Abstract
Fluoroquinolones can cause tendinitis and tendon rupture. However, toxicological as well as clinical information on quinolone-induced tendopathy is scarce. We performed extensive electron microscopic studies with Achilles tendon specimens from ofloxacin-treated rats. The drug was given at a dose of 1,200 mg/kg (body weight) orally. Juvenile Wistar rats received one or three oral doses each of 1,200 mg of ofloxacin/kg (body weight)/day. Three days after treatment, the tenocytes of their Achilles tendons showed degenerative alterations, such as multiple vacuoles and vesicles in the cytoplasm that had developed due to swellings and dilatations of cell organelles. Other indications of cell degradation were the occurrence of cell debris and cell detachment from the extracellular matrix accompanied by a loss of cell-matrix interaction. The tenocytes of juvenile Wistar rats that had been treated at day 36 with a single oral dose of 1,200 mg of ofloxacin/kg (body weight) and sacrificed either 3 or 6 months later exhibited similar degenerative alterations. The number of degenerative alterations of tenocytes after ofloxacin treatment was considerably higher in rats that had received a magnesium-deficient diet than in rats with normal magnesium status. Of the adult rats that had been treated once, 5 times, and 10 times with ofloxacin and killed 1 day later, only those with the 10-times treatment showed a significantly increased number of degeneratively altered tenocytes. In summary, effects observed in tendons show similar pathological features as described earlier in cartilage, indicating that quinolone-induced arthropathy and quinolone-induced tendopathy probably are different clinical manifestations of the same toxic effect on cellular components of connective tissue structures.
Musculoskeletal adverse effects represent a small but significant fraction of the adverse effects observed during therapy with fluoroquinolones (0.5 to 2%). Besides arthralgia and myalgia, cases of tendinitis and tendon ruptures have also been described. As with quinolone-induced arthropathy, most cases of tendon disorders have occurred with pefloxacin. Tendinitis associated with other drugs such as ciprofloxacin, ofloxacin, norfloxacin, and enoxacin has been reported, but the incidence appears to be much lower. In trying to estimate the overall clinical significance of this effect, it should be kept in mind that probably quite a number of unidentified cases exist (2, 3, 22).
Clinical information on quinolone-induced tendopathy is scarce. Selective clinical studies regarding this adverse effect have not been published, and toxicological data are very limited. Some studies in rats that have been published so far do not seem to reflect the clinical situation because the alterations are detectable in juvenile rats only and are preventable by dexamethasone (8–10).
We performed extensive electron microscopic studies with Achilles tendon specimens from ofloxacin-treated rats. Our experiments were designed to answer several questions. First, are multiple doses necessary to induce the lesions or is one dose sufficient? Second, are juvenile rats more susceptible than adults? Third, are the effects reversible over a period of several months? Finally, is the toxic effect enhanced by magnesium deficiency?
(This research was conducted in part for the doctoral thesis of Kerstin Pfister to be submitted to the Fachbereich Humanmedizin, Freie Universität Berlin, Berlin, Germany.)
MATERIALS AND METHODS
Quinolone treatment.
Wistar rats were kept in Macrolon cages at room temperature of 21 ± 1°C, a relative humidity of 50 ± 5%, and a constant light-dark schedule (light from 9:00 to 21:00 hours). Rats received a standard diet (Altromin 1324; Altromin, Lage, Germany; Mg2+ content, ca. 2 g/kg). For treatment with ofloxacin, commercially available tablets (Tarivid) containing 200 mg of the drug were suspended in 2% starch suspension. The freshly prepared suspension was administered by gastric intubation at a volume of 10 ml per kg of body weight. Body weights of the juvenile rats (28 to 36 days) were 68 ± 11 g; those of the adult rats were 268 ± 18 g (mean values ± the standard deviation).
At various times after treatment the rats were sacrificed and Achilles tendon samples were collected and studied by electron microscopy. Information on the various groups of rats studied including dose, duration of treatment, and time of ultrastructural evaluation is given in Table 1.
