, No = 0 |
| Use of multi-compartment medicines compliance aid | Yes =1, No = 0 | |
#Median grip strength of study population stratified by gender used, to allow differentiation in a hospitalised and likely deconditioned population. About 77.4% of females and 68.1% of males would be described as sarcopenic according to EWGSOP 2010 [25] cutoff of <20 kg and <30 kg, respectively.
* Barthel index; 1 = dependent/needs help, 0 = independent.
Figure 1.
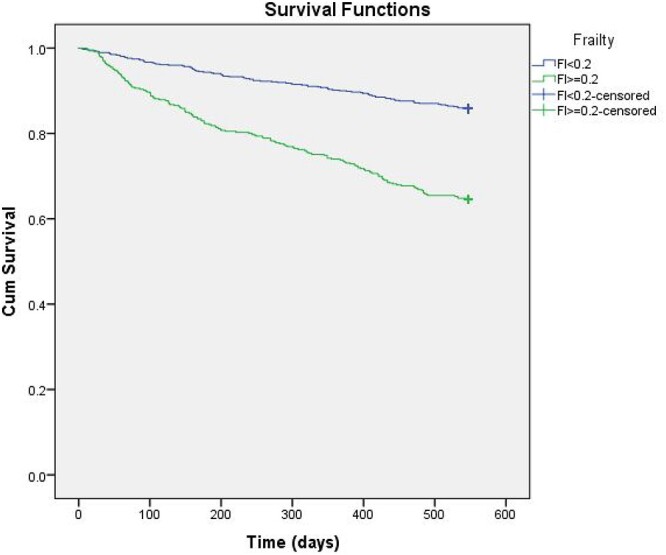
Kaplan–Meier plot comparing 18-month survival of frail (FI ≥ 0.2) and non-frail (FI < 0.2) patients.
Analysis
To investigate the extent of how frailty influences healthcare utilisation following MRH, we developed a logistic regression model to estimate their association, considering concurrent influences of age, gender and number of medicines. We followed recommended procedures [26] for checking model misspecifications (Stata: linktest), need for non-linear terms (Stata: boxtid), multicollinearity (Stata: collin), Hosmer and Lemeshow’s goodness-of-fit test (Stata: lfit), and influential observations (Pearson residual, deviance residual and Pregibon leverage). We used average marginal effects at representative values [27] to illustrate how probabilities of healthcare service use for MRH vary with number of medicines and frailty in each gender. Marginal estimation was carried out using the margins command that was introduced in Stata [28]. These estimates were bootstrapped 1,000 times to obtain confidence intervals.
Results
Baseline demographics
The PRIME study recruited 1,280 older adults at hospital discharge of which 17 (1.3%) died without follow-up, and 147 participants (11.5%) were lost to follow-up because no post-discharge information was available via readmission, GP records, or follow-up telephone call [2]. There was no clinically significant difference between those participants included or excluded from the final analysis (Table 2).
Table 2.
