Abstract
The United States healthcare system underperforms in healthcare access, quality, and cost resulting in some of the poorest health outcomes among comparable countries, despite spending more of its gross national product on healthcare than any other country in the world. Within the United States, there are significant healthcare disparities based on race, ethnicity, socioeconomic status, education level, sexual orientation, gender identity, and geographic location. COVID-19 has illuminated the racial disparities in health outcomes.
This article provides an overview of some of the main concepts related to health disparities generally, and in orthopaedics specifically. It provides an introduction to health equity terminology, issues of bias and equity, and potential interventions to achieve equity and social justice by addressing commonly asked questions and then introduces the reader to persistent orthopaedic health disparities specific to total hip and total knee arthroplasty.
The U.S. healthcare system underperforms in healthcare access, quality, and cost resulting in some of the poorest health outcomes among comparable countries, despite spending more of its gross national product on healthcare than any other country in the world (Commonwealth Fund, 2021; Salmond & Echevarria, 2017). Within the United States, there are significant healthcare disparities based on race, ethnicity, socioeconomic status, education level, sexual orientation, gender identity, and geographic location (Healthy People 2020, n.d.a). Although the issue of health disparities is not new, the coronavirus disease-2019 (COVID-19) pandemic has illuminated these inequities.
Over 35 years ago, the U.S. government convened a group of experts to study the health status of “minorities” (more modern nomenclature, which will be used whenever possible in this article, is Black, indigenous and other people of color, or BIPOC) (see Box 1). The results, released in the Report of the Secretary's Task Force on Black and Minority Health (Heckler Report), documented the existence of shocking racial disparities in health outcomes in the United States. Margaret Heckler, the U.S. Department of Health and Human Services (HHS; 1985) secretary at the time, responded to the report stating that the plague of health inequities is “an affront both to our ideals and to the ongoing genius of American medicine” (Gracia, 2015). The report put reduction of health inequities, especially among BIPOC, on the national agenda. Key policy and research centers such as the HHS Office of Minority Health and the National Center on Minority Health and Health Disparities, which ultimately became the National Institute on Minority Health and Health Disparities, were established. During the nearly four decades since the publication of the Heckler Report, Healthy People, which guides national health promotion and disease prevention efforts to improve the health of the nation, has set national strategy to address disparities. Healthy People's progressive goals include (1) reduce health disparities (Healthy People 2000, n.d.a), (2) eliminate health disparities (Healthy People 2010), (3) achieve health equity and eliminate disparities (Healthy People 2020, n.d.a) and, in the latest version, Healthy People 2030, to (4) expand health equity focus to consider social determinants of health (the conditions in which people are born, grow, live, work, and age that shape health), health literacy, and well-being. Other key policy initiatives have been the promulgation of the National Culturally and Linguistically Appropriate Services Standards, HHS Action Plan to Reduce Racial and Ethnic Health Disparities, The National Partnership for Action to End Health Disparities, and the Affordable Care Act.
Box 1. Language Used in the Article.
| In this article, we have used the term “people of color” or “patient of color” to refer to individuals who do not identify as White. “White,” as a race, is defined using the U.S. Census Bureau definition “A person having origins in any of the original peoples of Europe, the Middle East, or North Africa.” Note that an individual's response to a question about race is based on self-identification. |
| Language is dynamic and is constantly changing, and we considered using the term “BIPOC” (black, indigenous, and people of color) but this is a relatively new term and is currently not without its critics. We do recognize that “people of color” includes people of many ethnicities and races including African Americans, Asian Americans, Native Americans, Pacific Islander American, multiracial Americans, and some Latino Americans, and that members of these communities are more likely to identify through these communities than as “people of color.” “People of color,” however, does emphasize the common experiences of systemic racism faced by most, if not all, non-White communities. |
| Throughout the narrative and in the evidence tables, we used the term “Black” to refer to populations or individuals referred in the corresponding studies as “African American” and/or “Black.” As the published studies rarely record or report the richness of ethnic and racial information beyond labels, we too were limited by the racial categories used in the primary articles. |
Prioritization and interest in the reduction of health disparities extend well beyond the federal level. In 2003, the Institute of Medicine (now the National Academy of Medicine, NAM) published the landmark report, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (Institute of Medicine, 2003). This report charted the history of unequal healthcare for BIPOC in the United States, and highlighted the existence and persistence of racial and ethnic health disparities even after controlling for disease severity, socioeconomic status, education, and access. Significant findings included BIPOC were less likely to receive preventive health services, often received lower-quality care across the care continuum, and had poorer health outcomes including lower quality of life and a higher incidence of functional impairment and mortality than their White counterparts. Nearly 10 years later, the follow-up report, “How Far Have We Come in Reducing Health Disparities? Progress Since 2000” (Institute of Medicine, 2012) documented the persistence of stark health disparities over time and across the life course, despite the dramatic increase in public and governmental awareness of health. Recognizing these disparities, numerous organizations have prioritized working toward health equity through community action and research, including Robert Wood Johnson Foundation, the Kellogg Foundation, and the Kaiser Family Foundation.
Health Disparities in Orthopaedics
The field of orthopaedics is not immune to health inequities. There is clear documentation of disparities in the treatment of musculoskeletal disorders, which collectively are a leading cause of disability accounting for nearly 70 million physician office visits per year and an estimated 130 million total health care encounters including outpatient, hospital and emergency room visits (Centers for Disease Control and Prevention [CDC], n.d.a). For example, significant disparities are seen in access to care for, and outcomes associated with, hip and knee arthroplasty, fracture treatment, and pain management (Amen et al., 2020; Dykes & White, 2009). Responding to data on inequities in orthopaedic care and outcomes, the American Academy of Orthopaedic Surgeons, the Orthopaedic Research Society, and the Association of Bone and Joint Surgeons sponsored a research symposium in May 2010 to better understand musculoskeletal healthcare disparities and initiated a “call to arms” to eliminate these disparities. The subsequent 2011 report, Movement Is Life: A Catalyst for Change prioritized an action plan for eliminating racial disparities in musculoskeletal care (Movement Is Life Caucus, 2011). That action plan includes three recommendations: the first, “Sound the Alarm” recommends researching and disseminating information to energize people to address musculoskeletal health disparities; the second recommendation, “Mirror Success,” encourages the acceleration of development and adoption of solutions that address disparities; and the third, “Open Communication Lines” recommends the facilitation of better patient and provider understanding of the unique needs of women and BIPOC.
As well, the orthopaedic community has begun to acknowledge and address the lack of diversity of the orthopaedic workforce as a potential contributor to inequities. Orthopaedics has the lowest percentage of both BIPOC and female providers of all medical and surgical subspecialties (Day et al., 2019). Increased diversity of healthcare providers leads to improved access to and utilization of care by underrepresented groups, such as racial/ethnic minorities, as well as improved patient satisfaction (Smedley et al., 2004). Improving the diversity of the orthopaedic workforce may have a significant impact on patient outcomes, especially among historically underrepresented groups.
The Role of Nurses in Addressing Health Inequities
Working to lessen disparities is an essential part of the nursing role and reflects the values, vision and mission of the profession. In the past year, multiple major nursing organizations have acknowledged that racism is a public health threat, and made statements about the overdue need to address bias, stigma, and discrimination in our profession, organizations, and institutions. Despite inclusion in major nursing documents, such as the American Nurses Association and International Council of Nurses Codes of Ethics, health disparities and inequities, and the underlying roots of these differences including racism, have been underaddressed in nursing. The publishing of the National Academy of Medicine Report, The Future of Nursing 2020–2030: Charting a Path to Achieve Health Equity (National Academies of Sciences, Engineering, and Medicine, 2021) addresses building the capacity of the nursing workforce to engage in advancing health equity, addressing social determinants of health, and meeting social needs of individuals and families (National Academies of Sciences, Engineering, and Medicine, 2021). It provides eight recommendations and 37 subrecommendations that are needed to strengthen the nursing workforce to significantly contribute to advancing health equity. These recommendations are outlined in the article, The Future of Nursing: Application of Health Equity in Orthopaedics, in this issue. The Future of Nursing report calls on all nurses to stop and reflect on the state of health disparities in the United States, understand the contributing factors, and how we as nurses can take action to promote health equity for all.
This article provides an overview of some of the main concepts related to health disparities generally, and in orthopaedics specifically. It provides an introduction to health equity terminology, issues of bias and equity, and potential interventions to achieve equity and social justice by addressing commonly asked questions and then introduces the reader to persistent orthopaedic health disparities specific to arthritis and knee and hip surgical intervention (total hip and total knee arthroplasty). As one article cannot address all of orthopaedics, this article focuses on the management of osteoarthritis and the use of total hip arthroplasty (THA) and total knee arthroplasty (TKA) as exemplars for addressing orthopaedic inequities. Differential outcomes according to race/ethnicity and gender are examined and issues related to implicit biases that may impact payment, patient preference, and body type are explored. The hope is that this article will serve as a starting point for deeper reflection, conversation, and action around health disparities.
What Are Health Differences, Disparities, and Inequities?
Health Differences
Football players have a higher incidence of knee injuries than those who do not play football.
Due to static postures, dentists are more apt to develop cervical herniated intervertebral disc compared with the general population.
Adults between 50 and 70 years have higher incidence rates of symptomatic osteoarthritis of either the hand, knee, or hip as compared with younger cohorts.
These are differences, but not disparities. Differences can be identified through epidemiologic studies that examine prevalence and incidence. However, they are not related to systematic social, economic, or environmental disadvantage (see later).
Health Disparities and inequities
The following are examples of health disparities:
Black, American Indian, and Alaska Native people are two to three times more likely to die from pregnancy-related causes than White people (CDC, 2019).
The mortality rate for Black infants is twice that of infants born to non-Hispanic White mothers (Office of Minority Health, n.d.a,n.d.b).
Sexual minority adolescents (lesbian, gay, and bisexual) report a greater incidence of depression, anxiety, and suicidal behaviors than heterosexual adolescents (Healthy People 2020, n.d.b).
The rural south leads in mortality rates for nearly all top 10 causes of death (Rural Health Information Hub, n.d.).
Women are diagnosed later than men for more than 700 diseases (Westergaard et al., 2019).
There is strong evidence of racial/ethnic disparities in pain burden and pain management in both cancer pain and noncancer pain (Samuel et al., 2019).