TABLE 1.
Oral ofloxacin treatment of experimental animalsa
| Group | No. of rats | Dose (mg/kg) | Dietb | Age of rats at:
|
|
|---|---|---|---|---|---|
| Beginning of treatment | Examination of Achilles tendon | ||||
| I | 3 | 1 × 1,200 | Reg | 30 days | 34 days |
| II | 3 | 3 × 1,200 | Reg | 28 days | 34 days |
| III | 3 | 1 × 1,200 | Reg | 36 days | 3 months |
| IV | 3 | 1 × 1,200 | Mg-def | 36 days | 3 months |
| V | 5 | 1 × 1,200 | Reg | 36 days | 6 months |
| VI | 5 | 1 × 1,200 | Mg-def | 36 days | 6 months |
| VII | 3 | 1 × 1,200 | Reg | Adult | 1 day after treatment |
| VIII | 3 | 5 × 1,200 | Reg | Adult | 1 day after treatment |
| IX | 3 | 10 × 1,200 | Reg | Adult | 1 day after treatment |
In addition, groups of juvenile and adult rats were treated with the vehicle (starch solution) and investigated at corresponding times.
Reg, regular diet; Mg-def, Mg-deficient diet.
Magnesium deficiency.
Juvenile rats were fed a magnesium-deficient diet (Altromin C1035) starting on postnatal day 29 and treated with a single dose of ofloxacin (1,200 mg/kg) by gastric intubation on day 36. Atomic absorption spectrophotometric examination of the diet revealed a Mg2+ content of 84 mg/kg.
Transmission electron microscopy.
Achilles tendon samples were prepared from the right foot of animals from each dosing group. Tangential sections were made from the distal part of the tendon by using a razor blade. Subsequently, these tendons were cut crosswise for the preparation of ultrathin sections. All samples were fixed in 3% paraformaldehyde plus 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and then postfixed in 2% OsO4 in 0.1 M phosphate buffer. After a rinsing and dehydration step in ethanol, the samples were embedded in Epon (Plano, Marburg, Germany), cut with an Ultracut E (Reichert), contrasted with 2% uranyl acetate/lead citrate, and investigated under a transmission electron microscope (Zeiss EM 10).
Quantitative evaluation of tenocytes with pathological alterations.
Ultrathin sections of the tendons of the untreated and ofloxacin-treated rats from groups III, IV, V, VI, VII, VIII, and IX were prepared and evaluated under an electron microscope. The number of degenerated cells was determined by scoring a total of 50 cells from 15 different microscopic fields.
RESULTS
Rats treated at the juvenile stage.
The tenocytes of the control animals did not exhibit any pathological features. Longitudinal sections revealed an oblong contour and an oval nucleus. They were arranged between the collagenous fibrils of the extracellular matrix. In cross-sections, they showed the typical, triangular, irregular shape of tenocytes with long, wing-like cellular processes that extended into the extracellular matrix and attached to collagenous fibrils. The cell organelles were intact; the rough endoplasmic reticulum had a regular appearance. Some tenocytes of the control group showed a few vacuole-like structures or individual vesicles (Fig. 1a and b).
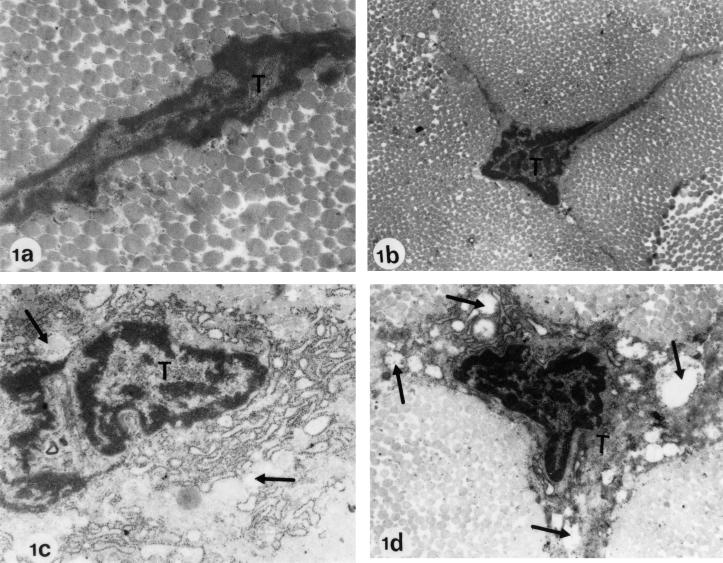
FIG. 1.
