Abstract
Intervertebral disc (IVD) herniation and degeneration contributes significantly to low back pain (LBP), of which the molecular pathogenesis is not fully understood. Disc herniation may cause LBP and radicular pain, but not all LBP patients have disc herniation. Degenerated discs could be the source of pain, but not all degenerated discs are symptomatic. We previously found that disc degeneration and herniation accompanied by inflammation. We further found that anti‐inflammatory molecules blocked immune responses, alleviated IVD degeneration and pain. Based on our recent findings and the work of others, we hypothesize that immune system may play a prominent role in the production of disc herniation or disc degeneration associated pain. While the nucleus pulposus (NP) is an immune‐privileged organ, the damage of the physical barrier between NP and systemic circulation, or the innervation and vascularization of the degenerated NP, on one hand exposes NP as a foreign antigen to immune system, and on the other hand presents compression on the nerve root or dorsal root ganglion (DRG), which both elicit immune responses induced by immune cells and their mediators. The inflammation can remain for a long time at remote distance, with various types of cytokines and immune cells involved in this pain‐inducing process. In this review, we aim to revisit the autoimmunity of the NP, immune cell infiltration after break of physical barrier, the inflammatory activities in the DRG and the generation of pain. We also summarize the involvement of immune system, including immune cells and cytokines, in degenerated or herniated IVDs and affected DRG.
Keywords: cytokines, degeneration, herniation, immune, inflammation, intervertebral disc, pain
1. INTRODUCTION
Low back pain (LBP) is a common symptom 1 affecting approximately 40% of the population worldwide, 2 producing a significant burden on society and the medical system. 3 Lumbar disc herniation (LDH) is the major cause of radicular pain, 4 which radiates into the lower extremity directly along the course of a spinal nerve root. In addition to LDH, nonherniated degenerating intervertebral discs (IVDs) can cause radicular pain in some patients. 5 The protrusion or extrusion of anIVDleadingto contact with or the compression of anerve root or dorsal root ganglion (DRG) is a common cause of LBP with or without sciatica. However, evolving evidence has demonstrated that disc herniation‐induced radicular pain may persist even after surgical interventions. Starkweather et al. 6 studied neural‐immune interactions in patients with LBP and sciatica. They suggested that the neuroimmune system was activated during disc herniation‐induced radicular pain and that activated immune cells release proinflammatory cytokines, which signal the brain through humoral and neural routes, resulting in pain and functional changes in neural activity. LDH also contributes to LBP by playing a role in spinal stenosis 7 or acting as a primary source of discogenic LBP, 8 , 9 , 10 which is defined as chronic LBP induced by degenerative disc disease.
However, not all LBP patients have obviousdisc protrusion or nerve root compression. In somepatients, the severity of pain is not related to the degree of nerve compression. 11 In addition to the mechanical compression of the nerve root or DRG, emerging evidence suggests that the degenerated disc itself could be a source of LBP. IVD degeneration starts from 10 years of age, when the number of notochordal cells drops to below detectable levels in the human NP, and develops with aging. 12 IVD degeneration is closely associated with LBP, especially discogenic LBP. 13 , 14 However, not all degenerated discs are painful. Some people have degenerated discs without any signs of LBP. The reason this difference has not yet been fully revealed. We previously found that disc degeneration is accompanied by inflammation 15 , 16 , 17 and fibrotic changes 18 , 19 as a result of chronic inflammation. We further found that anti‐inflammatory molecules, such as LIM mineralization protein‐1, 20 transforming growth factor‐β (TGF‐β), 21 , 22 or Wnt5a, 23 can suppress C‐C motif chemokine 4 (CCL4) expression and impede tumor necrosis factor‐α (TNF‐α)‐activated immune cascades, while melatonin can disrupt interleukin‐1β (IL‐1β) signaling, 24 , 25 thus alleviating IVD degeneration and pain. Therefore, we hypothesize that inflammation may be the key difference between symptomatic and asymptomatic IVD degeneration. 26
Since Naylar et al. 27 proposed the autoimmunity of IVDs in 1975, meaning that cells in IVDs will be recognized by the immune system as foreign antigens and elicit immune reactions, in the past few decades, studies about the relationship between autoimmunity and disc degeneration and LBP have received growing attention. A number of studies have focused on the role of molecular immunology and the immune‐related inflammatory response in LBP. 28 Currently, it is of practical significance to fully understand the relationship between the immune response and inflammatory factors and the role of molecular immunology in the process of LBP in the hope of designing effective biological treatments for disc degeneration and LBP. Based on this, we review the current literature related to the natural structure of the discs and the involvement and roles of immune cells and cytokines in pain production to highlight the necessity to treat against pain progression in IVD degeneration and to facilitate future studies on and clinical applicationsfor disc regeneration.
2. STRUCTURE AND IMMUNE PRIVILEGE OF THE NUCLEUS PULPOSUS
The IVD is composed of the annulus fibrosus (AF), nucleus pulposus (NP), and cartilaginous endplate (EP) adjacent to vertebral bodies (Figure 1). The main components of the NP are NP cells and a gelatinous extracellular matrix that keeps the NP moistured. In the maturated disc, the NPis wrapped by the outer AF and covered by the upper and lower EPs. Under healthy conditions, there are neither blood vessels nor nerve cells in the NP. This unique architecture makes the NP exempt from the development of immunological tolerance during fetal development and an immune‐privileged organ with no access to the systemic circulation, similar to other immune‐privileged organs, such as the nails, eyes, and brain. 29 , 30 For this reason, immune cells or inflammatory cytokines have not been found in healthy NP. In addition to the physical barriers, recent studies have found that a variety of molecular biological mechanisms are also involved in the maintenance of immune privilege. 31 For example, Fas ligand (FasL), which is predominantly expressed in the activated T lymphocytes of immune‐privileged sites, could induce the apoptosis of Fas‐expressing T lymphocytes and macrophages. FasL has been noted to be expressed in healthy NP and thus may play an important role in the maintenance of NP immune privilege. 32 , 33
FIGURE 1.
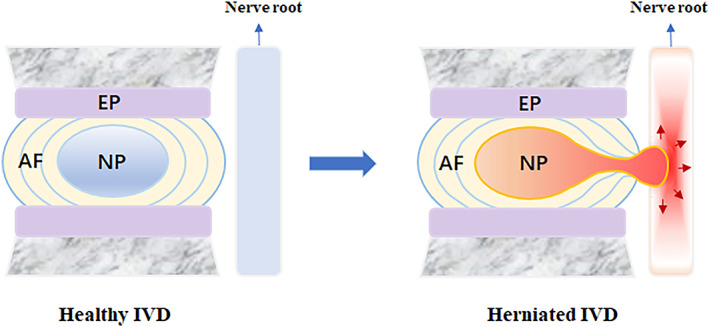
Structure of a healthy IVD and herniated IVD. In the case of disc herniation, the protruding disc may compress the concomitant spinal nerve, sensitizing the peripheral neurons in the DRG, eliciting the secondary immune response and finally generate pain. AF, annulus fibrosus; DRG, dorsal root ganglion; NP, nucleus pulposus
3. BROKEN PHYSICAL BARRIERS LEAD TO IMMUNE CELL INFILTRATION
Degenerated IVDs, ruptured AF and extruded NP are the basic pathological anatomies of disc herniation (Figure 1). Disc herniation can be caused by abnormal mechanical overloading 34 and trauma 35 , 36 and is closely associated with IVD degeneration. 37 , 38 When adisc is herniated, the physical barrier between the IVD and the immune system becomes damaged, which exposes the NP to the immune system. Then, the systemic immune system will recognize the “immune‐privileged” NP as a “foreign antigen” andinduce the initial immune response (primary response). In the subsequent stage, with the repair process of the damaged NP and AF, granulation tissue will form, followed by the ingrowth of blood vessels, further exposing NP tissues to immune cells in the bloodstream. In this case, some of the NP matrix is recognized as an autoantigen, which elicits the secondary immune response, which is mediated by cytotoxic T cells. In addition, nondegenerated NP cells strongly express FasL, which can lead to the apoptosis of infiltrated Fas‐positive cytotoxic T lymphocytes. However, FasL expression significantly decreases in degenerated NP cells, 32 which weakens the ability to clear T lymphocytes 39 , 40 and further destroys the intradiscal environment.
