ABSTRACT
Diabetic retinopathy is a microangiopathy resulting from the chronic effects of diabetes mellitus. Healthcare professionals often work in isolation to deliver highly specialised care efficiently and effectively for people living with diabetes. It is not uncommon for people with diabetes to be making frequent visits to community and hospital clinics to see a variety of specialists and healthcare professionals, with seemingly little opportunity for coordination of this complex health management programme between the wider team. In a field that is so diverse and rapidly changing, healthcare professionals of all specialties need to be aware of developments across all aspects of diabetes management. In this article, we discuss the epidemiology and natural history of diabetic retinopathy and describe an approach to its assessment and diagnosis. We provide an overview of the principles of diabetic retinopathy management and outline possible future treatments for diabetic retinopathy.
KEYWORDS: diabetic retinopathy, diabetic macular oedema, laser photocoagulation, intravitreal injections, vitrectomy
Key points
Diabetic retinopathy is the most common retinal vascular disease.
The risk factors for diabetic retinopathy are sub-optimal glycaemic control, hypertension, dyslipidaemia, longer duration of diabetes and genetic factors.
Identification and management of modifiable risk factors is important for preventing the onset and progression of diabetic retinopathy.
Diabetic eye screening programmes reduce the risk of sight loss among people with diabetes.
Ophthalmological treatments for diabetic retinopathy now include laser photocoagulation, vitrectomy and intravitreal pharmacotherapeutics.
Introduction
Diabetic retinopathy (DR) is a microangiopathy resulting from the chronic effects of diabetes mellitus (DM). It is the most common retinal vascular disease. DR affects three out of four people living with diabetes after 15 years of disease duration.1 DR was globally the fifth most common cause of preventable blindness and the fifth most common cause of moderate to severe visual impairment in those aged 50 years and above in 2020.2 DR is significantly associated with future risk of cerebrovascular accident, myocardial infarction and congestive heart failure.3
Who gets diabetic retinopathy?
Extraocular factors associated with risk of DR and its progression are poor glycaemic control, hypertension, dyslipidaemia, duration of DM, pregnancy and genetic factors.4–8
A recent meta-analysis showed that, in people with diabetes aged between 20 and 79 years, the overall prevalence was 34.6% for any DR and 7.0% for proliferative DR (PDR).8 The prevalence of any DR was higher in people with type 1 DM rather than type 2 DM (77.3% vs 25.2%, respectively), and was ‘highest among African Americans and lowest among Asians’.8
How is diabetic retinopathy classified?
DR is classified into non-proliferative and proliferative stages (Table 1).9 Non-proliferative DR (NPDR) involves progressive intraretinal microvascular alterations. PDR is characterised by the growth of newly formed vessels on the retina or optic disc. Diabetic macular oedema (DMO) refers to retinal thickening in the posterior pole and may occur in either NPDR or PDR.10
Table 1.
Classification of diabetic retinopathy
| Diabetic retinopathy severity scale | Findings observable on dilated ophthalmoscopy |
|---|---|
| No retinopathy | No abnormalities |
| Mild non-proliferative diabetic retinopathy | Microaneurysms only |
| Moderate non-proliferative diabetic retinopathy | More than just microaneurysms but less than severe non-proliferative diabetic retinopathy |
| Severe non-proliferative diabetic retinopathy | One or more of the following, in the absence of proliferative diabetic retinopathy:
|
| Proliferative diabetic retinopathy | One or more of the following:
|
| Mild diabetic macular oedema | Some retinal thickening or hard exudates in posterior pole but distant from the centre of the macula |
| Moderate diabetic macular oedema | Retinal thickening or hard exudates approaching the centre of the macula but not involving the centre |
| Severe diabetic macular oedema | Retinal thickening or hard exudates involving the centre of the macula |
Adapted from the proposed international diabetic retinopathy and diabetic macular oedema disease severity scales.9 NVD = neovascularisation at the disc; NVE = neovascularisation of the retina elsewhere.
What is the natural course of diabetic retinopathy?