Electron microscopy of Achilles tendons from Wistar rats treated at the juvenile stage. (a) Regular diet, no ofloxacin (control; age at investigation, 6 months). (b) Regular diet, no ofloxacin (control; age at investigation, 3 months). Electron micrographs a and b show two typical tenocytes (T) with intact cell organelles. The longitudinal section (a) reveals a regular cell contour. The cross-section (b) shows the typical triangular, irregular shape of tenocytes with wing-like cellular processes. (c) Regular diet plus treatment with one dose of 1,200 mg of ofloxacin/kg (body weight) (age at investigation, day 34). (d) Regular diet plus treatment with one dose of 1,200 mg of ofloxacin/kg (body weight) (age at investigation, 6 months). In panels c and d, pathological alterations after ofloxacin treatment are recognizable. The tenocytes show numerous, partly communicating dilatations and vacuoles in the cytoplasm (arrows). The cell organelles resemble vesicular or piston-like dilatations. Magnification: a, ×34,000; b to d, ×12,750.
Pathological alterations were seen in tenocytes (“wing cells”) of the juvenile rats of groups I, III, and V (treated once with ofloxacin), as well as those of group II (treated three times). These alterations were more pronounced in group II (3-day-treatment) than in groups I, III, and V (1-day-treatment). The most striking difference to the controls were numerous, partly communicating dilatations and vacuoles in the cytoplasm that had developed due to swellings of the rough endoplasmic reticulum and the mitochondria. The cell organelles resembled vesicular or piston-like dilatations (Fig. 1c and d). The overall appearance of the rough endoplasmic reticulum was less regular than that of cells not showing pathological features. Cell debris could occasionally be found in the cytoplasm of the tenocytes.
Almost all tenocytes of those rats that had received ofloxacin plus an Mg-deficient diet (groups IV and VI) exhibited the described degenerative alterations, whereas these alterations occurred less frequently in the tenocytes of rats treated with ofloxacin without the Mg-deficient diet (groups III and V).
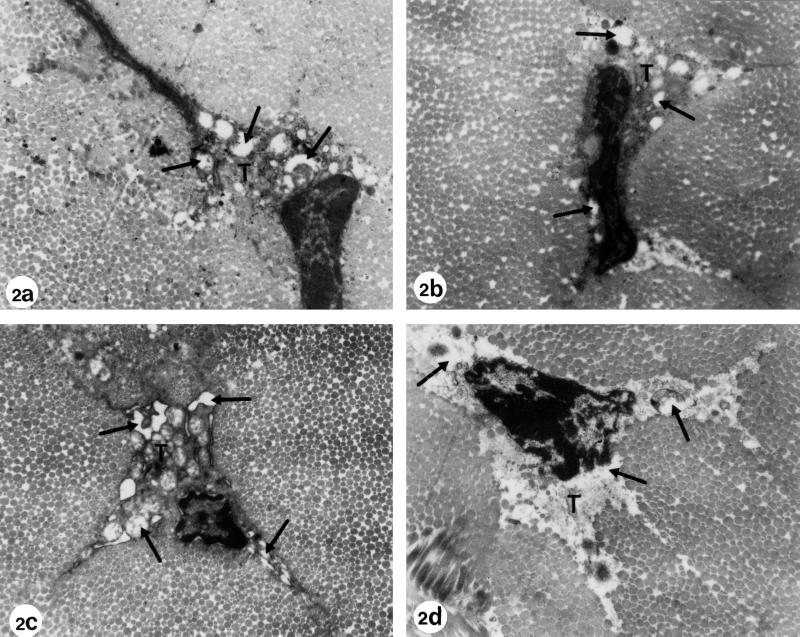
In addition to the described vacuoles and vesicles in the cytoplasm, the preparations of group VI exhibited small microvillus-like protrusions of the cell membrane. Individual heavily damaged tenocytes that had detached from the surrounding extracellular matrix were recognizable in specimens of groups V and VI. The cell-matrix interaction of these tenocytes was interrupted. Their nucleus was surrounded by cell debris without recognizable intact cell organelles (Fig. 2).