Several studies have found autoantibodies in degenerated NP. The team of Capossela 41 found a specific immunoglobulin G (IgG) antibody persisting in degenerated or injured discs, and this IgG antibody was reactive to the matrix proteins in the NP, especially to collagen II and aggrecan, suggesting that such antibodies were one of the factors contributing toIVD degeneration. The research of Mihn 42 also confirmed that autoantibodies were significantly higher in human degenerative IVDs than in nondegenerated IVDs. The detection of a humoral response, represented by the immunopositivity to factor VIII and IgG 43 and the number of immune cells, 44 was much higher in sequestered IVDs than protruding IVDs, indicating that the immune reactions against IVDs are the consequence, but not the initiating cause, of disc herniation.
4. DRG COMPRESSION BY OR IN CONTACT WITH THE NP INDUCES PAIN
Disc herniation may cause radicular pain, which is induced by pathological changes in the nerve root or DRG after direct contact with or compression by a herniated disc. The DRG is the primary processing center of pain generation and transmission. Traditionally, mechanical compression has been thought to serve as theprimary factor that leads to ischemia, edema, or demyelination in the DRG, which sufficiently induces spontaneous pain that may arise from abnormal production of proinflammatory molecules secreted by both AF and NP cells. 45 , 46 In the case of disc herniation, the protruding disc may compress the concomitant spinal nerve, sensitizing the peripheral neurons in the DRG and leading to pain. 47
In addition, evidence suggests that NP tissue could cause excitatory changes in the DRG even in the absence of mechanical compression. 48 , 49 Takebayashi et al. 50 used neurophysiological techniques in a rat model in vivo to investigate the role of the DRG in radicular pain in LDH. They found that after the application of NP tissue to the nerve root, the DRG demonstrated increased excitability and mechanical hypersensitivity when compared to the control group with the application of fat to the nerve root. Likewise, the application of NP tissue to the nerve root without compression could increase endoneurial fluid pressure and decrease blood flow in the dorsal root ganglia, which was closely related to the subsequent immune and inflammatory reactions. 51
Neural‐immune interactions play a crucial role in the pain‐producing process, 52 with the participation of immune cells, chemokines, and cytokines. Under healthy conditions, macrophages and a small number of T lymphocytes and satellite glial cells reside in the DRG. DeLeo et al. 46 demonstrated that satellite glial cells in DRG were activated by the immune system within 24 h, and local macrophages in DRG were activated approximately 1 week later under the influence of activated glial cells. Furthermore, satellite glial cells and macrophages together release mediators such as histamine, prostaglandins, cytokines, and chemokines, which in turn aggregate the infiltration of other immune cells, including neutrophils, macrophages, and lymphocytes. 53 This reaction reached its peak in approximately 3 weeks and could last for several months. DeLeo 46 and Moalem 54 found that even after the exposed NP was removed or absorbed, the pain continued. This suggests that as a result of a cascade of neural‐immune responses, the systemic immune reactions are not mitigated even after the stimulus is removed.
Disc degeneration or herniation could lead to increased inflammatory activities in the DRG. In a rat disc degeneration model, nuclear factor kappa B and cyclooxygenase 2 (COX‐2) levels were increased in the DRG to the left and/or right ofthe disc. 55 In a rabbit model of torsional injury, a significant increase in most DRG neurotransmitter values was observed 60–90 days later. 56 TNF‐α injectionin rat discs also led to increased substance P in DRG. 57 In a rat disc herniation model, the M1 macrophage markers chemokine ligand 3 (CCL3) and CD86 markedly increased on Day 14 after the surgery and decreased on Day 28 compared to very low expression in naive DRG. 58 In contrast, the M2 macrophage markers arginase 1 (Arg1) and CD206 were markedly increased on Day 28 compared to their low expression in naive DRG. 58
In addition, activated DRG can release inflammatory cytokines that affect remote uninjured DRG. For example, activated DRG neurons release monocyte chemoattractant protein‐1 (MCP‐1) after peripheral nerve injury. 59 Axonal damage in rats significantly increased the activation of genes expressed by immune and inflammatory cells, as revealed by oligonucleotide microarray analysis. 60 These factors secreted by the compressed or injured neurons can affect the uninjured DRG over a long distance. In a neuropathic pain model, both compressed and noncompressed DRG neurons showed increased CCR2 ligand and MCP‐1 expression by Day 5. 61
5. DEGENERATED NP ITSELF CONTRIBUTES TO DISCOGENIC PAIN
In addition to radicular pain, the degenerated disc itself can contribute to LBP. Inherently, the native attempt to address IVD injury or tears is through the process of vascularization and innervation into the disc. In healthy discs, nerve fibers, which include perivascular nerves, sensory nerves independent of blood vessels, and mechanoreceptors, appeared only on the surface of the AF. 62 Healthy discs have no substance P‐expressing nerve fibers in the inner AF and NP, which were detected only in degenerated discs. 62 , 63 The invasion of nerves may be the consequence of increased nerve growth factor expression during degeneration. 64 Since these nerve fibers are unmyelinated and use substance P as the neurotransmitter, their appearance is closely associated with pain. In healthy discs, the NP is avascular, while the EPs and AF have blood supplies in early life that diminish with aging. 65 Studies have shown that the density of blood vessels and nerves is positively associated with the degree of the degeneration of the discs. 66 Genome‐wide analysis has revealed that the expression of well‐recognized nerve‐related genes is much higher in degenerated human AF, accompanied by increased expression of proinflammatory cytokine‐ and chemokine‐related genes. 67 In degenerated and herniated discs, blood vessels and nerves were mostly localized indisrupted tissues with local proteoglycan loss. 66 In addition, the vascularization of the inner AF or NP creates conditions for immune cell infiltration, further deteriorating the situation.
6. IMMUNE CELLS INVOLVED IN IVD DEGENERATION
In this section, we revisit the findings about immune cells that have been found to be involved in disc degeneration or herniation, which include macrophages, T cells, B cells, and NK cells.
6.1. Macrophages
Macrophages can play a role in immune defense by phagocytosing bacteria or cell debris. In addition, macrophages also play an important role in immune reactions by secreting cytokines that can regulate the immune response. The detection of macrophages in degenerated or herniated discs has been reported in human and animal models. The infiltration of macrophages could occur in mouse IVDs at Days 1–4 afterdisc injury. 68 , 69 In human herniated discs, macrophages were detected in 37% of all 205 specimens. 44 In 25% of protruded human IVDs, macrophages, but no other inflammatory cells, were found. 44 In a rat NP explant study, the infiltration of macrophages into nondegenerated NP transplanted under the abdominal skin was detectable. 70 Moreover, a significantly higher NP cell survival rate was found when the recipient was immunedeficient rather than wild‐type. 70 Furthermore, the function of macrophages in herniated discs is different from that of macrophages in nonherniated discs. 71 While the main function of the former was to promote the reabsorption of prominent tissue and participate in the process of blood vessel ingrowth, the latter's main function was to remove the necrotic tissue and secrete inflammatory cytokines. 71 For example, in the IVDs of patients with discogenic pain, macrophages release a variety of inflammatory cytokines (such as IL‐1, IL‐6, and TNF‐α). 72 In vitro, the coculture of NP cells with macrophages promoted the expression of TNF‐α, IL‐6, IL‐8, and COX‐2. 73 These inflammatory cytokines have a significant effect on inducing hyperalgesia and are the most likely to be involved in the occurrence of discogenic pain.
