Abstract
Background
Disorders of the intervertebral disc (IVD) are widely known to result in low back pain; one of the most common debilitating conditions worldwide. As a multifaceted condition, both inflammatory environment and mechanical factors can play a crucial role in IVD damage, and in particular, in the annulus fibrosus (AF), the highly collagenous outer ring of the IVD. As a result, a better understanding of how cells from the IVD, and specifically the AF, interact and respond to their environment is imperative.
Goal
The goal of this study is to use collagen type I as an in vitro three‐dimensional extracellular matrix for AF cells of IVD and briefly examine both the cellular and mechanical effect of exposure to an inflammatory stimulant.
Methods
We utilized type I collagen as a 3D in vitro model material for culturing AF cells of Sprague Dawley rat tail IVDs.
Results
We showed that the cultured cells are viable and metabolically active; these cells also induced a distinct and significant contraction on their collagen matrix. Furthermore, to demonstrate potential versatility of our model our model and its versatility, we used lipopolysaccharide (LPS), as a known inflammatory stimulant in IVDs, to manipulate the cells and their interaction. LPS treatment resulted in detectable changes to the contraction cells induced on the collagen matrix and affected the mechanical properties of these constructs.
Keywords: annulus fibrosus cells, inflammation, intervertebral disc degeneration, lipopolysaccharide (LPS), type I collagen
In this paper, we used type I collagen as a 3D model material for culturing annulus fibrosus cells of intervertebral discs of Sprague Dawley rat tails and showed that these cells are viable. These viable cells also induced a robust and repeatable contraction on the surrounding matrix. This work demonstrates type I collagen as an in vitro model material for intervertebral disc research and explores the interactions of these cells with their 3D matrix.
1. INTRODUCTION
Low back pain is one of the top five main causes of disability worldwide. 1 The total direct and indirect costs associated with it is estimated to be between $100 and $200 billion per year in the United States. 2 The etiology of low back pain is vast; however, a significant proportion is a result of damage or disorders of the intervertebral disc (IVD), 3 the soft load‐bearing tissue located between vertebrae within the spine. 4 The IVD contains a centrally located highly hydrated nucleus pulposus (NP) which acts to resist compression, and is peripherally surrounded by the annulus fibrosus (AF). The AF is composed of 15–25 concentric circles called lamellae. 5 , 6 , 7 Each lamella contains parallel collagen fibers (types I and II) at an oblique angle relative to the spinal column, and neighboring lamellae alternate the collagen fiber angles at approximately 60°. 8 , 9 With its unique and highly organized structure, the AF is mainly responsible for resisting tensile loading 10 , 11 and is also a primary location of damage and a potential source of low back pain. AF damage tends to accompany various IVD disorders and is therefore the focus of this paper.
IVDs are immune‐privileged tissue and their degeneration is a complex process involving multiple factors. In some cases, degeneration is initiated following IVD herniation when NP material migrates outside the boundaries of the damaged AF. 12 , 13 This disruption can occur as a result of mechanical wear (due to aging or trauma, 14 , 15 or even specific types of infection, 16 and can induce changes in the behavior of resident cells 15 , 17 , 18 and their subsequent interaction with their surrounding extracellular matrix. Given the unique structural organization of the AF (highly collagenous concentric lamellae), the interaction of the AF cells with their surrounding matrix could have a significant impact on the overall function of the IVD.
AF cells are thin, elongated cells that resemble fibroblasts and can attach to collagen fibers. 19 Cells in the inner regions of the AF have been described as fibrochondrocytes, 9 , 20 resulting in a morphological transition zone between the AF and NP containing both type I and type II collagen. 6 Average density of AF cells is about cells/cm3, with higher number of cells in outer AF and lower density in inner AF. 10 , 18
IVDs are avascular tissues. 10 With the initiation of the degeneration cascades, an innate immune response starts which triggers the resident cells to secrete pro‐inflammatory and catabolic markers. 21 These factors, such as TNF‐α and IL‐1 and 2, can not only contribute to the degenerative process, 22 , 23 but also induce nerve growth and contribute to discogenic pain. 24 , 25 The balance between catabolic and anabolic factors secreted from the cells is a major factor in determining the state of the degeneration process. 15 , 26 With increased catabolic factor expression during degeneration, the extracellular matrix of the IVD, and particularly the AF, becomes degraded. 27 , 28 The fragments of the degraded AF can, in turn, act as danger associated molecular patterns or alarmins. 29 Danger associated molecular patterns subsequently activate toll‐like receptors, a family of receptors that function as sentinels for the innate immune response, and whose downstream effector responses further contribute to the degeneration process. 29 Toll‐like receptors also recognize foreign components referred to as pathogen‐associated molecular patterns. Activation of toll‐like receptors through pathogen‐associated molecular patterns also activate inflammatory cascades in IVDs. 30 Specifically, Toll‐like receptors 2 and 4 have been previously shown to be triggered by both danger associated molecular patterns and pathogen‐associated molecular patterns in IVD degeneration cascades. 29 , 31 , 32 Toll‐like receptor 2 has been previously shown to also induce nerve growth in IVD. 33
The complex mechanical loading profile applied to the spine plays an important role in the homeostasis of the IVD tissue. 34 , 35 , 36 While inflammatory cascades in IVD degeneration have been previously studied for the IVD, there have not been many studies focused on the effect of inflammation on the mechanical properties and integrity of the IVD extracellular matrix in vitro and at the cellular level. 37 The first step for broadening this field is to find a substrate, preferably a three‐dimensional (3D) material, which can best resemble the native tissue.
The purpose of this study was therefore two‐fold: (1) to examine the use of type I collagen as a 3D matrix to culture AF cells of the IVD. While collagen fibers in native AF tissue are more organized than self‐assembled in vitro collagen matrix, using a natural material that cells are mostly exposed to in native tissue, especially as a 3D scaffold, highly increases the relevancy of the model; and (2) to measure the mechanical properties of the cells + collagen constructs following exposure to an inflammatory stimulant.