DR progresses from mild abnormalities (characterised by vascular hyperpermeability) to moderate and severe NPDR (characterised by progressive retinal capillary leakage or loss resulting in retinal ischaemia) to PDR (characterised by the development of new vessels on the optic disc and retina). These new vascular growths are frequently accompanied by fibrous tissue formation, subsequent contraction of which leads to vitreous haemorrhage and tractional retinal detachments (TRDs). Invariably, untreated or treated, PDR will eventually reach an involutional quiescent stage. The subsequent level of visual acuity (VA) is dependent on the degree of damage to critical structures that has occurred by that point. Laser panretinal photocoagulation (PRP) of PDR induces this quiescent state earlier.11
What are the symptoms of diabetic retinopathy?
Patients with NPDR are typically asymptomatic. If PDR develops, the patient may present with a sudden loss of vision due to a vitreous haemorrhage. Patients may notice a more gradual loss of vision if DMO develops.
How is diabetic retinopathy assessed and diagnosed?
A comprehensive eye examination in a person with DR includes measurement of VA and intraocular pressure, evaluation of the anterior segment by slit-lamp biomicroscopy, gonioscopy when warranted (such as in the setting of elevated intraocular pressure, glaucoma or iris neovascularisation), and dilated funduscopic examination.
Funduscopy can be performed by a non-specialist using a handheld direct ophthalmoscope. The clinical diagnosis and characterisation of DR is mainly based on typical abnormal findings on a fundal examination. The key signs of NPDR are microaneurysms (saccular outpouchings of the retinal capillary wall due to pericyte loss), dot-blot intraretinal haemorrhages and exudates (Fig 1). These may be accompanied by cotton wool spots (small grey-white linear or serpentine lesions with fimbriated borders in the superficial retina that develop secondary to obstruction of a retinal arteriole with resultant ischaemia; Fig 1b), venous beading (segmental dilation of retinal veins that represent foci of venous endothelial cell proliferation), intraretinal microvascular abnormalities (dilated capillary remnants that occur secondary to extensive closure of capillary networks between arterioles and venules; Fig 1a). PDR is characterised by the presence of new vessels on the optic disc or elsewhere (more than one-disc diameter away from the optic disc; Fig 2). In advanced PDR, proliferation of fibrous tissue may lead to extensive TRDs with macula distortion (Fig 3).
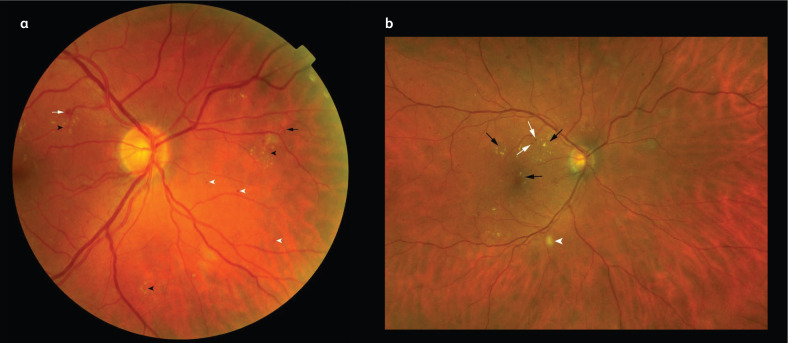
Fig 1.
Colour fundus photography of diabetic retinopathy lesions. a) A photograph of an eye with severe non-proliferative diabetic retinopathy showing dot (white arrowheads) and blot (white arrow) haemorrhages, exudates (black arrowheads) and intraretinal microvascular abnormalities (segments of dilated and tortuous retinal vasculature amid retinal vessels; black arrow). b) A close-up photograph of an eye with diabetic macular oedema showing exudates (black arrows) and microaneurysms (white arrows) at the macula, a cotton wool spot is shown just outside the inferotemporal vascular arcade (white arrowhead).
Fig 2.