6.2. T cells
The presence of T cells has been reported in degenerated or herniated discs. In a human disc study, abundant activated T cells were detected in 17% of all 205 herniated discs. 44 In widely used TNF‐α transgenic mice with evidenced spontaneous annular tears and disc herniation, neutrophil, macrophage, and mast cell infiltration was found in extruded discs, whereas the additional presence of CD4+ and CD8+ T cells was found in pronounced herniated discs. 74 In rat herniated NP, the number of Th1 cells was greatly increased on Day 14 but decreased on Day 28, while the number of Th2 cells was increased on Day 28. 58 In a porcine study, the proportion of activated T cells (CD4+ and CD8+) was significantly higher in the exudate of the perforated titanium chamber containing nondegenerated porcine NP explantsthan in that of empty chambers. 75 Geiss et al. 76 further found in porcine models that 3 weeks after the exposure of autologous NP to the systemic immune system, T lymphocytes were primed into IL‐4‐producing CD4+ Th2 cells and promoted the autoimmune response in the disc through released IL and TNF‐α, leading to the occurrence of pain. The infiltration of T lymphocytes was detectable at Day 3 and reached the reaction peak at approximately Day 21. 76
6.3. B cells
The presence of B cells has been reported in human discs. In Virri's study, B cells were detected in 16% of all the examined human herniated discs. 44 In an NP explant study that placed nondegenerated porcine NP in perforated titanium chambers subcutaneously in recipient pigs, the proportion of immunoglobulin kappa‐expressing activated B cells was significantly increased in the exudate of the NP‐filled chambers compared to empty chambers. 75
6.4. NK cells
The detection of NK cells in herniated or degenerated discsis rarely reported. We found only one study, in which Murai et al. reported the infiltration of NK cells into the nondegenerated NP transplanted under the abdominal skin of recipient rats. 70
7. IMMUNE CYTOKINES INVOLVED IN IVD DEGENERATION/HERNIATION
Cytokines are small proteins or peptides constitutively expressed on the cell surface in precursor forms. They are synthesized and secreted by immune or other types of cells and participate in immune activation and inflammatory reactions. Currently, cytokines are classified into two opposing categories, as demonstrated in Table 1. One category is proinflammatory cytokines, including IL‐1β, TNF‐α, IL‐6, and interferon‐gamma (IFN‐γ). The other category is anti‐inflammatory cytokines, such as IL‐4, IL‐10, and TGF‐β. To date, various cytokines have been identified in degenerated IVDs. 77 Wang et al. 78 found that treatment with TNF‐α or IL‐1β led to increased secretion of CCL3, but not CCL4, in degenerated NP cells, which in turn promoted macrophage infiltration that could be blocked by an antagonist of its ligand CCR1. In this section, we summarize the immune cytokines related to IVD degeneration/herniation and discuss how they may be involved in pain production.
TABLE 1.
Proinflammatory and anti‐inflammatory cytokines in discogenic pain
| Cytokines | Category | Primary source | Function in neuroimmunologic pain | Expression during IVD degeneration |
|---|---|---|---|---|
| TNF‐α | Proinflammatory | Schwann cells, macrophages, mast cells, and neutrophils | Sensitize and enhance the excitability of neurons; promote sustained inflammatory response | Increased |
| IL‐1β | Proinflammatory | Macrophages, monocytes, dendritic cells | Increase excitability of neurons | Increased |
| IL‐6 | Proinflammatory | Mast cells, macrophages, lymphocytes, neurons, and glial cells | Decrease thermal activation and pain threshold; increase excitability of neurons | Increased |
| IFN‐γ | Proinflammatory | Th1 cells; astrocytes and damaged neurons | Induce spontaneous pain and pain hypersensitivity | Increased |
| IL‐10 | Anti‐inflammatory | T cells, B cells, macrophages, and mast cells | Inhibit the release IL‐1β, IL‐6, and TNF‐α | Reduced |
| TGF‐β | Anti‐inflammatory | Activated T cells and B cells | Inhibit proinflammatory cytokine (IL‐1β, IL‐6, and TNF‐α) release and promote expression of endogenous opioids | Increased |
| IL‐4 | Anti‐inflammatory | T cells, mast cells, and granulocytes | Suppress the expression of IL‐1β, IL‐6, and TNF‐α | Increased |
Abbreviations: IFN‐γ, interferon‐gamma; IL, interleukin; TGF‐β, transforming growth factor‐β; TNF‐α, tumor necrosis factor‐α.
7.1. TNF‐α
TNF‐α is recognized as a major proinflammatory cytokine. It is also known as a pain‐inducing factor, with the ability to promote a cascade of immune reactions and cytokine production. Patients with degenerated IVDs showed elevated TNF‐α levels in IVDs and peripheral serum. 79 TNF‐α overexpression or treatment led to spontaneous IVD herniation 80 and COX‐2 expression, 81 while TNF‐α inhibition at the time of IVD puncture limited degeneration and pain in animal models. 82 In animal models, changes in neuronal properties can be caused by topical application of TNF‐α to the nerve root and DRG, which decreased the pain threshold required to activate nerve C‐fibers. 83 At the initial stage of injury, macrophages, mast cells, and glial cells in the DRG‐released endogenous TNF‐α to induce a rapid immune response, leading to a subsequent cascade of inflammation. 84 In the next 3–5 days, immune cells (macrophages, neutrophils) infiltrated from the circulation released additional TNF‐α, forming a positive feedback loop to promote immune inflammation and decrease pain thresholds. 85 Then, a systemic immune response was initiated, and the expression of TNF‐α in remote DRG was also increased in distant areas of the body. 86 In summary, TNF‐α is mainly released by immune cells around neurons and immune glial cells, which can sensitize and enhance the excitability of neurons and promote a sustained inflammatory response at various levels of the nervous system. However, TNF‐α is required for the production of IL‐6 and prostaglandin E2 (PGE2), an inflammatory mediator to induce pain and enhance pain sensitivity, but not for IL‐8 production 73 in IVD autografts; therefore, TNF‐α is not the sole cytokine that initiates all immune reactions in the disc.
7.2. IL‐1β
IL‐1 is secreted by a variety of immune cells or immune‐like glial cells, including macrophages, monocytes, and dendritic cells. Global IL‐1α/β knockout in mice resulted in a more degenerative phenotype in the AF and changes in collagen type and maturity, accompanied by alterations in systemic cytokine levels and vertebral bone morphology. 87 Studies have shown that IL‐1β expression was correlated with, or upregulated, the expression of chemokines, such as CCL5, 88 CCL3, and CCL4, 89 indicating that IL‐1β can activate monocytes‐macrophages and aggravate inflammatory cell infiltration. Similar to TNF‐α, IL‐1β has also been demonstrated to increase the excitability of neurons. DRG neurons are susceptible to IL‐1β, with a short period of application resulting in the potentiation of heat‐activated inward currents and a shift of activation thresholds toward lower temperature. 90 When NP cells isolated from herniated discs were stimulated with IL‐1β, a significant increase in the production of PGE2 was observed. 91 In addition, IL‐1 can also elevate the expression level of intercellular cell adhesion molecule‐1, which has a chemotactic effect on promoting the recruitment of inflammatory cells, leading to neuropathic pain. 92 However, IL‐1β differs from TNF‐α in that IL‐1β, but not TNF‐α, stimulates matrix degradation. 93 Taken together, the evidence for IL‐1β in enhancing synaptic transmission and neuronal activity at several locations of the nervous system is strong, suggesting its prominent role in inflammatory cascades.
7.3. IL‐6
IL‐6 is a proinflammatory cytokine mainly released by mast cells, macrophages, lymphocytes (activated T cells and B cells), neurons, and glial cells. Patients with degenerated IVDs had increased levels of IL‐6 in serum 94 as well as in IVDs. 95 IL‐1β/TNF‐α stimulated IL‐6 levels in cultured human AF cells. 96 Recently, Sainoh et al. 97 found that the injection of IL‐6 receptor antibody reduced pain in LBP patients. Moreover, IL‐6 is also an effective serum marker of LBP. Weber investigated the serum levels of various cytokines in LBP patients. 98 Among IL‐1β, IL‐2, IL‐4, IL‐6, IL‐8, IL‐10, IL‐12p70, IL‐13, IFN‐γ, TNF‐α, matrix metalloproteinase (MMP)‐1, MMP‐3, and MMP‐9, only IL‐6 showed significantly higher serum levels in LBP subjects than in control subjects. 98 Haddadiet al. found that IL‐6 serum levels were greatly reduced in LDH patients with radicular pain after lumbar disc surgery. 99 All these findings suggest that IL‐6 is a strong pain indicator in discogenic LBP. However, the role of IL‐6 in modulating acute pain is less clear than that of TNF‐α and IL‐1β. For instance, IL‐6 did not show an effect on themechanical threshold 100 or thermal hypoalgesia. 101 It was not until Obreja et al. 102 found that the application of IL‐6 in vitro in combination with its soluble receptor directly potentiated heat‐activated inward currents in cultured DRG neurons and resulted in a decreased thermal activation threshold that the association of IL‐6 with discogenic pain was realized. IL‐6 can induce the aggregation of inflammatory cells, activate the release of inflammatory mediators, and promote the process of IVD degeneration. Brazda et al. 103 used sciatic nerve ligature to investigate temporal changes in IL‐6 and its receptor gp130 in both ipsilateral and contralateral DRG in rats. They found increased IL‐6 expression not only in the DRG associated with the damaged nerve but also in those not associated with nerve injury in the experimental neuropathic pain model. 103 Furthermore, the research of Koerner 104 provided evidence that substance P led to the activation of the inflammatory pathway by increasing IL‐6 expression, suggesting that IL‐6 may be an important link between IVD degeneration and LBP.