Whereas health differences are just that—differences—disparities are measures of inequities, or differences in health access and/or outcomes between socially advantaged and socially disadvantaged or marginalized population groups (Agency for Health Care Research and Quality, n.d.; Braveman, 2006). Disadvantage is a systematic experience of unfavorable social, economic, environmental, or political conditions based on relative position in a hierarchy (Braveman, 2014b). Groups higher in position (considered dominant groups) have more assets and resources, and this translates to improved opportunity, access, and health outcomes. Nondominant groups are devalued and disempowered and have less allocation of societal opportunities and may be regarded as inferior (Bonilla-Silva, 2006; Williams et al., 2019). This applies to many groups of people, based on demographic or identity characteristics such as age, race/ethnicity, sex, sexual orientation and gender identity, socioeconomic class (educational level, income, and occupation), country of birth, disability status, and geographic location as well as other characteristics associated with discrimination or marginalization (Agency for Health Care Research and Quality, n.d.; Braveman, 2014a; Braveman et al., 2011).
Health disparities are measured by comparing health indicators between the “dominant” or more advantaged group and the less dominant or more disadvantaged group (Braveman, 2006; Dean et al., 2016). Table 1 illustrates this concept for common identities based on the dominant discourse and the ultimate consequences of this social status in creating “isms” (Robinson, 1999) that perpetuate the status quo and contribute to inequities and disparities. It is essential to note that individuals often belong to more than one group, and therefore, may have overlapping health and social inequities, as well as overlapping strengths and assets. The concept of intersectionality helps us understand the exponential impact of multiple, intersecting marginalized identities on health and health outcomes (Kapilashrami & Hankivsky, 2018).
Table 1. Visible and Invisible Identities.
| Race | Gender Identity | Sexual Orientation | Physical Ability | Class |
|---|---|---|---|---|
| Dominant United States discourses: Advantage | ||||
| Whites | Cisgender men (i.e., people who were both born male and currently identify as men) | Heterosexuals | Able-bodied | Middle class or higher |
| Dominant United States discourses: Disadvantage | ||||
| Black, indigenous, people of color (BIPOC) | Cisgender women and gender minorities, including transgender and nonbinary and gender nonconforming persons | Sexual minorities, including lesbian, gay, bisexual, and others | Persons with disabilities | People with lower incomes, people experiencing poverty, people who are unhoused |
| Consequences of dominant discourses | ||||
| Racism | Sexism, transphobia | heterosexism, homo-and bi-phobia | Ableism | Classism |
Social Justice
The World Health Organization (WHO) further expands the definition of disparities to identify disparities as an issue of social justice. The WHO puts forth that health inequities (the underlying contributing factor(s) or structural or institutional patterns that result in disparities) are “differences in health status or in the distribution of health resources between different population groups, arising from the social determinants of health or the conditions in which people are born, grow, live, work and age.” The WHO goes on to point out that “health inequities are unfair and could be reduced by the right mix of government policies” (WHO, 2018).
Overcoming inequities or systemic, preventable, and unjust differences in health outcomes require tackling the root causes for these differences (American Medical Association, 2021). Getting at the roots means understanding the social, economic, environmental, and structural disparities that make people sick (Castrucci & Auerbach, 2019). It requires both an acknowledgment of the harmful effects of the past and a sincere and in-depth examination of contemporary inequities in the healthcare system and other social institutions. Only by changing community conditions will we truly create health equity.
What Are Health Equity and Social Justice? Why Equity and Not Equality?
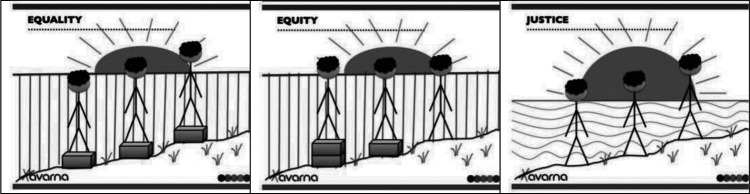
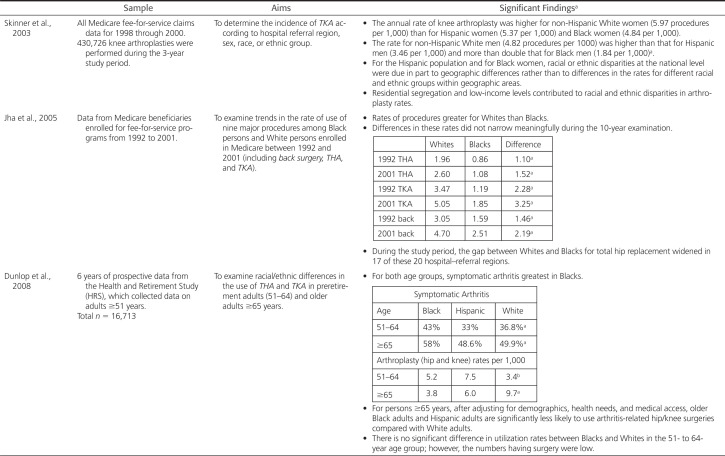
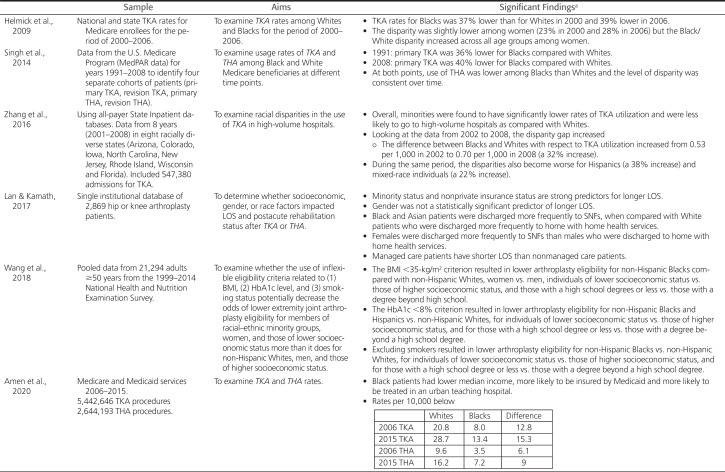
Health equity means that every individual has a fair and just opportunity to be as healthy as possible and no one is disadvantaged from achieving this potential because of their social position or other socially determined circumstances (Brennan Ramirez et al., 2008). Underlying health equity is the basic tenet that health is a human right and calls for action to equalize opportunities to be healthy (Braveman et al., 2011). Equality, a core value of the United States, suggests that resources or opportunities should be distributed equally across individuals and groups. This type of equality can result in further continuation of differential outcomes—outcomes that do not address the underlying inequity. This concept (equality) is illustrated in the first image in Figure 1—all of the stick figures are the same height, symbolizing that all people are equally valuable human beings, despite being different from each other. However, the foundation on which these three stick figures stand, is unequal. In the first image in Figure 1, the first stick figure still cannot see over the fence that obscures the sunrise over the ocean, whereas the second and third figures can see over the fence. In the middle image in Figure 1, the wooden boxes on which the stick figures stand take into consideration that persons and groups have different circumstances (as symbolized by the hill on which the stick figures stand). To this end, different resources and opportunities (as symbolized by the two boxes under one stick figure and the single box under the other) are provided to reach an equal outcome (i.e., each of the individuals can now see over the fence and view the sunrise on the other side). In the third image in Figure 1, efforts to remove barriers (in this case the fence that obscured the sunset has been removed) bring justice to all. The removal of the fence is symbolic of efforts that might include, for example, rebuilding a criminal justice system that is restorative rather than punitive, establishing educational systems that are excellent regardless of neighborhood, and removing discrimination that acts as a barrier to education or jobs.
Figure 1.
Equality versus equity and social justice. Image credit: Xavarna. “And ... Here's yet another equity v., equality (v. justice) image series.” https://theavarnagroup.com/and-heres-yet-another-equity-v-equality-v-justice-image-series/
Health disparities are the metrics we use to assess our progress toward achieving health equity. Examining how disparities change over time—whether they are consistent, narrowing, or widening—is critical to planning priorities around health equity. Social justice goes beyond allocating resources differentially and focuses on removing the sources of the inequities or “fixing systems in a way that leads to long-term, sustainable, equitable access for generations to come” (Milken Institute School of Public Health, 2020).
How Did the COVID-19 Crisis Draw Attention to Health Disparities and Inequities?
Despite the recognition and documentation of U.S. health disparities for decades, many disparities have persisted, and in some cases, widened over the past 20 years. The COVID-19 global pandemic brought the issue of disparities to the forefront of the national scene. Although it was originally thought that COVID might be a “great equalizer”—meaning that everyone was more or less at the same risk of COVID morbidity and mortality—data show that people at greater social disadvantage have experienced a far greater burden of disease than their more advantaged counterparts. For example, people of color fared worse compared with their White counterparts, with greater mortality, intensive care needs, suffering, and sacrifice (Kapilashrami & Bhui, 2020). This is due to the complex relationship between physical health, mental health, and social health/opportunity. Understanding COVID-19 as both a pandemic and a syndemic helps to explain these disparate outcomes as a consequence of economic and social marginalization.
The science of syndemics examines the biosocial complexities of disease. It suggests that comorbidities are not simply coexisting illnesses. Rather, the science of syndemics examines the linkages and adverse interactions among comorbidities, and between comorbidities and social determinants of health. In the case of COVID, we saw that underlying comorbidities, such as hypertension, diabetes, and asthma, put people at higher risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Horton, 2020). BIPOC not only tend to have a higher prevalence of these underlying comorbidities, but are also often employed in essential worker settings such as healthcare facilities, farms, factories, grocery stores, and public transportation, placing them in higher risk settings and in positions where working from home was not an option. The aggregation of underlying comorbidities on a background of social and economic disparity, combined with barriers to accessing healthcare, exacerbates the adverse effects of each separate disease or social condition and places the individual and population at greater susceptibility to harm and more negative health outcomes (Gravlee, 2020; Horton, 2020; Nikiphorou et al., 2019).
It is imperative to understand that these diseases are not independent of the social contexts in which they are found. In fact, these linkages, and the subsequent health inequities, are a direct result of “poverty, stigmatization, discrimination, marginalization, stress, structural racism, or structural violence” (Mendenhall & Like, 2020; Singer et al., 2017, p. 941). For example, in the case of COVID-19, morbidity and mortality increased among essential workers, many of whom were people with less social capital than those who were able to quarantine safely at home (Khazanchi et al., 2020). To effectively respond to this complexity requires not only attention to the biologic interaction of multiple comorbidities, but also to the social determinant context of people's lives that may contribute to adversity, healthcare access, and outcomes. This necessitates a truly holistic and personalized approach to thinking about how we promote health, and prevent and treat illness.
