Abstract
Background:
Currently, there is no approved treatment for the management of coronavirus disease (COVID-19). Drug repurposing of existing medications could be a possible way to find out a novel therapeutic entity to combat the COVID-19.
Aim:
To determine the clinical efficacy and safety of an Ayurveda intervention (Guduchighana Vati) in asymptomatic and mild-to-moderate cases of COVID-19.
Materials and methods:
This was an open-label randomized controlled pilot study with a sample size of 30 participants (15 in each arm). The participants were asymptomatic or mild to moderate cases of COVID-19. Guduchighana Vati 500 mg twice daily for 10 days was administered in the study group and Hydroxychloroquine for 5 days in the control group. Paracetamol, Vitamin C, Multivitamin, and Zinc were also provided in the control group. The main outcome measures were to negative real-time reverse transcription–polymerase chain reaction (RT-PCR) assay for COVID-19, proportion of participants with negative RT-PCR for COVID-19 at 5th and 10th day, proportion of participants with clinical recovery, improvement in laboratory parameters, and incidence of adverse drug reaction/adverse event (ADR/AE). The results of RT-PCR and clinical recovery were compared between groups using Chi-square test. The data related to laboratory parameters were compared within group using paired sample t-test/Wilcoxon signed-rank test and between groups using independent sample t-test/Mann–Whitney test.
Results:
The proportion of participants with negative RT-PCR for COVID-19 in the Guduchighana Vati group (93.3%) was better as compared to the control group (66.6%) till 10th day of the study period. Though, the results are statistically not significant (P = 0.068). All the symptomatic patients in the Guduchighana Vati group clinically recovered whereas one patient remained symptomatic in the control group on the 5th day. No symptoms of COVID-19 were observed at 10th day in both the groups. No ADR/serious adverse event were observed during the study period in either of the groups.
Conclusion:
In this study on asymptomatic and mild to moderate cases of COVID-19, Guduchighana Vati showed numerically better proportion of participants with negative RT-PCR assay for COVID-19 and reduced time to clinical improvement which requires confirmation through studies with larger sample size. Although, the study outcomes are statistically not significant which may be due to small sample size.
Keywords: Coronavirus disease-19, Guduchi, Guduchighana Vati, severe acute respiratory syndrome coronavirus 2, Tinospora cordifolia
Introduction
The pandemic caused by Coronavirus disease (COVID-19), affected more than 181 million people globally with around 3.9 million reported deaths as per the information updated by World Health Organization on June 30, 2021.[1] The global scale of transmission, considerable morbidity and mortality, and increased risk of complications and death in high-risk individuals have been the major cause of concern related to this pandemic.[2] Till date, there is no proven treatment for the management of COVID-19, although several pharmacological options are being explored as per the clinical condition. Significant numbers of clinical trials are going on in conventional as well as traditional medicine systems to find out a safe and effective treatment of COVID-19. Repurposing of existing medications could be a possible way to overcome the time limitation of research and development required to find out a novel therapeutic entity to combat the COVID-19. It can facilitate prompt clinical decisions at lower costs as compared to new drug development. Ayurveda is probably the oldest medicine system having a description of infectious diseases and epidemics and their various modes of transmission.[3,4,5] Ayurveda has time-tested interventions to improve the immune response which have been further evaluated for their immune-modulator, anti-inflammatory, and anti-viral properties.[6,7,8,9,10,11,12,13,14,15,16,17] These interventions may arrest the progression of COVID-19 and helpful in reducing morbidity and mortality associated with this pandemic. Government of India has incorporated Ayurveda interventions also in the National clinical management protocol based on Ayurveda, Yoga for management of COVID-19.[18]
Guduchi (Tinospora cordifolia [Willd.] Miers) is a widely used medicinal plant in Ayurveda indicated for Jwara (febrile illness) among other indications.[19] Several research studies have established the immune-modulatory, anti-inflammatory, and anti-microbial properties exhibited by T. cordifolia.[20,21,22,23,24,25,26] Guduchighana Vati (formulation prepared from powdered aqueous extract of T. cordifolia) has been widely prescribed in the management of febrile illnesses of infective origin. However, there is no evidence to recommend its use in the management of COVID-19. Therefore, the present study was designed to determine the efficacy and safety of Guduchighana Vati in the management of asymptomatic and mild-to-moderate cases of COVID-19.
Materials and methods
The study was executed in accordance with the principles of Declaration of Helsinki and the ICMR ethical guidelines for biomedical research on human participants (2017). The study was reviewed and approved by the Institutional Ethics Committee of Pt. Khushilal Sharma Government Ayurveda College and Institute, Bhopal, Madhya Pradesh, India vide letter no. KLSGACI/IEC/2020/06 dated July 24, 2020. The clinical trial was registered prospectively at Clinical Trial Registry of India vide CTRI/2020/07/026840 dated July 27, 2020. The CONSORT guidelines were followed while reporting the study results.
Study design
This was an open-label randomized active-controlled pilot study. No deviation from study protocol occurred after the commencement of the study.
Study setting and participants
The study was conducted at Pt. Khushilal Sharma Government Ayurveda College and Institute, Bhopal, Madhya Pradesh, India. The study site was a designated COVID care center notified by the State Government of Madhya Pradesh. Real-time reverse transcription–polymerase chain reaction (RT-PCR) for COVID-19 of all the participants included in the study was done at ICMR authorized laboratory.[27] The participants included in the study were kept in isolation and regular clinical monitoring and check-up were done at the study site. The study was conducted from July 2020 to September 2020.