7.4. IFN‐γ
IFN‐γ is released by Th1 cells, which infiltrate into damaged neurons. IFN‐γ has been implicated in many chronic pain states, including neuropathic pain. 105 The application of IFN‐γ can cause the spontaneous firing of dorsal horn neurons in vivo and increase the response to stimulation. 106 Luchting et al. 107 investigated the systemic T cell subset responses and profiles of T cell‐related cytokines, such as macrophage inflammatory protein‐1α, TNF‐α, IFN‐γ, and IL‐4, in patients with chronic neuropathic pain. They found that T cell subsets and their related cytokines played a role in anti‐inflammation. IFN‐γ appears to induce central sensitization by several mechanisms and is a potent proinflammatory cytokine implicated in the pathogenesis of neuropathic pain. In a study of the application of NP tissue to spinal dorsal nerve roots, the level of IFN‐γ in exposed NP tissue was increased relative to native tissue, and a positive correlation between IFN‐γ and the macrophage marker CD68 in NP tissue was found. 108 The serum level of IFN‐γ in LBP patients is not significantly different from that in healthy controls. 98 Interestingly, IFN‐γ antibodies could prevent the elevation of IL‐6 in NP exposed to DRG, 109 indicating a role of IFN‐γ in IL‐6 signaling.
7.5. IL‐10
IL‐10 is known as an anti‐inflammatory cytokine. IL‐10 is released by activated T cells, B cells, macrophages, and mast cells. 110 Reduced IL‐10 expression was found in rat disc degeneration models. 111 IL‐10 treatment suppresses the expression of IL‐1β and TNF‐α as a consequence of the impeded development of inflammatory responses. 112 Moreover, serum levels of IL‐10 are reported to be lower in LBP patients than controls. 113 Zhou et al. 114 demonstrated that IL‐10 led to a reduction in pain sensitivity in the spinal dorsal horn induced by formalin injection. Following injury to the sciatic nerve and DRG in a mouse model, the expression level of IL‐10 was increased. 115 Taken together, increasing the expression of IL‐10 by gene therapy or drugs may result insubstantial inhibitory effects on acute disc‐related pain.
7.6. TGF‐β
TGF‐β is mainly produced by activated T cells and B cells. TGF‐β is mainly regarded as an anti‐inflammatory cytokine with a wide variety of functions, including promoting cell survival, inhibiting apoptosis, stimulating cell proliferation or inducing cell differentiation. 116 For example, TGF‐β can downregulate the TNF‐α expression induced by IFN‐γ and IL‐1β, antagonizethe MMP3 expression induced by TNF‐α, 21 downregulate CCL4 expression and reduce pain behavior in rats. 22 TGF‐β1 was reported to be upregulated in NP tissues of patients and rats with IDD. 117 In a rat model, intradiscal TGF‐β1 injection prevented the inflammatory response in DRG and pain development. 22 Similar to IL‐10, TGF‐β treatment suppressed the expression of IL‐1β and TNF‐α and inhibited the development of inflammatory responses in degenerated IVD cells. 112 In degenerated IVDs, when combined with carboxymethylcellulose as a scaffold, TGF‐β3 stimulated IVD cell proliferation and extracellular matrix production in vitro. 118 In a model of neuropathy, TGF‐β significantly attenuated the development of pain hypersensitivity and reversed previously established pain. 119 In both the glial cells and the neurons of DRG, TGF‐β suppressed their activation and proliferation, inhibited proinflammatory cytokine release, and reduced sensitivity to pain. 120
7.7. IL‐4
IL‐4 is an anti‐inflammatory cytokine thatis released by activated T cells, mast cells and granulocytes. IL‐4 is known as an inhibitor of IL‐1β, IL‐6, and TNF‐α. IL‐4 can stimulate the activation of B cells, promote T cells to differentiate into the Th2 phenotype, and suppress the activation of macrophages. IL‐4 was virtually nonexistent in healthy discs, while the immunoreactivity was increased in degenerated and herniated IVD tissue. 121 A meta‐analysis found significantly more IL‐4 expression in the IVDs but not in the blood samples of IDD patients. 122 IL‐4 treatment downregulated LPS‐stimulated inflammatory responses, including the production of IFN‐β, IL‐12, IL‐6, and IL‐8 in IVD cells. 123 Similarly, the overexpression of IL‐4 in vivo 124 suppressed c‐Fos immunoreactivity in the dorsal horn of the spinal cord and impededthe upregulation of spinal PGE2, IL‐1β, and phosphorylated‐p38 MAP kinase. Further investigation would be desirable to elucidate therole of IL‐4 in IVD degeneration and herniation.
7.8. IL‐8
IL‐8 is also known as chemokine CXCL8. IL‐8 is a cytokine secreted by macrophages and epithelial cells. IL‐1β/TNF‐α stimulation enhanced the production of IL‐8 from cultured human AF cells. 96 In a trauma model induced by overloading, injured human IVDs with broken EPs secreted increased IL‐8. 125 Among IL‐8, TNF‐α, and IL‐1α, which are strongly expressed in human degenerated disc tissues, IL‐8 had the strongest association with pain scores. 126 In the cerebrospinal fluid of chronic LBP patients with disc degeneration, IL‐8 was elevated compared to that in pain‐free subjects with or without disc degeneration. 127 In the injured sciatic nerve, a significant increase in IL‐8 was observed following partial sciatic ligation. 128 Furthermore, anti‐IL‐8 antibodies can reduce the release of nerve growth factor. 129 Together, these results indicate that the suppression of IL‐8 may be beneficial for relieving disc‐associated pain.
8. CONCLUSION
Even though there is growing evidence for immune and glial cells and their mediators playingan important role in maintaining the immune privilege status of the NP, as well as being involved in the pathogenesis of IVD degeneration, the complex interactions of these participants remain unclear. Understanding the role of the immune system in disc‐related pain may lead to a better appreciation of the nature of pain and therapeutic approaches.
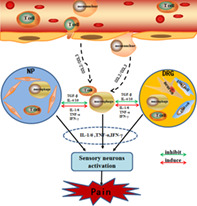
In this review, we discuss possible immune events during disc herniation and degeneration. The summary of the procedure is illustratedin Figure 2. In brief, the NP is an immune‐privileged tissue protected from immune tolerance during fetal development due to its avascular nature. At times of trauma, long‐term abnormal loading or gradual disc degeneration occurs when the AF ring is weakened, the AF is ruptured and the NP leaks out. The protruded NP may compress the DRG and activate the macrophages, T lymphocytes and glial cells in the DRG, which secrete chemokines to attract more immune cells and release more inflammatory cytokines, leading to further inflammation and radicular pain. At the same time, the systemic circulation recognizes the NP as a foreign antigen and initiates an immune reaction to attack it. As a native attempt to repair injured tissue, nerves and blood vessels, which are distributed in the outer AF under healthy circumstances, grow into the inner AF or even the NP. Thus, immune cells can directly contact the NP during disc herniation or infiltrate into the NP during disc degeneration, probably through the established vascularization, and react with the NP to produce autoantibodies, elicit immune reactions and release cytokines to amplify inflammation, which act on the invaded nerve, resulting in local pain or referred pain caused by sinuvertebral nerve irritation. However, this theory may not explain all typesof disc‐related pain observed in the clinic, as in some patients with discogenic pain, no sign of neurovascular ingrowth in the NP can be observed.
FIGURE 2.