Racial Reckoning and Health Equity
At the same time as the COVID pandemic was illuminating the role of economic, environmental, and social inequities on health and health disparities, the murder of George Floyd, a Black man, accused of using a counterfeit $20 bill brought the reality of racial injustice in the United States into the living rooms of everyone in the country. The tragedy of his senseless arrest and murder brought renewed and long overdue attention to the societal and public health crisis of racism.
Racism, not race, is a root cause or critical driver of health disparities (Boyd et al., 2020). Race is a social categorization, not a biological category. We now know that genes have little to do with the racial patterns of health and disease. Instead, disparities reflect ongoing social and economic inequity patterns stemming from long-standing social policy—laws, rules, and practices, sanctioned and embedded in our economic, cultural and social system and norms (Bailey et al., 2021). These policies and practices constitute structural racism and maintain a racial hierarchy with BIPOC more frequently residing in neighborhoods with significant socioeconomic challenges, greater exposures to environmental toxins, lower tax bases, fewer jobs, and fewer services (Gee, 2016). Ongoing exposure to overt racism, subtler forms of racism such as implicit bias and microaggressions (everyday verbal, nonverbal, and environmental slights, snubs, or insults, whether intentional or unintentional, which communicate hostile, derogatory, or negative messages to target persons based solely upon their marginalized group membership), and discrimination, has a detrimental impact on health and overall well-being (Williams & Mohammed, 2013). This limited access to opportunity, greater exposure to risk and stress, and less opportunity for preventive activities place these individuals and communities at greater risk (Boyd et al., 2020). Understanding this requires a shift in thinking from a framework where the focus is on proximal causes for disease (diet, cholesterol levels, exercise, substance use, etc.) to a framework where one considers what puts people at risk. These social conditions—the conditions in the places where people live, learn, work, and play—are also referred to as “upstream” factors or conditions. By examining upstream conditions for social determinants of health and investing resources and support targeting these upstream issues, it is more likely to result in real health outcome change (Link & Phelan, 1995).
By acknowledging racism as a public health crisis that contributes to disparities and health inequities, it focuses attention on the underlying structures and systems contributing to disparities rather than blaming health differences on individual behaviors. Confronting racism as a public health crisis will require individuals, organizations, and municipalities to critically reflect on race, power, and privilege and its contribution to inequity. Such a reflection needs to then be followed by allocation of resources and strategic action. The American Nurses Association, the American Academy of Nursing, the American Association of Colleges of Nursing, and the American Medical Association among many other organizational groups have published position papers highlighting racism as a public health crisis that impacts the mental, spiritual, and physical health of all people (American Nurses Association, 2020). All have called for active antiracist strategies addressing the continuum of interpersonal racism and unconscious bias to systemic and institutional racism, known to perpetuate ongoing disparities.
Orthopaedic Health Disparities
Arthritis is a leading cause of disability in the United States and osteoarthritis is a leading cause of arthritis-related disability (CDC, n.d.a). An estimated 45% of adults in the United States are at risk for developing symptomatic knee osteoarthritis. Impacting about 32.5 million U.S. adults, the incidence of osteoarthritis is similar or greater among Black and Hispanic populations as compared with Whites (Dunlop et al., 2001; Helmick et al., 2009) although the disabling effects of arthritis in terms of activity and work limitations and presence of severe pain are disproportionately prevalent in racial/ethnic minorities (CDC, n.d.b; Arthritis Health Disparity Statistics). Total hip arthroplasty and TKA are cost-effective, efficacious treatments for more severe arthritis with outcomes of decreased pain, improved physical function, and quality of life (Zhang et al., 2008). Despite the consensus of the value of arthroplasty, racial and ethnic minority groups in the United States have lower utilization of this procedure (Dunlop et al., 2008; Emejuaiwe et al., 2007; Skinner et al., 2003). One of the objectives of Healthy People 2010 calls for eliminating racial disparities in the rate of TKA among persons older than 65 years.
As arthroplasty can potentially improve the health of many Americans, it is important that we understand, track, and resolve racial and/or ethnic disparities (Zhang et al., 2016). Racial disparities associated with arthroplasty have been identified in: (1) rates in use of arthroplasty; (2) hospital metric outcomes; and (3) postoperative outcomes (Amen et al., 2020). The majority of studies examining these disparities have focused on a comparison of Black and White populations, with little data on other minority groups (Mehta et al., 2018; Wu et al., 2021). Unfortunately, much of the literature examining outcomes THA and TKA does not examine race.
Prevalence Disparities in Use of TKA and THA
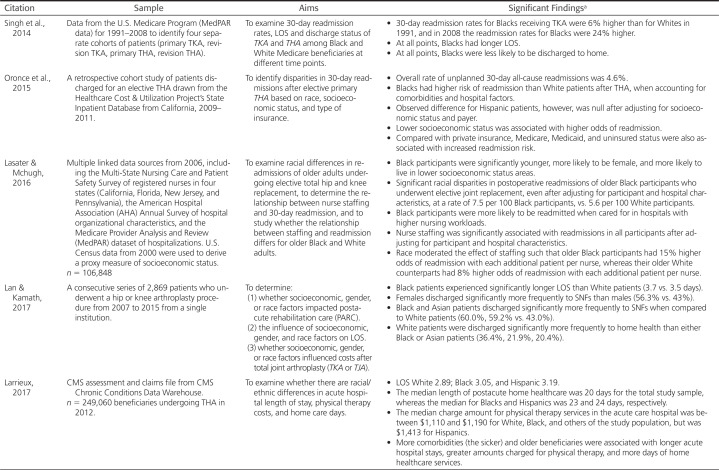
Black, Hispanic, Asian, Native American, and mixed-race patients are less likely than their White counterparts to receive joint arthroplasty treatment for advanced hip and knee osteoarthritis (Dunlop et al., 2008). These disparities are not recent but have been noted since the 1980s and 1990s (Baron et al., 1996; Katz et al., 1996; McBean & Gornick, 1994). Dunlop et al. (2003) reported that Whites were 1.5 times more likely than Black or Hispanic individuals to be treated with TKA even after controlling for economic access (income, assets, education, and health insurance) and functional levels. Similarly, Skinner et al. (2003) examined rates of TKA by race using Medicare fee-for-service claims from 1998 through 2000 and found the same trends—the annual rate of TKA was higher for non-Hispanic White women compared with Hispanic and Black women. The rate for White men was higher than that of Hispanic men and more than double that for Black men. In an early study by Jones et al. (2005), these similar disparities were found in a universally insured population of patients from the Veterans Affairs system. African American patients were significantly less likely than White patients to undergo TKA. Tables 2–4 provide evidence from the last 20 years examining disparities in rates, hospital metrics, and patient outcomes for THA and TKA by race.
Table 2. Prevalence of Arthroplasty by Race/Ethnicity.
Table 4. Postoperative Outcomes: Complication, Mortality, and Functioning .
| Citation | Sample | Aims | Significant Findingsa |
|---|---|---|---|
| Kamath et al., 2010 | Sample of 185 patients receiving TKA in 2004. | To determine the influence or race, gender, and BMI on primary TKA functional scores and ROM before gender-specific implants and whether comorbidities influence ROM and functional scores. |
|
| Lavernia et al., 2010 | Patients with a diagnosis of end-stage osteoarthritis who were scheduled for either primary or revision hip or knee arthroplasty. | (1) To determine and compare function and quality of life between Blacks and Whites at clinical presentation and at an average follow-up of 5 years after surgery; (2) To determine whether differences in fear and anxiety of pain exist between races before surgery; and (3) To explore the relationship of anxiety and fear of pain before surgery with function and quality of life before and after surgery as a function of race. |
|
| Singh et al., 2014 | Data from the U.S. Medicare Program (MedPAR data) for 1991–2008 to identify four separate cohorts of patients (primary TKA, revision TKA, primary THA, revision THA). | To examine 30-day mortality following TKA and THA among Black and White Medicare beneficiaries at different time points. |
|
| Lavernia & Villa, 2015 | Retrospective sample using an institutional arthroplasty registry. 2,010 arthroplasties of which 1,446 were TKA and 564 were THA. | (1) To examine whether Black patients have more severe or more frequent preoperative pain, well-being, general health, and disease-specific scores when compared with White patients. (2) To examine whether there are differences between Black patients and White patients after hip or knee arthroplasty on those same measures. |
|
| Goodman et al., 2016 | 4,035 patients undergoing TKA enrolled in a hospital-based registry between 2007 and 2011 who provided 2-year outcomes and lived in New York, Connecticut, or New Jersey. | (1) Are race and socioeconomic factors at the individual level associated with patient-reported pain and function 2 years after TKA? (2) What is the interaction between race and community poverty and patient-reported pain and function 2 years after TKA? |
|
| Goodman et al., 2018 | 4,170 THA cases who agreed to be part of an institutional registry for THA between May 1, 2007, and February 5, 2011, and with complete data. | Determine whether neighborhood socioeconomic factors have a differential effect in Blacks and Whites on WOMAC pain and function 2 years after undergoing THA at the same high-volume hospital. |
|
| Zhang et al., 2016 | Using all-payer state inpatient databases. | Examined racial disparities in the TKA outcomes including mortality and complications. |
|
| Larrieux, 2017 | CMS assessment and claims file from CMS Chronic Conditions Data Warehouse. n = 249,060 beneficiaries undergoing THA in 2012 and receiving postacute care physical rehabilitation through a Medicare-certified home healthcare agency. |
To examine whether there are racial disparities in functional outcomes (transfer abilities, ambulation) and hospital outcomes (home care days) in patients undergoing THA. |
|
| Okike et al., 2019 | U.S. health-care system total joint replacement registry of persons undergoing elective primary THA between 2001 and 2016. | To assess whether racial/ethnic disparities in THA outcomes persist in a universally insured population of patients enrolled in an integrated healthcare system. |
|
| Amen et al., 2020 | Medicare and Medicaid services 2006–2015. 5,442,646 TKA procedures 2,644,193 THA procedures. |
To use a nationally representative sample to investigate trends in racial disparities in TJA (TKA and THA) complications between Black and White patients from 2006 to 2015. |
|
| Cavanaugh et al., 2020 | Data drawn from the Women's Health Initiative prospective study linked with Medicare claims data. Total sample size 10,325 women who underwent TKA between October 1, 1993, and December 31, 2014. | To examine trajectories of physical functioning (PF) by race/ethnicity before and after TKA among older women. |
|
| Trivedi et al., 2020 | American College of Surgeons National Surgical Quality Improvement Program—all Black patients who underwent primary elective TKA between 2011 and 2017. n = 19,496 | To examine recent annual trends in 30-day outcomes after primary elective TKA in a sample over time of Black patients. |
|
Note. BMI = body mass index; CMS = Centers for Medicare & Medicaid Services; DVT = deep vein thrombosis; LOS = length of stay; PE = pulmonary embolism; PF = physical functioning; ROM = range of motion; SES = socioeconomic status; THA = total hip arthroplasty; TJA = total joint arthroplasty; TKA = total knee arthroplasty; UTI = urinary tract infection; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
aAll reported results are statistically significant unless specifically reported as nonsignificant.