Inclusion and exclusion criteria
Participants of either sex aged 18 years and above who were RT-PCR positive for COVID-19 and categorized as asymptomatic or mild-to-moderate cases as per the guidelines of Ministry of Health and Family Welfare (MoHFW), Government of India guidelines were included in the study.[28] Participants with severe COVID-19 as per 8-point ordinal score,[29] i.e., hospitalized, on invasive mechanical ventilation or extracorporeal membrane oxygenation, participants with comorbidities, participants with severe vomiting which would make oral administration of medicine difficult, with alanine amino transaminase (ALT) or aspartate amino transaminase (AST) more than two times the upper limit of normal, and pregnant or lactating women were excluded from the study.
Among the 32 individuals screened as per the inclusion and exclusion criteria, 30 patients of COVID-19 confirmed by RT-PCR and fulfilling the inclusion criteria were included in the study. The detailed information related to the research study was provided to the study participants and written informed consent in their regional language was taken before enrolling them in the study.
Study intervention
Guduchighana Vati (Batch no.: HTW011; Manufacturing date: April 2020; Expiry date: March 2023) two tablets (250 mg each) twice daily after food with water for 10 days was administered to the participants in the Guduchighana Vati group. Hydroxychloroquine (Batch no: GPD080012BH; Manufacturing date: March 2020; Expiry date: February 2024) was given in the maintenance dose of 800 mg for 1st day followed by 400 mg per day for the next 4 days in divided doses in the control group as per the recommendations of the MoHFW, Madhya Pradesh, India. Paracetamol, Vitamin C, Multivitamin, and Zinc were also provided in the control group whereas Paracetamol only was provided in the Guduchighana Vati group as per the clinical condition of the participant if required. All the study participants were also instructed to follow respiratory and hand hygiene measures.
Guduchighana Vati was procured from Pharmanza Herbal Private Limited, Gujarat, India. It is an Ayurvedic classical formulation included in the Ayurvedic Formulary of India.[30] It is prepared from the powdered aqueous extract of T. cordifolia (Willd.) Miers in tablet form. This dosage form has several advantages such as accuracy of dosage, stability, patient palatability, and easy storage.
Outcomes measures
Primary outcome measure
Time to negative RT-PCR for COVID-19 (from the day of trial drug administration) and proportion of participants with negative RT-PCR assay at 5th and 10th day was the primary outcome measure. Two-day continuous real-time RT-PCR test was done on 5th day and 10th day (in case the study participant was found RT-PCR positive on 5th day assessment).
Secondary outcome measures
The proportion of participants with clinical recovery on 5th and 10th day; changes in laboratory parameters (from baseline to 10th day of the study period) such as differential and Total leukocyte count, erythrocyte sedimentation rate, immunoglobulin M and immunoglobulin G (IgM and IgG), inflammatory markers such as interleukin-6 (IL-6), serum ferritin, changes in liver enzymes, renal function parameters, proportion of participants advanced to severe stage of COVID-19 (i.e., participants referred due to onset of complications or required invasive or noninvasive oxygen therapy during the intervention period) and incidence of adverse drug reaction/adverse event (ADR/AE) on 10th day (end of the trial).
Criteria of “clinical recovery”
Normal body temperature (≤36.6°C axilla or ≤37.2°C oral), absence of cough or mild cough, absence of dyspnea on routine activity or respiratory rate <30 breaths per minute without supplemental oxygen, absence of any other symptom/sign attributed to COVID-19, normal SpO2 by standard peripheral oximetry (above 93%) and recovery should be sustained for at least 48 h under physician observation.
The study participants who remain RT-PCR negative at the end of the study period were discharged as per the existing MoHFW, Government of India guidelines.[31]
Safety assessment
The study participants were assessed daily for the presence of ADR/AE till discharge. Laboratory parameters such as aspartate transaminase (AST), alanine amino transaminase (ALT), serum alkaline phosphatase, serum bilirubin, serum creatinine, blood urea, and serum uric acid were assessed on baseline and 10th day of the study period.
Sample size and randomization
A sample size of 30 participants (15 in each group) was taken in a pilot study. Thirty eligible participants were randomized into two parallel arms in the ratio of 1:1. A statistician generated a randomized list using theSPSS 15.0 for Windows, 233 South Wacker Drive, 11th Floor, Chicago, Illinois, U.S.A.
Statistical analysis
The categorical variables (such as results of RT-PCR and clinical recovery) have been represented as number (percentage) and compared using Chi-square test. The continuous data (related to laboratory parameters) has been represented as mean (standard deviation), and median (min-max) for data not following normal distribution. This continuous data has been compared within group by using paired sample t-test/Wilcoxon signed-rank test and between groups analysis has been done using independent sample t-test/Mann–Whitney test. A P < 0.05 has been considered as statistically significant. Analysis has been done using the Statistical Package for the Social Sciences SPSS 15.0 for Windows, 233 South Wacker Drive, 11th Floor, Chicago, Illinois, U.S.A.