Not only are type I collagen fibers what AF cells are mostly in contact with in native tissue, but this type of collagen matrix also provides the benefits of a 3D substrate, which is more akin to the native environment of cells, as opposed to traditional 2D cultures. Collagen has been previously used for IVD cell culturing. Neidlinger‐Wilke et al. 38 has shown the effect of static and dynamic mechanical loading on IVD cells using 3D collagen matrices and Bowles et al. 39 used a collagen matrices to demonstrate that AF cells can realign collagen fibers. Other synthetic matrices have been previously used for culturing IVD cells for mechanical studies such as alginate 40 , 41 , 42 and PGA/PLLA. 43
For the second aim, lipopolysaccharides (LPS) was used as a pathogen‐associated molecular pattern to examine manipulation of this model. LPS is an endotoxin abundantly found in the cell wall of gram‐negative bacteria that is known to activate Toll‐like receptor 4 in IVDs as an inflammatory stimulant. 30 , 31 , 44 , 45 , 46 While previous studies have used pro‐inflammatory cytokines (e.g., TNF‐α 47 , 48 and IL‐1β 23 to induce an inflammatory environment, we chose an upstream inflammatory stimulant (specifically LPS) in order to create a strong innate immune response which, in turn, activates the inflammatory cascade and the production of a spectrum of pro‐inflammatory cytokines. We stimulated these cells + collagen constructs and studied how LPS affects the mechanical properties. This study provides a proof‐concept for utilizing type I collagen as a relatively simple and versatile 3D model substrate for in vitro studies of IVD degeneration. Studying IVD disruption using this model allows for controlled assessment of different variables in this otherwise very complex condition.
2. MATERIALS AND METHODS
2.1. AF cell isolation and culture
AF tissues were collected aseptically from the IVDs of 4‐month‐old female Sprague Dawley rat tails (Charles River) immediately following euthanization. Animal utilization protocols were reviewed and approved by the institutional Animal Care Committee in compliance with the Canadian Council on Animal Welfare. In total, six rats were used and cells from different animals were not pooled together. Collected tissues were washed 3× in DPBS (HyClone) with 2% penicillin–streptomycin (Thermo Fisher Scientific), cut into small pieces and cultured in vented T75 cell culture flasks with DMEM (HyClone) supplemented with 10% FBS (Sigma‐Aldrich) and 1% penicillin–streptomycin. Cells were allowed to grow out of the tissue and reach confluency (3–4 weeks). Cells were incubated at 37°C, 5% CO2, and 95% humidity, and culture medium was changed every 2–3 days. Cells were used between Passages 1 and 4.
2.2. 3D cells + collagen constructs
High Concentration type I Collagen (from rat tail tendon; 8–11 mg/ml; Corning) was chosen as the 3D extracellular matrix for this study. 3D cells + collagen constructs were prepared by diluting high concentration collagen to a final concentration of 3 mg/ml with 1 N NaOH, 10× DPBS, and sterile distilled water. This concentration was chosen based on previously published research 38 , 39 and dilutions were calculated according to manufacturer's protocols. A number of 1.5 × 106 cells were then mixed with the collagen solution, immediately placed in custom built silicon molds and allowed to gel for 30 min at 37°C. Collagen handling and sample preparation was performed on ice and collagen solutions were used immediately following the dilution procedure. Complete cell culture medium was then added to the molds and images of cells+collagen constructs were taken at 24‐h intervals and processed by ImageJ (NIH). Cells+collagen constructs were cultured for a maximum of 6 days at 37°C, 5% CO2, and 95% humidity inside the molds before being used in various testing platforms. Culture medium was changed every 2–3 days. Initial thickness is calculated based on the volume of the collagen solution in each mold. Initial mold dimensions were 60.75 mm (length) × 16.70 mm (width) × 3.94 mm (thickness) for 4 ml of collagen solution.
2.3. Cell viability analysis
Cell viability was assessed using two fluorescent indicator dyes: alamarBlue viability assay (Invitrogen) and via 5‐carboxyfluorescein diacetate (5‐CFDA‐AM; Invitrogen). 40
Resazurin is the active component of the alamarBlue assay and is a nontoxic compound reduced by live cells and is an effective measure of cellular metabolism. Following incubation of the cells, cells+collagen constructs were washed twice with DPBS, and a 5% vol/vol alamarBlue solution in DPBS was added to the constructs. Constructs were then incubated at 37°C for 1 and 2 h in the dark and fluorescence was measured using a Synergy HT plate reader (BioTek) by transferring 50 μl of the media into a new 96 well culture plate.
To study cell membrane integrity of the AF cells encapsulated in 3D collagen, 5‐CFDA‐AM was diluted in DPBS and constructs were incubated with the diluted solution for 30 minutes at 37°C. Images of cells at different locations (including depth) within the constructs were taken by an inverted confocal laser scanning microscope (Olympus Fluoview 1000; Olympus Life Sciences).
Collagen fibers were imaged using the same microscope by confocal reflectance microscopy technique. Samples were illuminated by an Argon laser ( = 488 nm) and the same wavelength was collected to image the collagen fibers.
2.4. LPS treatment
To induce sterile inflammation on cells+collagen constructs, lipopolysaccharide (LPS; Sigma‐Aldrich) was used as the pathogen‐associated molecular patterns agent at 10 concentration. Complete culture medium containing LPS was added directly to the constructs right after the gelation process was finished (30 min at 37°C) and cells in collagen were exposed to LPS for the duration of the culture period (up to 6 days).
AlamarBlue assay was also used to assess the viability of the AF cells cultured inside type I collagen treated with LPS. Three samples were used for this test: acellular collagen, live cells in collagen with no treatment, and live cells in collagen treated with LPS. The assay was incubated with the samples for both 1 and 2 h at 37°C to allow the assay to penetrate within the 3D matrix and fluorescence was measured using a Synergy HT plate reader.
2.5. Actin filament staining
Cytoskeleton structures of AF cells were examined via fluorescent staining of actin filaments. Cells embedded in 3D collagen matrix were fixed with 4% formaldehyde (Thermo Fisher Scientific) solution in DPBS and were permeabilized with 0.1% Triton X‐100 (MilliporeSigma) in DPBS. One percent solution of bovine serum albumin (MilliporeSigma) in DPBS was used to reduce nonspecific binding. Alexafluor‐488 phalloidin (Invitrogen) was used to stain actin filaments. In order to image cells that were inside the 3D collagen matrix, images of cells were again taken using an inverted confocal laser scanning microscope (Olympus Fluoview 1000; Olympus Life Sciences).
2.6. Mechanical testing
Displacement controlled uniaxial tensile tests were performed using the Biotester (CellScale) on cells+collagen constructs to investigate the mechanical properties of the samples. Each test is performed with at least n = 3, each sample was cut in half immediately before testing and both pieces were tested to account for any potential variability along the samples. Samples were mounted via custom 5‐prong tungsten rakes with an inter‐rake spacing of 2.2 mm. Tests were performed at tensile strain rate of 10%/s and a 2.5‐N load cell was used to measure the force during the tests. Sample thickness necessary for normalizing to stress and strain were determined using a laser displacement sensor (IL‐065; Keyence).