Baseline sample characteristics of patient cohort
| Characteristic | Included patients† (n = 1,116) | Excluded patients (n = 164) | P-value |
|---|---|---|---|
| Age, median (IQR), years | 81.9 (75.5–86.9) | 80.5 (74.7–86.2) | 0.123 |
| Gender, n (%) | |||
| Women | 652 (58.4) | 93 (56.7) | 0.673 |
| Men | 464 (41.6) | 71 (43.3) | |
| Hospital stay, median (IQR), days | 7 (3–14) | 7 (3–13) | 0.595 |
| Number of Charlson Index co-morbidities (%) | |||
| 0–1 | 541 (48.5) | 88 (53.7) | |
| ≥2 | 575 (51.5) | 76 (46.3) | 0.242 |
| Selected co-morbidities, n(%) | |||
| Hypertension | 611 (54.7) | 86 (52.4) | 0.615 |
| CLD | 326 (29.2) | 56 (34.1) | 0.202 |
| Atrial fibrillation | 279 (25.0) | 43 (26.2) | 0.773 |
| Diabetes | 269 (24.1) | 31 (18.9) | 0.167 |
| IHD | 224 (20.1) | 38 (23.2) | 0.352 |
| CKD | 153 (13.7) | 21 (12.8) | 0.808 |
| CCF | 150 (13.4) | 20 (12.2) | 0.713 |
| Depression | 95 (8.5) | 12 (7.3) | 0.762 |
| Dementia | 51 (4.6) | 6 (3.7) | 0.839 |
| Charlson Index, median (IQR) | 2 (1–3) | 1 (1–3) | 0.087 |
| Bathel Score, median (IQR) | 17 (13–20) | 18 (14–20) | 0.035 |
| Hand grip strength*, median (IQR) | 18 (12–24) | 18 (12–26) | 0.345 |
| Falls* (2 or more in last year), n (%) | 401 (36.4) | 57 (35.2) | 0.794 |
| Number of discharge medicines, median (IQR) | 9 (7–12) | 9 (6–12) | 0.393 |
| Multi-compartment compliance aid, n (%) | 371 (33.2) | 43 (26.2) | 0.074 |
| Discharge to care home, n (%) | 30 (2.7) | 8 (4.9) | 0.136 |
| Living alone after discharge, n (%) | 551 (49.4) | 80 (48.8) | >0.999 |
IQR: Interquartile range; CLD: chronic lung disease; IHD: ischaemic heart disease; CKD: chronic kidney disease; CCF: congestive cardiac failure.
†Ten patients were included following readmission which was not associated with MRH, for whom GP records were not available and were uncontactable at 8-weeks (median follow-up 29 days after recruitment).
* missing data: hand grip strength, n = 164; falls, n = 15.
Mann–Whitney U test for continuous variables and Fisher’s Exact test for categorical variables.
The median age of the PRIME study cohort was 82 years old (IQR 75.5–86.9 years) with more female participants (58%). The study population had a high level of multi-morbidity (52% had a Charlson Comorbidity Index of ≥2), with cardiac and respiratory conditions, and diabetes dominating. The level of multi-morbidity was reflected in the number of discharge medicines (median number of discharge drugs = 9, IQR 7–12, range 0–27) and 1/3 of study participants (33.2%) used a multi-compartment medicines compliance aid to support medicines use. There was a median of two drug changes per participant between admission and discharge. Participants had an average length of stay of 1 week (median 7, IQR 3–14 days).
Median hand grip strength for the study population was 18 kg (IQR 12–24 kg) and when differentiated by gender 77.4% of females and 68.1% of males would be described as sarcopenic [25], with median hand grip strength of 14 kg (IQR 10–18 kg) and 24 kg (IQR 19–31 kg), respectively. Using a clinically relevant cutpoint of 0.2 (13 out of 55 possible deficits in our FI) [17], 446 patients (40%) were frail with a FI range from 0 to 0.44.
Out of 1,112 participants (4 were excluded due to incomplete MRH outcome data), 413 (37%) participants experienced MRH in the 8-week follow-up period and 52% of the MRH events were potentially preventable. Healthcare utilisation secondary to MRH was required in 328 (29%) participants, equating to 96 hospital admissions and 316 GP consultations. Further details, including types of MRH and medications involved have been published elsewhere [2].
Those requiring MRH-related healthcare use tended to be older, female, taking more medicines and frailer (Table 3).
Table 3.
Sample description
| Sample characteristics (reported as mean (SD) unless otherwise stated) | MRH-related healthcare use (n = 328) | No MRH-related healthcare use (n = 784) |
|---|---|---|
| Age (years) | 82.1 (7.2) | 80.9 (7.9) |
| Female (%) | 66.8 | 55.1 |
| Number of discharge medications | 10.3 (4.0) | 8.9 (4.1) |
| Frailty index | 0.20 (0.1) | 0.17 (0.1) |
Frailty associated with MRH
Healthcare utilisation due to MRH showed statistically significant associations with frailty (OR = 10.06, 95% CI 2.06–49.26, P = 0.004), controlling for the concurrent influence of age, gender and number of medicines (Table 4). The odds of healthcare service utilisation for MRH were increased with the number of medicines and frailty but were lower in males. These odds were not associated with age, possibly because this study’s population was an older cohort.