Table 2 presents data examining prevalence rates of arthroplasty, particularly THA and TKA, by race. This evidence shows that despite many national initiatives to reduce this gap, racial disparities persist and are more pronounced among men than women (Hanchate et al., 2008). Even with similar incidences in osteoarthritis among White, Black, and Hispanic patients, and in some cases greater symptomatic incidence among those who were Black (Dunlop et al., 2008), the access to hip and knee arthroplasty continues when comparing White with Black patients (12–50% difference) or White with Hispanic patients (5–40% difference) (CDC, 2009; Helmick et al., 2009; Jha et al., 2005; Lan & Kamath, 2017; Skinner et al., 2006; Singh et al., 2014). Regrettably, evidence shows the gap in usage is not only continuing, but worsening (Amen et al., 2020; Best et al., 2021; Helmick et al., 2009; Singh et al., 2014; Zhang et al., 2016). Klemt et al. (2021) report similar disparities in THA/TKA revision with underutilization by ethnic minority groups despite higher failure rates as compared with White patients.
Of particular note is the study by Kim et al. (2021) who examined differences in prevalence rates before and after the enactment of the Comprehensive Care for Joint Replacement (CJR) model through Medicare. Comprehensive Care for Joint Replacement provides bundled payment and quality measurement for TKA and THA with the expectation that the bundled and quality approach will enhance coordination of care. They found continuation of racial disparities in receipt of elective hip or knee replacement among Black beneficiaries, not Hispanic beneficiaries. Similar findings were reported by Thirukumaran et al. (2021) who found that CJR did not impact THA rates but was associated with modest reductions in TKA use for Black in comparison to White beneficiaries, and no difference in rates among those who were Hispanic.
Hospital Metric Outcome Disparities
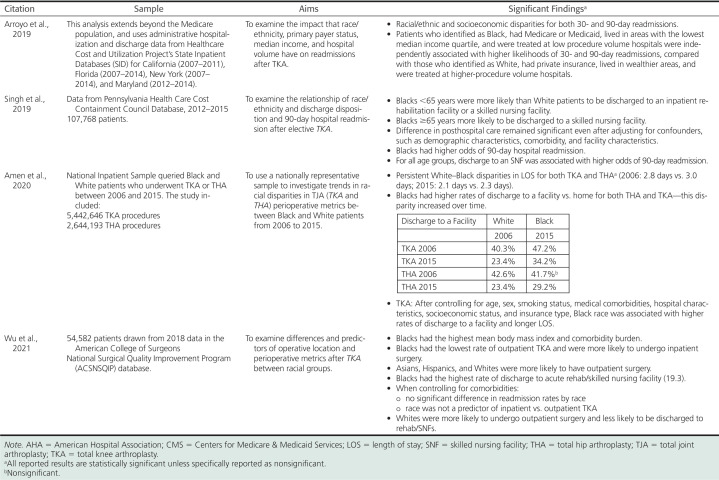
The literature examining racial variations in hospital metrics is reported in Table 3. Black patients have more negative hospital metrics as compared with White patients with longer length of stay (LOS) (Amen et al., 2020; Lan & Kamath, 2017; Larrieux, 2017; Singh et al., 2014). There is a greater likelihood that Black patients will be discharged to a skilled nursing facility as compared with home/home health (Amen et al., 2020; Lan & Kamath, 2017; Lasater & Mchugh, 2016; Singh et al., 2014, 2019), which is of concern, as discharge to locations other than home is associated with lower patient satisfaction and poorer clinical outcomes (Wu et al., 2021). Also, 30- and 90-day readmissions are greater for Black patients (Arroyo et al., 2019; Oronce et al., 2015; Wu et al., 2021).
Table 3. Hospital Metrics by Race/Ethnicity (LOS, Discharge Status, and Readmissions).
There are other influencing and interacting factors contributing to these disparities. Older age and greater comorbidity burden were significantly associated with longer LOS (Larrieux, 2017). Comorbidity burden also influenced whether surgery was done as an inpatient or outpatient, and was a factor in readmission rates. Wu et al. (2021) found that Black patients were more likely than White, Asian, and Hispanic patients to have TKA in the inpatient versus outpatient setting and reported that, after controlling for comorbidities, there was no significant difference in readmission rates by race and race was not a predictor of inpatient versus outpatient TKA. The following variables also increased the odds of readmission: lower socioeconomic status (Arroyo et al., 2019; Oronce et al., 2015), Medicare, Medicaid, or uninsured status (Oronce et al., 2015; Xu et al., 2017), being treated at a low-procedure volume hospital (Arroyo et al., 2019), and being discharged to a skilled nursing facility (Wu et al., 2021).
Of note is that Black populations have greater social disadvantage as compared with Whites. These unfavorable social and economic conditions are factors in the health disparities experienced, potentially contributing to the more negative hospital metrics. Additionally, delayed timing in surgical intervention results in patients presenting with greater pain and dysfunction than patients receiving surgery earlier in the course of osteoarthritis. A number of authors found that African Americans experienced significantly greater pain, lower physical function, and lower well-being in THA/TKA preoperative function (Goodman et al., 2018; Kamath et al., 2010; Lavernia et al., 2010; Lavernia & Villa, 2015; Slover et al., 2010). Slover et al. (2010) report that lower physical functioning was also present in Hispanic patients.
Postoperative Outcome Disparities
Although total joint arthroplasties generally have low complication rates, low readmission rates, and overall high patient satisfaction, racial disparities in postoperative outcomes are well documented with complication rates higher for patients from minority groups than for Whites, as shown in Table 4. Black patients have been shown to have higher postoperative pain (Lavernia & Villa, 2015; Mehta et al., 2018) and poorer functional outcomes (Goodman et al., 2018; Lavernia et al., 2010; Larrieux, 2017; Mehta et al., 2018; Shahid & Singh, 2016). Larrieux (2017) similarly found postoperative functional differences among Hispanic patients undergoing THA with Hispanic patients five times more likely to be dependent with transfer compared with Whites. Okike et al. (2019) examined whether racial/ethnic disparities in THA outcomes persist in a universally insured population of patients enrolled in a large, integrated healthcare system. Of note, they found similar or better THA outcomes in patients of color (Black, Hispanic, and Asian) compared with White patients. Lifetime all-cause revision was significantly lower for all minority groups. There was no difference in 90-day complications (90-day deep infection, venous thromboembolism, readmission, and mortality) when comparing minority groups with the White group.
The similarity in outcomes between Black and White patient groups is especially important considering that Black patients had several risk factors for adverse outcomes such as lower income, lower educational attainment, and a greater number of comorbidities. The authors suggest that these positive results can be linked to universal access to care associated with membership in the health maintenance organization, standardized protocols associated with treatment in a managed care system that minimizes care variability, and the majority of THAs were performed by high-volume surgeons and facilities. The only disparity found in the study was higher rates of 90-day emergency department visits among Black and Hispanic patients compared with White patients.
Factors Associated With Racial Disparity in Arthroplasty Utilization
The research is clear that racial and ethnic disparities in THA and TKA metrics exist. However, the contributing factors associated with these differences are not as clear and not as well researched. We do know that differences cannot be explained based on prevalence of osteoarthritis, as osteoarthritis of the hip and knee is equally prevalent across all racial and ethnic groups. Rather, there appears to be a multifaceted web of potential reasons (patient-level, provider-level, and system-level) that might explain why these racial and ethnic disparities in joint replacement have been so intransigent. Although multifaceted and complex, the outcomes reported by Okike et al. (2019) and Aseltine et al. (2019) are a testament to the reality that eliminating racial disparities is possible. Categories of factors attempting to explain variation among racial/ethnic groups have been identified as patient-specific, provider-specific, or system-related. This provides a mechanism for discussing relevant factors, but it must be noted that they are closely related (Irgit & Nelson, 2011).
Patient-Level Factors
As THA or TKA is an elective procedure, it seems apparent that patient preference plays an important role in decision-making on utilization of THA/TKA and time to THA/TKA. Hausmann et al. (2010) used patient survey data rating sociodemographic and clinical variables that could influence preferences for osteoarthritis treatment and concluded that there were racial differences in preferences for total joint replacement that accounted for disparities in utilization rates. This difference in preference has been linked with understanding, expectations, coping approaches, and fear.
Ibrahim et al. (2001) examined variations in self-care practices between elderly Black and White patients and found that those who identified as Black relied more heavily on home remedies, complementary care, and coping with the use of prayer for managing the symptoms of osteoarthritis. Figaro et al. (2004) held focus groups of Black patients with advanced osteoarthritis and reported that the participants expressed preferences for natural remedies and against undergoing surgery.
Compared with Whites, Ibrahim et al. (2002a) and Cavanaugh et al. (2020) found that Black patients were less likely to have a comprehensive understanding of joint replacement as a form of treatment. Black patients also were less likely to have a family member or friend who had undergone the procedure (Cavanaugh et al., 2020; Ibrahim et al., 2002a; Mingo et al., 2013). Several studies show differing expectations of arthroplasty among White and Black patients, reporting that those who identified as Black did not believe TKA would improve knee pain or improve walking (Cavanaugh et al., 2020; Figaro et al., 2004; Ibrahim et al., 2001, 2002a; 2002b). Ang (2009) also found that Black patients perceived fewer benefits and greater risks from THA/TKA than White patients.