FIG. 2.
Electron microscopy of Achilles tendons from Wistar rats treated at the juvenile stage and fed with a magnesium-deficient diet. (a) Regular diet plus treatment with one dose of 1,200 mg of ofloxacin/kg (body weight) (age at investigation, 3 months). (b) Regular diet plus treatment with one dose of 1,200 mg of ofloxacin/kg (body weight) (age at investigation, 6 months). Electron micrographs a and b show the typical pathological alterations after ofloxacin treatment as described in Fig. 1. The dilatations and vacuoles (arrows) in the cytoplasm of the tenocytes (T) developed due to swellings of the rough endoplasmic reticulum and the mitochondria. Some of the vesicles contain electron-dense material. (c) Magnesium-deficient diet plus treatment with one dose of 1,200 mg of ofloxacin/kg (body weight) (age at investigation, 3 months). (d) Magnesium-deficient diet plus treatment with one dose of 1,200 mg of ofloxacin/kg (body weight) (age at investigation, 6 months). The alterations, such as vacuole formation and dilatation of cell organelles, were more pronounced in rats that had received ofloxacin plus an Mg-deficient diet (panels c and d). The heavily damaged tenocyte in panel d is detached from the surrounding matrix and contains cell debris. Magnification, ×12,750.
Hence, the degenerative alterations of the tenocytes brought about by the ofloxacin (group V) and the ofloxacin-plus-Mg-deficient diet (group VI) were still present almost 5 months after treatment.
Rats treated at the adult stage.
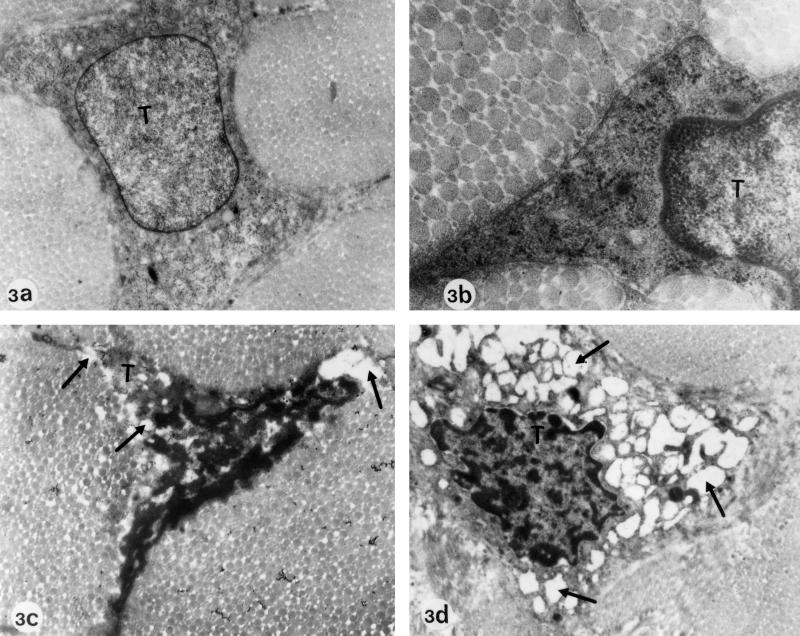
While the Achilles tendons of the control group as well as of group VII (treated once with ofloxacin) and group VIII (treated five times) of the adult rats did not exhibit any pathological alterations (Fig. 3a and b), those of group IX (treated 10 times) showed indications of tenocyte degeneration similar to those observed in the treated juvenile rats. These alterations included above all heavy vesicle and vacuole formation in the cytoplasm of the tenocytes, pronounced dilatations and ballooning of cell organelles such as rough endoplasmic reticulum and mitochondria, and occasionally cell debris and detachment from the surrounding collagenous fibrils of the extracellular matrix (Fig. 3c and d).