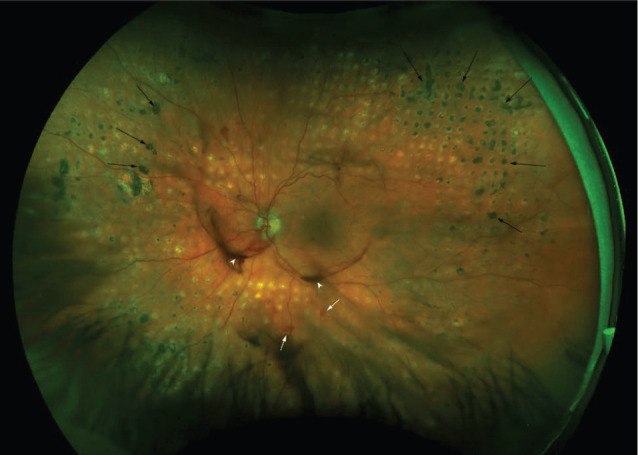
Ultra-widefield fundus photography of an eye with active proliferative diabetic retinopathy showing laser photocoagulation scars (black arrows), new vessels elsewhere (white arrows) and a vitreous haemorrhage (white arrowheads).
Fig 3.
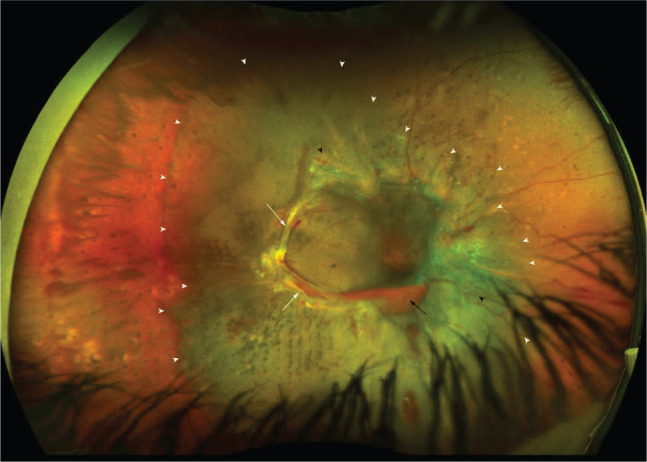
Ultra-widefield fundus photography of an eye with advanced proliferative diabetic retinopathy showing fibrovascular proliferations (white arrows), new vessels elsewhere (black arrowhead), subhyaloid haemorrhage (black arrow) and tractional retinal detachment (within area of the white arrowheads) involving the macula.
What are the principles of diabetic retinopathy management?
The principles of management can be broadly divided into prevention, early detection and ophthalmological treatments to reduce the risk of visual loss in eyes with sight-threatening complications.
Prevention
Control of hyperglycaemia is critical to minimising risk of onset and progression of DR. Two landmark randomised controlled trials, the Diabetes Control and Complications Trial that involved patients with type 1 diabetes and the UK Prospective Diabetes Study (UKPDS) that involved patients with type 2 diabetes, showed that tight control of glycated haemoglobin levels leads to a reduced risk of developing DR and its progression.4,5 Systematic review with meta-analysis and trial sequential analysis of clinical trials suggest that intensive glycaemic control leads to a 20% reduction in risk of DR.12
Control of hypertension is also beneficial in lowering risk of progression of DR.6 Hypertensive participants with type 2 diabetes in the UKPDS were randomly assigned to more intensive blood pressure control (<150/85 mmHg) and less intensive blood pressure control (<180/105 mmHg). After 7.5 years of follow-up, there was a 34% reduction in the rate of progression of DR by two or more steps using a modified DR severity scale and a 47% reduction in the deterioration of VA by three lines or more using standardised logMAR charts.