Suarez-Almazor et al. (2005) and Kwoh et al. (2015) examined determinants of preferences for TKA in patients with symptomatic osteoarthritis. Suarez-Almazor et al. examined White, Hispanic, and Black patients and Kwoh et al. examined White and Black patients. Willingness to undergo TKA was significantly higher among Whites. Shared factors across studies that contributed to the willingness among Black or Hispanic patients to undergo TKA included knowledge or a better understanding of the procedure, and more positive expectations about procedural outcomes—perceptions of a short hospital course, less postsurgical pain, and preoperative walking difficulty. Kwoh et al. also reported that Black patients who were less religious and tended to trust physicians were more willing to undergo TKA.
Although patient-level factors are often used to explain disparities, it is critical that nurses and other health providers stop placing the blame or rationale for deficits primarily at the patient level. It is time to ask additional questions to uncover the underlying causes for patient-level preferences. If a patient is unfamiliar with or unknowledgeable about a procedure, is the underlying problem the patient or the fact that there has not been appropriate individual or community education to inform and discuss expectations? Is the underlying problem the fact that only 12% of U.S. adults have proficient health literacy (HHS, n.d.) and the education provided to them does not take this into account? If the patient delays care and opts out of a surgical intervention, could the problem be that there is a lack of patient–provider racial and ethnic concordance? Minorities are underrepresented in the ranks of orthopaedic surgery—only 4.3% of orthopaedic surgeons are Black and 4.4% Hispanic or Latino (Poon et al., 2019). Or is the prevalence disparity a result of “mistrust, resignation, helplessness, hopelessness, and other manifestations of internalized racism based in the patient—s real or perceived mistreatment within healthcare” (Dykes & White, 2009, p. 2599)? If the problem is lack of trust in the provider or the healthcare system, is the real problem the patient or the fact that for many ethnic minorities there have been unethical abuses in medicine, ongoing mistreatment along with structural and institutional drivers that have allowed this mistreatment to occur, which contribute to hesitancy, lack of trust, and fear?
Provider-Level Factors
Provider-level factors that have been identified include referral patterns, problems in patient–provider communication, and decision-making, which may be influenced by implicit bias.
Ineffective communication between patients and providers is a critical factor contributing to healthcare disparities. A systematic review by Shen et al. (2018) examined the effects of race and racial concordance on patient–physician communication. They found that Black patients experienced poorer communication quality, information giving, patient participation, and participatory decision-making in comparison to their White counterparts. Stereotype threat, the activation of negative stereotypes about stigmatized groups, can disrupt communication by creating patient anxiety and negative expectations impairing patients— communication abilities, which may result in discounting of information from the provider, and failure to seek care (Williams & Mohammed, 2013).
Ibilibor and Moses (2021) note that shorter visit times, less rapport-building behaviors and statements, and lower participatory decision-making are unfortunate characteristics of patient–physician interactions for Black patients in racially discordant pairs. As there is a link between patient-centered communication and patient trust in their physician, poor-quality interactions may contribute to lower postvisit trust in physicians that Black patients report. Hamel et al. (2021) summarize problems in discordant physician–patient communication to include the patient—s tendency to “ask fewer questions and participate in decision making, and the physicians— tendency to be less patient-centered, more verbally dominant, more contentious, exhibit fewer rapport building non-verbal behaviors and provide less information” (p. 1080). Using a nonverbal coding system to examine video recordings, Hamel et al. examined the data for nonverbal synchrony or the coordination of physical movement that occurs between two individuals during an interaction. They found that positive interactions occurred when physicians focus on immediacy behaviors such as smiling, gazing, and laughter, as these behaviors elicit a favorable and matching response from patients.
Shen et al. (2018) stressed the importance of educating providers to improve communication by focusing on patient-centeredness, information giving, partnership building, and patient engagement in communication. Provider communication that demonstrates cultural humility, a process of being aware of how people—s culture can impact their health behaviors and in turn using this awareness to cultivate sensitive approaches in treating patients (Prasad et al., 2016), has been linked with deeper connections and understanding between patients and providers.
Necessary for both cultural humility and reducing implicit bias is reflection on one—s own identity and its impact on how we view and receive others. Implicit bias operates on a largely unintentional basis and many providers may not even be aware of how it influences their behavior (Dykes & White, 2009), yet implicit bias is a factor in racial, socioeconomic, and gender inequities in healthcare. Implicit bias may unconsciously alter one—s perceptions and consequently affect behaviors, interactions, and decision-making (Marcelin et al., 2019). Chapman et al. (2013) suggest that uncertainty and time pressure may contribute to reliance on stereotypes during decision-making. They further identify that these stereotypes may be reinforced through a preservice educational approach that emphasizes population-level risk factors that may portray some minority groups in unfavorable circumstances. As these generalizations may be presented as research-based, the reader may believe that their perspective or actions based on those generalizations are objective.
Even in the absence of direct measurement of implicit bias, there are compelling data around pain medication treatment decisions for patients with musculoskeletal disorders that providers divide patients with similar clinical presentations along lines of race or ethnicity. Studies have shown that Hispanic and Black patients receive less analgesia compared with White patients even after adjusting for confounders (Dickason et al., 2015; Heins et al., 2006; Miner et al., 2006; Terrell et al., 2010). Although the assessment of pain severity was accurate, less analgesia was prescribed/administered to Black and Hispanic patients. Moreover, the perception of whether a patient was exaggerating symptoms was associated with the patient—s ethnic background and the perceived quality of both the physician—s and patient—s perception of their interaction. It is essential to acknowledge how our implicit biases about our patients can contribute to unintended healthcare disparities.
Similarly, studies have shown the presence of implicit gender bias among physicians that unknowingly may sway decisions about arthroplasty treatment. Women are three times less likely than men to receive knee arthroplasty when clinically appropriate (Chapman et al., 2013). Although most physicians would deny that gender influences their decision-making, Borkhoff and colleagues— study (Borkhoff et al., 2008) challenged this. In the study, orthopaedic surgeons and family practitioners received identical vignettes of a patient with moderate unilateral knee pain and a radiograph revealing osteoarthritis with the exception that some vignettes involved a female patient and others a male patient. The odds of an orthopaedic surgeon recommending TKA to a male patient was 22 times that for a female patient. The odds of a family physician recommending TKA to a male patient was two times greater. Explanations for these differences were grounded in the implicit assumptions that men are more stoic than women or that men were more apt to engage in rigorous activities that would benefit from joint replacement.
System-Level Factors
Healthcare system factors that lead to disparities are diverse in themselves and include factors such as lack of systems to screen for or consider social determinants of health, lacking a diverse workforce, lack of interpreters, poor access to care, and time constraints of practitioners. Decades of adverse effects of social determinants of health have influenced disparate health outcomes (Owen et al., 2020). We know that populations burdened by adverse social determinants face disproportionately greater challenges and have more negative outcomes. Poverty overly affects people of color and most significantly Black patients (Noonan et al., 2016). Black and White patients living in communities with little poverty have similar patient-reported TKA outcomes, whereas in communities with high levels of poverty, there are important racial disparities with those in poverty experiencing more pain and worse function. Within these same communities with high levels of poverty, greater percentages of the population have earned no more than a high school degree. Education plays a role in mitigating the effect of poverty on TKA outcomes. In examining populations with advanced osteoarthritis, those with no college have worse pain and function and the interaction of education and community poverty produces even greater deficits (Goodman et al, 2018).
Communities experiencing greater social determinant barriers and poverty have a higher prevalence of obesity, a comorbid risk factor that may influence arthroplasty outcomes. It is critical to recognize that in these communities the social deprivations make it more difficult to lose weight. These areas have inadequate resources in terms of fresh produce and healthy food, often referred to as food deserts, but are rich in fast food options that are energy-dense, low-nutrient but more affordable. Moreover, the communities generally have higher rates of crime making them less safe for outdoor exercise, often recommended as an adjuvant to weight loss. Cleary modifying body mass index (BMI) is a greater challenge for these individuals due to the social and financial situations. Other social determinant factors found to be associated with disparities include built environment (i.e., transportation, healthcare systems, and informational networks), which can be lacking in neighborhoods with greater poverty. Although it is imperative that individuals be assessed for social needs, if we are to change the conditions that create disparities, it requires attention to social policy and laws across all governmental levels regarding allocation of resources. Also important are the development of guidelines for accreditation of institutions, the review of criteria for initial issuance, and renewal of professional licensing, as well as promulgation of Medicare (Centers for Medicare & Medicaid Services) regulations that recommend professional education on approaches to ensure health equity and the passing of local, state, and national laws and policies that promote health equity.
Insurance regulations and bundled payments are examples of regulations that may have a differential impact on health equity. Cost-containment strategies may discourage hospitals/surgeons from performing surgery for patients with preexisting risk factors such as smokers, those with high BMI, those with high hemoglobin A1c (HbA1c), or a high comorbidity index (Wang et al., 2018). These risk factors do not appear in equal proportions across the population, with higher proportions in the Black and Hispanic population. The question must be asked, if we use inflexible criteria for lower extremity joint arthroplasty to select those patients most likely not to have problems are we potentially widening the disadvantage or disparities for existing racial–ethnic, gender, and socioeconomic groups? Are we denying access to an operation that can improve health and quality of life for those most in need (Leopold, 2018; Wang et al., 2018)? Is this true medical justification or is this decision-making driven by a system of bundled payment models where the incentive is to care for the healthier patients and avoid the sicker ones? These “benign cutoffs are in fact complex issues intertwined with economic, social, and racial biases that can restrict access to total joint arthroplasty in underrepresented populations” (Chun et al., 2021, p. 2).
The National Collaborating Centre for Chronic Conditions (2008) in the U.K. in collaboration with the National Institute for Health and Care Excellence recommends that patient-specific factors (including age, gender, smoking, obesity, and comorbidities) should not be barriers to referral for surgery. They advise that decisions on referral thresholds should be based on discussions between patients and clinicians rather than on scoring tools. This decision should take place in the context of a conversation about risks and benefits that varies from patient to patient and occurs within a shared decision-making construct. Although optimization programs have met with some success, it must be asked what optimization and to what degree to ensure that they are not a deterrent to surgery (Zhang et al., 2016). Many of these risk factors may be much less modifiable than practitioners assume, leaving persons with moderate to advanced arthritic symptoms not being offered surgery (Leopold, 2018).
Conclusion
Healthcare inequities are shockingly prevalent in the U.S. healthcare system, including among patients with a wide range of musculoskeletal issues. Nurses have a professional responsibility to work to lessen disparities through individual, provider, and structural-level interventions. This includes the ethical mandate for nurses to understand how racism and other forms of discrimination and bias lead to poorer health outcomes. This article sought to define and explore the main concepts of health equity and apply them to orthopaedic nursing. It is meant to serve as an introduction to concepts and processes that truly take a lifetime to fully understand and work to change. As the bedrock of the healthcare system, nurses have the power to make an extraordinary contribution to the health of our country by working individually and collectively to maximize the health of all people.