FIG. 3.
Electron microscopy of Achilles tendons from Wistar rats treated at the adult stage. (a and b) Regular diet, no ofloxacin (controls). Magnifications: a, ×26,775; b, ×52,700. Panels a and b show typical tenocytes (T) with a well-demarcated cell surface and regular cell organelles. (c and d) Regular diet plus 10-day treatment with daily doses of 1,200 mg of ofloxacin/kg (body weight). Magnification: ×12,750. Electron micrographs c and d show tenocytes with signs of degeneration similar to those observed in rats that had been treated at the juvenile stage. Vesicle and vacuole formation in the cytoplasm and dilatation and ballooning of cell organelles can be seen (arrows).
Quantitative evaluation.
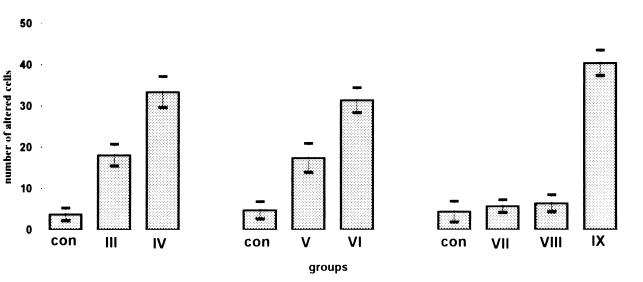
The quantitative evaluation revealed clearly that, in samples from juvenile rats of groups III and V treated with ofloxacin once, the number of pathologically altered cells slightly increased, while these cells were markedly increased in those rats of groups IV and VI that had additionally received an Mg-deficient diet. The differences between these groups and controls were statistically significant (t test, P > 0.05) (Fig. 4).
FIG. 4.
Effect of ofloxacin with or without magnesium deficiency on tenocytes. Rats were treated with ofloxacin and fed a magnesium-deficient diet (Table 1). The number of pathologically altered cells increased in samples from juvenile rats of groups III and V (treated once with ofloxacin), while the number of pathologically altered cells was markedly increased in those rats of groups IV and VI that had additionally received an Mg-deficient diet. The evaluation of tendons from the adult control rats and rats of groups VII and VIII (treated once or five times with ofloxacin) did not reveal a significant number of pathologically altered cells, while the rats of group IX (treated ten times with ofloxacin) showed pathological alterations similar to those observed in ofloxacin-treated juvenile animals. The number of cells was counted by scoring 50 cells from 15 different microscopic fields. The diagram shows the number of pathologically altered cells (mean values and standard deviations) of the results obtained from three different rats.
Quantification of the pathologically altered cells from adult rats showed that only those rats of group IX (treated 10 times with ofloxacin) exhibited a statistically significant increase (t test, P > 0.05) in pathologically altered cells similar to those observed in treated juvenile rats (Fig. 4).
DISCUSSION
As a result of our findings, one might assume that the tendon cells are the main target for quinolones to induce tendon toxicity. However, besides an effect on intracellular structures, it is also possible that quinolones primarily disturb the physiological interaction between cells and matrix, for example, by chelating divalent ions. It is well known that cells of connective tissue undergo morphological alterations when the interaction between the cell and matrix is disturbed. A persistently harmed matrix would provide an explanation as to why the observed effects persisted for several months (19, 20).
