Abstract
Background
Widespread lifestyle risk reduction at the community level is considered effective in decreasing Alzheimer’s disease (AD). To address the limited use of risk deduction in AD, this study aimed to explore the feasibility of community-level implementation. Diverse older adults (60+) living in Richmond, VA, with incomes below $12,000/year and managing diabetic/cardiovascular symptoms were offered weekly lifestyle telephone-health coaching for 12-weeks in 2019–2020 (Phase 1). The health coaching sessions were framed to provide AD lifestyle risk reduction education, goal setting, and support: motivations and self-efficacy. The study sample (n=40, mean age 68 years (range: 60–76 years)) was 90% African American/Black (n=36), 100% Non-Hispanic, and 45% males (n=18). Twenty-five participants (60%) reported experiencing some/often memory problems in the last 12-months. Thirty-nine (95%) of subjects successfully participated in coaching sessions; on average, 11 (91.9%) sessions per subject were completed. Participants provided positive anecdotal feedback and stated the need for continued health coaching. Consequently, n=30 (75%) of the original sample consented to continued health coaching during the 2020–2021 COVID-19 pandemic (Phase 2). All study subjects were examined at baseline (Time 1), 3-month (Time 2), covid-baseline (Time 3), and 3-months postcovid-baseline (Time 4). Repeated Measures ANOVAs were done to examine Time and Time*Memory Status effects.
Results
There was a total risk reduction at Phase 1 (F=9.26; p=.004; effect size=.19). At Phase 2, alcohol use decreased (p=.05), quadratic time effects were observed in physical activity (p=.01–.02), and cubic time effects were observed in depression (p=.02). Overall, total risk reduction in Phase 2 was observed at F=5.05; p=.03 effect size=.16. Pre/post-test analyses indicated improvement in Memory Problem Time Interaction (p=.007), AD knowledge (p=.01–.03), and Tired Days (p=.04) across Phase 1. There was also improvement in Social Isolation Time Interaction (p=.03); Tobacco Addiction (p=.001); Poor Mental Health Days (p=.05), and Worried Days Time Interaction (p=.02−.01) across Phase 2. Between subject Memory Status effects, indicating poorer baseline levels for individuals reporting memory problems had greater improvement seen in memory complaints (p=.001), poor mental health days (.02), and tired days (.003−.01).
Conclusions
This preliminary work creates the impetus for future large-scale lifestyle AD risk reduction investigations to mitigate and improve modifiable AD risk among low-income, diverse older adults, including individuals reporting memory problems. Our findings surrounding participant engagement and positive trends in AD risk reduction support the hypothesis that telephone-based health coaching is a practical and feasible AD risk reduction intervention.
Key words: Covid, Alzheimer’s disease, risk reduction, memory, minority health
Introduction
About a third of Alzheimer’s disease (AD) cases worldwide are attributed to modifiable risk factors, particularly psycho-behavioral risk factors: memory/cognitive ability, depression, stress/anxiety, and lifestyle factors: excessive alcohol use, physical inactivity, smoking, and social isolation (1). Preclinical stage AD risk reduction should be fully explored and tested to address increasing AD rates. Public health programming for AD risk reduction needs to address the existing barriers in knowledge, self-efficacy, and expectations for psycho-behavioral change (2). Lifestyle health coaching shows promise in potentially reducing AD but requires more testing (3, 4). Low-income urban-dwelling adults are a high-priority risk group due to having some of the greatest frequency of lifestyle risk factors, high levels of morbidity and disability, and shorter lifespans (5, 6). Additionally, this population experiences barriers to innovative intervention (7). Although susceptibility to AD is not determined by income, it is influenced by personal history of income-related modifiable lifestyle risk factors (5). In response to these barriers, we propose to address AD lifestyle risk factors in low-income groups.
AD Lifestyle Risk Factors
The main AD psycho-behavioral risk factors are cognitive ability, depression, anxiety, and lifestyle behaviors: excessive alcohol use, physical inactivity, smoking, and social isolation (3, 4). Psychological variables such as cognitive ability, depression, and anxiety have long been identified as risk factors for AD. Cognitive problems are typically one of the first warning signs of cognitive impairment related to AD and dementia (8). Depression/anxiety also has shown an increased risk of dementia in late-life (9), attributed to disturbed sleep and stress-induced cardiovascular effects (10). Change in psychological AD risk factors are more amenable to clinical and pharmaceutical-related interventions than other lifestyle factors.
AD lifestyle risk factor changes are relatively more amenable to community-level interventions and can have a distal effect on AD-disease risk and AD-psychological risk factors. For instance, in the case of excessive alcohol use, there are detrimental neuro-psychological effects from heavy levels (hazardous/intoxicating) of intake, and it is a risk factor for AD (11, 12), especially among APOE e4 allele carriers (13). Physical inactivity is one of the most consistent AD risk factors because of influences on cognition, vascular health, and brain metabolism (14). There is an overall AD risk reduction in physically active adults compared to non-active counterparts, extending into old age (15). Smoking increases the risk of AD (16), explained by vascular and neurological detriments (17). Lonely persons also have a higher AD risk; cognitive decline and the onset of AD can be delayed/prevented with frequent participation in social activities (18), partially explained by the vascular and oxidative stress benefits (19).
AD Lifestyle Health Coaching
Prevention at the preclinical stage is currently the most effective way to decrease the incidence of AD and its associated burden for individuals and society (20). Health coaching, a lifestyle intervention, operates by supporting lifestyle change with trained, motivational coaches who systematicaly encourage, support, plan, and address individual goals, barriers, and motivators, ultimately creating sustained lifestyle changes within their clients (21, 22). Health coaching also removes attitude, belief, and knowledge barriers by engaging participants in discussions around health literacy, adherence, and self-efficacy (23, 24). Health literacy and knowledge barriers are specifically relevant because, while AD is often a major concern for adults (25), there is frequently an individual failure to connect lifestyle behaviors and AD (2). Precision education which teaches the association between behavior risk, lifestyle change, and AD can be psychologically beneficial, motivating, and programmatically concrete (26), especially among individuals with poor AD health risk appraisal (27). Furthermore, there is evidence for health coaching efficacy in low-income and underserved populations (28, 29). Health coaching is especially beneficial for lifestyle change in people with numeracy and literacy deficits because it facilities participant interpretation of health risk information (30). Previous findings have indicated that health coaches should personalize the coaching experience to balance competing and conflicting needs in limited-resource groups to maximize lifestyle change (31).