For additional nursing continuing professional development activities on orthopaedic nursing topics, go to nursingcenter.com/ce.
The authors have disclosed that they have no financial interests to any commercial company related to this educational activity.
Contributor Information
Susan Salmond, Email: salmonsu@sn.rutgers.edu.
Caroline Dorsen, Email: caroline.dorsen@rutgers.edu.
References
- Agency for Health Care Research and Quality. (n.d.). Disparities. https://www.ahrq.gov/topics/disparities.html
- Amen T. B., Varady N. H., Rajaee S., Chen A. F. (2020). Persistent racial disparities in utilization rates and perioperative metrics in total joint arthroplasty in the U.S.: A comprehensive analysis of trends from 2006 to 2015. Journal of Bone and Joint Surgery, 102(9), 811–820. 10.2106/JBJS.19.01194 [DOI] [PubMed] [Google Scholar]
- American Medical Association. (2021). Organizational strategic plan to embed racial justice and advance health equity 2021–2023. https://www.ama-assn.org/system/files/2021-05/ama-equity-strategic-plan.pdf
- American Nurses Association. (2020, August 4). The American Academy of Nursing and the American Nurses Association call for social justice to address racism and health equity in communities of color. (2020) https://www.nursingworld.org/news/news-releases/2020/the-american-academy-of-nursing-and-the-american-nurses-association-call-for-social-justice-to-address-racism-and-health-equity-in-communities-of-color/
- Ang D. C., Monahan P. O., Cronan T. A. (2008). Understanding ethnic disparities in the use of total joint arthroplasty: Application of the health belief model. Arthritis and Rheumatism, 59(1), 102–108. 10.1002/art.23243 [DOI] [PubMed] [Google Scholar]
- Arroyo N. S., White R. S., Gaber-Baylis L. K., La M., Fisher A. D., Samaru M. (2019). Racial/ethnic and socioeconomic disparities in total knee arthroplasty 30-and 90-day readmissions: A multi-payer and multistate analysis, 2007–2014. Population Health Management, 22(2), 175–185. https://doi.org/10.1089/pop.2018.0025 [DOI] [PubMed] [Google Scholar]
- Aseltine R. H., Jr, Wang W., Benthien R. A., Katz M., Wagner C., Yan J., Lewis C. G. (2019). Reductions in race and ethnic disparities in hospital readmissions following total joint arthroplasty from 2005 to 2015. The Journal of Bone and Joint Surgery, 101(22), 2044–2050. 10.2106/JBJS.18.01112 [DOI] [PubMed] [Google Scholar]
- Bailey Z. D., Feldman J. M., Bassett M. T. (2021). How structural racism works—racist policies as a root cause of U.S. racial health inequities. The New England Journal of Medicine, 384(8), 768–773. https://doi.org/10.1056/NEJMms2025396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J. A., Barrett J., Katz J. N., Liang M. H. (1996). Total hip arthroplasty: Use and select complications in the US Medicare population. American Journal of Public Health, 86(1), 70–72. 10.2105/AJPH.86.1.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best M. J., McFarland E. G., Thakkar S. C., Srikumaran U. (2021). Racial disparities in the use of surgical procedures in the US. JAMA Surgery, 156(3), 274–281. 10.1001/jamasurg.2020.6257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Silva E. (2006). Racism without racists: Color-blind racism and the persistence of racial inequality in the United States. Rowman and Littlefield Publishers, Inc. [Google Scholar]
- Borkhoff C. M, Hawker G. A., Kreder H. J., Glazier R. H., Mahomed N. N., Wright J. G. (2008). The effect of patients' sex on physicians' recommendations for total knee arthroplasty. CMAJ: Canadian Medical Association Journal, 178(6), 681–687. 10.1503/cmaj.071168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R. W., Lindo E. G., Weeks L. D., McLemore M. R. (2020, July 2). On racism: A new standard for publishing on racial health inequities. Retrieved August 31, 2020, from https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/ [Google Scholar]
- Braveman P. (2006). Health disparities and health equity: Concepts and measurement. Annual Review of Public Health, 27, 167–194. [DOI] [PubMed] [Google Scholar]
- Braveman P. (2014a). What are health disparities and health equity? We need to be clear. Public Health Reports, 129(Suppl. 2), S5–S8. 10.1177/00333549141291S203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P. (2014b). What is health equity: And how does a life-course approach take us further toward it? Maternal and Child Health Journal, 18(2), 366–372. 10.1007/s10995-013-1226-9 [DOI] [PubMed] [Google Scholar]
- Braveman P. A., Kumanyika S., Fielding J., LaVeist T., Borrell L. N., Manderscheid R., Troutman A. (2011). Health disparities and health equity: The issue is justice. American Journal of Public Health, 101(Suppl. 1), S149–S155. 10.2105/AJPH.2010.300062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan Ramirez L. K, Baker E. A, Metzler M. Promoting health equity: A resource to help communities address social determinants of health. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- Castrucci B. C., Auerbach J. (2019). Health Affairs: Meeting individual social needs falls short of addressing social determinants of health. deBeaumont. https://debeaumont.org/news/2019/meeting-individual-social-needs-falls-short-of-addressing-social-determinants-of-health/ [Google Scholar]
- Cavanaugh A. M., Rauh M. J., Thompson C. A., Alcaraz J., Mihalko W. M., Bird C. E., Corbie-Smith G., Rosal M. C., Li W., Shadyab A. H., Gilmer T., LaCroix A. Z. (2020). Racial/ethnic disparities in physical function before and after total knee arthroplasty among women in the United States. JAMA Network Open, 3(5), e204937. 10.1001/jamanetworkopen.2020.4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (n.d.a) Work-related musculoskeletal disorders & ergonomics. https://www.cdc.gov/workplacehealthpromotion/health-strategies/musculoskeletal-disorders/index.html
- Centers for Disease Control and Prevention. (n.d.b). Arthritis health disparity statistics. https://www.cdc.gov/arthritis/data_statistics/disparities.htm
- Centers for Disease Control and Prevention. (2009). Racial disparities in total knee replacement among Medicare enrollees—United States, 2000–2006. JAMA, 302(14), 1525–1526. [Google Scholar]
- Centers for Disease Control and Prevention. (2019). Racial and ethnic disparities continue in pregnancy-related deaths. https://www.cdc.gov/media/releases/2019/p0905-racial-ethnic-disparities-pregnancy-deaths.html
- Chapman E. N., Kaatz A., Carnes M. (2013). Physicians and implicit bias: How doctors may unwittingly perpetuate health care disparities. Journal of General Internal Medicine, 28(11), 1504–1510. 10.1007/s11606-013-2441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S. C., Leonard A. K., Enchill Z., Suleiman L. I. (2021). Racial disparities in total joint arthroplasty. Current Reviews in Musculoskeletal Medicine, 14(6), 434–440. 10.1007/s12178-021-09718-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commonwealth Fund. (2021). Mirror, mirror 2021: Reflecting poorly: Health care in the U.S. compared to other high-income countries. https://www.commonwealthfund.org/publications/fund-reports/2021/aug/mirror-mirror-2021-reflecting-poorly
- Day M. A., Owens J. M., Caldwell L. S. (2019). Breaking barriers: A brief overview of diversity in orthopedic surgery. The Iowa Orthopaedic Journal, 39(1), 1–5. [PMC free article] [PubMed] [Google Scholar]
- Dean H. D., Roberts G. W., Bouye K. E., Green Y., McDonald M. (2016). Sustaining a focus on health equity at the Centers for Disease Control and Prevention through organizational structures and functions. Journal of Public Health Management and Practice, 22(Suppl. 1), S60–S67. 10.1097/PHH.0000000000000305 [DOI] [PubMed] [Google Scholar]
- Dickason R. M., Chauhan V., Mor A., Ibler E., Kuehnle S., Mahoney D., Armbrecht E., Dalawari P. (2015). Racial differences in opiate administration for pain relief at an academic emergency department. The Western Journal of Emergency Medicine, 16(3), 372–380. 10.5811/westjem.2015.3.23893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop D. D., Manheim L. M., Song J., Chang R. W. (2001). Arthritis prevalence and activity limitations in older adults. Arthritis and Rheumatism, 44(1), 212–221. [DOI] [PubMed] [Google Scholar]
- Dunlop D. D., Manheim L. M., Song J., Sohn M. -W., Feinglass J. M., Chang H. J., Chang R. W. (2008). Age and racial/ethnic disparities in arthritis-related hip and knee surgeries. Medical Care, 46(2), 200–208. 10.1097/MLR.0b013e31815cecd8 [DOI] [PubMed] [Google Scholar]
- Dunlop D. D., Song J., Manheim L. M., Chang R. W. (2003). Racial disparities in joint replacement use among older adults. Medical Care, 41(2), 288–298. 10.1097/01.MLR.0000044908.25275.E1 [DOI] [PubMed] [Google Scholar]
- Dykes D. C., White A. A., 3rd. (2009). Getting to equal: Strategies to understand and eliminate general and orthopaedic Healthcare disparities. Clinical Orthopaedics and Related Research, 467(10), 2598–2605. 10.1007/s11999-009-0993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emejuaiwe N., Jones A. C., Ibrahim S. A., Kwoh C. K. (2007). Disparities in joint replacement utilization: A quality of care issue. Clinical and Experimental Rheumatology, 25(6, Suppl- 47), 44–49. [PubMed] [Google Scholar]
- Figaro M. K., Russo P. W., Allegrante J. P. (2004). Preferences for arthritis care among urban African Americans: “I don't want to be cut.” Health Psychology, 23(3), 324–329. 10.1037/0278-6133.23.3.324 [DOI] [PubMed] [Google Scholar]
- Gee G. C. (2016, June 1–2). Racism as a social determinant of health inequities. Paper presented at Robert Wood Johnson Foundation Convening: Leveraging the social determinants to build a culture of health, Philadelphia, PA. [Google Scholar]
- Goodman S. M., Mandl L. A., Parks M. L., Zhang M., McHugh K. R., Lee Y. -Y., Nguyen J. T., Russell L. A., Bogardus M. H., Figgie M. P., Bass A. R. (2016). Disparities in TKA outcomes: Census tract data show interactions between race and poverty. Clinical Orthopaedics and Related Research, 474(9), 1986–1995. 10.1007/s11999-016-4919-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. M., Mehta B., Zhang M., Szymonifka J., Nguyen J. T., Lee L., Figgie M. P., Parks M. L., Dey S. A., Crego D., Russell L. A., Mandl L. A., Bass A. R. (2018). Disparities in total hip arthroplasty outcomes: Census tract data show Interactions between race and community deprivation. Journal of the American Academy of Orthopaedic Surgeons, 26(21), e457–e464. 10.5435/JAAOS-D-17-00393 [DOI] [PubMed] [Google Scholar]
- Gracia J. N. (2015). Now is the time to answer the call. Office of Minority Health. https://minorityhealth.hhs.gov/Blog/BlogPost.aspx?BlogID=68 [Google Scholar]
- Gravlee C. C. (2020). Systemic racism, chronic health inequities, and COVID-19: A syndemic in the making? American Journal of Human Biology, 32(5), e23482. 10.1002/ajhb.23482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel L. M., Moulder R., Harper F. W. K., Penner L. A., Albrecht T. L., Eggly S. (2021). Examining the dynamic nature of nonverbal communication between Black patients with cancer and their oncologists. Cancer, 127(7), 1080–1090. 10.1002/cncr.33352 [DOI] [PubMed] [Google Scholar]
- Hanchate A. D., Zhang Y., Felson D. T., Ash A. S. (2008). Exploring the determinants of racial and ethnic disparities in total knee arthroplasty: Health insurance, income, and assets. Medical Care, 46(5), 481–488. 10.1097/MLR.0b013e3181621e9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann L.R., Mor M., Hanusa B. H., Zickmund S., Cohen P. Z., Grant R., Kresevic D. M., Gordon H. S., Ling B. S., Kwoh C. K., Ibrahim S. A. (2010). The effect of patient race on total joint replacement recommendations and utilization in the orthopedic setting. Journal of General Internal Medicine, 25(9), 982–988. 10.1007/s11606-010-1399-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthy People 2020. (n.d.a). Foundation health measures. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. https://www.healthypeople.gov/2020/about/foundation-health-measures
- Healthy People 2020. (n.d.b). Lesbian, gay, bisexual, and trangender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health.