Observation and Results
Patient enrollment
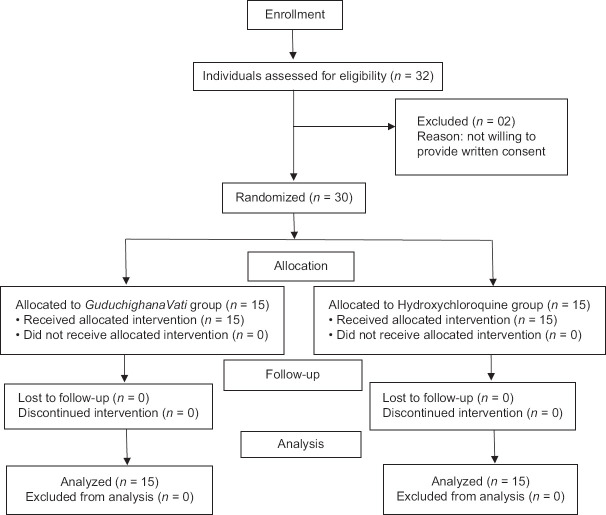
A total of 32 patients were screened as per the inclusion and exclusion criteria of the study from July 28, 2020. Two patients were not willing to provide written consent to participate in the study and thus were excluded from the study. A total of 30 participants were included in the study (15 each in Guduchighana Vati group and control group). Outflow of the study participants is shown as CONSORT flow diagram in Figure 1.
Figure 1.
CONSORT flow diagram
Baseline clinical characteristics of study participants
The demographic data of the participants are shown in Table 1. The mean age of participants in the Guduchighana Vati group and control group was (30.27 ± 8.83) and (32.27 ± 7.35) years, respectively. The distribution of age of the participants was similar between the two groups (P = 0.506). In the present study, 53.3% participants were found symptomatic in each group (P = 0.991). At baseline, fever was present in 40%, cough in 26.7%, sore throat in 20%, and body-ache (myalgia) in 33.3% of participants in the Guduchighana Vati group whereas it was 20%, 6.7%, 13.3%, and 6.7% respectively in the control group. The demographic and clinical characteristics were comparable between the two groups at baseline.
Table 1.
Baseline clinical characteristics of the study participants
| Variable | Parameters | GVG (n=15) | CG (n=15) | P $ |
|---|---|---|---|---|
| Age:Mean±SD | - | 30.27±8.836 | 32.27±7.353 | 0.506 |
| Gender, n (%) | Male | 12 (80.0) | 11 (73.3) | 0.666 |
| Female | 3 (20.0) | 4 (26.7) | ||
| Clinical features, n (%) | Asymptomatic | 7 (46.7) | 7 (46.7) | 0.991 |
| Symptomatic | 8 (53.3) | 8 (53.3) |
$ Compared using Chi-square test. SD: Standard deviation, GVG: GuduchighanaVati Group, CG: Control group
Efficacy outcomes
In the Guduchighana Vati group, 66.6% participants turned RT-PCR negative for COVID-19 on 5th day and 93.3% participants on 10th day from the administration of trial drug. While it was 53.3% and 66.6% on 5th and 10th day in the control group [Table 2]. However, the results were statistically insignificant (P = 0.068). The remaining patients with positive RT-PCR assay in both the study groups were asymptomatic at the end of the study period and discharged as per the existing guidelines. On the 5th day of study period, all the symptomatic patients in the Guduchighana Vati group clinically recovered whereas one patient remained symptomatic in the control group. No symptoms were observed in any of the patients at the end of the study period, i.e., 10th day in both the groups [Table 3].
Table 2.
Result of reverse transcription-polymerase chain reaction assay in Guduchighana Vati group and control group
| Day to assess RT-PCR for COVID-19 |
GVG (n=15), n (%) | CG (n=15), n (%) | P $ |
|---|---|---|---|
| 5th day | 10 (66.6) | 8 (53.3) | 0.456 |
| 10th day | 14 (93.3) | 10 (66.6) | 0.068 |
$ Compared using Chi-square test. RT-PCR: Reverse transcription-polymerase chain reaction, COVID-19: Coronavirus disease-2019, GVG: GuduchighanaVati Group, CG: Control group
Table 3.
Chief complaints in Guduchighana Vati group and control group
| Chief complaints | Group | Baseline | 5th day | 10th day |
|---|---|---|---|---|
| Fever at evaluation | GVG | 6 (40.0) | 0 | 0 |
| CG | 3 (20.0) | 0 | 0 | |
| P # | 0.232 | - | - | |
| Cough | GVG | 4 (26.7) | 0 | 0 |
| CG | 1 (6.7) | 0 | 0 | |
| P # | 0.142 | - | - | |
| Sore throat | GVG | 3 (20.0) | 0 | 0 |
| CG | 2 (13.3) | 1 (6.7) | 0 | |
| P # | 0.624 | - | - | |
| Bodyache/myalgia | GVG | 5 (33.3) | 0 | 0 |
| CG | 1 (6.7) | 0 | 0 | |
| P # | 0.068 | - | - | |
| Anorexia | GVG | 2 (13.3) | 0 | 0 |
| CG | 1 (6.7) | 0 | 0 | |
| P # | 0.543 | |||
| Headache | GVG | 7 (46.7) | 0 | 0 |
| CG | 3 (20.0) | 0 | 0 | |
| P # | 0.245 | - | - |
# Compared using Chi-square test. GVG: GuduchighanaVatiGroup,, CG: Control group
Vital parameters such as SpO2, pulse rate, respiratory rate, and blood pressure were within normal limits in both the groups during the study period. No complications such as pneumonia, acute respiratory distress syndrome, sepsis, septic shock, arrhythmia developed in any of the participants during the study period in both the groups. The values of laboratory parameters such as IL-6, IgG, IgM, serum ferritin, and SpO2 are depicted in Table 4. The reduction in IL-6 levels after the treatment was statistically significant in both the groups. The level of IgG, IgM, Serum Ferritin, and SpO2 were found within the normal limits during the study period in both the groups. None of the participants enrolled in the trial required invasive/noninvasive oxygen therapy during the intervention period.