2.7. Statistical analysis
Statistical analysis was performed using R Studio (Version 1.1.453, RStudio, Inc.). Analysis of variance (ANOVA) followed by a single‐step multiple comparison Tukey's honestly significant difference (HSD) test was used to perform multiple comparisons for the mechanical testing results as well as cells+collagen contraction data (n = 3). Fisher exact test was performed on mechanical failure contingency table. The significance level was set at 0.05. Cohen's d effect sizes were calculated for AlamarBlue assay results.
3. RESULTS
3.1. AF cells were viable and metabolically active inside collagen matrices
Viability of AF cells cultured in collagen was assessed using the fluorescent indicator dye, alamarBlue. AlamarBlue was used to assess metabolic activity of various cells. Here, Figure 1 shows the relative fluorescent units (RFUs) received from the alamarBlue assay. Comparing the fluorescence values, it can be seen that increasing incubation time (Figure 1, 1 h vs. 2 h) as well as culture period (24 vs. 48 h) increased these values. With fixed cells, acellular collagen, and empty wells (blank) as our controls, the increase in the RFUs (Cohen's d effect size >2.15) could be considered to be an indication of AF cells proliferating inside collagen matrices.
FIGURE 1.
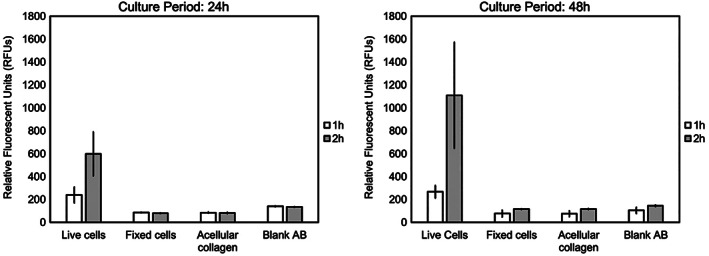
AF cell viability inside collagen matrices using the fluorescent indicator dye, alamarBlue. Besides live cells+collagen constructs, fixed cells cultured in collagen, acellular collagen, and empty wells (blank) were also used to illustrate the proliferation of cells in collagen matrix. The y axis shows the relative fluorescence units (RFUs). Distinct increased fluorescence expression is seen as culture period and incubation time increased. This indicates AF cells are viable and potentially proliferating inside collagen matrices. Cohen's d effect size for live versus fixed cells >2.15. Error bars indicate standard error
5‐CFDA‐AM, a measure of cell membrane integrity, was also used to study the viability of cells inside collagen matrices after longer culture period of up to 6 days. Figure 2 shows cells cultured inside collagen matrices at two different magnifications (A and B) as well as a 3D reconstructed view (Figure 2C).
FIGURE 2.
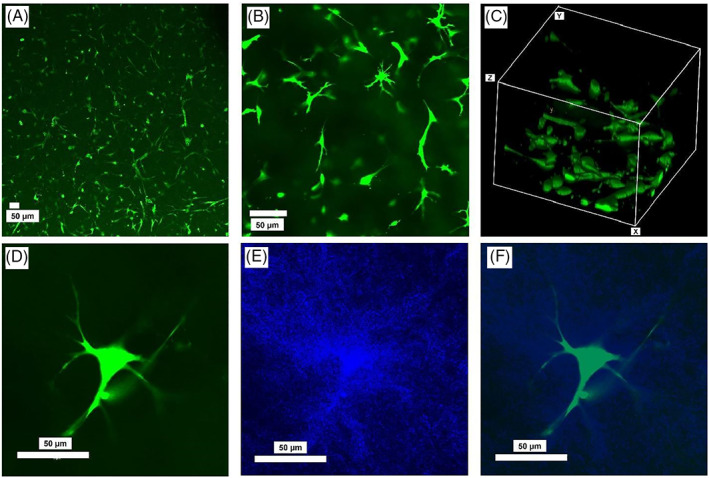
Representative images of AF cells cultured inside type I collagen for 6 days, and then stained with 5‐CFDA‐AM at (A) ×10 and (B) ×20 magnification, and (C) a 3D view, as well as (D) a single AF cell inside collagen, (E) collagen fibers around the cell, and (F) overlayed image of the cell and the collagen fibers (blue). Images were acquired by an inverted confocal laser scanning microscope (Olympus Fluoview 1000; Olympus Life Sciences)
Collagen fibers were also imaged using confocal reflectance microscopy technique. Figure 2 also shows an AF cell inside collagen (Figure 2D), at 6 days postculture, as well as the collagen fibers around that cell (Figure 2E) and the merged image of the cell and collagen fibers (Figure 2F). Higher density of collagen fibers immediately around the cell, compared to collagen density further away from the cell, is visible here.
3.2. AF cells induced a significant contraction on collagen matrices
As the first indication of AF cells interacting with collagen, AF cells induced a robust contraction on the collagen that was clear as early as 24 h postculture (Figure 3). To ensure the contraction was the result of AF cells interacting with collagen, constructs with fixed cells (using 70% ethanol) were also made and contraction was measured. Live cells induced contraction while constructs containing fixed cells did not show any sign of contraction (Panel B in Figure 3).
FIGURE 3.
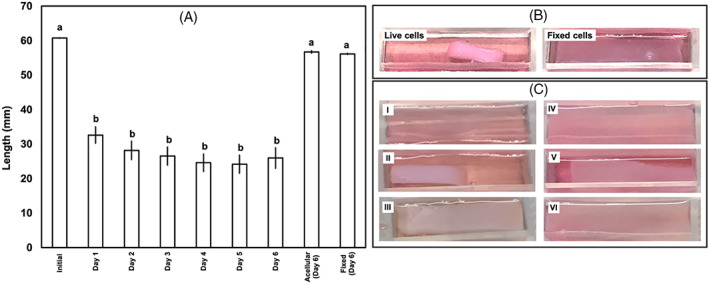
(A) Induced contraction on collagen matrices by AF cells over 6 days postculture. Initial calculated thickness = 3.94 cm, collagen concentration = 3 mg/ml. Length was measured by imaging the samples and measuring the length using ImageJ. n = 3 for each of Day 1–6 measurements. One measurement was made for the acellular collagen sample at Day 6 and one measurement for the sample with fixed cells at Day 6. Bars with different letters are significantly different (p < 0.05; Cohen's d > 9.5 for all comparisons). Error bars indicate standard error. (B) Representative images of cells+collagen constructs for both live and fixed cells. Images are taken 48 h postculture. Constructs with cultured fixed cells show no contraction whereas live cells in collagen show significant contraction. (C) Representative images of cells+collagen constructs with varying thickness: (I) acellular collagen; (II) 3.94 cm (4 ml collagen solution); (III) 4.92 cm (5 ml collagen solution) – culture period = 24 h; (IV) acellular collagen; (V) 5.91 cm (6 ml collagen solution); (VI) 7.88 cm (8 ml collagen solution) culture period = 48 h. Increasing thickness of the collagen matrix decreases the amount of contraction induced by AF cells
Cells cultured inside constructs having higher thickness were not able to effectively induce any significant contraction (Panel C in Figure 3: Images I–III). Even increasing culture period to 48 hours for samples with significantly higher thickness did not affect the amount of contraction (Panel C in Figure 3: Images IV–VI). Based on these results, we chose 3.94 cm (4 ml of collagen solution) with 3 mg/ml concentration for collagen for all further experiments.