Table 4.
Odds ratios (ORs) from logistic regression model (dependent variable: healthcare service use due to medication-related harm)
| Independent variable | OR | 95%CI | P-value | |
|---|---|---|---|---|
| Age | 1.01 | 0.99 | 1.03 | 0.305 |
| Gender | 0.63 | 0.48 | 0.84 | 0.001 |
| Number of medicines | 1.07 | 1.03 | 1.10 | <0.001 |
| Frailty | 10.06 | 2.06 | 49.26 | 0.004 |
| Model intercept | 0.08 | 0.02 | 0.38 | 0.001 |
Female gender tended to be associated with elevated risks for MRH healthcare utilisation, particularly when the number of medications was in the range of 7–13 (Figure 2) and the frailty index was in the range of 0.15–0.25 (Figure 3). Detailed estimates and bootstrapped confidence intervals are reported in the online supplement. With increasing number of medicines, the need for healthcare utilisation as a result of MRH increases from a probability of around 0.2–0.4. This is also the case for frailty. Frailty appears to matter as much as the number of medicines in its association with MRH.
Figure 2.
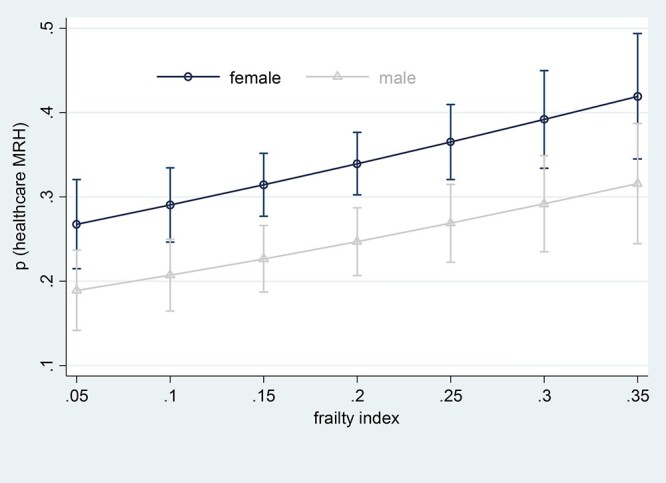
Average marginal estimates (95% confidence intervals) of probabilities of healthcare service use due to medication-related harm at representative values of frailty index.
Figure 3.
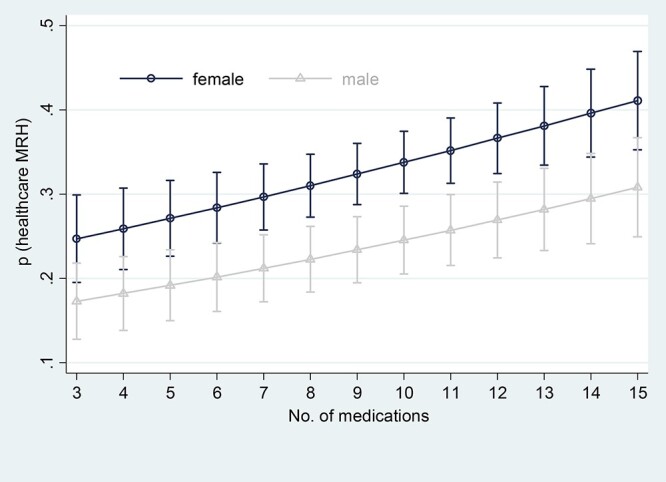
Average marginal estimates (95% confidence intervals) of probabilities of healthcare service use due to medication-related harm at representative values of number of medicines.
Sensitivity analysis
Reflective of clinical practice, and in line with similar research in this area, we also modelled frailty as a binary variable, using 0.2 as the cutpoint. Using this approach resulted in a marked difference in the association between frailty and MRH healthcare use, when compared to modelling frailty as a continuous variable: frailty binary OR = 1.37 (95%CI: 1.04–1.81, P = 0.027) (online supplement), frailty continuous OR = 10.06 (95%CI: 2.06–49.26, P = 0.004; Table 4).