Table 4.
Effect on interleukin-6, immunoglobulin G, immunoglobulin M, serum ferritin and oxygen saturation in Guduchighana Vati group and control group
| Parameters | Mean±SD | Between group P# | |
|---|---|---|---|
|
| |||
| GVG (n=15) | CG (n=15) | ||
| IL-6 (pg/mL) (0-5.9 pg/mL) | |||
| Baseline | 6.59 (1.0-19.4)a | 4.10 (2.0-12.9)a | 0.983 |
| 10th day | 3.15 (1.20-9.86)a | 2.80 (0.20-5.20)a | 0.297 |
| P$ | 0.027* | 0.001* | |
| IgG (g/L) (5.4-18.22 g/L) | |||
| Baseline | 13.42±1.99 | 11.54±1.64 | 0.009* |
| 10th day | 13.39±1.94 | 12.28±1.41 | 0.965b |
| P$ | 0.951 | 0.002* | |
| IgM (g/L) (0.22-2.40 g/L) | |||
| Baseline | 0.93±0.27 | 1.08±0.38 | 0.235 |
| 10th day | 1.09±0.28 | 1.10±0.24 | 0.732 |
| P$ | 0.007* | 0.722 | |
| Serum ferritin (ng/mL) (20-400 ng/mL) | |||
| Baseline | 83.76±45.08 | 104.89±84.15 | 0.399 |
| 10th day | 97.16±43.81 | 103.97±72.64 | 0.758 |
| P$ | 0.395 | 0.929 | |
| SpO2 (%) | |||
| Baseline | 95.82±1.54 | 96.08±1.31 | 0.620 |
| 10th day | 97.64±0.67 | 96.73±1.38 | 0.029* |
| P$ | 0.006* | 0.433 | |
*P <0.05 has been considered as significant, $ Within group P value, compared using paired sample t -test/Wilcoxon signed rank test, # Between group P value, compared using independent sample t -test/Mann-Whitney test, a Data has been reported as median (minimum-maximum), b Adjusted data at baseline through ANCOVA. SD: Standard deviation, IgG: Immunoglobulin G, IgM: Immunoglobulin M, IL-6: Interleukin-6, SpO2: saturation of peripheral oxygen, GVG: GuduchighanaVati Group, CG: Control group
Safety outcomes
ADR/AE was not observed or reported by any of the study participants. Hematological tests, liver function test and kidney function test were found to be within the normal limits throughout the study period in both the groups [Tables 5 and 6].
Table 5.
Effect on Hematological parameters in Guduchighana Vati Group and control group
| Parameters | Mean±SD | Between group P# | |
|---|---|---|---|
|
| |||
| GVG (n=15) | CG (n=15) | ||
| Total leukocyte count (per mm3) | |||
| Baseline | 5793.33±1087.24 | 6980.00±1993.63 | 0.053 |
| 10th day | 7273.33±1447.39 | 7506.67±1582.70 | 0.677 |
| P$ | 0.001 | 0.375 | - |
| Neutrophils (%) | |||
| Baseline | 54.07±9.88 | 66.40±9.02 | 0.001 |
| 10th day | 56.53±8.35 | 63.13±7.28 | -0.74 |
| P$ | 0.208 | 0.270 | - |
| Eosinophils (%) | |||
| Baseline | 3.87±1.30 | 4.27±0.96 | 0.347 |
| 10th day | 4.40±1.35 | 3.60±2.06 | 0.220 |
| P$ | 0.251 | 0.290 | - |
| Lymphocytes (%) | |||
| Baseline | 39.20±10.04 | 26.87±8.63 | 0.001 |
| 10th day | 36.00±8.05 | 30.67±6.58 | 0.057 |
| P$ | 0.144 | 0.162 | - |
| ESR (mm/Ist hour) | |||
| Baseline | 9.40±2.72 | 10.07±4.32 | 0.617 |
| 10th day | 8.47±2.85 | 9.67±4.08 | 0.359 |
| P$ | 0.224 | 0.373 | - |
$ Within group P value, compared using paired sample t-test, # Between group P value, compared using independent sample t-test, P<0.05 has been considered as significant. SD: Standard deviation, ESR: Erythrocyte sedimentation rate, GVG: Guduchighana Vati group, CG: Control group
Table 6.