3.3. Cells+collagen matrices had higher strength compared to acellular collagen
Uniaxial tensile tests were performed to determine the effect of contraction on the mechanical properties of the collagen matrices as well as cells+collagen constructs. As expected, and shown in force displacement graphs (Figure 4), cells+collagen constructs had higher strength compared to acellular collagen constructs.
FIGURE 4.
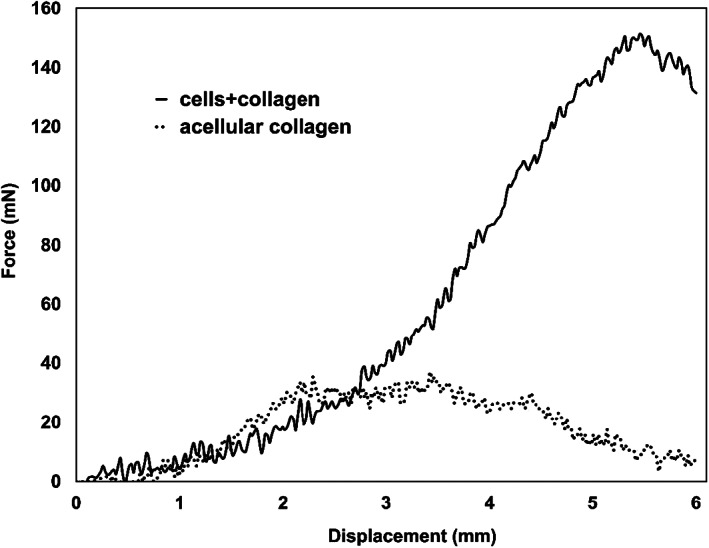
Representative force‐displacement curves for cells+collagen constructs as well as acellular collagen for simple tensile test. As expected, cells+collagen constructs show higher mechanical properties compared to acellular collagen
3.4. LPS, as an inflammatory treatment, affected cells+collagen constructs properties
To further show the capability of our model to be easily manipulated, we treated the constructs with LPS as an inflammatory stimulant that has been shown to trigger Toll‐like receptor 4 in IVDs. 30 , 31 , 44 , 45 , 46 LPS significantly affected the contraction cells induced on collagen matrices (Figure 5). At 6 days postculture, constructs treated with LPS show less contraction when compared to untreated constructs (Figure 5).
FIGURE 5.
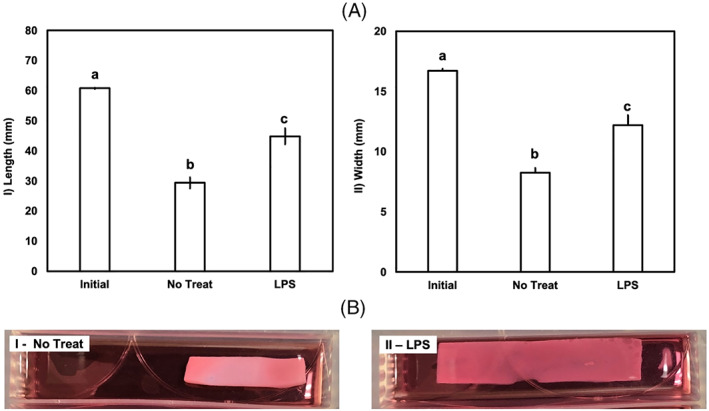
The effect of LPS on the cells+collagen construct model. Panel (A): (I) length and (II) width are reported for n = 3. Initial, initial dimensions; LPS, LPS treated; No Treat, no treatment. Bars with different letters are significantly different (p < 0.05; Cohen's d > 3.1 for all comparisons). Cells+collagen constructs treated with LPS show noticeably less contraction. Error bars indicate standard error. Panel B shows representative images of cells+collagen. Differences in contraction induced by AF cells can also be seen in the images of the constructs
3.5. LPS negatively affected the strength of the constructs
LPS treatment affected the mechanical properties of cells+collagen constructs. Elastic modulus was not statistically affected (no LPS vs. LPS: p = 0.28, Figure 6A); however, strength was significantly decreased after LPS treatment (no LPS, p = 0.005; Figure 6B). The variations between strength and elastic modulus of different samples can also be seen in their representative stress strain curves (Figure 6C).
FIGURE 6.
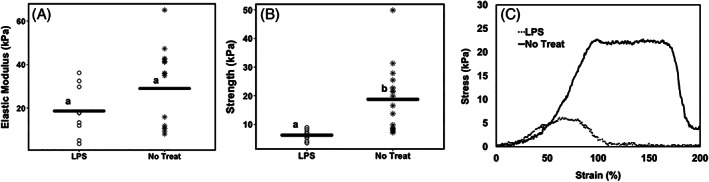
Mechanical properties of cells+collagen constructs, (A) the effect of LPS treatment on elastic modulus of cells+collagen constructs at 6 days postculture. Graph shows the scatter plot with all measurements; horizontal line shows the average for each group. LPS slightly decreased the elastic modulus, however the changes are not statistically significant; (B) the effect of LPS treatment on strength of cells+collagen constructs at 6 days postculture. Graph shows the scatter plot with all measurements; horizontal line shows the average for each group. LPS significantly decreased the strength. Minimum n = 3 (at least two measurements per replicate). Bars with different letters are significantly different (p < 0.05; Cohen's d = 1.52). (C) Representative stress strain curves for cells+collagen constructs, not treated with any inflammatory stimulants as well as treated with LPS. These graphs also demonstrate similar elastic moduli between samples and higher strength for no inflammation versus LPS‐treated constructs. LPS, LPS treated; No Treat, no treatment
3.6. LPS treatment decreased the ductility of the cells+collagen constructs
Investigating stress–strain curves of the constructs with and without LPS treatment revealed that ductility, or elongation to failure, seemed to have decreased after constructs were exposed to inflammatory stimulant (Figure 6C). Since not all samples fully failed, samples were categorized based on whether failure had occurred by 150% strain (150% was chosen based on the spread of data). Categorized samples were then statistically tested using a Fisher exact test on the generated contingency table (Table 1); a significant effect of condition (no inflammation vs. LPS) was found (p = 0.0019) such that more samples treated with LPS failed by 150% strain compared to untreated samples.