Since quinolones are well known for their chelating properties (11, 12, 15), some in vitro findings with other chelators on the integrity of tendons are of interest. Steven (24) showed more than 30 years ago that an EDTA solution can be used to release polymerized collagen from interfibrillar matrix of bovine or human tendons. The action of the chelating agent was rapid and reversible by the addition of calcium. Possibly, quinolones act similarly on tendons in vivo.
We have shown before that, in juvenile rats, magnesium deficiency causes lesions in immature joint cartilage similar to those caused by quinolone treatment and that integrins, the function of which depends on divalent cations, were reduced on the cell surface (4, 21). Also, by means of electron microscopy, the effects of quinolone treatment and magnesium deficiency on chondrocytes from joint cartilage of immature rats were very similar, and pathological changes induced by these two conditions were not discernible. The main findings were swollen mitochondria and widened endoplasmic reticulum—effects which were now also found in tendon cells (20).
The first data on structural changes in tendon and tendon-associated tissues in rats were published by Kato and coworkers (10). They found that single doses of pefloxacin (at 300 and 900 mg/kg) or ofloxacin (at 900 mg/kg) affected the Achilles tendon in juvenile, 4-week-old rats, but not in 12-week-old rats. The quinolone-induced lesions were characterized by edema and mononuclear cell infiltration in the inner sheath of the inner Achilles tendon, with infiltration into the adjacent synovial membrane and joint space. As a result of their electron microscopic examination, these researchers describe an increased number of fibroblasts and macrophages and collagen disposition in the matrix of the synovial membrane and tendon sheath. The authors concluded that the observed alterations differ from the disorders reported in humans because older patients (>60 years) have been reported to be especially sensitive to the effects of quinolones, whereas in this animal model only young subjects were sensitive (9, 10).
In a follow-up study the authors showed that, of a series of 10 fluoroquinolones, pefloxacin and fleroxacin were the most toxic derivatives whereas, for example, sparfloxacin, norfloxacin, and ciprofloxacin were less toxic or produced no lesions even after rather high oral doses (8). These findings are explainable by the kinetics of the compounds in rats. The absorption of fluoroquinolones from the gastrointestinal tract in rats differs considerably for various quinolones: for example, fleroxacin is well absorbed, whereas the absorption rates of sparfloxacin or ciprofloxacin are extremely low (23; R. Schwabe, U. Zippel, C. Förster, I. Baumann-Wilschke, and R. Stahlmann, Naunyn-Schmiedeberg Arch. Pharmacol. 354(Suppl.):R28, abstr. 110, 1996).
Interestingly, partial reduction of the incidence of tendopathies after pefloxacin was obtained by administration of L-NAME (N-nitro-l-arginine methyl ester, a nitric oxide synthase inhibitor). This suggests that nitric oxide partly mediates the induction of lesions, which is in agreement with the finding of Hayem and coworkers that radical formation is an important step in the pathogenesis of quinolone-induced arthropathy (5, 8).
Furthermore, the pefloxacin-induced tendon lesions were completely inhibited by the coadministration of dexamethasone. At first glance, this finding stands in contrast to the clinical experience that patients undergoing corticosteroid therapy are prone to quinolone-induced tendon disorders, but it could be explained by the fact that patients are usually on continuous therapy, whereas the animals had been treated for a short period only (8, 16).
Quinolones exhibit a pronounced affinity for connective tissues. Concentrations in cartilage, bone, and other tissues shortly after dosing exceed those measured simultaneously in plasma (see, for example, references 13, 17, and 23). Although no specific data are available, it appears reasonable to assume that in tendons also these drugs reach high concentrations. This peculiar pharmacokinetic behavior is one important aspect explaining why connective tissue structures are rather sensitive to the action of these drugs.