Effect on kidney function test and liver function test in Guduchighana Vati group and control group
| Parameters | Mean±SD | Between group P# | |
|---|---|---|---|
|
| |||
| GVG (n=15) | CG (n=15) | ||
| Blood urea (mg/dl) | |||
| Baseline | 27.40±4.81 | 28.16±2.91 | 0.605 |
| 10th day | 27.76±3.13 | 27.43±4.13 | 0.809 |
| P$ | 0.730 | 0.402 | - |
| Serum uric acid (mg/dl) | |||
| Baseline | 5.07±0.82 | 5.49±0.67 | 0.136 |
| 10th day | 5.08±0.49 | 5.41±0.67 | 0.138 |
| P$ | 0.952 | 0.592 | - |
| Serum creatinine (mg/dl) | |||
| Baseline | 1.11±1.36 | 0.75±0.13 | 0.326 |
| 10th day | 0.69±0.23 | 0.64±4.13 | 0.476 |
| P$ | 0.314 | 0.035* | - |
| AST (IU/L) | |||
| Baseline | 26.47±4.20 | 28.31±2.29 | 0.146 |
| 10th day | 26.55±3.98 | 26.24±3.45 | 0.820 |
| P$ | 0.894 | 0.006* | - |
| ALT (IU/L) | |||
| Baseline | 33.89±3.22 | 36.25±3.26 | 0.055 |
| 10th day | 33.38±3.83 | 35.66±3.13 | 0.085 |
| P$ | 0.716 | 0.555 | - |
| Total protein (g/dl) | |||
| Baseline | 6.04±0.87 | 6.43±0.75 | 0.204 |
| 10th day | 5.58±0.72 | 5.88±0.78 | 0.282 |
| P$ | 0.017* | 0.001* | - |
| Serum albumin (g/dl) | |||
| Baseline | 4.31±0.54 | 4.44±0.70 | 0.33 |
| 10th day | 4.26±0.28 | 4.40±0.41 | 0.29 |
| P$ | 0.735 | 0.804 | - |
| Serum globulin (g/dl) | |||
| Baseline | 1.73±0.68 | 1.98±0.62 | 0.298 |
| 10th day | 1.32±0.69 | 1.48±0.58 | 0.498 |
| P$ | 0.035* | 0.003* | - |
| Conjugated bilirubin (mg/dl) | |||
| Baseline | 0.11±0.02 | 0.10±0.00 | 0.326 |
| 10th day | 0.11±0.05 | 0.12±0.10 | 0.643 |
| P$ | 0.702 | 0.334 | - |
| Unconjugated bilirubin (mg/dl) | |||
| Baseline | 0.68±0.17 | 0.65±0.14 | 0.563 |
| 10th day | 0.53±0.17 | 0.51±0.15 | 0.729 |
| P$ | 0.035* | 0.002* | - |
| Serum alkaline phosphatase (U/L) | |||
| Baseline | 156.91±34.87 | 169.39±34.39 | 0.332 |
| 10th day | 158.04±38.15 | 155.01±32.62 | 0.817 |
| P$ | 0.874 | 0.166 | - |
*P <0.05 has been considered as significant, $ group P value, compared using paired sample t-test, # Between group P value, compared using independent sample t-test. SD: Standard deviation, AST: Aspartate transaminase, ALT: Alanine transaminase , GVG: GuduchighanaVati Group, CG: Control group
Discussion
This randomized, active-controlled pilot study evaluated the efficacy and safety of Ayurvedic intervention, Guduchighana Vati in asymptomatic and mild to moderate COVID-19. In this study, both the groups were comparable in clinical characteristics and disease severity at baseline. Improvement in terms of better proportion of negative RT-PCR for COVID-19was observed in the Guduchighana Vati group. Although the proportion of participants with negative RT-PCR test was numerically better in the Guduchighana Vati group at each scheduled clinical evaluation, however, it was statistically not significant (P = 0.068). It may be attributed to the small sample size for the study. Clinical recovery in terms of fever, cough, sore throat, and body-ache was within 5 days in all the symptomatic participants of Guduchighana Vati group whereas one patient remained symptomatic in the control group on 5th day. No symptoms were observed on10th day in both the groups. Statistically significant reduction in the inflammatory markers such as IL-6 in both the groups is noteworthy as elevated levels of inflammatory markers can be associated with heightened cytokine release, leading to COVID-19 associated systemic inflammation and hypoxic respiratory failure.[32,33] The elevated level of IL-6 has also been associated with high case fatality from COVID-19.[34] The level of serum ferritin was within normal range in both the groups. Ferritin is a key mediator of immune system deregulation through pro-inflammatory and immunosuppressive effects. It has been observed that the serum ferritin levels might be influencing the severity of COVID-19.[35] The level of SpO2 was within normal range in both the groups indicating no requirement of invasive or noninvasive oxygen therapy during the study period. Notably, oxygen saturation to fraction of inspired oxygen ratio (SpO2/FiO2) has been considered a noninvasive prognostic marker of adverse outcome for COVID-19.[36] Liver Function Test and Kidney function Test were also found within normal limits after the study period and there was absence of any ADR or serious adverse event in both the study groups. Further, Tinospora cordifoilia (Guduchi) has established safety profile as observed in earlier published studies[37,38,39] and this study also substantiate the safety of the trial drug. Although no ADR/serious adverse event was observed in the control group also in this study, United States Food and Drug Administration reported that Hydroxychloroquine has certain known side-effects such as cardiac arrhythmia, blood and lymph system disorders, kidney injuries and liver failure.[40]