TABLE 1.
Contingency table for the number of samples (frequency) that failed at 150% strain under simple tensile test
The number of samples for each group that failed at 150% strain.
The number of samples for each group that did not fail at 150% strain.
3.7. AF cells in collagen treated with LPS show lower viability
To further understand the effect of LPS on the AF cells in this model, alamarBlue viability assay was performed on the constructs. Three samples were chosen to study this effect: acellular collagen, live cells in collagen with no treatment and live cells in collagen treated with LPS for 6 days. Figure 7 shows florescence readings for incubation on matrices with Alamar blue for both 1 and 2 h.
FIGURE 7.
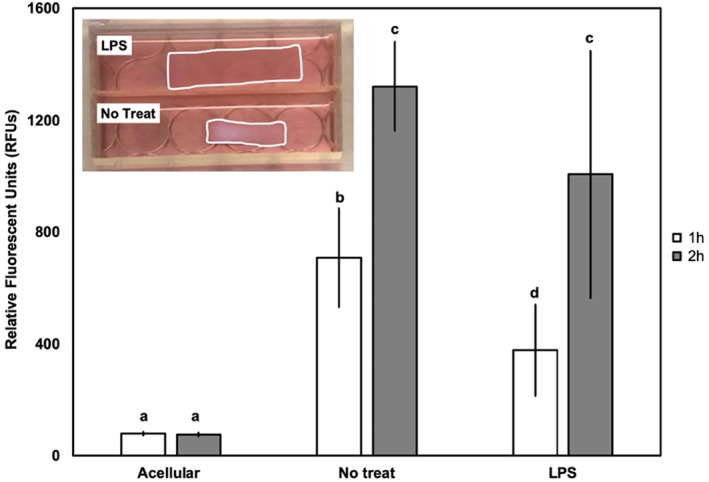
AF cells viability inside collagen matrices was studied using a fluorescent indicator dye, alamarBlue assay. Acellular collagen, live cells in collagen no treatment, and live cells in collagen treated with LPS are studied after 6 days in culture. Also, a representative image of the samples and contraction of the samples shown for reference. The y‐axis is RFUs. n = 3. Bars with different letters are significantly different (p < 0.05; Cohen's d > 1.1 for all comparisons); specifically RFUs were compared across condition (acellular versus no treatment versus treatment with LPS) as well as between incubation times (1 h vs. 2 h). Error bars indicate standard error
3.8. AF cells cultured inside collagen and treated with LPS showed shorter and fewer protrusions
In order to understand how AF cells are inducing this contraction, the actin filaments of AF cells were stained using Alexa Fluor conjugated phalloidin, both for cells cultured in glass bottom culture dishes (Figure 8) as well as cells cultured inside collagen matrices (Figure 9). For cells cultured in glass bottom dishes, actin filaments appeared unaffected by LPS treatment (Figure 8B compared to A).
FIGURE 8.
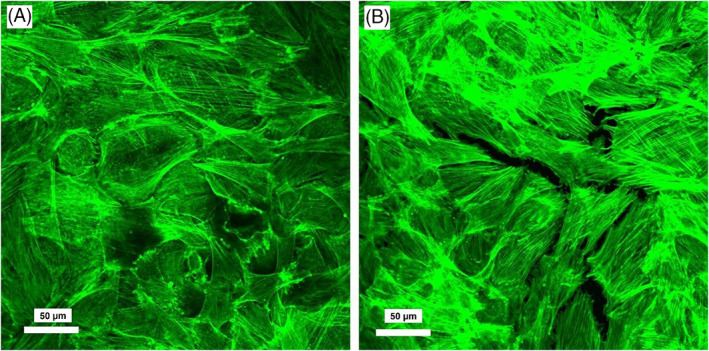
Representative images of AF cells cultured in glass bottom confocal dishes for 6 days. (A) Cells with no treatment; (B) cells treated with LPS. Images were acquired by an inverted confocal laser scanning microscope (Olympus Fluoview 1000; Olympus Life Sciences). Actin filaments formation and organization appeared unaffected by LPS
FIGURE 9.
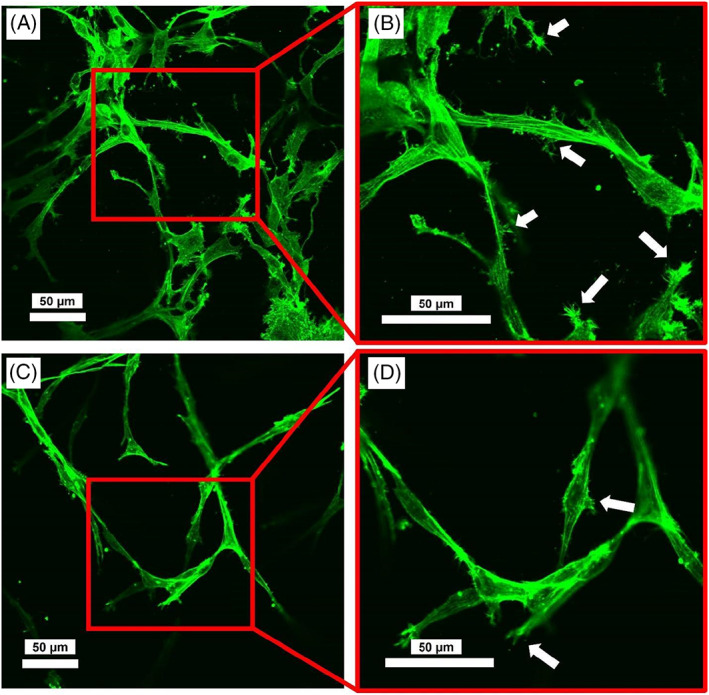
Representative images of cells+collagen constructs 6 days postculture; (A and B) Constructs without LPS treatment at different magnifications (×20 and ×40); (C and D) constructs treated with LPS. Images were acquired by an inverted confocal laser scanning microscope (Olympus Fluoview 1000; Olympus Life Sciences). Arrows indicate the cellular protrusions. Actin filaments formation and organization were not affected; however, cell processes/protrusions appeared to be shorter and less visible for constructs treated with LPS
On the other hand, for cells cultured inside collagen matrices, while actin filaments were formed and were visible, we observed that cell processes/protrusions might have been affected by LPS (Figure 9). Protrusions are cell membrane extensions containing actin filaments involved in cell adhesion and migration. 49 For cells in constructs that were not exposed to LPS, cells had more visible processes than cells in constructs exposed to LPS.