Discussion
Statement of principal findings
This study demonstrated that frailty is a predictor of healthcare utilisation secondary to MRH in the 8 weeks post-hospital discharge, independent of the number of medicines. The need for healthcare intervention reflects both the severity of MRH, and the vulnerability of older adults living with frailty to the harmful effects associated with medicines. Furthermore, our findings suggest that frailty is an important driver of MRH, in addition to the number of medicines, which should prompt review of current approaches to tackling the global challenge of MRH in older adults.
Strengths and weaknesses of the study
The major strengths of this study were the large number of participants, its prospective multi-centre design, a robust multistage, multidisciplinary process for verifying MRH, and use of an internally validated frailty index. However, an important limitation was measuring frailty using only a cumulative deficits model which had not been externally validated, and this may impact on the generalisability of our findings. A routine measurement of frailty, neither cumulative deficits or phenotype model, were not available and so efforts were made in the development of our model to minimise this limitation by following the standardised approach to developing a Frailty Index, and through inclusion of a larger number of variables than the recommended minimum: an approach which has been reported to improve precision [15].
Strengths and weaknesses in relation to other studies
Traditionally, the number of medicines has been used as a trigger for medicines review in clinical practice [29], and deprescribing interventions, including the application of medicines appropriateness criteria as recommended by NHS England [30]. Whilst these interventions have reduced the number of medicines prescribed, and increased their appropriateness, their long-term impact on clinical outcomes has been limited [10, 11]. Polypharmacy is logically and evidently a dominant driver of MRH, but our study demonstrates that frailty is similarly important. On-going research to understand the risk factors associated with MRH has illustrated the importance of not only biological or medicines-related variables but also the impact of psychological and social variables on MRH. ‘Living alone’, a social determinant, was identified as an important predictor of MRH in our development of an MRH risk-prediction tool [13]. Hence MRH should be viewed through a holistic bio-psychosocial lens [31]. The complexity of the relationship between MRH, frailty and the number of medicines is also seen in clinical practice; not all patients with multiple co-morbidities and polypharmacy will experience MRH. Similarly patients with few medicines, but multiple social and psychological challenges may experience harm. As with frailty and other geriatric syndromes, viewing MRH from this perspective recognises the multifactorial nature of the problem, and the need for a more sophisticated and holistic approach to its mitigation.
Our results resonate with the findings of Lattanzio et al. [32] who investigated the association between Geriatric Conditions and ADRs in 506 hospitalised older adults. Adverse drug reactions were experienced by 11.5% of inpatients (mean age 80.1 years, SD 6.0 years; 54.3% female; mean number of medicines 10.6, SD 5.5). Whilst there was no association between individual Geriatric Conditions and ADRs, the combined variables of history of falls and dependency in at least one activity of daily living (ADL) was significantly correlated with ADRs (OR 2.18, 95% CI 1.13–4.19) and upon multivariable analysis ADR was independent of the number of medicines used. It may be argued that whilst singular geriatric conditions are markers of frailty, individually they do not reflect the global loss of homeostatic reserve seen in frailty that limits an individual’s ability to withstand a situational challenge presented by a medicine. The presence of more than one geriatric condition, in particular, dependency in ADLs in the context of an acute episode of care may reflect a more globally compromised functional reserve system, thus making these individuals more vulnerable to an ADR.
Our study identified an elevated risk of MRH requiring healthcare utilisation for females. This may be due to an increased opportunity to identify and record MRH requiring healthcare in females, as they are more likely to seek healthcare advice. [33] Also, older females are reported to have increased difficulties in performing instrumental activities of daily living, which includes medication-taking, compared to men [34], which may provide some explanation of our finding. As is now the case with frailty, future interventions to reduce MRH risk must consider the sociodemographic risk factors.