Guduchi (T. cordifolia [Willd.] Miers) is one of the most popular and important Ayurvedic Rasayana (medicines used to improve health and longevity) widely used in practice for a variety of infective ailments.[41] T. cordifolia has established anti-inflammatory and immunomodulatory properties as observed in several experimental and clinical studies.[42,43,44,45] It modulates the response of CD4+ and CD 8+ T-cells, activates macrophages via TLR6 signaling and nuclear factor kappa B translocation, inhibit lipoxygenase, and cyclooxygenase enzymes, inhibit tumor necrosis factor-a and cytokine production and alters the level of enzymes such as catalase to stimulate lymphocytes.[42,43,44,45] T. cordifolia also alters the activity of immune system through the dynamic regulation of cytokines.[46] Furthermore, one of the active compounds of T. cordifolia, Tinocordiside is capable of inhibiting the interface between host angiotensin-converting enzyme 2 receptor and Receptor Binding Domain of severe acute respiratory syndrome coronavirus 2 spike protein, which is crucial for virus entry into the host respiratory epithelium.[47] Research studies also showed significant anti-infective and antipyretic properties of T. cordifolia.[48,49]
Hydroxychloroquine was considered a candidate for COVID-19 prophylaxis and management due to its immunomodulatory property. The immune responses in COVID-19 are different between severe and mild to moderate infected patients. In mild-to-moderate COVID-19, before resolution of symptoms, increase in the activated CD4+ helper T-cells, CD8+ killer T-cells, follicular helper T cells, IgG, and IgM were observed.[50] Whereas, in severe COVID-19 cases, lymphocytopenia, substantial fall in CD3+ T-cells, CD4+ helper T-cells, CD8+ killer T cells, increase in neutrophil-to-lymphocyte ratio and elevated levels of C-reactive protein, pro-inflammatory cytokines, and chemokines were observed.[32,51,52] This hyperactive immune response along with impaired adaptive immune response may trigger complications such as pulmonary injury, sepsis and organ failure, and even death in some patients. Further, lung opacities have been observed as sequelae in asymptomatic COVID-19 patients also.[53] In such circumstances, Guduchi can be considered in the management of asymptomatic and mild to moderate COVID-19 as it may be beneficial in inducing optimum immunomodulation and anti-inflammatory activities which are the key targets to COVID-19 management and aid in preventing long term effects of COVID-19.
Limitations of the study
Since it was a randomized controlled pilot study, it had some limitations which should be considered while interpreting the study results. The small sample size was one of the main limitations. Further, patients with asymptomatic and mild-to-moderate COVID-19 were included in the study, so the findings cannot be extrapolated to patients with severe disease or with co-morbidities.
Conclusion
Guduchighana Vati can be considered as safe and potential therapeutic option in the management of asymptomatic and mild cases of COVID-19. Guduchighana Vati also demonstrated the potential in reducing the levels of pro-inflammatory markers such as IL-6. Nevertheless, randomized controlled trials with a sufficient sample size are required to confirm these preliminary findings, and to make conclusions for the efficacy of this Ayurveda intervention in managing the mild to moderate cases of COVID-19.
Financial support and sponsorship
Directorate of AYUSH, Government of Madhya Pradesh, India
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to Central Council for Research in Ayurvedic Sciences (CCRAS), Ministry of AYUSH, Government of India for the technical support for this study. The authors are also thankful to Dr. Rama Jayasundar, Professor and Head, Department of Nuclear Magnetic Resonance (NMR), All India Institute of Medical Sciences, New Delhi, for providing intellectual insights while finalizing the manuscript. The authors are thankful to Pharmanza Herbal Private Limited, Gujarat, India for providing Guduchighana Vati for this study.
References
- 1.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. [Last accessed on 2021 Jul 01]. Available from: https://covid19.who.int/
- 2.Chatterjee P, Nagi N, Agarwal A, Das B, Banerjee S, Sarkar S, et al. The 2019 novel coronavirus disease (COVID-19) pandemic: A review of the current evidence. Indian J Med Res. 2020;151:147–59. doi: 10.4103/ijmr.IJMR_519_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shastri AD, editor. Sushruta Samhita of Sushruta, Nidana Sthana. Ch. 5. Reprint edition. Varanasi: Chaukhambha Sanskrit Sansthan; 2007. pp. 246–51. [Google Scholar]
- 4.Shastri AD, editor. Sushruta Samhita of Sushruta, Nidana Sthana. Ch. 12. Reprint edition. Varanasi: Chaukhambha Sanskrit Sansthan; 2007. pp. 275–81. [Google Scholar]
- 5.Mishra BS, editor. Bhavaprakasha of Sri Bhavamishra Part II. Reprint edition. Varanasi: Chaukhambha Sanskrit Sansthan; 2005. pp. 562–3. [Google Scholar]
- 6.Patwardhan B, Chavan-Gautam P, Gautam M, Tillu G, Chopra A, Gairola S, et al. Ayurveda rasayana in prophylaxis of COVID-19. Curr Sci. 2020;118:1158–60. [Google Scholar]