4. DISCUSSION
In this study, we used type I collagen as a 3D substrate material in which AF cells could grow, in vitro. We showed that this model can be manipulated and utilized to advance our understanding of the fundamental cellular processes that can occur in the extracellular matrix of the IVD. The most notable observation was a distinct and significant contraction that AF cells induced on the collagen. Further, we used LPS, as a known inflammatory stimulant in IVD, as a treatment within our model. Interestingly, we observed that AF cells were viable within our collagen constructs and that LPS negatively altered the mechanical properties of these constructs.
To study the viability of our AF cells cultured inside collagen, alamarBlue and 5‐CFDA‐AM paired with confocal microscopy was utilized. While cells were metabolically active and viable, an interesting observation was a higher density of fibers around the cell compared to more distance locations which may indicate a mechanical effect by the cells on the surrounding fibers compared to more distant locations. Similarly, Bowles et al. 39 previously showed that AF cells can realign collagen fibers around them. We also observed a distinct and significant contraction that cells induced on the collagen. In fact, the first sign of AF cells interacting with collagen was a robust contraction induced by the cells on their matrix. Cells decreased the length of the construct by almost 50% within the first 24 h and retained this contraction over the following 6 days in culture. Similar collagen contraction has been previously reported for fibroblasts 50 , 51 , 52 which was hypothesized to be linked to cell attachment to the matrix and traction forces being applied by cells. 53 , 54 AF cells are in direct contact with type I collagen fibers in native tissue, therefore, once cultured in collagen, they can form focal adhesions and attach to this matrix and subsequently apply traction forces that cause this distinct contraction. Since we found that fixed cells did not cause the contraction, we conclude that it is due to the cells directly interacting with this matrix. We also showed that cells inside collagen substrates with higher thickness were not capable of effectively inducing the contraction; it is hypothesized that cells within these thicker constructs were not able to access nutrients and rid their waste which appeared to negatively affect their ability to mechanically interact with the surrounding collagen. Another possibility could also be that cells were not able to physically contract higher amount of collagen fibers effectively.
While alginate is a very common material for in vitro studies of AF cells, 40 , 41 , 55 collagen has not been widely used for AF cells before. Collagen is a natural biomaterial and mimics the native tissue better than alginate. Bowles et al. 39 and Takai et al. 54 have previously shown that AF cells can induce contraction on collagen matrices and that can be used to manipulate the alignment of collagen fibers. As an application of our model, we treated the cells+collagen constructs with LPS, as a known inflammatory stimulant. This treatment negatively, and significantly, affected collagen contraction. Studying the viability of AF cells cultured in collagen and treated with LPS 6 days postculture revealed that after incubation with the assay for 1 h, cells show significantly lower viability), which could potentially be a reason for lower contraction in these samples. However, after 2 h of incubation with alamarBlue, cells viability for samples with no treatment and treated with LPS were not significantly different. This could be either because the assay had enough time to fully penetrate in collagen or it could simply be because the readings are reaching the saturation values.
The cytoskeleton structure of cells inside collagen may provide insight into these observed changes. Although imaging used in the current study was limited, LPS‐treated cells inside collagen appeared to show fewer cell protrusions and the protrusions and extensions present appeared to be shorter compared to untreated samples. Collagen contraction, specifically by fibroblasts, has been previously linked to cell extension formation. 54 It is possible that a similar mechanism exists here. The fact that these subtle changes were not observed when cells were cultured in glass bottom confocal dishes may reveal more details about these cells. When cultured on glass, AF cells are adhering to a much stiffer substrate compared to collagen. As a result, subtle changes in cell protrusions were likely obscured by the interaction of the cells and stiff substrate, which could, on its own, be an indication of the AF cells sensitivity to the stiffness of their surroundings. It is unclear whether the reduced viability in the LPS‐treated samples affected the changes observed in the cellular protrusions, although likely so. While Figure 8 shows that cells cultured in monolayer treated with LPS have very similar viability, morphology, and cytoskeleton structure compared to untreated cells, when inside a 3D matrix, cellular viability, and protrusions could have been affected. It is difficult to say if decreased viability is the cause of lower contraction or whether LPS is affecting cell attachment. More comprehensive image analysis is needed to confirm this notion. Either way, these experiments demonstrate versatility of this model. While cells, 56 , 57 including IVD cells, 38 , 39 have been previously cultured in collagen in various forms, our model explains a relatively easy and versatile matrix that can be used for not only mechanical testing but also the interplay between inflammation and mechanics for AF cells, which then provides insight into the functional impact of structural changes. Another hypothesis could be that adding LPS to cells+collagen constructs has caused an increase in matrix metalloproteinases (MMPs) secretion which in turn has affected matrix molecules. MMPs have been previously shown to affect collagen contraction by lung fibroblasts. 58
Studying the mechanical properties of the cells+collagen constructs showed that contracted collagen matrices have higher strength and deform more than acellular collagen. Constructs treated with LPS had the lowest elastic modulus, though not statistically significant, from untreated constructs. However, strength was found to be significantly affected by LPS with the lowest strength in samples treated with LPS. Furthermore, the LPS‐treated constructs failed at a lower deformation indicating reduced ductility. All these changes are expected based on lower cell viability and reduced contraction as well as potentially fewer cellular protrusions. Also, increased MMP secretion could also play a role in mechanical changes of the constructs. While our substrate does not have the organized structure of the native AF extracellular matrix, we can speculate that in IVD tissue, the presence of strong inflammatory agents could likely also affect the mechanical properties of the IVD through directly affecting cell‐substrate adhesion. This can then affect the mechanical performance of the IVD under inflammation.