- Heins J. K., Heins A., Grammas M., Costello M., Huang K., Mishra S. (2006). Disparities in analgesia and opioid prescribing practices for patients with musculoskeletal pain in the emergency department. Journal of Emergency Nursing, 32(3), 219–224. 10.1016/j.jen.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Helmick C., Murphy L., Croft J., Cisternas M. (2009). Racial disparities in total knee replacement among Medicare Enrollees—United States, 2000–2006. Morbidity and Mortality Weekly Report, 58(6), 133–138. [PubMed] [Google Scholar]
- Horton R. (2020). COVID-19 is not a pandemic. The Lancet, 396(10255), 874. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32000-6/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibilibor C., Moses K. A. (2021). Putting nonverbal communication under a lens: An examination of the dynamic interplay of patient–provider interactions between Black patients and non-Black physicians. Cancer, 127(7), 1008–1009. 10.1002/cncr.33353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S. A., Siminoff L. A., Burant C. J., Kwoh C. K. (2001). Variation in perceptions of treatment and self-care practices in elderly with osteoarthritis: A comparison between African American and White patients. Arthritis and Rheumatism, 45(4), 340–345. 10.1002/1529-0131(200108)45:43.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- Ibrahim S. A., Siminoff L. A., Burant C. J., Kwoh C. K. (2002a). Understanding ethnic differences in the utilization of joint replacement for osteoarthritis: The role of patient-level factors. Medical Care, 40(1 Suppl.), I44–I51. 10.1097/00005650-200201001-00006 [DOI] [PubMed] [Google Scholar]
- Ibrahim S.A., Siminoff L. A., Burant C. J., Kwoh C. K. (2002b). Differences in expectations of outcome mediate African American/White patient differences in “willingness” to consider joint replacement. Arthritis and Rheumatism, 46(9), 2429–2435. 10.1002/art.10494 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2003). Unequal treatment: Confronting racial and ethnic disparities in health care. The National Academies Press. 10.17226/12875 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2012). How far have we come in reducing health disparities? Progress since 2000: Workshop Summary. National Academies Press. https://www.ncbi.nlm.nih.gov/books/NBK114232/ [PubMed] [Google Scholar]
- Irgit K., Nelson C. L. (2011). Defining racial and ethnic disparities in THA and TKA. Clinical Orthopaedics and Related Research, 469(7), 1817–1823. 10.1007/s11999-011-1885-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A. K., Fisher E. S., Li Z., Orav E. J., Epstein A. M. (2005). Racial trends in the use of major procedures among the elderly. The New England Journal of Medicine, 353(7), 683–691. 10.1056/NEJMsa050672 [DOI] [PubMed] [Google Scholar]
- Jones A., Kwoh C. K., Kelley M. E., Ibrahim S. A. (2005). Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis and Rheumatism, 53(6), 979–981. 10.1002/art.21596 [DOI] [PubMed] [Google Scholar]
- Kamath A. F., Horneff J. G., Gaffney V., Israelite C. L., Nelson C. L. (2010). Ethnic and gender differences in the functional disparities after primary total knee arthroplasty. Clinical Orthopaedics and Related Research, 468(12), 3355–3361. 10.1007/s11999-010-1461-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapilashrami A., Bhui K. (2020). Mental health and COVID-19: Is the virus racist? British Journal of Psychiatry, 217(2), 405–407. 10.1192/bjp.2020.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapilashrami A., Hankivsky O. (2018). Intersectionality and why it matters to global health. The Lancet, 391(10140), 2589–2591. 10.1016/S0140-6736(18)31431-4 [DOI] [PubMed] [Google Scholar]
- Katz B. P., Freund D. A., Heck D. A., Dittus R. S., Paul J. E., Wright J., Coyte P., Holleman E., Hawker G. (1996). Demographic variation in the rate of knee replacement: A multi-year analysis. Health Services Research, 31(2), 125–140. [PMC free article] [PubMed] [Google Scholar]
- Khazanchi R., Evans C. T., Marcelin J. R. Racism, not race, drives inequity across the COVID-19 continuum. JAMA Network Open, 2020;3(9), e2019933. 10.1001/jamanetworkopen.2020.19933 [DOI] [PubMed] [Google Scholar]
- Kim H., Meath T. H. A., Quiñones A. R., McConnell K. J., Ibrahim S. A. (2021). Association of Medicare mandatory bundled payment program with the receipt of elective hip and knee replacement in White, Black, and Hispanic beneficiaries. JAMA Network Open, 4(3), e211772. 10.1001/jamanetworkopen.2021.1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemt C., Walker P., Padmanabha A., Tirumala V., Xiong L., Kwon Y. M. (2021). Minority race and ethnicity is associated with higher complication rates after revision surgery for failed total hip and knee joint arthroplasty. The Journal of Arthroplasty, 36(4), 1393–1400. https://doi.org/10.1016/j.arth.2020.10.043 [DOI] [PubMed] [Google Scholar]
- Kwoh C. K., Vina E. R., Cloonan Y. K., Hannon M. J., Boudreau R. M., Ibrahim S. A. (2015). Determinants of patient preferences for total knee replacement: African-Americans and whites. Arthritis Research & Therapy, 17(1), 348–348. 10.1186/s13075-015-0864-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R. H., Kamath A. F. (2017). Post-acute care disparities in total joint arthroplasty. Arthroplasty Today, 3(3), 187–191. 10.1016/j.artd.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieux S. L. (2017). Functional outcome after total hip replacement: The effect of race and ethnicity among Medicare beneficiaries. Doctoral dissertation, Northeastern University. [Google Scholar]
- Larrieux S. L. (2017). Functional outcome after total hip replacement: The effect of race and ethnicity among Medicare beneficiaries. Doctoral dissertation, Northeastern University. [Google Scholar]
- Lavernia C. J., Villa J. M. (2015). Does race affect outcomes in total joint arthroplasty? Clinical Orthopaedics and Related Research, 473(11), 3535–3541. 10.1007/s11999-015-4481-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavernia C. J., Alcerro J. C., Rossi M. D. (2010). Fear in arthroplasty surgery: The role of race. Clinical Orthopaedics and Related Research, 468(2), 547–554. 10.1007/s11999-009-1101-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold S. S. (2018). Editor's spotlight/take 5: Eligibility criteria for lower-extremity joint replacement may worsen racial and socioeconomic disparities. Clinical Orthopaedics and Related Research, 476(12), 2297–2300. 10.1097/CORR.0000000000000522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link B. G., Phelan J. (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, Spec No, 80–94. 10.2307/2626958 [DOI] [PubMed] [Google Scholar]
- MacFarlane L. A., Kim E., Cook N. R., Lee I., Iversen M. D., Katz J. N., Costenbader K. H., (2018). Racial variations in total knee replacement in a diverse nationwide clinical trial. Journal of Clinical Rheumatology, 24(1), 1–5. 10.1097/rhu.0000000000000613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin J. R., Siraj D. S., Victor R., Kotadia S., Maldonado Y. A. (2019). The impact of unconscious bias in healthcare: How to recognize and mitigate it. The Journal of Infectious Diseases, 220(Suppl. 2), S62–S73. 10.1093/infdis/jiz214 [DOI] [PubMed] [Google Scholar]
- McBean A. M., Gornick M. (1994). Differences by race in the rates of procedures performed in hospitals for Medicare beneficiaries. Health Care Financing Review, 15(4), 77–90. [PMC free article] [PubMed] [Google Scholar]
- Mehta B. Y., Bass A. R., Goto R., Russell L. A., Parks M. L., Figgie M. P., Goodman S. M. (2018). Disparities in outcomes for Blacks versus Whites undergoing total hip arthroplasty: A systematic literature review. Journal of Rheumatology, 45(5), 717–722. 10.3899/jrheum.170855 [DOI] [PubMed] [Google Scholar]
- Mendenhall E., Like R. (2020). COVID-19 pandemic 7: Both pandemic and syndemic—how clusters of preexisting comorbid conditions have driven up fatalities. Podcast episode 43. Movement is Life. https://www.movementislifecaucus.com/mil_podcast/covid-19-pandemic-7-both-pandemic-and-syndemic-how-clusters-of-preexisting-comorbid-conditions-have-driven-up-fatalities-featuring-dr-emily-mendenhall-and-dr-robert-like/
- Milken Institute School of Public Health. (2020, November 5). Equity vs. equality: What's the difference. https://onlinepublichealth.gwu.edu/resources/equity-vs-equality/
- Miner J., Biros M. H., Trainor A., Hubbard D., Beltram M. (2006). Patient and physician perceptions as risk factors for oligoanalgesia: A prospective observational study of the relief of pain in the emergency department. Academic Emergency Medicine, 13(2), 140–146. 10.1197/j.aem.2005.08.008 [DOI] [PubMed] [Google Scholar]
- Mingo C. A., McIlvane J. M., Jefferson M., Edwards L. J., Haley W. E. (2013). Preferences for arthritis interventions: Identifying similarities and differences among African Americans and Whites with osteoarthritis. Arthritis Care & Research, 65(2), 203–211. 10.1002/acr.21781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movement Is Life Caucus. (2011). Movement is life: A catalyst for change. https://www.movementislifecaucus.com/wp-content/uploads/Movement-Is-Life-A-Catalyst-For-Change-Proceedings-Report.pdf [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2021). The future of nursing 2020–2030: Charting a path to achieve health equity. The National Academies Press. 10.17226/25982 [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Chronic Conditions (UK). (2008). Osteoarthritis: National clinical guideline for care and management in adults. Royal College of Physicians (UK). https://pubmed.ncbi.nlm.nih.gov/21290638/ [PubMed] [Google Scholar]