The first cases of tendinitis in association with norfloxacin therapy were published as early as 1983 (1). Meanwhile, several hundred cases have been reported in the literature. The exact incidence of quinolone-induced tendopathy is unknown, but probably a considerable number of unidentified cases exist. It seems noteworthy that in patients with Achilles tendon problems, lag periods of several months were reported between quinolone treatment and manifestation of clinical symptoms (2, 6, 7, 14).
Tendopathy due to pefloxacin has mainly been described in patients older than 60 years, whereas arthropathy predominantly occurred in juveniles (16, 22). A reasonable explanation for this finding is that the differences in the incidence primarily reflect the differences in use. Because quinolone treatment is contraindicated in juveniles, treatment with these drugs is very limited. Fluoroquinolones are mainly used in adults, which explains the predominance of adult patients in the case reports. Our studies show that in principle at least the cellular components of the juvenile tendon seem to be even more susceptible than the tenocytes in the adult tendon, which is in agreement with the findings by Kato and coworkers (10).
Cellular pathological effects observed in cartilage and tendons show many identical features, indicating that quinolone-induced arthropathy and quinolone-induced tendopathy probably are different clinical manifestations of the same toxic effect on cellular components of connective tissue structures. However, additional factors which determine the susceptibility for such adverse effects remain unclear. So far there is no explanation of why these effects are seen so rarely. A thoroughly conducted clinical study in patients with tendon disorders, including a detailed history of drug use, might shed some new light on this issue.
ACKNOWLEDGMENTS
We thank Irmela Baumann-Wilschke for her skillful technical assistance. We also thank Ingrid Wolff for her photographic assistance and Barbara Steyn for her help in preparing the manuscript.
The study was supported by a grant from Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Bailey R R, Kirk J A, Peddie B A. Norfloxacin-induced rheumatic disease. N Z Med J. 1983;96:590. [PubMed] [Google Scholar]
- 2.Carrasco J M, Garcia B, Andujr C, Garrote F, de Juana P, Bermejo T. Tendinitis associated with ciprofloxacin. Ann Pharmacother. 1997;31:120. doi: 10.1177/106002809703100122. [DOI] [PubMed] [Google Scholar]
- 3.Christ W, Esch G. Adverse reactions to fluorochinolones in adults and children. Infect Dis Clin Pract. 1994;3:S168–S176. [Google Scholar]
- 4.Förster C, Kociok K, Shakibaei M, Merker H-J, Vormann J, Günther T, Stahlmann R. Integrins on joint cartilage chondrocytes and alterations by ofloxacin or magnesium deficiency in immature rats. Arch Toxicol. 1996;70:261–270. doi: 10.1007/s002040050272. [DOI] [PubMed] [Google Scholar]
- 5.Hayem G, Petit P X, Levacher M, Gaudin C, Kahn M F, Pocidalo J J. Cytofluorometric analysis of chondrotoxicity of fluoroquinolone antimicrobial agents. Antimicrob Agents Chemother. 1994;38:243–247. doi: 10.1128/aac.38.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayem G, Carbon C. A reappraisal of quinolone tolerability. Drug Safety. 1995;13:338–342. doi: 10.2165/00002018-199513060-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kahn M F, Carbon C. Tendinopathies et fluoroquinolones. Concours Med. 1993;115:819–823. [Google Scholar]