Health coaching is an under-tested strategy for AD risk reduction (32). There have been calls for lifestyle change health coaching to be more fully explored and evaluated (33, 34). While findings are mixed (33) relevant to AD, health coaching interventions have shown improvement in alcohol/smoking use (35), physical activity (36, 37), and social isolation (38). In addition to lifestyle behaviors, health coaching indirectly supports psychological and emotional health, improving stress levels (37) and mental health (39). Evidence indicates that health coaching enhances participant care and satisfaction compared to standard treatment, reducing depressive symptoms and hospitalizations (40). In a sample with Type 2 Diabetes Mellitus, health coaching increased self-management skills and reduced 30-day readmissions (41). Health coaching also distally improves healthy quality of life, i.e., healthy days, through lifestyle management (42). Lifestyle change can be difficult due to motivation, self-efficacy, and knowledge barriers (43, 44). In turn, improving individual understanding of barriers/benefits of supporting lifestyle change within an AD context can improve attempts to induce positive change in AD-risk populations.
Lifestyle health coaching is a promising yet underresearched strategy for reducing AD risk factors (32). Programmatically connecting individual-level lifestyle health coaching requires testing to address AD risk reduction outcomes (26). Accordingly, we proposed to offer AD targeted lifestyle health coaching to explore reductions in AD modifiable psycho-behavioral risk factors. This study’s primary aim is to examine change in AD modifiable risk factors (e.g., memory/cognitive ability, stress/anxiety, depression, and lifestyle) in high-risk older adults. Twelve telephone-based health coaching contacts (once/week) are offered to 60+ aged adults, using a participant preference strategy, in which the participant decides the lifestyle factor to target. This research occurred in collaboration with partnering urban subsidized housing unit contexts (45), as part of the Virginia Commonwealth University (VCU) iCubed Health and Wellness in Aging Population Core. We aimed to recruit n=40 high-AD risk adults as indicated by aged 60+, low-income (below $12,000/year), and managing diabetes or cardiovascular symptoms. The overall hypothesis of this research is that AD risk can be reduced by implementing a telephone-based health coaching intervention.
Methods
The study sample (n=40, mean age 68 years (range: 60–77 years) was 90% African American/Black (n=36), 100% Non-Hispanic, and 45% males (n=18)). All study recruitment and research activities occurred in Richmond, VA, focused on senior public housing settings. With appropriate permissions, study staff set up recruitment/research tables in common areas where advertisements were viewable and flyers were made available for dissemination. The study coordinator staffed recruitment events. Participants affirmed their eligibility to participate to the study coordinator: English proficiency, denial of cognitive impairment, actively managing diabetic or cardiovascular health symptoms, aged 60+ years, income below $12,000/year, and an accessible telephone. For individuals interested, consent and the baseline interviews could be completed immediately. After the onset of the COVID-19 pandemic, recruitment and assessment activities took place via telephone or mail. Once the baseline assessment was complete, the health coach was assigned to complete the study intervention. After the subjects completed their 12-week intervention, they were contacted by study staff to complete their follow-up assessment (Phase 1). At the conclusion of the 12-week intervention program, study staff offered participants an additional 6 weeks of follow-up health coaching (Phase 2). The additional coaching was added in response to the COVID-19 pandemic and focused on pandemic knowledge, prevention, and safety. Thirty of the original participants (75%) agreed to participate in the COVID-19 Phase 2 follow-up coaching. Of the phase 2 sample (n=30, 86% African American/Black and 36% male), 66% of participants had minimally completed high school.
In Phase 1, the health battery was administered at baseline (prior to coaching, Time 1) and again at exit (after 12 weeks of health coaching, Time 2). Prior to starting the COVID-19 coaching (Phase 2), participants who re-enrolled were given a Phase 2 (Time 3) health assessment. At the end of the COVID-19 six-coaching sessions, the final (Time 4) health battery was administered.
Study Battery
All selected instruments are state dynamic to assess change over the intervention period.
AD Knowledge: AD Knowledge Scale (46), 34-item self-report survey, includes health disparities and behavioral health risk factor information.
Alcohol Use: Audit-C (47). Brief 3-item self-report alcohol screen that reliably identifies alcohol quantity, hazardous drinkers, or active alcohol use disorders.
- Cognitive Ability: Memory and Speed abilities are robust and malleable AD predictors).
- Memory ability: memorization and recall of meaningful language units (48).
- ■ Immediate Recall (IR), study, and recall a list of 20 words for 3.5 minutes.
- ■ Delayed Recall (DR), recall the same 20-word list of IR words after an hour of other activities.
- Psychomotor speed: Trail Making Test (TMT) TMT Part A. Part A is a measure of rote memory.
- Speed of executive functioning: TMT Part B (49). Part B is sensitive to executive functioning since the test requires multiple abilities to complete.
- Cognitive Telephone Instrument (COGTEL) (50) for no-contact Cognitive Ability. Performance assessment in 6-item/cognitive domains (prospective, short-term, long-term working memory, verbal fluency, and reasoning). It can be applied in a face-to-face session and over the phone and takes only about 15 minutes. Appropriate for use during COVID-19 like situations.
Depression: Patient Health Questionnaire-9 (51). A nine-item self-report instrument for screening, diagnosing, monitoring, and measuring the severity of depression.
Health History/Status: Quality of Life assessed by the CDC HRQOL-14 “Healthy Days” (52).
Physical Activity: Leisure Time Physical Activity Instrument (53). Self-report 4-item instrument measuring activity in populations predominantly engaging in low-intensity activities.
Smoking/Nicotine Use: Fagerstrom (54). A standard 6-item self-report instrument for assessing the intensity of smoking and physical addiction to nicotine.
Social Isolation/Support: De Jong Loneliness scale (55), Self-report 6-item scale, three statements about ‘emotional loneliness’ and three about ‘social loneliness.’ Social loneliness (SL) addresses the broader social network, and emotional loneliness (EL) addresses intimate relationships.