- 7.Upadhyaya R, Pandey RP, Sharma V, Verma Anita K. Assessment of the multifaceted immunomodulatory potential of the aqueous extract of Tinospora cordifolia. Res J Chem Sci. 2011;1:71–9. [Google Scholar]
- 8.Tiwari R, Chakraborty S, Saminathan M, Dhama K, Singh SV. Ashwagandha (Withania somnifera): Role in safeguarding health, immunomodulatory effects, combating infections and therapeutic applications: A review. J Biol Sci. 2014;14:77–94. [Google Scholar]
- 9.Naik SR, Hule A. Evaluation of immunomodulatory activity of an extract of and rographolides from Andographis paniculata. Planta Med. 2009;75:785–91. doi: 10.1055/s-0029-1185398. [DOI] [PubMed] [Google Scholar]
- 10.Mondal S, Varma S, Bamola VD, Naik SN, Mirdha BR, Padhi MM, et al. Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. J Ethnopharmacol. 2011;136:452–6. doi: 10.1016/j.jep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Manu KA, Kuttan G. Immunomodulatory activities of Punarnavine, an alkaloid from Boerhaavia diffusa. Immunopharmacol Immunotoxicol. 2009;31:377–87. doi: 10.1080/08923970802702036. [DOI] [PubMed] [Google Scholar]
- 12.Sunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. andpiperine. J Ethnopharmacol. 2004;90:339–46. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Borse S, Joshi M, Saggam A, Bhat V, Walia S, Sagar S, et al. Ayurveda botanicals in COVID-19 management: An in silico-multitarget approach. PLoS ONE. 2021;16(6):e0248479. doi: 10.1371/journal.pone.0248479. doi.org/10.1371/journal.pone.0248479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rege AA, Chowdhary AS. Evaluation of some medicinal plants as putative HIV - protease inhibitors. Indian Drugs. 2013;50:24–8. [Google Scholar]
- 15.Gundeti MS, Bhurke LW, Mundada PS, Murudkar S, Surve A, Sharma R, et al. AYUSH 64, a polyherbal Ayurvedic formulation in Influenza-like illness - Results of a pilot study. J Ayurveda Integr Med. 2020 doi: 10.1016/j.jaim.2020.05.010. S0975-9476(20)30025-5. doi: 10.1016/j.jaim.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurya VK, Kumar S, Prasad AK, Bhatt MLB, Saxena SK. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virusdisease. 2020;31:179–93. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi AJ, Rupareliya JD, Shukla VJ, Donga SB, Acharya R. An Ayurvedic perspective along with in silico study of the drugs for the management of SARS-Cov-2. J Ayurveda Integr Med. 2020 doi: 10.1016/j.jaim.2020.07.002. doi: 10.1016/j.jaim.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of AYUSH, Govt. of India. National Clinical Management Protocol Based on Ayurveda and Yoga for Management of Covid-19. [Last accessed on 2020 Nov 12]. Available from: https://www.ayush.gov.in/docs/ayush-Protocol-covid-19.pdf .
- 19.1st ed. Vol. 1. New Delhi: Department of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 2001. The Ayurvedic Pharmacopoeia of India. Part I; pp. 53–5. [Google Scholar]
- 20.Upadhyay AK, Kumar K, Kumar A, Mishra HS. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) – validation of the Ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res. 2010;1:112–21. doi: 10.4103/0974-7788.64405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhama K, Sachan S, Khandia R, Munjal A, Iqbal HM, Latheef SK, et al. Medicinal and beneficial health applications of Tinospora cordifolia (Guduchi): A miraculous herb countering various diseases/disorders and its immunomodulatory effects. Recent Pat Endocr Metab Immune Drug Discov. 2017;10:96–111. doi: 10.2174/1872214811666170301105101. [DOI] [PubMed] [Google Scholar]
- 22.Bishayi B, Roychowdhury S, Ghosh S, Sengupta M. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl 4 intoxicated mature albino rats. J Toxicol Sci. 2002;27:139–46. doi: 10.2131/jts.27.139. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian M, Chintalwar GJ, Chattopadhyay S. Antioxidant properties of a Tinospora cordifolia polysaccharide against iron-mediated lipid damage and gamma-ray induced protein damage. Redox Rep. 2002;7:137–43. doi: 10.1179/135100002125000370. [DOI] [PubMed] [Google Scholar]
- 24.More P, Pai K. In vitro NADH-oxidase, NADPH-oxidase and myeloperoxidase activity of macrophages after Tinospora cordifolia (guduchi) treatment. Immunopharmacol Immunotoxicol. 2012;34:368–72. doi: 10.3109/08923973.2011.606324. [DOI] [PubMed] [Google Scholar]
- 25.Sudhakaran DS, Srirekha P, Devasree LD, Premsingh S, Michael RD. Immunostimulatory effect of Tinospora cordifolia Miers leaf extract in Oreochromis mossambicus. Indian J Exp Biol. 2006;44:726–32. [PubMed] [Google Scholar]
- 26.Tambekar DH, Khante BS, Chandak BR, Titare AS, Boralkar SS, Aghadte SN. Screening of antibacterial potentials of some medicinal plants from Melghat forest in India. Afr J Tradit Complement Altern Med. 2009;6:228–32. doi: 10.4314/ajtcam.v6i3.57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Indian Council of Medical Research, Govt. of India. [Last assessed on 2020 Nov 23]. Available from: https://covid.icmr.org.in/16-private-laboratories/579-molecular-lab-l-nmedical-college-j-k-hospital-bhopal .