- 8.Kashida Y, Kato M. Characterization of fluoroquinolone-induced Achilles tendon toxicity in rats: comparison of toxicities of 10 fluorochinolones and effects of anti-inflammatory compounds. Antimicrob Agents Chemother. 1997;41:2389–2393. doi: 10.1128/aac.41.11.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashida Y, Kato M. Toxic effects of quinolone antibacterial agents on the musculoskeletal system in juvenile rats. Toxicol Pathol. 1997;25:635–643. doi: 10.1177/019262339702500615. [DOI] [PubMed] [Google Scholar]
- 10.Kato M, Takada S, Kashida Y, Nomura M. Histological examination on Achilles tendon lesions induced by quinolone antibacterial agents in juvenile rats. Toxicol Pathol. 1995;23:385–392. doi: 10.1177/019262339502300315. [DOI] [PubMed] [Google Scholar]
- 11.Kawai Y, Matsubayashi K, Hakusui H. Interaction of quinolones with metal cations in aqueous solution. Chem Pharmacol Bull. 1996;44:1425–1430. [Google Scholar]
- 12.Lecomte S, Baron M H, Chenon M T, Coupry C, Moreau M J. Effect of magnesium complexation by fluoroquinolones on their antibacterial properties. Antimicrob Agents Chemother. 1994;38:2810–2816. doi: 10.1128/aac.38.12.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meissner A, Borner K, Koeppe P. Concentrations of ofloxacin in human bone and cartilage. J Antimicrob Chemother. 1990;26:69–74. doi: 10.1093/jac/26.suppl_d.69. [DOI] [PubMed] [Google Scholar]
- 14.Pierfitte C, Royer R J. Tendon disorders with fluoroquinolones. Therapie. 1996;51:419–420. [PubMed] [Google Scholar]
- 15.Ross D, Riley R. Physicochemical properties of the fluoroquinolone antimicrobials. V. Effect of fluoroquinolone structure and pH on complexation of various fluoroquinolones with magnesium and calcium ions. Int J Pharmacol. 1993;93:121–129. [Google Scholar]
- 16.Royer R J, Pierfitte C, Netter P. Features of tendon disorders with fluoroquinolones. Therapie. 1994;49:75–76. [PubMed] [Google Scholar]
- 17.Schwabe R, Lozo E, Baumann-Wilschke I, Stahlmann R. Quinolone-induced arthropathy: concentrations of ofloxacin in plasma and cartilage of juvenile rats after multiple doses. Teratology. 1997;56:388. [Google Scholar]
- 18.Shakibaei M, Schröter-Kermani C, Merker H-J. Matrix changes during long-term cultivation of cartilage (organoid or high-density culture) Histol Histopathol. 1993;8:463–470. [PubMed] [Google Scholar]
- 19.Shakibaei M, Abou-Rebyeh H, Merker H-J. Integrins in ageing cartilage tissue in vitro. Histol Histopathol. 1993;8:715–723. [PubMed] [Google Scholar]
- 20.Shakibaei M, Kociok K, Förster C, Vormann J, Günther T, Stahlmann R, Merker H-J. Comparative evaluation of ultrastructural changes in articular cartilage of ofloxacin-treated and magnesium-deficient immature rats. Toxicol Pathol. 1996;24:580–587. doi: 10.1177/019262339602400507. [DOI] [PubMed] [Google Scholar]
- 21.Stahlmann R, Förster C, Shakibaei M, Vormann J, Günther T, Merker H-J. Magnesium deficiency induces joint cartilage lesions in juvenile rats which are identical with quinolone-induced arthropathy. Antimicrob Agents Chemother. 1995;39:2013–2018. doi: 10.1128/aac.39.9.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stahlmann R, Lode H. Safety overview: Toxicity, adverse effects, and drug interactions. In: Andriole V T, editor. The quinolones. 2nd ed. San Diego, Calif: Academic Press; 1998. pp. 369–414. [Google Scholar]
- 23.Stahlmann R, Zippel U, Förster C, Schwabe R, Shakibaei M, Merker H-J, Borner K. Chondrotoxicity and toxicokinetics of sparfloxacin in juvenile rats. Antimicrob Agents Chemother. 1998;42:1470–1475. doi: 10.1128/aac.42.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steven F S. The effect of chelating agents on collagen interfibrillar matrix interactions in connective tissue. Biochim Biophys Acta. 1967;140:522–528. doi: 10.1016/0005-2795(67)90526-0. [DOI] [PubMed] [Google Scholar]
- 25.Szarfman A, Chen M, Blum M D. More on fluoroquinolone antibiotics and tendon rupture. N Engl J Med. 1995;332:193. [PubMed] [Google Scholar]