Total Risk Score is an aggregate score calculated from affirmation to 1) any depression symptoms, 2) drinking more than 1 drink/day, 3) any smoking, 4) any social isolation symptoms, 5) reporting more than 5 medications, and 6) reporting <1.5 hours physical activity/exercise each week.
Memory Problem Status is a dichotomous score calculated from the following question: “In the past 12 months, how much of the time have you had any of the following problems? Memory Problems”. Never/rarely, sometimes, and often. Sometimes and often were coded as 1=Yes for Memory problems. Never/rarely was coded as 0=No for Memory Problems.
Intervention
Twelve telephone-based health coaching contacts (weekly over 3-months) were offered as the study intervention. Health coaching calls lasted an average of 15–20 minutes per session and were structured with learning objectives. The first call focused on scheduling, developing familiarity/comfort, AD education/learning objectives, lifestyle change goal setting/planning, coaching-creating change, identifying barriers/motivators, problem-solving, and summarization/learning verification. The subsequent ten sessions consisted of education/learning objectives, goal achievements/reidentification, lifestyle change coaching, progress/planning on barriers/motivators/problem solving, and summarization/learning verification. The final session was a closure call, focusing on continued goal setting and planning for sustained lifestyle change for AD prevention and highlighting and celebrating achievements. For participants who chose to re-enroll in the COVID-19 coaching program, the health coaching calls were similar. However, they included COVID-19 learning objectives and supported overcoming COVID-related barriers to sustaining lifestyle risk reduction.
The intervention protocol was conceptually designed to provide health coaching to target AD-Lifestyle risk via education, personal risk assessment, attitudes, and lifestyle behavioral change, to reduce AD risk. Health coaching praxis supports individual lifestyle change using trained individuals that serve as coaches to systematically encourage, support, plan, and address goals, barriers, and motivators to create learning objectives and sustain lifestyle change (21, 22). The Health Belief Model56 and the Information-Motivation-Behavioral Skills Model (57) are guiding health coaching models. Theoretical principles including information-education, threat, motivation, skills, and barrier/ motivator domains were foundational concepts integrated into the intervention protocol (58).
A participant preference lifestyle change approach framed the health coaching sessions. Participant preference approaches are cost-effective, feasible, and support knowledge, evaluation, and understanding of health concepts (59, 60). Increasing these skills is critical in low-income populations, who are at higher risks of health literacy challenges and may need support to synthesize health information effectively (61). Based on Maslow’s hierarchy, low-income populations are one of the most challenging groups in which to create lifestyle change, partly from competing basic needs and increased vulnerability (62). Employing the participant preference approach generates understanding between the health coach and the participant. It empowers the participant to choose which lifestyle behavior issues are most pressing and/or feasible for sustained change.
Analyses
Repeated Measures Analysis of Variance, with time as the independent variable and outcome scores (e.g., cognitive functioning, alcohol use, depression, physical inactivity, smoking, social isolation, and medication risk), as the primary dependent variable, effects of self-reported memory problem status on the dependent variables are also explored. Change is evaluated at 2–4 time points, depending on if participants re-enrolled in COVID-19 coaching. Statistical significance is set at (p<.05), trends in cognitive change are identified as (p<.10).
Results
The study sample (n=40, mean age 67.85 years (range: 60–77 years, 4.78 standard deviation) was 90% African American/Black (n=36), 100% Non-Hispanic, 55% females (n=22), and 60% of the sample reported memory problems sometimes or often in the past 12 months. Approximately 70% of the sample had minimally completed high school (GED earned). 87% (n=35) of the sample had monthly incomes equal to or less than $1,000. 82% of the sample (n=33) lived in senior housing or apartments. The rest (n=7) of the sample lived in a single-family home. 93% of participants lived alone. Table 1 shows the demographic distribution across memory status (MS), indicating no demographic differences across memory.
Table 1.
Demographics
| No Memory Problems (40%) | Memory Problems (60%) | p-value | |
|---|---|---|---|
| Age (mean (standard deviation)) | 69.8 (5.1) | 67.8 (5.1) | .225 |
| Gender (% female) | 69% | 54% | .356 |
| Race (% African American/Black) | 81% | 96% | .132 |
| Income Monthly | $1.4K | $1.1K | .259 |
| Education (% less than high school) | 25% | 33% | .557 |
| Housing (% Single-family home) | 19% | 25% | .311 |
| Living Alone (% Living alone) | 81% | 92% | .203 |
Overall, the subjects were able to engage in telephone health coaching. Thirty-nine (95%) of subjects successfully participated in coaching sessions; on average, 91.9% (11) sessions/subject were completed during Phase 1. On a scale of 0–100 (higher scores= more positivity), they rated their experience a 93.3. On a scale of 0–10 (higher scores= more improvement), rated their health improved 8.36. There was a total risk reduction at Phase 1 (F=9.26; p=.004; effect size=.19). Significant improvement in depression risk status was seen at Phase 1 (F=4.89; p=.03; effect size=.11). The other risk factors showed trends for improvement and no smoking change. At Phase 2, there was a quadratic time effect for Total Risk (F=5.05; p=.03; effect size=.16), indicating improvement that was not sustained. Phase 2 total risk change was driven by cubic changes in depression (F=6.58; p=.02; effect size=.20), alcohol improvement (F=4.20; p=.05; effect size=14), social isolation improvement (F=5.34; p=.03; effect size=.17), and quadratic physical activity change (F=6.45; p=.02; effect size =.19).
Table 2.