Treatment of dyslipidaemia may be beneficial to DR. The Action to Control Cardiovascular Risk in Diabetes randomised study showed that people with type 2 diabetes with elevated triglycerides who received simvastatin and fenofibrate treatment had a lower rate of progression of DR at 4 years compared with placebo.7 These findings are consistent with the findings from the Fenofibrate Intervention and Event Lowering in Diabetes study, a randomised trial of monotherapy with fenofibrate that showed a significant reduction in the need for laser therapy for PDR in the fenofibrate treatment group as compared with the placebo group.13
Early detection
Sight-threatening retinopathy may not cause symptoms prompting evaluation until the disease is advanced. Treatment to reduce risk of vision loss in eyes with sight-threatening complications of DR is most effective when initiated before severe vision loss has occurred. These facts underpin the importance of DR screening and surveillance. In the UK, all people with diabetes aged 12 years and above are offered annual screening for the presence of retinopathy.14 All diabetic screening programmes require digital fundus photographs to be taken. The fundus photographs acquired are reviewed by trained image graders. Those manifesting findings indicative of a certain level of retinopathy and those for whom adequate images cannot be obtained are referred to the hospital eye service for full evaluation and management.
In the UK, screening uptake has been excellent but variable. Screening has resulted in significant reduction of visual loss so that DR is no longer the commonest cause of visual loss in the working population, having been overtaken by inherited retinal disease.14 This is a clear indicator of the success of the retinal screening programmes in the UK.
Over the past few years, the diagnostic accuracy of artificial intelligence systems in identifying DR have been shown to be comparable with the grading of DR by retinal specialists on retinal images.15 Artificial intelligence systems could improve DR screening by reducing the reliance on manual work and providing savings in resources and cost, and may be incorporated into future screening programmes that are currently implemented or routinely practised.
Ophthalmological treatments
Laser photocoagulation
Evidence from randomised controlled trials performed in the 1970s and 1980s supported the use of PRP in PDR.11,16,17 The Diabetic Retinopathy Study (DRS), which compared PRP with no photocoagulation in people with PDR, demonstrated that PRP reduces the risk of severe visual loss from PDR by 50% or more.11,16,18 The Early Treatment for Diabetic Retinopathy Study (ETDRS) group recommended that PRP should not be used in eyes with mild to moderate NPDR but can be considered for eyes with severe NPDR.17 Additionally, the ETDRS demonstrated that focal photocoagulation of clinically significant DMO reduced the risk of moderate visual loss by 50%.17 The mechanisms by which PRP mediates its benefits are not fully understood. One possible mechanism is that an ischaemic retina, which produces growth factors, is destroyed by PRP thus reducing the angiogenic stimulus. Another possible mechanism is that PRP increases oxygenation from the choroid to the inner retina that occurs through the laser scars due to thinning of the retina in the treated area.19
Vitrectomy
Pars plana vitrectomy has been the standard treatment for non-resolving vitreous haemorrhage associated with PDR since the 1970s.20 Today, vitrectomy has an established role in the management of TRDs associated with severe fibrovascular proliferations in PDR.
Intravitreal pharmacotherapeutics
Today, the treatment paradigm for centre-involved DMO has shifted towards intravitreal pharmacotherapeutics, including anti-vascular endothelial growth factor (anti-VEGF) therapies.10 The introduction of such intravitreal pharmacotherapeutics has had a significant effect on the reduction of visual loss in patients with DMO. Early on in the treatment of DMO, patients often require monthly intravitreal injections and follow-up. Once the disease has stabilised, the interval between injections may be extended, permitting less frequent injections and follow-up.
What new ophthalmological treatments can we expect for DR?