- Nikiphorou E., Lempp H., Kohrt B. A. (2019). Treatment failure in inflammatory arthritis: Time to think about syndemics? Rheumatology, 58(9), 1526–1533. 10.1093/rheumatology/kez222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan A. S., Velasco-Mondragon H. E., Wagner F. A. (2016). Improving the health of African Americans in the USA: An overdue opportunity for social justice. Public Health Reviews, 37(1), 12. 10.1186/s40985-016-0025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Minority Health. (n.d.a). Infant mortality and African Americans. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=23
- Office of Minority Health. (n.d.b) Now is the time to answer the call. https://minorityhealth.hhs.gov/Blog/BlogPost.aspx?BlogID=68
- Okike K., Chan P. H., Prentice H. A., Navarro R. A., Hinman A. D., Paxton E. W. (2019). Association of race and ethnicity with total hip arthroplasty outcomes in a universally insured population. Journal of Bone and Joint Surgery: American Volume, 101(13), 1160–1167. 10.2106/JBJS.18.01316 [DOI] [PubMed] [Google Scholar]
- Oronce C. I. A., Shao H., Shi L. (2015). Disparities in 30-day readmissions after total hip arthroplasty. Medical Care, 53(11), 924–930. 10.1097/MLR.0000000000000421 [DOI] [PubMed] [Google Scholar]
- Owen W. F., Jr, Carmona R., Pomeroy C. (2020). Failing another national stress test on health disparities. JAMA, 323(19), 1905–1906. 10.1001/jama.2020.6547 [DOI] [PubMed] [Google Scholar]
- Poon S., Kiridly D., Mutawakkil M.,Wendolowski S., Gecelter R., Kline M., Lane L. B. (2019). Current trends in sex, race, and ethnic diversity in orthopaedic surgery residency. Journal of the American Academy of Orthopaedic Surgeons, 27(16), e725–e733. 10.5435/JAAOS-D-18-00131 [DOI] [PubMed] [Google Scholar]
- Prasad S. J., Nair P., Gadhvi K., Barai I., Danish H. S., Philip A. B. (2016). Cultural humility: Treating the patient, not the illness. Medical Education Online, 21, 30908. 10.3402/meo.v21.30908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. L. (1999). The intersections of dominant discourses across race, gender, and other identities. Journal of Counseling and Development, 77(1), 73–79. 10.1002/j.1556-6676.1999.tb02423.x [DOI] [Google Scholar]
- Rural Health Information Hub. (n.d.). Rural disparities. https://www.ruralhealthinfo.org/topics/rural-health-disparities
- Salmond S. W., Echevarria M. (2017). Healthcare transformation and changing roles for nursing. Orthopaedic Nursing, 36(1), 12–25. 10.1097/NOR.0000000000000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. A., Corbie-Smith G., Cykert S. (2019). Racial/ethnic disparities in pain burden and pain management in the context of opioid overdose risk. Current Epidemiology Reports, 6(2), 275–289. [Google Scholar]
- Shahid H., Singh J. A. (2016). Racial/ethnic disparity in rates and outcomes of total joint arthroplasty. Current Rheumatology Reports, 18(4), 20. 10.1007/s11926-016-0570-3 [DOI] [PubMed] [Google Scholar]
- Shen M. J., Peterson E. B., Costas-Muñiz R., Hernandez M. H., Jewell S. T., Matsoukas K., Bylund C. L. (2018). The effects of race and racial concordance on patient-physician communication: A systematic review of the literature. Journal of Racial and Ethnic Health Disparities, 5(1), 117–140. 10.1007/s40615-017-0350-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Bulled N., Ostrach B., Mendenhall E. (2017). Syndemics and the biosocial conception of health. The Lancet, 389(10072), 941–950. 10.1016/S0140-6736(17)30003-X [DOI] [PubMed] [Google Scholar]
- Singh J. A., Kallan M. J., Chen Y., Parks M. L., Ibrahim S. A. (2019). Association of race/ethnicity with hospital discharge disposition after elective total knee arthroplasty. JAMA Network Open, 2(10), e1914259. 10.1001/jamanetworkopen.2019.14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J. A., Lu X., Rosenthal G. E., Ibrahim S., Cram P. (2014). Racial disparities in knee and hip total joint arthroplasty: An 18-year analysis of national Medicare data. Annals of the Rheumatic Diseases, 73(12), 2107–2115. 10.1136/annrheumdis-2013-203494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J., Weinstein J. N., Sporer S. M., Wennberg J. E. (2003). Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. The New England Journal of Medicine, 349(14), 1350–1359. 10.1056/NEJMsa021569 [DOI] [PubMed] [Google Scholar]
- Skinner J., Zhou W., Weinstein J. (2006). The influence of income and race on total knee arthroplasty in the United States. Journal of Bone and Joint Surgery: American Volume, 88(10), 2159–2166. 10.2106/JBJS.E.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slover M., Walsh M. G., Zuckerman J. D. (2010). Sex and race characteristics in patients undergoing hip and knee arthroplasty in an urban setting. The Journal of Arthroplasty, 25(4), 576–580. 10.1016/j.arth.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Smedley B., Butler A., Bristow L. (2004). The Nation's compelling interest: Ensuring diversity in the health care workforce. National Academies Press (US). [PubMed] [Google Scholar]
- Suarez-Almazor M. E., Souchek J., Kelly P. A., O—Malley K., Byrne M., Richardson M., Pak C. (2005). Ethnic variation in knee replacement: Patient preferences or uninformed disparity? American Medical Association, 165(10), 1117–1124. 10.1001/archinte.165.10.1117 [DOI] [PubMed] [Google Scholar]
- Terrell K. M., Hui S. L., Castelluccio P., Kroenke K., McGrath R. B., Miller D. K. (2010). Analgesic prescribing for patients who are discharged from an emergency department. Pain Medicine, 11(7), 1072–1077. 10.1111/j.1526-4637.2010.00884.x [DOI] [PubMed] [Google Scholar]
- Thirukumaran C. P., Kim Y., Cai X., Ricciardi B. F., Li Y., Fiscella K. A., Mesfin A., Glance L. G. (2021). Association of the Comprehensive Care for Joint Replacement model with disparities in the use of total hip and total knee replacement. JAMA Network Open, 4(5), e2111858. 10.1001/jamanetworkopen.2021.11858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi A., Ezomo O. T., Gronbeck C., Harrington M. A., Halawi M. J. (2020). Time trends and risk factors for 30-day adverse events in Black Patients undergoing primary total knee arthroplasty. The Journal of Arthroplasty, 35(11), 3145–3149. 10.1016/j.arth.2020.06.013 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Task Force on Black and Minority Health (1985). Report of the Secretary's Task Force on Black and Minority Health (Heckler Report). https://archive.org/details/reportofsecretar00usdepar
- Wang A. Y., Wong M. S., Humbyrd C. J. (2018). Eligibility criteria for lower extremity joint replacement may worsen racial and socioeconomic disparities. Clinical Orthopaedics and Related Research, 476(12), 2301–2308. 10.1097/CORR.0000000000000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard D., Moseley P., Sørup F. K. H., Baldi P., Brunak S. (2019). Population-wide analysis of differences in disease progression patterns in men and women. Nature Communications, 10(1), 666. 10.1038/s41467-019-08475-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., Mohammed S. A. (2013). Racism and health I: Pathways and scientific evidence. American Behavioral Scientist, 57(8). 10.1177/0002764213487340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., Lawrence J. A., Davis B. A. (2019). Racism and health: Evidence and needed research. Annual Review of Public Health, 40(1), 105–125. 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2018, February 22). Health inequities and their causes. https://www.who.int/news-room/facts-in-pictures/detail/health-inequities-and-their-causes
- Wu M., Belay E., Cochrane N., O'Donnell J., Seyler T. (2021). Comorbidity burden contributing to racial disparities in outpatient versus inpatient total knee arthroplasty. Journal of the American Academy of Orthopaedic Surgeons, 29(12), 537–543. 10.5435/JAAOS-D-20-01038 [DOI] [PubMed] [Google Scholar]
- Xu H. F., White R. S., Sastow D. L., Andreae M. H., Gaber-Baylis L. K., Turnbull Z. A. (2017). Medicaid insurance as primary payer predicts increased mortality after total hip replacement in the state inpatient databases of California, Florida and New York. Journal of Clinical Anesthesia, 43, 24–32. 10.1016/j.jclinane.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Lyman S., Boutin-Foster C., Parks M. L., Pan T. J., Lan A., Ma Y. (2016). Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. Journal of Bone and Joint Surgery, 98(15), 1243–1252. 10.2106/JBJS.15.01009 [DOI] [PubMed] [Google Scholar]
- Zhang W., Moskowitz R. W., Nuki G., Abramson S., Altman R. D., Arden N., Bierma-Zeinstra S., Brandt K. D., Croft P., Doherty M., Dougados M., Hochberg M., Hunter D. J., Kwoh K., Lohmander L. S., Tugwell P. (2008). OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis and Cartilage, 16(2), 137–162. 10.1016/j.joca.2007.12.013 [DOI] [PubMed] [Google Scholar]