Health Coaching Effects on Targets
| Sample size | Time 1 | Time 2 | Time 3 | Time 4 | TIME f-value (p-value) effect size | Time*Memory Problem Status f-value (p-value) effect size | Between- Subjects Memory Problem Status f-value (p-value) effect size | |
|---|---|---|---|---|---|---|---|---|
| Memory Complaints | n=40 | .80 | .60 | — | — | 2.79 (.10).07 | 8.27 (.007).18 | 80.98 (.001).68 |
| n=28 | .82 | .64 | .64 | .57 | 2.78 (.11).09 | 2.97 (.10).10 | 37.7 (<.001).59 | |
| DeJong Score | n=40 | 1.70 | 1.37 | — | — | 2.54 (.12).06 | 3.46 (.07).08 | 0.50 (.49).01 |
| n=28 | 1.75 | 1.54 | 1.57 | 1.57 | 0.31 (.58).01 | 4.91 (.04).16 | 0.73 (.40).03 | |
| Smoking Score | n=11 | 6.27 | 6.09 | — | — | 0.48 (.51).05 | 0.16 (.70).02 | 0.35 (.57).04 |
| n=6 | 6.16 | 6.0 | 6.33 | 3.33 | 46.7 (.001).90 | 0.16 (.71).04 | 3.64 (.13).48 | |
| Alzheimer’s Knowledge* | n=40 | 22.25 | 25.37 | — | — | 6.86 (.01).15 | 4.82 (.03).11 | 2.38 (.13).06 |
| Poor mental health in past 30 days | n=35 | 13.31 | 9.94 | — | — | 1.56 (.22).04 | 0.02 (.90).001 | 8.18 (.007).20 |
| n=26 | 13.92 | 10.76 | 8.54 | 8.62 | 4.13 (.05).14 | 1.76 (.20).07 | 5.77 (.02).19 | |
| Tired Days in past 30 days | n=37 | 12.86 | 8.05 | — | — | 4.38 (.04).11 | .91 (.35).03 | 9.99 (.003).22 |
| n=25 | 13.16 | 8.12 | 8.76 | 9.24 | 1.01 (.32).04 | 0.41 (.53).02 | 7.07 (.01).23 | |
| Worried Days over past 30 days | n=38 | 12.68 | 8.65 | — | — | 2.37 (.13).06 | .17 (.68).005 | 5.46 (.03).13 |
| n=27 | 13.70 | 11.03 | 9.44 | 6.44 | 6.47 (.02).20 | 7.09 (.01).22 | 3.85 (.06).13 | |
| Depression | n=40 | .92 | .75 | — | — | 4.89 (.03).11 | 0.02 (.90).00 | 1.23 (.28).03 |
| n=28 | .96 | .79 | .93 | .86 | 6.58 (.02).20∧ | 2.16 (.15).08 | 0.76 (.39).03 | |
| Alcohol Use | n=40 | .52 | .47 | — | — | 1.00 (.32).03 | 0.04 (.84).001 | 0.11 (.74).003 |
| n=28 | .57 | .54 | .46 | .43 | 4.20 (.05).14 | 0.55 (.46).02 | 0.04 (.84).002 | |
| Social Isolation | n=40 | .75 | .62 | — | — | 2.35 (.13).06 | 1.59 (.22).04 | 2.92 (.10).07 |
| n=28 | .71 | .68 | .64 | .64 | 0.39 (.54).01 | 5.34 (.03).17 | 0.52 (.48).02 | |
| Physical Inactivity | n=40 | .35 | .28 | — | — | 1.00 (.32).03 | 0.02 (.89).00 | 0.16 (.69).004 |
| n=28 | .39 | .32 | .29 | .54 | 6.45 (.02).19* | 6.90 (.01).21 | .001 (.98).00 | |
| Total Risk Index | n=40 | 3.50 | 3.03 | — | — | 9.26 (.004).19 | 0.21 (.65).01 | .03 (.86).001 |
| n=28 | 3.67 | 3.32 | 3.357 | 3.53 | 5.05 (.03).16* | 3.10 (.09).10∧ | .75 (.40).03 |
Note. (*) Was not measured at COVID follow up; (*) Quadratic Curve; (∧) Cubic Curve
Pre/post-test analyses indicated improvement in Memory Problem MS Interaction (F=8.27; p=.007; effect size=.18); AD knowledge (F=6.86; p=.01; effect size=.15), AD knowledge MS Interaction (F=4.82; p=.03; effect size=.11), and Tired Days (F=4.38; p=.04; effect size=.11) across Phase 1.
There was also improvement in Social Isolation MS Interaction (F=5.34; p=.03; effect size=.17); Tobacco Addiction (F=46.7; p=.001; effect size=.90); Poor Mental Health Days (F=4.13; p=.05; effect size=.14), and Worried Days (F=6.47; p=.02; effect size=.20)/Worried Days MS Interaction (F=7.09; p=.01; effect size=.22) across Phase 2.
Between subject MS effects indicated poorer baseline levels for individuals reporting memory problems, but often with more significant improvement. Between subjects effects for memory status were seen in memory problems (F=80.98; p=.001), poor mental health days (F=5.77; p=.02), and tired days (F=9.99; p=.003). Figure 1 depicts Memory status time interaction effects.
Figure 1.
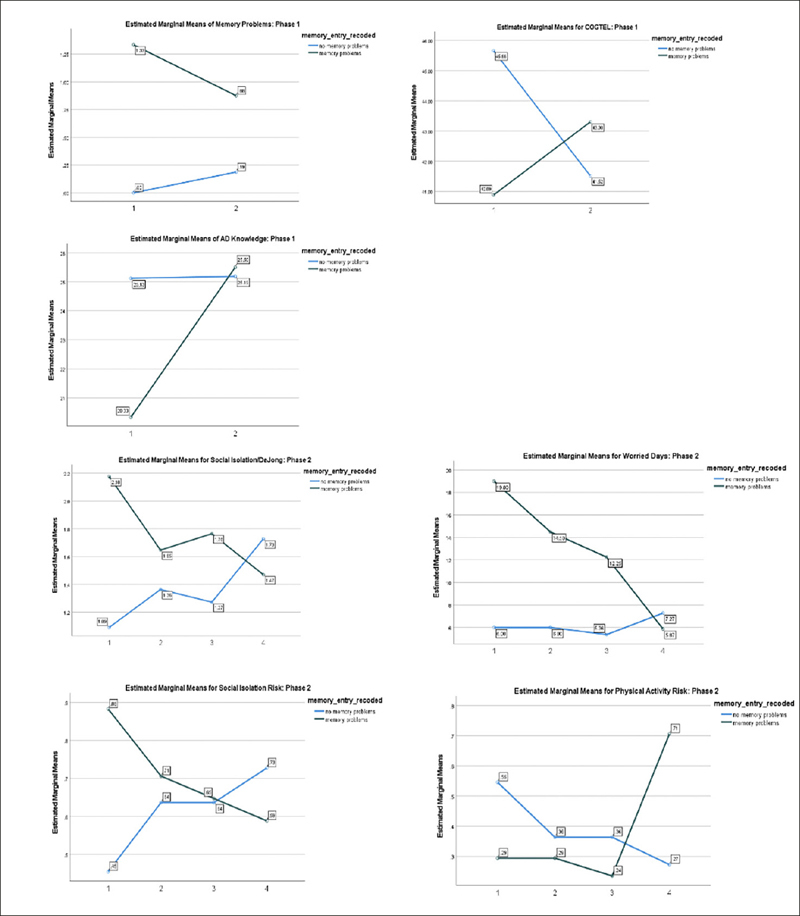
Significant Memory Status Interaction Change effects
There was no clear indication that the intervention impacted cognitive ability due to COVID-19 IRB restrictions which limited face-to-face cognitive assessments. There were some trends indicating improvement in the Trails A (F=3.72; p=07; effect size=.16/ MS Between Subjects F=2.87;p=.10;effect size=.13) and COGTEL (F=3.33; p=09; effect size=.18) performance over time.