Intravitreal anti-VEGF agents may play a future role in the management of PDR. The Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol S trial showed that people with PDR who received intravitreal ranibizumab treatment had a non-inferior VA outcome at 2 years compared with PRP.21 In the CLARITY study, a multicentre randomised non-inferiority trial of 232 patients with PDR, intravitreal aflibercept produced VA improvement at 1-year compared with those treated with PRP standard care.22 More recently, in the DRCR.net Protocol W study, intravitreal aflibercept was compared with sham control treatment in patients who have moderate to severe NPDR, with the objective of seeing whether aflibercept can prevent the development of PDR and benefit visual acuity.23 The investigators found that at 2 years, the proportion of patients developing PDR was lower in the aflibercept group than in the sham group. However, the mean VA change from baseline to 2 years was similar between the two groups. Although anti-VEGF therapies have been shown in clinical trials to be efficacious in treating PDR, the National Institute for Health and Care Excellence has not recommended its use in the NHS. Furthermore, the risk of PDR progression associated with the risk of non-compliance to treatment and follow-up is significant. PRP currently remains the standard treatment for PDR in the UK.11
Conflicts of interest
Emma G Wilmot has received personal fees from Abbott Diabetes Care, Dexcom, Eli Lilly, Insulet, Medtronic, Novo Nordisk and Sanofi Aventis. Winfried MK Amoaku has received honoraria for advisory board memberships from AbbVie, Alcon, Alimera, Allergan, Apellis, Bayer, Bausch + Lomb, Bioeq, Novartis and Pfizer; speaker Fees from Alimera, Allergan, Bayer, Novartis and Pfizer; and educational travel grants from Alimera, Allergan, Bayer, Novartis and Pfizer. He has undertaken clinical research sponsored by Allergan, Bayer, Gyroscope and Novartis. His institution has received research funding from Allergan, Bayer, Boehringer Ingelheim, CenterVue, Novartis and Optos.
References
- 1.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol 1989;107;244–9. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019 Blindness and Visual Impairment Collaborators . Causes of blindness and visual impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to vision 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health 2021;9;e144–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modjtahedi BS, Wu J, Luong TQ, et al. Severity of diabetic retinopathy and the risk of future cerebrovascular disease, cardiovascular disease, and all-cause mortality. Ophthalmology 2021;128;1169–79. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial Research Group , Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and prevention of long-term complications in insulin-dependent diabetes mellitus. N Eng J Med 1993;329;977–86. [DOI] [PubMed] [Google Scholar]
- 5.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001;44;156–63. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317;703–13. [PMC free article] [PubMed] [Google Scholar]
- 7.The ACCORD Study Group, ACCORD Eye Study Group . Effects of medical therapies on retinopathy progression in type 2 diabetes. N Eng J Med 2010;363;233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35;556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110;1677–82. [DOI] [PubMed] [Google Scholar]
- 10.Amoaku WM, Ghanchi F, Bailey C, et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK consensus working group. Eye (Lond) 2020;34;1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Diabetic Retinopathy Study Research Group . Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 1978;85;82–106. [DOI] [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352;837–53. [PubMed] [Google Scholar]
- 13.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007;370;1687–97. [DOI] [PubMed] [Google Scholar]
- 14.Scanlon PH. The English national screening programme for diabetic retinopathy 2003-2016. Acta Diabetol 2017;54;515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol 2020;8;337–47. [DOI] [PubMed] [Google Scholar]
- 16.The Diabetic Retinopathy Study Research Group . Indications for photocoagulation treatment of diabetic retinopathy: Diabetic Retinopathy Study Report no. 14. Int Ophthalmol Clin 1987;27;239–53. [DOI] [PubMed] [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group . Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology 1991;98;766–85. [PubMed] [Google Scholar]
- 18.The Diabetic Retinopathy Study Research Group . Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology 1981;88;583–600. [PubMed] [Google Scholar]
- 19.Weiter JJ, Zuckerman R. The influence of the photoreceptor-RPE complex on the inner retina. An explanation for the beneficial effects of photocoagulation. Ophthalmology 1980;87;1133–9. [DOI] [PubMed] [Google Scholar]
- 20.Machemer R, Blankenship G. Vitrectomy for proliferative diabetic retinopathy associated with vitreous haemorrhage. Ophthalmology 1981;88;643–6. [DOI] [PubMed] [Google Scholar]
- 21.The Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation vs intravitreal ranibizumab for proliferative diabetic retinopathy. A randomized clinical trial. JAMA 2015;314;2137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 2017;389;2193–203. [DOI] [PubMed] [Google Scholar]
- 23.Maturi RK, Glassman AR, Josic K, et al. Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: The protocol W randomised clinical trial. JAMA Ophthalmol 2021;139;701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]