Meaning of the study
We found that frailty substantially increases the risk of older adults experiencing MRH that requires further management. It would seem sensible and imperative then to target primary care interventions at those with the highest levels of frailty. Although frail older adults may already benefit from geriatrician intervention, where medication review forms part of the Comprehensive Geriatric Assessment, our research suggests that more needs to be done, for example community based follow-up, responsive to the dynamic nature of frailty. Future strategies in primary care should also adopt a more proactive approach to mitigating MRH by focusing on individuals who are approaching frailty or living with mild frailty, and not yet known to specialist geriatric services. The increasing prevalence of frailty in younger years [8], and the Health Secretary’s ambition to add ‘years to life, and life to years’ should motivate this change.
The findings of our study also stress the importance of avoiding the dichotomisation of continuous variables due to the risk of loss of valuable data [35]. Dichotomisation of frailty in this study resulted in loss of information about its influence on MRH requiring healthcare, which when applied in practice may incorrectly influence resource allocation. Furthermore, as highlighted by the proposal to apply frailty scales to determine the access of older people to healthcare during the COVID-19 pandemic, we are reminded that frailty is not a binary state, but a bi-directional continuum [36].
Unanswered questions and future research
Frailty is a risk factor for MRH requiring healthcare utilisation, independent of the number of medicines. Further exploration of the interactions between these two geriatric syndromes using different frailty models, in particular those used in routine clinical care, is required to inform future health and social care policy and practice to mitigating MRH. Holistic and individualised assessments and interventions are required to reduce the impact of the multitude of factors contributing to the frailty state. These, alongside strategies to improve medicines appropriateness, may reduce the incidence of MRH and subsequent healthcare utilisation.
Supplementary Material
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
NIHR Research for Patient Benefit (PB-PG-0711-25,094) and Guy’s and St. Thomas’ Charity (G100716). The study funders/sponsors played no role in study design, data collection, analysis, interpretation of data, writing report or decision to submit the article for publication. All researchers were independent from study funders.
References
- 1. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 2007; 55: 780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parekh N, Ali K, Stevenson JMet al. . Incidence and cost of medication harm in older adults following hospital discharge: a multicentre prospective study in the UK. Br J Clin Pharmacol 2018; 84: 1789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Medication Without Harm - Global Patient Safety Challenge on Medication Safety. Geneva: World Health Organization, 2017. Available from:https://apps.who.int/iris/bitstream/handle/10665/255263/WHO-HIS-SDS-2017.6-eng.pdf;jsessionid=137E2C6ACE784A9238A497EA2FEB1417?sequence=1 (cited 25 July 2019).
- 4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston Jet al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56. [DOI] [PubMed] [Google Scholar]
- 6. Rockwood K, Song X, MacKnight Cet al. . A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005; 173: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gale CR, Cooper C, Aihie SA. Prevalence of frailty and disability: findings from the English longitudinal study of ageing. Age Ageing 2015; 44: 162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blodgett JM, Rockwood K, Theou O. Changes in the severity and lethality of age-related health deficit accumulation in the USA between 1999 and 2018: a population-based cohort study. Lancet Healthy Longev 2021; 2: e96–104. [DOI] [PubMed] [Google Scholar]
- 9. Cullinan S, O’Mahony D, O’Sullivan D, Byrne S. Use of a frailty index to identify potentially inappropriate prescribing and adverse drug reaction risks in older patients. Age Ageing 2016; 45: 115–20. [DOI] [PubMed] [Google Scholar]
- 10. Rankin A, Cadogan CA, Patterson SMet al. . Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2018; CD008165. 10.1002/14651858.CD008165.pub4. (cited 12 January 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tecklenborg S, Byrne C, Cahir C, Brown L, Bennett K. Interventions to reduce adverse drug event-related outcomes in older adults: a systematic review and meta-analysis. Drugs Aging 2020; 37: 91–8. [DOI] [PubMed] [Google Scholar]