Discussion
We conclude that telephone-based AD Health Coaching is a feasible community health intervention. Participants provided positive anecdotal feedback and the need for continued health coaching. Participants tolerated the intervention well, as demonstrated by 97% of participants completing all or most of Phase 1 and 93% of participants completing phase 2. Likely, the ease of accessing the intervention (participants only needed a telephone) combined with the health coaches’ patient preference strategy helped retain participants in the program.
Table 3.
Health Coaching Effects on Cognition*
| Sample size | Time 1 | Time 2 | Time 3 | Time 4 | Time f-value (p-value) effect size | Time*Memory Problem Status f-value (p-value) effect size | Between- Subjects Memory Problem Status f-value (p-value) effect size | |
|---|---|---|---|---|---|---|---|---|
| Trail A | n=20 | 65.21 | 54.49 | — | — | 3.72 (.07).16 | 0.17 (.68).01 | 2.87 (.10).13 |
| Trail B | n=20 | 136.17 | 120.07 | — | — | 1.51 (.23).07 | 0.03 (.86).00 | 4.26 (.05).19 |
| Short-term Recall | n=18 | 7.78 | 8.50 | — | — | 2.01 (.18).11 | 0.81 (.38).05 | 2.12 (.16).12 |
| Delayed Recall | n=18 | 5.11 | 5.66 | — | — | 0.83 (.37).05 | 0.12 (.74).01 | 2.62 (.12).14 |
| COGTEL | n=17 | 43.41 | 42.35 | — | — | 0.30 (.59).02 | 3.33 (.09).18 | .13 (.73).008 |
| n=24 | — | — | 39.34 | 37.67 | 1.28 (.27).05 | 0.03 (.86).00 | 2.94 (.10).12 | |
| n=10 | 42.67 | 41.79 | 43.72 | 42.51 | 0.02 (.89).00 | 0.07 (.80).01 | 0.33 (.58).04 |
Note. Due to IRB COVID-19 precautions Sample groups for cognition instruments are uneven
There were significant improvements in overall risk, memory complaints, social isolation, smoking, Alzheimer’s Knowledge, and poor mental health/tired/worried days across timepoints. Memory complaints and tired days did not have continued significant changes during Phase 2, suggesting that the COVID-19 pandemic may have undermined continued improvement. The Phase 2 improvements observed in social isolation, smoking, poor mental health days, and worried days suggest that some AD risk factors may need higher dosages of health coaching for improvement to be observed. Time 3 measurement indicated higher Fagerstrom smoking scores than Time 1 measurement. Tobacco use is of particular interest because smoking increased in some users during the pandemic. Still, tobacco users may have been uniquely motivated to reduce or stop their use due to fears related to vulnerability to the virus (Rigotti et al., 2021). Although the Fagerstrom smoking scores increased to above baseline levels at the Time 3 COVID-19 entry, the additional 6 health coaching sessions successfully reduced tobacco use. Despite our intervention occurring during the pandemic lockdown, participants in the health coaching program had reduced social isolation, smoking, poor mental health days, and worried days during the COVID-19 pandemic.
The decrease in memory complaints is particularly exciting, as cognitive difficulty/memory complaints are frequently the first sign of AD. Furthermore, the group differences seen for memory status indicate greater levels of improvement in the memory complaint group. Study cognitive ability trends also showed patterns for improvement. Future research is needed to address how AD health coaching can be used as a remedial intervention to address memory complaints prior to a formal AD diagnosis.
There are some limitations to our findings to be noted. The COVID-19 pandemic began after recruiting approximately half of our participants, forcing us to adjust our instruments and recruiting methods. Our original recruitment strategy focused on an in-person presence within Richmond, Virginia’s senior public housing communities. After pandemic onset, we switched to snowball recruiting and select outdoor events, slowing recruitment efforts. We were unable to administer the TMT or the Memory Ability Test. Instead, we used the COGTEL, but it makes it difficult to compare memory changes across participants. We are also not able to adjust our scores in cognitive measures for social factors. The additional 6 sessions of COVID-19 (Phase 2) health coaching were not part of the original study design. The time difference between lapses in health coaching varies considerably among participants. Some participants re-enrolled for an additional 6 weeks of COVID-focused health coaching after a 2 week waiting period following the conclusion of the 12-session intervention. Other participants had been disenrolled for more extended periods. Finally, the majority of this study took place during 2020–2021 amid the COVID-19 pandemic. It is possible that testing this intervention during “normal” times may have yielded different results due to fewer demands on cognitive reserves and more resources for participants. Many participants provided anecdotal evidence that exercise programs, social activities, and transportation services were limited or halted during the pandemic.