- 28.Ministry of Health and Family Welfare, Govt. of India. Clinical Management Protocol: COVID-19 Version 5. [Last accessed on 2020 Nov 12]. Available from: https://www.mohfw.gov.in/pdf/UpdatedClinicalManagementProtocolforCOVID19dated03072020.pdf .
- 29.World Health Organization. COVID-19 Therapeutic Trial Synopsis. [Last assessed on 2020 Nov 23]. Available from: https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf .
- 30.The Ayurvedic Formulary of India Part II. 1st ed. New Delhi: Department of ISM and H, Ministry of Health and Family Welfare, Govt. of India; 2000. p. 183. [Google Scholar]
- 31.Ministry of Health and Family Welfare, Govt. of India. Revised Discharge Policy for COVID-19. [Last accessed on 2020 Nov 12]. Available from: https://www.mohfw.gov.in/pdf/ReviseddischargePolicyforCOVID19.pdf .
- 32.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–77. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J, Pang J, Ji P, Zhong Z, Li H, Li B, et al. Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis. J Med Virol. 2021;93:35–7. doi: 10.1002/jmv.26085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas-Vargas M, Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica. 2020;44:e72. doi: 10.26633/RPSP.2020.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu X, Jiang L, Chen T, Wang Y, Zhang B, Hong Y, et al. Continuously available ratio of SpO2/FiO2 serves as a noninvasive prognostic marker for intensive care patients with COVID-19. Respir Res. 2020;21:194. doi: 10.1186/s12931-020-01455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karkal YR, Bairy LK. Safety of aqueous extract of Tinospora cordifolia (Tc) in healthy volunteers: A double blind randomised placebo controlled study. Iran J Pharmacol Ther. 2007;6:59–61. [Google Scholar]
- 38.Chandrasekaran CV, Mathuram LN, Daivasigamani P, Bhatnagar U. Tinospora cordifolia, a safety evaluation. Toxicol In vitro. 2009;23:1220–6. doi: 10.1016/j.tiv.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Mishra S, Verma N, Bhattacharya S, Usman K, Reddy H, Verma N, et al. Efficacy and safety of Tinospora cordifolia (Tc) as an add-on therapy in patients with type-2 diabetes. Int J Res Med Sci. 2015;3:1109–13. [Google Scholar]
- 40.United States Food and Drug Administration. [Last accessed on 2021 Mar 22]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or .
- 41.Banerjee N, Saha B, Mukhopadhyay S. Intracellular ROS generated in chikungunya patients with persisting polyarthralgia can be reduced by Tinospora cordifolia leaf extract. Virusdisease. 2018;29:375–9. doi: 10.1007/s13337-018-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma U, Bala M, Kumar N, Singh B, Munshi RK, Bhalerao S. Immunomodulatory active compounds from Tinospora cordifolia. J Ethnopharmacol. 2012;141:918–26. doi: 10.1016/j.jep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 43.Jacob J, Babu BM, Mohan MC, Abhimannue AP, Kumar BP. Inhibition of proinflammatory pathways by bioactive fraction of Tinospora cordifolia. Inflammopharmacology. 2018;26:531–8. doi: 10.1007/s10787-017-0319-2. [DOI] [PubMed] [Google Scholar]
- 44.Nair PK, Melnick SJ, Ramachandran R, Escalon E, Ramachandran C. Mechanism of macrophage activation by (1,4)-alpha-D-glucan isolated from Tinospora cordifolia. Int Immunopharmacol. 2006;6:1815–24. doi: 10.1016/j.intimp.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 45.Aher V, Kumar Wahi A. Biotechnological approach to evaluate the immunomodulatory activity of ethanolic extract of Tinospora cordifolia stem (mango plant climber) Iran J Pharm Res. 2012;11:863–72. [PMC free article] [PubMed] [Google Scholar]
- 46.Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. Modulation of cytokine expression by traditional medicines: A review of herbal immunomodulators. Altern Med Rev. 2006;11:128–50. [PubMed] [Google Scholar]
- 47.Balkrishna A, Pokhrel S, Varshney A. Tinocordiside from Tinospora cordifolia (Giloy) May Curb SARS-CoV-2 contagion by disrupting the electrostatic interactions between host ACE2 and viral S-protein receptor binding domain? Comb Chem High Throughput Screen. 2021;24:1795–1802. doi: 10.2174/1386207323666201110152615. doi: 10.2174/1386207323666201110152615. [DOI] [PubMed] [Google Scholar]
- 48.Rege N, Bapat RD, Koti R, Desai NK, Dahanukar S. Immunotherapy with Tinospora cordifolia: A new lead in the management of obstructive jaundice. Indian J Gastroenterol. 1993;12:5–8. [PubMed] [Google Scholar]
- 49.Jeyachandran R, Xavier TF, Anand SP. Antibacterial activity of stem extracts of Tinospora cordifolia (Willd) Hook. f and Thomson. Anc Sci Life. 2003;23:40–3. [PMC free article] [PubMed] [Google Scholar]
- 50.Thevarajan I, Nguyen TH, Koutsakos M, Druce J, Caly L, van de Sandt CE, et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat Med. 2020;26:453–5. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5:e137799. doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cella L, Gagliardi G, Hedman M, Palma G. Injuries from asymptomatic COVID-19 disease: New hidden toxicity risk factors in thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2020;108:394–6. doi: 10.1016/j.ijrobp.2020.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]