5. LIMITATIONS AND FUTURE RESEARCH
In this research, we utilized type I collagen as a 3D model material and cultured AF cells of the IVD. We then studied the effect of an inflammatory stimulus, specifically LPS, on the mechanical properties of the final cells and collagen constructs. While this study has generated insight into the interaction between annulus cells and surrounding matrix, there are a number of limitations that need to be addressed as well as future directions.
The collagen used was made by self‐assembly of the collagen fibers and lacks the organization of collagen fibers in the native AF tissue. This may affect cell–matrix interactions.
We reported here that cells exposed to LPS may show fewer and smaller protrusions on their surface. To further study this observation and confirm the hypothesis that LPS negatively impacts cell interactions with the ECM, staining for focal adhesion and contractile proteins as well as mechanobiological factors associated with cellular tension (e.g., YAP/TAZ or MRTFa) should be considered.
Future experiments should consider the effect of time on both viability and mechanical properties of the constructs to help determine the time‐dependent behavior of AF cells and their interaction with collagen.
Studying the cellular catabolic and anabolic markers (such as various MMPs) should also be considered to further provide insight into the interaction of these cells and collagen.
6. CONCLUSIONS
Our findings demonstrate that type I collagen can be a useful model material for in vitro studies of IVD disruption, and in particular AF damage. Using this model, we report that AF cells induce a significant contraction on collagen substrates, which can then be used as an indicator for the health of these cells in the matrix. We also showed that this model can be easily manipulated for further detailed in vitro testing. For example, LPS, as a known inflammatory stimulant in IVDs, altered the mechanical properties of our constructs; an observation that may be a result of an altered capability of cells to produce cell extensions and protrusions.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Sara Molladavoodi and Diane E. Gregory designed the experiments in consultation with Stephanie J. Dewitte‐Orr. Sara Molladavoodi performed the experiments and analyzed the data. Stephanie J. Dewitte‐Orr and Diane E. Gregory provided experimental setup and laboratories. Stephanie J. Dewitte‐Orr provided technical insight. All the authors discussed the results and wrote the manuscript. Funding for this project was provided by the Natural Sciences and Engineering Research Council of Canada; grant held by Diane E. Gregory.
ACKNOWLEDGMENT
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Molladavoodi, S. , DeWitte‐Orr, S. J. , & Gregory, D. E. (2022). An in vitro 3D annulus fibrosus cell culture model with type I collagen: An examination of cell–matrix interactions. JOR Spine, 5(1), e1193. 10.1002/jsp2.1193
Funding information Natural Sciences and Engineering Research Council of Canada
REFERENCES
- 1. Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: a systematic analysis for the global burden of disease study 2016. The Lancet. 2017;390(10100):1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katz JN. Lumbar disc disorders and low‐back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21‐24. [DOI] [PubMed] [Google Scholar]
- 3. DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12:224‐233. [DOI] [PubMed] [Google Scholar]
- 4. Vergroesen PPA, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015;23:1057‐1070. [DOI] [PubMed] [Google Scholar]
- 5. Smith LJ, Elliott DM. Formation of lamellar cross bridges in the annulus fibrosus of the intervertebral disc is a consequence of vascular regression. Matrix Biol. 2011;30:267‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159‐171. [DOI] [PubMed] [Google Scholar]
- 7. Newell N, Little JP, Christou A, Adams MA, Adam CJ, Masouros SD. Biomechanics of the human intervertebral disc: a review of testing techniques and results. J Mech Behav Biomed Mater. 2017;69:420‐434. [DOI] [PubMed] [Google Scholar]
- 8. Holzapfel GA, Schulze‐Bauer C a J, Feigl G, Regitnig P. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech Model Mechanobiol. 2005;3:125‐140. [DOI] [PubMed] [Google Scholar]
- 9. Hsieh AH, Twomey JD. Cellular mechanobiology of the intervertebral disc: new directions and approaches. J Biomech. 2010;43:137‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012;221:480‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomaszewski KA, Saganiak K, Gładysz T, Walocha JA. The biology behind the human intervertebral disc and its endplates. Folia Morphol. 2015;74:157‐168. [DOI] [PubMed] [Google Scholar]
- 12. Gregory DE, Bae WC, Sah RL, Masuda K. Anular delamination strength of human lumbar intervertebral disc. Eur Spine J. 2012;21:1716‐1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams MA, Hutton WC. Gradual disc prolapse. Spine. 1985;10:524‐531. [DOI] [PubMed] [Google Scholar]
- 14. Adams MA, Freeman BJC, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625‐1636. [DOI] [PubMed] [Google Scholar]
- 15. Alkhatib B, Rosenzweig DH, Krock E, et al. Acute mechanical injury of the human intervertebral disc: link to degeneration and pain. Eur Cells Mater. 2014;28:98‐111. [DOI] [PubMed] [Google Scholar]
- 16. Alpantaki K, Katonis P, Hadjipavlou AG, Spandidos DA, Sourvinos G. Herpes virus infection can cause intervertebral disc degeneration: a causal relationship? J Bone Jt Surg. 2011;93:1253‐1258. [DOI] [PubMed] [Google Scholar]
- 17. Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691‐2699. [DOI] [PubMed] [Google Scholar]
- 19. Setton LA, Chen J. Cell mechanics and mechanobiology in the intervertebral disc. Spine. 2004;29:2710‐2723. [DOI] [PubMed] [Google Scholar]
- 20. Chen J, Yan W, Setton LA. Static compression induces zonal‐specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22:573‐583. [DOI] [PubMed] [Google Scholar]
- 21. Murai K, Sakai D, Nakamura Y, et al. Primary immune system responders to nucleus pulposus cells: evidence for immune response in disc herniation. Eur Cell Mater. 2010;19:13‐21. [DOI] [PubMed] [Google Scholar]
- 22. Ahn S‐H, Cho Y‐W, Ahn M‐W, Jang S‐H, Sohn Y‐K, Kim H‐S. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911‐917. [DOI] [PubMed] [Google Scholar]
- 23. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin‐1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732‐R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murata Y, Onda A, Rydevik B, Takahashi I, Takahashi K, Olmarker K. Changes in pain behavior and histologic changes caused by application of tumor necrosis factor‐alpha to the dorsal root ganglion in rats. Spine. 2006;31:530‐535. [DOI] [PubMed] [Google Scholar]