Time 3 measurement indicated higher Fagerstrom smoking scores than Time 1 measurement. Tobacco use is of particular interest because smoking increased in some users during the pandemic. Still, tobacco users may have been uniquely motivated to reduce or stop their use due to fears related to the virus (63). Although the Fagerstrom smoking scores increased to above baseline levels at the Time 3 COVID-19 entry, the additional 6 health coaching sessions successfully reduced tobacco use. Despite our intervention occurring during lockdown, participants in the health coaching program had reduced social isolation, smoking, poor mental health days, and worried days during the COVID-19 pandemic.
The challenges faced during the pandemic emphasize the strengths inherent in a telephonic, patient preference lifestyle intervention. Health coaches are trained to be cognizant of participant wishes and mindful of barriers, potential competing needs, and to respect voice and choice. The patient preference strategy encouraged both coach and participant to find methods and set feasible goals for risk reduction. From an operational and methodological standpoint, our intervention showed resilience and robust effects. Despite the pandemic, we continued providing uninterrupted health coaching to participants. We proceeded with our recruitment and measurement with only minor modifications. Using the telephone instead of videoconferencing or more modern telehealth approaches meant that participants could continue accessing coaching even after many of them lost access to the computer labs in their housing complexes and local libraries. We used the mail to obtain signatures and compensate participants. The consideration of delivering accessible interventions is relevant beyond the lens of pandemic preparedness. Lower educational attainment, lower-income, identifying as African American/Black, and being 65+ correlate with a lack of internet access (64). Our telephonic health coaching model could also be applied in rural populations, who also are more likely to be without internet access.
Conclusion
In conclusion, this preliminary work creates the impetus for future large-scale lifestyle AD risk reduction investigations to improve the lives of AD-risk, low-income, diverse older adults. These findings demonstrate that telephone-based health coaching is feasible, based on participant engagement, and practical, based on positive trends in reducing AD risk factors. The employment of a telephone-based model allowed for the continuation of coaching through pandemic shutdowns and made the intervention more accessible for participants with physical disabilities or those who did not have access to computers than traditional in-person or telehealth interventions. Health coaching interventions are cost-effective when compared to licensed professionals that work in behavior change. The relationship-building between participants and health coaches allowed coaches to be conscious of individual barriers to implementing change while continuously reinforcing the connections between participants, their susceptibility to AD, and their strengths in behavior change. To our knowledge, this is the first pilot study that has closely examined health coaching as an AD risk reduction strategy in low-income diverse populations.
Funding: This study received funding from Virginia Commonwealth University Presidential Research Quest Fund. The Alzheimer’s and Related Diseases Research Fund (ARDRAF). The Wright Center for Clinical and Translational Research (CTSA UL1TR002649), and the Institute of Inclusion Inquiry and Innovation at Virginia Commonwealth University.
Conflict of interest: The authors have no conflicts of interest.
Ethical standard: This study was reviewed and approved by the Institutional Review Board at Virginia Commonwealth University.
References
- 1.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 2.Clevenger CK, Cantey S, Quinn ME. Teaching Primary Prevention of Alzheimer’s Disease: Does It Make a Difference? J Prim Care Community Health. 2010;1(2):134–138. doi: 10.1177/2150131910367510. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher D, Kiss A, Lanctot KL, Herrmann N. Toward Prevention of Mild Cognitive Impairment in Older Adults With Depression: An Observational Study of Potentially Modifiable Risk Factors. J Clin Psychiatry. 2018;80(1). doi:10.4088/JCP.18m12331 [DOI] [PMC free article] [PubMed]
- 4.Isaacson RS, Ganzer CA, Hristov H, et al. The clinical practice of risk reduction for Alzheimer’s disease: A precision medicine approach. Alzheimers Dement. 2018;14(12):1663–1673. doi: 10.1016/j.jalz.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stępkowski D, Woźniak G, Studnicki M. Correlation of Alzheimer’s Disease Death Rates with Historical Per Capita Personal Income in the USA. Reddy H, ed. PLOS ONE. 2015;10 (5):e0126139. doi:10.1371/journal.pone.0126139 [DOI] [PMC free article] [PubMed]
- 6.Zanjani F, Smith R, Slavova S, et al. Concurrent alcohol and medication poisoning hospital admissions among older rural and urban residents. Am J Drug Alcohol Abuse. 2016;42(4):422–430. doi: 10.3109/00952990.2016.1154966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet Lond Engl. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva MVF, Loures C d M, Alves LCV, de Souza LC, Borges KBG, Carvalho M d G. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci. 2019;26(1):33. doi: 10.1186/s12929-019-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banks SJ, Raman R, He F, et al. The Alzheimer’s Disease Cooperative Study Prevention Instrument Project: Longitudinal Outcome of Behavioral Measures as Predictors of Cognitive Decline. Dement Geriatr Cogn Disord Extra. 2014;4(3):509–516. doi: 10.1159/000357775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke SL, Cadet T, Alcide A, O’Driscoll J, Maramaldi P. Psychosocial risk factors and Alzheimer’s disease: the associative effect of depression, sleep disturbance, and anxiety. Aging Ment Health. 2018;22(12):1577–1584. doi: 10.1080/13607863.2017.1387760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heymann D, Stern Y, Cosentino S, Tatarina-Nulman O N, Dorrejo J, Gu Y. The Association Between Alcohol Use and the Progression of Alzheimer’s Disease. Curr Alzheimer Res. 2016;13(12):1356–1362. doi: 10.2174/1567205013666160603005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piazza-Gardner AK, Gaffud TJB, Barry AE. The impact of alcohol on Alzheimer’s disease: A systematic review. Aging Ment Health. 2013;17(2):133–146. doi: 10.1080/13607863.2012.742488. [DOI] [PubMed] [Google Scholar]