- 12. Stevenson JM, Parekh N, Ali Ket al. . Protocol for a prospective (P) study to develop a model to stratify the risk (RI) of medication (M) related harm in hospitalized elderly (E) patients in the UK (the PRIME study). BMC Geriatr 2016; 16: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parekh N, Ali K, Davies JGet al. . Medication-related harm in older adults following hospital discharge: development and validation of a prediction tool. BMJ Qual Saf 2020; 29: 142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parekh N, Stevenson JM, Schiff Ret al. . Can doctors identify older patients at risk of medication harm following hospital discharge? A multicentre prospective study in the UK. Br J Clin Pharmacol 2018; 84: 2344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clegg A, Bates C, Young Jet al. . Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45: 353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Ser A 2007; 62: 722–7. [DOI] [PubMed] [Google Scholar]
- 18. Mitnitski A, Song X, Skoog Iet al. . Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc 2005; 53: 2184–9. [DOI] [PubMed] [Google Scholar]
- 19. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World Journal 2001; 1: 323–36. Available from:https://www.hindawi.com/journals/tswj/2001/321027/abs/ (cited 14 January 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol Ser A 2007; 62: 738–43. [DOI] [PubMed] [Google Scholar]
- 21. Kulminski A, Yashin A, Arbeev Ket al. . Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: results from analyses of the National Long Term Care Survey. Mech Ageing Dev 2007; 128: 250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kulminski AM, Ukraintseva SV, Akushevich IV, Arbeev KG, Yashin AI. Cumulative index of health deficiencies as a characteristic of long life. J Am Geriatr Soc 2007; 55: 935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. J Gerontol Ser A 2005; 60: 1046–51. [DOI] [PubMed] [Google Scholar]
- 24. Woo J, Goggins W, Sham A, Ho SC. Social determinants of frailty. Gerontology 2005; 51: 402–8. [DOI] [PubMed] [Google Scholar]
- 25. Cruz-Jentoft AJ, Baeyens JP, Bauer JMet al. . Sarcopenia: European consensus on definition and diagnosisReport of the European working group on sarcopenia in older people. Age Ageing 2010; 39: 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bruin, J. Newtest: command to compute new test. UCLA: Statistical Consulting Group. 2006. Available from: https://stats.idre.ucla.edu/stata/ado/analysis/. (cited 19 November 2020).
- 27. Cameron A, Trivedi P. Nonlinear Regression Methods. Microeconometrics Using Stata. 2nd edition. Oxford, UK: Taylor and Francis, 2010. [Google Scholar]
- 28. Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J 2012; 12: 308–31. [Google Scholar]
- 29.National Institute for Health and Care Excellence. Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. [NICE Guideline No. 05], 2015. Available from: https://www.nice.org.uk/guidance/ng5/chapter/recommendations#terms-used-in-this-guideline (cited 5 September 2020). [PubMed]
- 30. Toolkit for general practice in supporting older people living with frailty. Leeds: NHS England Long Term Conditions Team, 2017. Available from: https://www.england.nhs.uk/wp-content/uploads/2017/03/toolkit-general-practice-frailty-1.pdf (cited 5 September 2020).
- 31. Stevenson JM, Davies JG, Martin FC. Medication-related harm: a geriatric syndrome. Age Ageing 2020; 49: 7–11. [DOI] [PubMed] [Google Scholar]
- 32. Lattanzio F, Laino I, Pedone Cet al. . Geriatric conditions and adverse drug reactions in elderly hospitalized patients. J Am Med Dir Assoc 2012; 13: 96–9. [DOI] [PubMed] [Google Scholar]
- 33. Rogers RG, Everett BG, Onge JMS, Krueger PM. Social, behavioral, and biological factors, and sex differences in mortality. Demography 2010; 47: 555–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crimmins EM, Shim H, Zhang YS, Kim JK. Differences between men and women in mortality and the health dimensions of the morbidity process. Clin Chem 2019; 65: 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006; 332: 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hubbard RE, Maier AB, Hilmer SN, Naganathan V, Etherton-Beer C, Rockwood K. Frailty in the face of COVID-19. Age Ageing 2020; 49: 499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.