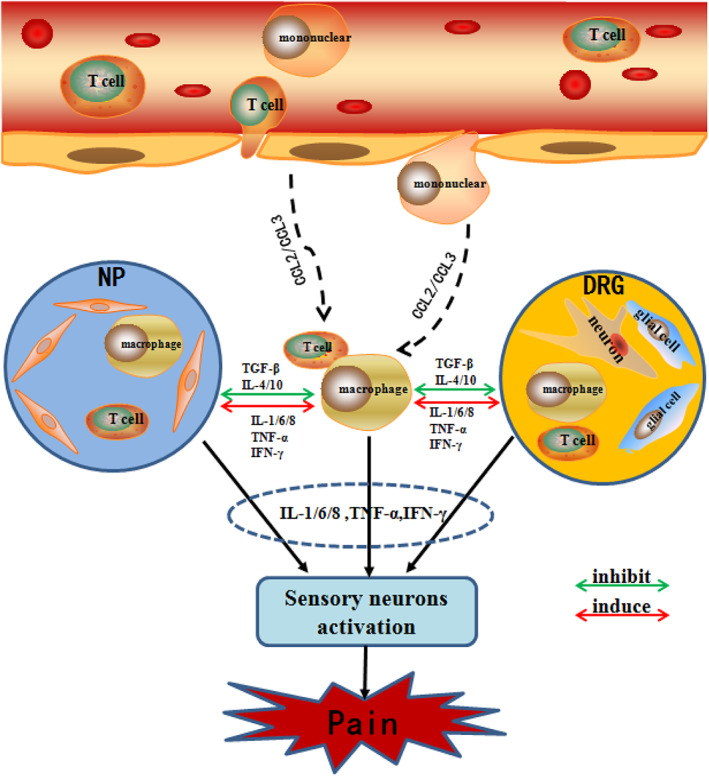
Schematic diagram demonstrates immune cascades in disc‐related pain producing. When the protruding nucleus pulposus tissue breaks through the immune barrier and is recognized by the immune system, the immune cells in the blood circulation (such as T cells and macrophages) are activated to release ; (CCL2/CCL3), and more immune cells in the blood (such as T cells and macrophages) are activated and aggregated toward NP and DRG. Simultaneously, the release of inflammatory mediators (TNF‐α, IFN‐γ, IL‐1/6, etc.) and inhibitory mediators (TGF‐β, IL‐4/10, etc.) activates the immune cells (such as T cells and macrophages) in NP and DRG tissues. Eventually, both immune cells from different sources jointly release inflammatory cytokines (IL‐1/6, TNF‐α, IFN‐γ, etc.) to activate sensory neurons and produce pain. IFN‐γ, interferon‐gamma; IL, interleukin; TGF‐β, transforming growth factor‐β; TNF‐α, tumor necrosis factor‐α
The administration of anti‐inflammatory drugs may help to dampen immune reactions and alleviate disc degeneration. As Kim reported, 130 the inhibition of IL‐1 by lactoferricin can deliver anti‐inflammatory and anticatabolic effects in culture models. We previously found that Wnt5a can inhibit TNF‐α‐induced inflammatory signaling and suppress IVD degeneration. 23 Nevertheless, caution should be taken when designing anti‐inflammatory therapies, since studies have shown that although the silencing of key proinflammatory cytokines may reduce inflammatory reactions in vivo, it does not always indicate a less degenerated IVD as a result. For example, IL‐1 knockout in mice resulted in reduced serum concentrations of inflammatory cytokines during agingcompared to those in wild‐type mice. 87 However, rather than protecting the animals from degeneration, IL‐1 knockout mice exhibited a more degenerated phenotype, represented by a less stable AF and smaller NP with alterations in collagen type and maturity. 87 This finding may indicate that the absolute absence of IL‐1 is not beneficial to disc development and, thus, that although a dampened immune reaction may be beneficial to patients, the dose and administration protocol of the drugs may matter and should be carefully designed to achieve the desired effect.
Many studies are underway to design regenerative strategies for discs, especially with mesenchymal stem cells as a tool. 131 , 132 In addition to their potential to give rise to NP‐like cells, 133 mesenchymal stem cells also have anti‐inflammatory and immunomodulatory effects, which may suppress inflammation in the disc. 134 However, the impact of the inflammatory condition inside the disc on the survival and function of these cells should be taken into consideration to maximize the effect. In addition, endogenous progenitor cells were recently identified in all three compartments of the IVD, 135 , 136 highlighting a novel cell source for disc repair. Knowledge on how these progenitor cells may help the herniated or degenerated discs to repair and how they react to the inflammatory microenvironment awaits further research.
CONFLICT OF INTEREST
The authors declare no conflic of interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (82072490; 81572175, 81772386), Natural Science Foundation of Fujian Province by Fujian Science and Technology Department (2021 J01394), Guangdong‐Hong Kong‐Macao Science and Technology Cooperation Project by Guangdong Science and Technology Department (2017A050506019), the Natural Science Foundation of Guangdong Province (2020A1515011031).
Ye, F. , Lyu, F.‐J. , Wang, H. , & Zheng, Z. (2022). The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine, 5(1), e1196. 10.1002/jsp2.1196
Fubiao Ye and Feng‐Juan Lyu contributed equally to this work and shared first authorship.
Funding information Natural Science Foundation of Guangdong Province, Grant/Award Number: 2020A1515011031; Guangdong‐Hong Kong‐Macao Science and Technology Cooperation Project, Grant/Award Number: 2017A050506019; Natural Science Foundation of Fujian Province by Fujian Science and Technology Department, Grant/Award Number: 2021 J01394; National Natural Science Foundation of China, Grant/Award Numbers: 82072490, 81772386, 81572175
REFERENCES
- 1. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356‐2367. [DOI] [PubMed] [Google Scholar]
- 2. Buchbinder R, van Tulder M, Öberg B, et al. Low back pain: a call for action. Lancet. 2018;391:2384‐2388. [DOI] [PubMed] [Google Scholar]
- 3. Maher C, Underwood M, Buchbinder R. Non‐specific low back pain. Lancet. 2017;389:736‐747. [DOI] [PubMed] [Google Scholar]
- 4. Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine. 2008;33:2464‐2472. [DOI] [PubMed] [Google Scholar]
- 5. Centeno C, Markle J, Dodson E, et al. Treatment of lumbar degenerative disc disease‐associated radicular pain with culture‐expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med. 2017;15:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Starkweather A, Witek‐Janusek L, Mathews HL. Neural‐immune interactions: implications for pain management in patients with low‐back pain and sciatica. Biol Res Nurs. 2005;6:196‐206. [DOI] [PubMed] [Google Scholar]
- 7. Murata K, Akeda K, Takegami N, Cheng K, Masuda K, Sudo A. Morphology of intervertebral disc ruptures evaluated by vacuum phenomenon using multi‐detector computed tomography: association with lumbar disc degeneration and canal stenosis. BMC Musculoskelet Disord. 2018;19:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cassidy JD, Cote P, Carroll LJ, Kristman V. Incidence and course of low back pain episodes in the general population. Spine. 2005;30:2817‐2823. [DOI] [PubMed] [Google Scholar]
- 9. Garcia‐Cosamalon J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leven D, Passias PG, Errico TJ, et al. Risk factors for reoperation in patients treated surgically for intervertebral disc herniation: a subanalysis of eight‐year SPORT data. J Bone Joint Surg Am. 2015;97:1316‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawakami M, Tamaki T, Hashizume H, Weinstein JN, Meller ST. The role of phospholipase A2 and nitric oxide in pain‐related behavior produced by an allograft of intervertebral disc material to the sciatic nerve of the rat. Spine. 1997;22:1074‐1079. [DOI] [PubMed] [Google Scholar]
- 12. Pazzaglia UE, Salisbury JR, Byers PD. Development and involution of the notochord in the human spine. J R Soc Med. 1989;82:413‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bogduk N, Aprill C, Derby R. Lumbar discogenic pain: state‐of‐the‐art review. Pain Med. 2013;14:813‐836. [DOI] [PubMed] [Google Scholar]
- 14. Lyu FJ, Cui H, Pan H, et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Z, Liu H, Yang H, et al. Both expression of cytokines and posterior annulus fibrosus rupture are essential for pain behavior changes induced by degenerative intervertebral disc: an experimental study in rats. J Orthop Res. 2014;32:262‐272. [DOI] [PubMed] [Google Scholar]
- 16. Li Z, Wang X, Pan H, et al. Resistin promotes CCL4 expression through toll‐like receptor‐4 and activation of the p38‐MAPK and NF‐kappaB signaling pathways: implications for intervertebral disc degeneration. Osteoarthr Cartil. 2017;25:341‐350. [DOI] [PubMed] [Google Scholar]
- 17. Liu C, Liang G, Deng Z, Tan J, Zheng Q, Lyu FJ. The Upregulation of COX2 in human degenerated nucleus pulposus: the Association of Inflammation with intervertebral disc degeneration. Mediators Inflamm. 2021;2021:2933199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lv FJ, Peng Y, Lim FL, et al. Matrix metalloproteinase 12 is an indicator of intervertebral disc degeneration co‐expressed with fibrotic markers. Osteoarthr Cartil. 2016;24:1826‐1836. [DOI] [PubMed] [Google Scholar]
- 19. Lv F, Lim FL, Cheung KMC, Leung VYL. Degenerated intervertebral discs contain increased proportion of α‐smooth muscle actin positive cells. Osteoarthr Cartil. 2016;24:S481‐S482. [Google Scholar]