- 13.Downer B, Zanjani F, Fardo DW. The Relationship Between Midlife and Late Life Alcohol Consumption, APOE e4 and the Decline in Learning and Memory Among Older Adults. Alcohol Alcohol. 2014;49(1):17–22. doi: 10.1093/alcalc/agt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maliszewska-Cyna E, Lynch M, Oore J, Nagy P, Aubert I. The Benefits of Exercise and Metabolic Interventions for the Prevention and Early Treatment of Alzheimer’s Disease. Curr Alzheimer Res. 2016;14(1):47–60. doi: 10.2174/1567205013666160819125400. [DOI] [PubMed] [Google Scholar]
- 15.Beckett MW, Ardern CI, Rotondi MA. A meta-analysis of prospective studies on the role of physical activity and the prevention of Alzheimer’s disease in older adults. BMC Geriatr. 2015;15(1):9. doi: 10.1186/s12877-015-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito E, Diaz N, Chung J, McMurtray A. Smoking history and Alzheimer’s disease risk in a community-based clinic population. J Educ Health Promot. 2017;6(1):24. doi: 10.4103/jehp.jehp_45_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durazzo TC, Meyerhoff DJ, Yoder KK. Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimer’s Disease. Drug Alcohol Depend. 2018;192:277–284. doi: 10.1016/j.drugalcdep.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiao YH, Chang CH, Gean PW. Impact of social relationships on Alzheimer’s memory impairment: mechanistic studies. J Biomed Sci. 2018;25(1):3. doi: 10.1186/s12929-018-0404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiao YH, Kuo JR, Chen SH, Gean PW. Amelioration of social isolation-triggered onset of early Alzheimer’s disease-related cognitive deficit by N-acetylcysteine in a transgenic mouse model. Neurobiol Dis. 2012;45(3):1111–1120. doi: 10.1016/j.nbd.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Han JY, Han SH. Primary Prevention of Alzheimer’s Disease: Is It an Attainable Goal? J Korean Med Sci. 2014;29(7):886. doi: 10.3346/jkms.2014.29.7.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson KE, Alencar MK, Coakley KE, et al. Telemedicine-Based Health Coaching Is Effective for Inducing Weight Loss and Improving Metabolic Markers. Telemed E-Health. 2019;25(2):85–92. doi: 10.1089/tmj.2018.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira JS, Sherrington C, Amorim AB, Dario AB, Tiedemann A. What is the effect of health coaching on physical activity participation in people aged 60 years and over? A systematic review of randomised controlled trials. Br J Sports Med. 2017;51(19):1425–1432. doi: 10.1136/bjsports-2016-096943. [DOI] [PubMed] [Google Scholar]
- 23.Dejonghe LAL, Becker J, Froboese I, Schaller A. Long-term effectiveness of health coaching in rehabilitation and prevention: A systematic review. Patient Educ Couns. 2017;100(9):1643–1653. doi: 10.1016/j.pec.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Tülüce D, Kutlutürkan S. The effect of health coaching on treatment adherence, self-efficacy, and quality of life in patients with chronic obstructive pulmonary disease. Int J Nurs Pract. 2018;24(4):e12661. doi: 10.1111/ijn.12661. [DOI] [PubMed] [Google Scholar]
- 25.Roberts JR, Maxfield M. Examining the Relationship Between Religious and Spiritual Motivation and Worry About Alzheimer’s Disease in Later Life. J Relig Health. 2018;57(6):2500–2514. doi: 10.1007/s10943-018-0635-x. [DOI] [PubMed] [Google Scholar]
- 26.Berkowitz C, Mosconi L, Scheyer O, Rahman A, Hristov H, Isaacson R. Precision Medicine for Alzheimer’s Disease Prevention. Healthcare. 2018;6(3):82. doi: 10.3390/healthcare6030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifan A, Ganzer CA, Vermeylen F, et al. Development and validation of the Alzheimer’s prevention beliefs measure in a multi-ethnic cohort—a behavioral theory approach. J Public Health. Published online January 9, 2017:jphm;fdw145v1. doi:10.1093/pubmed/fdw145 [DOI] [PMC free article] [PubMed]
- 28.Jordan M. Health Coaching for the Underserved. Glob Adv Health Med. 2013;2(3):75–82. doi: 10.7453/gahmj.2013.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruffin L. Health Coaching Strategy to Improve Glycemic Control in African-American Adults with Type 2 Diabetes: An Integrative Review. J Natl Black Nurses Assoc JNBNA. 2017;28(1):54–59. [PubMed] [Google Scholar]
- 30.Nouri SS, Damschroder LJ, Olsen MK, et al. Health Coaching Has Differential Effects on Veterans with Limited Health Literacy and Numeracy: a Secondary Analysis of ACTIVATE. J Gen Intern Med. 2019;34(4):552–558. doi: 10.1007/s11606-019-04861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace AM, Bogard MT, Zbikowski SM. Intrapersonal Variation in Goal Setting and Achievement in Health Coaching: Cross-Sectional Retrospective Analysis. J Med Internet Res. 2018;20(1):e32. doi: 10.2196/jmir.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaffe K, Barnes DE, Rosenberg D, et al. Systematic Multi-Domain Alzheimer’s Risk Reduction Trial (SMARRT): Study Protocol. Anstey K, Peters R, eds. J Alzheimers Dis. 2019;70 (s1):S207–S220. doi:10.3233/JAD-180634 [DOI] [PMC free article] [PubMed]
- 33.Gierisch JM, Hughes JM, Edelman D, et al. The Effectiveness of Health Coaching. Department of Veterans Affairs (US); 2017. Accessed November 29, 2021. http://www.ncbi.nlm.nih.gov/books/NBK487702/ [PubMed]
- 34.Kennel J. Health and Wellness Coaching Improves Weight and Nutrition Behaviors. Am J Lifestyle Med. 2018;12(6):448–450. doi: 10.1177/1559827618792846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ski CF, Vale MJ, Bennett GR, et al. Improving access and equity in reducing cardiovascular risk: the Queensland Health model. Med J Aust. 2015;202(3):148–152. doi: 10.5694/mja14.00575. [DOI] [PubMed] [Google Scholar]
- 36.Losina E, Collins JE, Deshpande BR, et al. Financial Incentives and Health Coaching to Improve Physical Activity Following Total Knee Replacement: A Randomized Controlled Trial. Arthritis Care Res. 2018;70(5):732–740. doi: 10.1002/acr.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiede M, Dwinger S, Herbarth L, Härter M, Dirmaier J. Long-term effectiveness of telephone-based health coaching for heart failure patients: A post-only randomised controlled trial. J Telemed Telecare. 2017;23(8):716–724. doi: 10.1177/1357633X16668436. [DOI] [PubMed] [Google Scholar]