- 20. Liu H, Pan H, Yang H, et al. LIM mineralization protein‐1 suppresses TNF‐alpha induced intervertebral disc degeneration by maintaining nucleus pulposus extracellular matrix production and inhibiting matrix metalloproteinases expression. J Orthop Res. 2015;33:294‐303. [DOI] [PubMed] [Google Scholar]
- 21. Yang H, Gao F, Li X, Wang J, Liu H, Zheng Z. TGF‐beta1 antagonizes TNF‐alpha induced up‐regulation of matrix metalloproteinase 3 in nucleus pulposus cells: role of the ERK1/2 pathway. Connect Tissue Res. 2015;56:461‐468. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Li Z, Chen F, et al. TGF‐beta1 suppresses CCL3/4 expression through the ERK signaling pathway and inhibits intervertebral disc degeneration and inflammation‐related pain in a rat model. Exp Mol Med. 2017;49:e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Z, Zhang K, Li X, et al. Wnt5a suppresses inflammation‐driven intervertebral disc degeneration via a TNF‐alpha/NF‐kappaB‐Wnt5a negative‐feedback loop. Osteoarthr Cartil. 2018;26:966‐977. [DOI] [PubMed] [Google Scholar]
- 24. Chen F, Jiang G, Liu H, et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL‐1beta/NF‐kappaB‐NLRP3 inflammasome positive feedback loop. Bone Res. 2020;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen F, Liu H, Wang X, et al. Melatonin activates autophagy via the NF‐kappaB signaling pathway to prevent extracellular matrix degeneration in intervertebral disc. Osteoarthr Cartil. 2020;28:1121‐1132. [DOI] [PubMed] [Google Scholar]
- 26. Peng Y, Lv F‐J. Symptomatic versus asymptomatic intervertebral disc degeneration: is inflammation the key? Crit Rev Eukaryot Gene Expr. 2015;25:13‐21. [DOI] [PubMed] [Google Scholar]
- 27. Naylor A, Happey F, Turner RL, Shentall RD, West DC, Richardson C. Enzymic and immunological activity in the intervertebral disk. Orthop Clin North Am. 1975;6:51‐58. [PubMed] [Google Scholar]
- 28. Evans C. Potential biologic therapies for the intervertebral disc. J Bone Joint Surg Am. 2006;88(suppl 2):95‐98. [DOI] [PubMed] [Google Scholar]
- 29. Lanna C, Cesaroni GM, Mazzilli S, et al. Nails as immune‐privileged sites: a case of disabling Acrodermatitis continua of Hallopeau successfully treated with Apremilast. Dermatol Ther. 2019;32:e12946. [DOI] [PubMed] [Google Scholar]
- 30. Al Qtaish N, Gallego I, Villate‐Beitia I, et al. Niosome‐based approach for in situ gene delivery to retina and brain cortex as immune‐privileged tissues. Pharmaceutics. 2020;12:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takada T, Nishida K, Doita M, Kurosaka M. Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine. 2002;27:1526‐1530. [DOI] [PubMed] [Google Scholar]
- 32. Kaneyama S, Nishida K, Takada T, et al. Fas ligand expression on human nucleus pulposus cells decreases with disc degeneration processes. J Orthop Sci. 2008;13:130‐135. [DOI] [PubMed] [Google Scholar]
- 33. Wang HQ, Yu XD, Liu ZH, et al. Deregulated miR‐155 promotes Fas‐mediated apoptosis in human intervertebral disc degeneration by targeting FADD and caspase‐3. J Pathol. 2011;225:232‐242. [DOI] [PubMed] [Google Scholar]
- 34. Paul CP, de Graaf M, Bisschop A, et al. Static axial overloading primes lumbar caprine intervertebral discs for posterior herniation. PLoS One. 2017;12:e0174278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robles LA, Curiel A. Posttraumatic cervical disc herniation: an unusual cause of near drowning. Am J Emerg Med. 2005;23:905‐907. [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi JT, Hsu WK. Intervertebral disc herniation in elite athletes. Int Orthop. 2019;43:833‐840. [DOI] [PubMed] [Google Scholar]
- 37. Naylor A. The biophysical and biochemical aspects of intervertebral disc herniation and degeneration. Ann R Coll Surg Engl. 1962;31:91‐114. [PMC free article] [PubMed] [Google Scholar]
- 38. Sollmann N, Weidlich D, Cervantes B, et al. T2 mapping of lumbosacral nerves in patients suffering from unilateral radicular pain due to degenerative disc disease. J Neurosurg Spine. 2019;30:750‐758. [DOI] [PubMed] [Google Scholar]
- 39. Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014;14:513‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tapia‐Perez H. Intervertebral disc pathologies from an immunological perspective. Rev Neurol. 2008;46:751‐757. [PubMed] [Google Scholar]
- 41. Capossela S, Schlafli P, Bertolo A, et al. Degenerated human intervertebral discs contain autoantibodies against extracellular matrix proteins. Eur Cell Mater. 2014;27:251‐263. [DOI] [PubMed] [Google Scholar]
- 42. Mihn DC, Kim TY. Presence of various autoantibodies demonstrated by autoimmune target test in the sera of patients with degenerated and herniated intervertebral disc: comment on the article by Shamji et al. Arthritis Rheum. 2011;63:862. [DOI] [PubMed] [Google Scholar]
- 43. Slowinski J, Pieniazek J, Szydlik W, et al. The study of the role of intervertebral disc neovascularization and immune response in the pathogenesis of lumbar discopathy. Neurol Neurochir Pol. 1998;32:341‐350. [PubMed] [Google Scholar]
- 44. Virri J, Gronblad M, Seitsalo S, Habtemariam A, Kaapa E, Karaharju E. Comparison of the prevalence of inflammatory cells in subtypes of disc herniations and associations with straight leg raising. Spine. 2001;26:2311‐2315. [DOI] [PubMed] [Google Scholar]
- 45. Liu DL, Lu N, Han WJ, et al. Upregulation of Ih expressed in IB4‐negative Adelta nociceptive DRG neurons contributes to mechanical hypersensitivity associated with cervical radiculopathic pain. Sci Rep. 2015;5:16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1‐6. [DOI] [PubMed] [Google Scholar]
- 47. Van Zundert J, Harney D, Joosten EA, et al. The role of the dorsal root ganglion in cervical radicular pain: diagnosis, pathophysiology, and rationale for treatment. Reg Anesth Pain Med. 2006;31:152‐167. [DOI] [PubMed] [Google Scholar]
- 48. Shamji MF, Allen KD, So S, et al. Gait abnormalities and inflammatory cytokines in an autologous nucleus pulposus model of radiculopathy. Spine. 2009;34:648‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murata Y, Onda A, Rydevik B, Takahashi K, Olmarker K. Distribution and appearance of tumor necrosis factor‐alpha in the dorsal root ganglion exposed to experimental disc herniation in rats. Spine. 2004;29:2235‐2241. [DOI] [PubMed] [Google Scholar]
- 50. Takebayashi T, Cavanaugh JM, Cuneyt Ozaktay A, Kallakuri S, Chen C. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine. 2001;26:940‐945. [DOI] [PubMed] [Google Scholar]
- 51. Yabuki S, Kikuchi S, Olmarker K, Myers RR. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine. 1998;23:2517‐2323. [DOI] [PubMed] [Google Scholar]
- 52. Olmarker K. Radicular pain ‐ recent pathophysiologic concepts and therapeutic implications. Schmerz. 2001;15:425‐429. [DOI] [PubMed] [Google Scholar]
- 53. Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112:116‐138. [DOI] [PubMed] [Google Scholar]
- 54. Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240‐264. [DOI] [PubMed] [Google Scholar]
- 55. Suh HR, Cho HY, Han HC. Development of a novel model of intervertebral disc degeneration by the intradiscal application of monosodium iodoacetate (MIA) in rat. Spine J. 2022;21:183‐192. [DOI] [PubMed] [Google Scholar]
- 56. Hadjipavlou AG, Simmons JW, Yang JP, Bi LX, Simmons DJ, Necessary JT. Torsional injury resulting in disc degeneration in the rabbit: II. Associative changes in dorsal root ganglion and spinal cord neurotransmitter production. J Spinal Disord. 1998;11:318‐321. [PubMed] [Google Scholar]
- 57. Lai A, Moon A, Purmessur D, et al. Annular puncture with tumor necrosis factor‐alpha injection enhances painful behavior with disc degeneration in vivo. Spine J. 2016;16:420‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yao Y, Xue H, Chen X, et al. Polarization of helper T lymphocytes maybe involved in the pathogenesis of lumbar disc herniation. Iran J Allergy Asthma Immunol. 2017;16:347‐357. [PubMed] [Google Scholar]
- 59. Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein‐1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772‐783. [DOI] [PubMed] [Google Scholar]
- 60. Costigan M, Befort K, Karchewski L, et al. Replicate high‐density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. White FA, Sun JH, Waters SM, et al. Excitatory monocyte chemoattractant protein‐1 signaling is up‐regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092‐14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Groh AMR, Fournier DE, Battie MC, Seguin CA. Innervation of the human intervertebral disc: a scoping review. Pain Med. 2021;22:1281‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coppes MH, Marani E, Thomeer RT, Oudega M, Groen GJ. Innervation of annulus fibrosis in low back pain. Lancet. 1990;336:189‐190. [DOI] [PubMed] [Google Scholar]
- 64. Richardson SM, Doyle P, Minogue BM, Gnanalingham K, Hoyland JA. Increased expression of matrix metalloproteinase‐10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther. 2009;11:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fournier DE, Kiser PK, Shoemaker JK, Battie MC, Seguin CA. Vascularization of the human intervertebral disc: a scoping review. JOR Spine. 2020;3:e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lama P, Le Maitre CL, Harding IJ, Dolan P, Adams MA. Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. J Anat. 2018;233:86‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gruber HE, Hoelscher GL, Ingram JA, Hanley EN Jr. Genome‐wide analysis of pain‐, nerve‐ and neurotrophin‐related gene expression in the degenerating human annulus. Mol Pain. 2012;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee S, Millecamps M, Foster DZ, Stone LS. Long‐term histological analysis of innervation and macrophage infiltration in a mouse model of intervertebral disc injury‐induced low back pain. J Orthop Res. 2020;38:1238‐1247. [DOI] [PubMed] [Google Scholar]
- 69. Nakawaki M, Uchida K, Miyagi M, et al. Changes in nerve growth factor expression and macrophage phenotype following intervertebral disc injury in mice. J Orthop Res. 2019;37:1798‐1804. [DOI] [PubMed] [Google Scholar]
- 70. Murai K, Sakai D, Nakamura Y, et al. Primary immune system responders to nucleus pulposus cells: evidence for immune response in disc herniation. Eur Cell Mater. 2010;19:13‐21. [DOI] [PubMed] [Google Scholar]
- 71. Kobayashi S, Meir A, Kokubo Y, et al. Ultrastructural analysis on lumbar disc herniation using surgical specimens: role of neovascularization and macrophages in hernias. Spine. 2009;34:655‐662. [DOI] [PubMed] [Google Scholar]
- 72. Koike Y, Uzuki M, Kokubun S, Sawai T. Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine. 2003;28:1928‐1933. [DOI] [PubMed] [Google Scholar]
- 73. Takada T, Nishida K, Maeno K, et al. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis Rheum. 2012;64:2601‐2610. [DOI] [PubMed] [Google Scholar]
- 74. Gorth DJ, Shapiro IM, Risbud MV. Transgenic mice overexpressing human TNF‐alpha experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death Dis. 2018;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Geiss A, Larsson K, Rydevik B, Takahashi I, Olmarker K. Autoimmune properties of nucleus pulposus: an experimental study in pigs. Spine. 2007;32:168‐173. [DOI] [PubMed] [Google Scholar]
- 76. Geiss A, Larsson K, Junevik K, Rydevik B, Olmarker K. Autologous nucleus pulposus primes T cells to develop into interleukin‐4‐producing effector cells: an experimental study on the autoimmune properties of nucleus pulposus. J Orthop Res. 2009;27:97‐103. [DOI] [PubMed] [Google Scholar]
- 77. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang J, Tian Y, Phillips KL, et al. Tumor necrosis factor alpha‐ and interleukin‐1beta‐dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013;65:832‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ding H, Wei J, Zhao Y, Liu Y, Liu L, Cheng L. Progranulin derived engineered protein Atsttrin suppresses TNF‐alpha‐mediated inflammation in intervertebral disc degenerative disease. Oncotarget. 2017;8:109692‐109702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gorth DJ, Shapiro IM, Risbud MV. Transgenic mice overexpressing human TNF‐α experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death Dis. 2018;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Du J, Pfannkuche JJ, Lang G, et al. Proinflammatory intervertebral disc cell and organ culture models induced by tumor necrosis factor alpha. JOR Spine. 2020;3:e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Evashwick‐Rogler TW, Lai A, Watanabe H, et al. Inhibiting tumor necrosis factor‐alpha at time of induced intervertebral disc injury limits long‐term pain and degeneration in a rat model. JOR Spine. 2018;1:e1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ozaktay AC, Kallakuri S, Takebayashi T, et al. Effects of interleukin‐1 beta, interleukin‐6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15:1529‐1537. [DOI] [PubMed] [Google Scholar]
- 84. Sacerdote P, Franchi S, Trovato AE, Valsecchi AE, Panerai AE, Colleoni M. Transient early expression of TNF‐alpha in sciatic nerve and dorsal root ganglia in a mouse model of painful peripheral neuropathy. Neurosci Lett. 2008;436:210‐213. [DOI] [PubMed] [Google Scholar]
- 85. Shubayev VI, Myers RR. Upregulation and interaction of TNFalpha and gelatinases a and B in painful peripheral nerve injury. Brain Res. 2000;855:83‐89. [DOI] [PubMed] [Google Scholar]
- 86. Jancalek R, Dubovy P, Svizenska I, Klusakova I. Bilateral changes of TNF‐alpha and IL‐10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J Neuroinflammation. 2010;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gorth DJ, Shapiro IM, Risbud MV. A new understanding of the role of IL‐1 in age‐related intervertebral disc degeneration in a murine model. J Bone Miner Res. 2019;34:1531‐1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kepler CK, Markova DZ, Dibra F, et al. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL‐1beta in painful human intervertebral discs. Spine. 2013;38:873‐880. [DOI] [PubMed] [Google Scholar]
- 89. Zhang Z, Bryan JL, DeLassus E, Chang LW, Liao W, Sandell LJ. CCAAT/enhancer‐binding protein beta and NF‐kappaB mediate high level expression of chemokine genes CCL3 and CCL4 by human chondrocytes in response to IL‐1beta. J Biol Chem. 2010;285:33092‐33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL‐1 beta potentiates heat‐activated currents in rat sensory neurons: involvement of IL‐1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16:1497‐1503. [DOI] [PubMed] [Google Scholar]
- 91. Miyamoto H, Saura R, Harada T, Doita M, Mizuno K. The role of cyclooxygenase‐2 and inflammatory cytokines in pain induction of herniated lumbar intervertebral disc. Kobe J Med Sci. 2000;46:13‐28. [PubMed] [Google Scholar]
- 92. Balbay Y, Tikiz H, Baptiste RJ, Ayaz S, Sasmaz H, Korkmaz S. Circulating interleukin‐1 beta, interleukin‐6, tumor necrosis factor‐alpha, and soluble ICAM‐1 in patients with chronic stable angina and myocardial infarction. Angiology. 2001;52:109‐114. [DOI] [PubMed] [Google Scholar]
- 93. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL‐1 and TNF in matrix degradation in the intervertebral disc. Rheumatology. 2008;47:809‐814. [DOI] [PubMed] [Google Scholar]
- 94. Deng X, Zhao F, Kang B, Zhang X. Elevated interleukin‐6 expression levels are associated with intervertebral disc degeneration. Exp Ther Med. 2016;11:1425‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Suzuki S, Fujita N, Fujii T, et al. Potential involvement of the IL‐6/JAK/STAT3 pathway in the pathogenesis of intervertebral disc degeneration. Spine. 2017;42:E817‐E824. [DOI] [PubMed] [Google Scholar]
- 96. Moon HJ, Kim JH, Lee HS, et al. Annulus fibrosus cells interact with neuron‐like cells to modulate production of growth factors and cytokines in symptomatic disc degeneration. Spine. 2012;37:2‐9. [DOI] [PubMed] [Google Scholar]
- 97. Sainoh T, Orita S, Miyagi M, et al. Single intradiscal injection of the interleukin‐6 receptor antibody tocilizumab provides short‐term relief of discogenic low back pain; prospective comparative cohort study. J Orthop Sci. 2016;21:2‐6. [DOI] [PubMed] [Google Scholar]