- 38.Jimison HB, Klein KA, Marcoe JL. A socialization intervention in remote health coaching for older adults in the home. In: 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE; 2013:7025–7028. doi:10.1109/EMBC.2013.6611175 [DOI] [PMC free article] [PubMed]
- 39.Almeida OP, Marsh K, Murray K, et al. Reducing depression during the menopausal transition with health coaching: Results from the healthy menopausal transition randomised controlled trial. Maturitas. 2016;92:41–48. doi: 10.1016/j.maturitas.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Thom DH, Willard-Grace R, Tsao S, et al. Randomized Controlled Trial of Health Coaching for Vulnerable Patients with Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2018;15(10):1159–1168. doi: 10.1513/AnnalsATS.201806-365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan VH, Hays MM, Alexander S. Health Coaching for Patients With Type 2 Diabetes Mellitus to Decrease 30-Day Hospital Readmissions. Prof Case Manag. 2019;24(2):76–82. doi: 10.1097/NCM.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 42.Cole S, Zbikowski SM, Renda A, Wallace A, Dobbins JM, Bogard M. Examining Changes in Healthy Days After Health Coaching. Am J Health Promot. 2019;33(5):774–777. doi: 10.1177/0890117118816286. [DOI] [PubMed] [Google Scholar]
- 43.Levy BR, Ferrucci L, Zonderman AB, Slade MD, Troncoso J, Resnick SM. A culture-brain link: Negative age stereotypes predict Alzheimer’s disease biomarkers. Psychol Aging. 2016;31(1):82–88. doi: 10.1037/pag0000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller WR, Rollnick S. The effectiveness and ineffectiveness of complex behavioral interventions: Impact of treatment fidelity. Contemp Clin Trials. 2014;37(2):234–241. doi: 10.1016/j.cct.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Parsons PL, Slattum PW, Bleich M. Mainstreaming health and wellness: The RHWP Innovation model to complement primary care. Nurs Forum (Auckl) 2019;54(2):263–269. doi: 10.1111/nuf.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carpenter BD, Balsis S, Otilingam PG, Hanson PK, Gatz M. The Alzheimer’s Disease Knowledge Scale: Development and Psychometric Properties. The Gerontologist. 2009;49(2):236–247. doi: 10.1093/geront/gnp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bush K. The AUDIT Alcohol Consumption Questions (AUDIT-C)An Effective Brief Screening Test for Problem Drinking. Arch Intern Med. 1998;158(16):1789. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 48.Zelinski EM, Gilewski MJ, Schaie KW. Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychol Aging. 1993;8(2):176–186. doi: 10.1037/0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]
- 49.Reitan RM. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept Mot Skills. 1958;8(3):271–276. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- 50.Kliegel M, Martin M, Jäger T. Development and Validation of the Cognitive Telephone Screening Instrument (COGTEL) for the Assessment of Cognitive Function Across Adulthood. J Psychol. 2007;141(2):147–170. doi: 10.3200/JRLP.141.2.147-172. [DOI] [PubMed] [Google Scholar]
- 51.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barile JP, Horner-Johnson W, Krahn G, et al. Measurement characteristics for two health-related quality of life measures in older adults: The SF-36 and the CDC Healthy Days items. Disabil Health J. 2016;9(4):567–574. doi: 10.1016/j.dhjo.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mannerkorpi K, Hernelid C. Leisure Time Physical Activity Instrument and Physical Activity at Home and Work Instrument. Development, face validity, construct validity and test-retest reliability for subjects with fibromyalgia. Disabil Rehabil. 2005;27(12):695–701. doi: 10.1080/09638280400009063. [DOI] [PubMed] [Google Scholar]
- 54.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 55.Gierveld J de J, van Tilburg T, Dykstra PA. Loneliness and Social Isolation. In: Vangelisti AL, Perlman D, eds. The Cambridge Handbook of Personal Relationships. Cambridge University Press; 2006:485–500. doi:10.1017/CBO9780511606632.027
- 56.Janz NK, Becker MH. The Health Belief Model: A Decade Later. Health Educ Q. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 57.Fisher WA, Fisher JD, Harman J. The Information-Motivation-Behavioral Skills Model: A General Social Psychological Approach to Understanding and Promoting Health Behavior. In: Suls J, Wallston KA, eds. Social Psychological Foundations of Health and Illness. Blackwell Publishing Ltd; 2003:82–106. doi:10.1002/9780470753552.ch4
- 58.Noar SM. A Health Educator’s Guide to Theories of Health Behavior. Int Q Community Health Educ. 2004;24(1):75–92. doi: 10.2190/DALP-3F95-GCT3-M922. [DOI] [PubMed] [Google Scholar]
- 59.Brennan PF, Strombom I. Improving Health Care by Understanding Patient Preferences: The Role of Computer Technology. J Am Med Inform Assoc. 1998;5(3):257–262. doi: 10.1136/jamia.1998.0050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koenigsberg MR, Corliss J. Diabetes Self-Management: Facilitating Lifestyle Change. Am Fam Physician. 2017;96(6):362–370. [PubMed] [Google Scholar]
- 61.Cho YI, Lee SYD, Arozullah AM, Crittenden KS. Effects of health literacy on health status and health service utilization amongst the elderly. Soc Sci Med. 2008;66(8):1809–1816. doi: 10.1016/j.socscimed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Avradinis N, Panayiotopoulos T, Anastassakis G. Behavior believability in virtual worlds: agents acting when they need to. SpringerPlus. 2013;2(1):246. doi: 10.1186/2193-1801-2-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rigotti NA, Chang Y, Regan S, et al. Cigarette Smoking and Risk Perceptions During the COVID-19 Pandemic Reported by Recently Hospitalized Participants in a Smoking Cessation Trial. J Gen Intern Med. Published online June 7, 2021. doi:10.1007/s11606-021-06913-3 [DOI] [PMC free article] [PubMed]
- 64.Perrin, A., Atske, S. 7% of Americans Don’t Use the Internet. Who Are They? Pew Research Center. https://www.pewresearch.org/fact-tank/2021/04/02/7-of-americans-dont-use-the-internet-who-are-they/