- 98. Weber KT, Alipui DO, Sison CP, et al. Serum levels of the proinflammatory cytokine interleukin‐6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Haddadi K, Abediankenari S, Alipour A, et al. Association between serum levels of Interleukin‐6 on pain and disability in lumbar disc herniation surgery. Asian J Neurosurg. 2020;15:494‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Czlonkowski A, Stein C, Herz A. Peripheral mechanisms of opioid antinociception in inflammation: involvement of cytokines. Eur J Pharmacol. 1993;242:229‐235. [DOI] [PubMed] [Google Scholar]
- 101. Flatters SJ, Fox AJ, Dickenson AH. Nerve injury alters the effects of interleukin‐6 on nociceptive transmission in peripheral afferents. Eur J Pharmacol. 2004;484:183‐191. [DOI] [PubMed] [Google Scholar]
- 102. Obreja O, Schmelz M, Poole S, Kress M. Interleukin‐6 in combination with its soluble IL‐6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96:57‐62. [DOI] [PubMed] [Google Scholar]
- 103. Brazda V, Klusakova I, Svizenska I, Veselkova Z, Dubovy P. Bilateral changes in IL‐6 protein, but not in its receptor gp130, in rat dorsal root ganglia following sciatic nerve ligature. Cell Mol Neurobiol. 2009;29:1053‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Koerner JD, Markova DZ, Schroeder GD, et al. The effect of substance P on an intervertebral disc rat organ culture model. Spine. 2016;41:1851‐1859. [DOI] [PubMed] [Google Scholar]
- 105. Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg. 2013;257:403‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vikman KS, Siddall PJ, Duggan AW. Increased responsiveness of rat dorsal horn neurons in vivo following prolonged intrathecal exposure to interferon‐gamma. Neuroscience. 2005;135:969‐977. [DOI] [PubMed] [Google Scholar]
- 107. Luchting B, Rachinger‐Adam B, Heyn J, Hinske LC, Kreth S, Azad SC. Anti‐inflammatory T‐cell shift in neuropathic pain. J Neuroinflammation. 2015;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Moen GH, Moen A, Schistad EI, Gjerstad J. Local up‐regulation of interferon‐gamma (IFN‐gamma) following disc herniation is involved in the inflammatory response underlying acute lumbar radicular pain. Cytokine. 2017;97:181‐186. [DOI] [PubMed] [Google Scholar]
- 109. Cuellar JM, Borges PM, Cuellar VG, Yoo A, Scuderi GJ, Yeomans DC. Cytokine expression in the epidural space: a model of noncompressive disc herniation‐induced inflammation. Spine. 2013;38:17‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Uceyler N, Schafers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:67‐78. [DOI] [PubMed] [Google Scholar]
- 111. de Campos MF, de Oliveira CP, Neff CB, Correa OM, Pinhal MA, Rodrigues LM. Studies of molecular changes in intervertebral disc degeneration in animal model. Acta Ortop Bras. 2016;24:16‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li W, Liu T, Wu L, et al. Blocking the function of inflammatory cytokines and mediators by using IL‐10 and TGF‐beta: a potential biological immunotherapy for intervertebral disc degeneration in a beagle model. Int J Mol Sci. 2014;15:17270‐17283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Banimostafavi ES, Fakhar M, Abediankenari S, et al. Determining serum levels of IL‐10 and IL‐17 in patients with low Back pain caused by lumbar disc degeneration. Infect Disord Drug Targets. 2021;21:e270421185135. [DOI] [PubMed] [Google Scholar]
- 114. Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV‐mediated transfer of interleukin‐10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther. 2008;15:183‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Uceyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun. 2007;21:553‐560. [DOI] [PubMed] [Google Scholar]
- 116. Bottner M, Krieglstein K, Unsicker K. The transforming growth factor‐betas: structure, signaling, and roles in nervous system development and functions. J Neurochem. 2000;75:2227‐2240. [DOI] [PubMed] [Google Scholar]
- 117. Li H, Li W, Liang B, Wei J, Yin D, Fan Q. Role of AP‐2alpha/TGF‐beta1/Smad3 axis in rats with intervertebral disc degeneration. Life Sci. 2020;263:118567. [DOI] [PubMed] [Google Scholar]
- 118. Gupta MS, Cooper ES, Nicoll SB. Transforming growth factor‐beta3 stimulates cartilage matrix elaboration by human marrow‐derived stromal cells encapsulated in photocrosslinked carboxymethylcellulose hydrogels: potential for nucleus pulposus replacement. Tissue Eng Part A. 2011;17:2903‐2910. [DOI] [PubMed] [Google Scholar]
- 119. Echeverry S, Shi XQ, Haw A, Liu H, Zhang ZW, Zhang J. Transforming growth factor‐beta1 impairs neuropathic pain through pleiotropic effects. Mol Pain. 2009;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tramullas M, Lantero A, Diaz A, et al. BAMBI (bone morphogenetic protein and activin membrane‐bound inhibitor) reveals the involvement of the transforming growth factor‐beta family in pain modulation. J Neurosci. 2010;30:1502‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shamji MF, Setton LA, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62:1974‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hanaei S, Abdollahzade S, Sadr M, et al. The role of interleukin 4 and IL‐4RA in intervertebral disc degeneration: investigation of single nucleotide polymorphisms in genes and a systematic review & meta‐analysis of IL‐4 expression level. Br J Neurosurg. 2020;34:66‐71. [DOI] [PubMed] [Google Scholar]
- 123. Kedong H, Wang D, Sagaram M, An HS, Chee A. Anti‐inflammatory effects of interleukin‐4 on intervertebral disc cells. Spine J. 2020;20:60‐68. [DOI] [PubMed] [Google Scholar]
- 124. Hao S, Mata M, Glorioso JC, Fink DJ. HSV‐mediated expression of interleukin‐4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Alkhatib B, Rosenzweig DH, Krock E, et al. Acute mechanical injury of the human intervertebral disc: link to degeneration and pain. Eur Cell Mater. 2014;28:98‐110. [DOI] [PubMed] [Google Scholar]
- 126. Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911‐917. [DOI] [PubMed] [Google Scholar]
- 127. Krock E, Millecamps M, Anderson KM, et al. Interleukin‐8 as a therapeutic target for chronic low back pain: upregulation in human cerebrospinal fluid and pre‐clinical validation with chronic reparixin in the SPARC‐null mouse model. EBioMedicine. 2019;43:487‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Khan J, Hassun H, Zusman T, Korczeniewska O, Eliav E. Interleukin‐8 levels in rat models of nerve damage and neuropathic pain. Neurosci Lett. 2017;657:106‐112. [DOI] [PubMed] [Google Scholar]
- 129. Kossmann T, Stahel PF, Lenzlinger PM, et al. Interleukin‐8 released into the cerebrospinal fluid after brain injury is associated with blood‐brain barrier dysfunction and nerve growth factor production. J Cereb Blood Flow Metab. 1997;17:280‐289. [DOI] [PubMed] [Google Scholar]
- 130. Kim JS, Ellman MB, Yan D, et al. Lactoferricin mediates anti‐inflammatory and anti‐catabolic effects via inhibition of IL‐1 and LPS activity in the intervertebral disc. J Cell Physiol. 2013;228:1884‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408‐1419. [DOI] [PubMed] [Google Scholar]
- 132. Leung VY, Aladin DM, Lv F, et al. Mesenchymal stem cells reduce intervertebral disc fibrosis and facilitate repair. Stem Cells. 2014;32:2164‐2177. [DOI] [PubMed] [Google Scholar]
- 133. Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243‐256. [DOI] [PubMed] [Google Scholar]
- 134. Borem R, Madeline A, Bowman M, Gill S, Tokish J, Mercuri J. Differential effector response of amnion‐ and adipose‐derived Mesenchymal stem cells to inflammation; implications for Intradiscal therapy. J Orthop Res. 2019;37:2445‐2456. [DOI] [PubMed] [Google Scholar]
- 135. Lyu FJ, Cheung KM, Zheng Z, Wang H, Sakai D, Leung VY. IVD progenitor cells: a new horizon for understanding disc homeostasis and repair. Nat Rev Rheumatol. 2019;15:102‐112. [DOI] [PubMed] [Google Scholar]
- 136. Sakai D, Schol J, Bach FC, et al. Successful fishing for nucleus pulposus progenitor cells of the intervertebral disc across species. JOR Spine. 2018;1:e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]