- 25. Hayashi S, Taira A, Inoue G, et al. TNF‐alpha in nucleus pulposus induces sensory nerve growth: a study of the mechanism of discogenic low back pain using TNF‐alpha‐deficient mice. Spine. 2008;33:1542‐1546. [DOI] [PubMed] [Google Scholar]
- 26. Matsui Y, Maeda M, Nakagami W, Iwata H. The involvement of matrix metalloproteinases and inflammation in lumbar disc herniation. Spine. 1998;23:863‐869. [DOI] [PubMed] [Google Scholar]
- 27. Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998;23:1612‐1626. [DOI] [PubMed] [Google Scholar]
- 28. Teixeira GQ, Pereira CL, Ferreira JR, et al. Immunomodulation of human mesenchymal stem/stromal cells in intervertebral disc degeneration: insights from a proinflammatory/degenerative ex vivo model. Spine. 2018;43:E673‐E682. [DOI] [PubMed] [Google Scholar]
- 29. Krock E, Rosenzweig DH, Currie JB, Bisson DG, Ouellet JA, Haglund L. Toll‐like receptor activation induces degeneration of human intervertebral discs. Sci Rep. 2017;7:17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajan NE, Bloom O, Maidhof R, et al. Toll‐like receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration. Spine. 2013;38:1343‐1351. [DOI] [PubMed] [Google Scholar]
- 31. Qin C, Zhang B, Zhang L, et al. MyD88‐dependent toll‐like receptor 4 signal pathway in intervertebral disc degeneration. Exp Ther Med. 2016;12:611‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shah BS, Burt KG, Jacobsen T, et al. High mobility group box‐1 induces pro‐inflammatory signaling in human nucleus pulposus cells via toll‐like receptor 4‐dependent pathway. J Orthop Res. 2019;37:220‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krock E, Currie JB, Weber MH, et al. Nerve growth factor is regulated by toll‐like receptor 2 in human intervertebral discs. J Biol Chem. 2016;291:3541‐3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gawri R, Moir J, Ouellet J, et al. Physiological loading can restore the proteoglycan content in a model of early IVD degeneration. PLoS ONE. 2014;9:e101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang D‐L, Jiang S‐D, Dai L‐Y. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine. 2007;32:2521‐2528. [DOI] [PubMed] [Google Scholar]
- 36. Abbott RD, Howe AK, Langevin HM, Iatridis JC. Live free or die: stretch‐induced apoptosis occurs when adaptive reorientation of annulus fibrosus cells is restricted. Biochem Biophys Res Commun. 2012;421:361‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tisherman R, Coelho P, Phillibert D, et al. NF‐κB signaling pathway in controlling intervertebral disk cell response to inflammatory and mechanical stressors. Phys Ther. 2016;96:704‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neidlinger‐Wilke C, Würtz K, Liedert A, et al. A three‐dimensional collagen matrix as a suitable culture system for the comparison of cyclic strain and hydrostatic pressure effects on intervertebral disc cells. J Neurosurg Spine. 2005;2:457‐465. [DOI] [PubMed] [Google Scholar]
- 39. Bowles RD, Williams RM, Zipfel WR, Bonassar LJ. Self‐assembly of aligned tissue‐engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng Part A. 2010;16:1339‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. le Maitre CL, Frain J, Fotheringham AP, Freemont AJ, Hoyland JA. Human cells derived from degenerate intervertebral discs respond differently to those derived from non‐degenerate intervertebral discs following application of dynamic hydrostatic pressure. Biorheology. 2008;45:563‐575. [PubMed] [Google Scholar]
- 41. Korecki CL, Kuo CK, Tuan RS, Iatridis JC. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res. 2009;27:800‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. le Maitre CL, Frain J, Millward‐Sadler J, Fotheringham AP, Freemont AJ, Hoyland JA. Altered integrin mechanotransduction in human nucleus pulposus cells derived from degenerated discs. Arthritis Rheum. 2009;60:460‐469. [DOI] [PubMed] [Google Scholar]
- 43. Reza AT, Nicoll SB. Hydrostatic pressure differentially regulates outer and inner annulus fibrosus cell matrix production in 3D scaffolds. Ann Biomed Eng. 2008;36:204‐213. [DOI] [PubMed] [Google Scholar]
- 44. Yi W, Wen Y, Tan F, et al. Impact of NF‐kappaB pathway on the apoptosis‐inflammation‐autophagy crosstalk in human degenerative nucleus pulposus cells. Aging. 2019;11:7294‐7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qin C, Lv Y, Zhao H, Yang B, Zhang P. MicroRNA‐149 suppresses inflammation in nucleus pulposus cells of intervertebral discs by regulating MyD88. Med Sci. 2019;25:4892‐4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu M‐H, Sun J‐S, Tsai S‐W, Sheu S‐Y, Chen M‐H. Icariin protects murine chondrocytes from lipopolysaccharide‐induced inflammatory responses and extracellular matrix degradation. Nutr Res. 2010;30:57‐65. [DOI] [PubMed] [Google Scholar]
- 47. Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFα in intervertebral disc degeneration: a non‐recoverable catabolic shift. Biochem Biophys Res Commun. 2013;433(1):151‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Du J, Pfannkuche J‐J, Lang G, et al. Proinflammatory intervertebral disc cell and organ culture models induced by tumor necrosis factor alpha. Jor Spine. 2020;3:e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alexandrova AY, Arnold K, Schaub S, et al. Comparative dynamics of retrograde Actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PloS One. 2008;3:e3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bell E, Ivarsson B, Merrill C. Production of a tissue‐like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci. 1979;76:1274‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bellows CG, Melcher AH, Aubin JE. Contraction and organization of collagen gels by cells cultured from periodontal ligament, gingiva and bone suggest functional differences between cell types. J Cell Sci. 1981;50:299‐314. [DOI] [PubMed] [Google Scholar]
- 52. Schiro JA, Chan BM, Roswit WT, et al. Integrin alpha 2 beta 1 (VLA‐2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991;67:403‐410. [DOI] [PubMed] [Google Scholar]
- 53. Coelho NM, Arora PD, van Putten S, et al. Discoidin domain receptor 1 mediates myosin‐dependent collagen contraction. Cell Rep. 2017;18:1774‐1790. [DOI] [PubMed] [Google Scholar]
- 54. Grinnell F. Fibroblast‐collagen‐matrix contraction: growth‐factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362‐365. [DOI] [PubMed] [Google Scholar]
- 55. Wenger KH, Woods JA, Holecek A, Eckstein EC, Robertson JT, Hasty KA. Matrix remodeling expression in anulus cells subjected to increased compressive load. Spine. 2005;30:1122‐1126. [DOI] [PubMed] [Google Scholar]
- 56. Mikami Y, Matsuzaki H, Takeshima H, Makita K, Yamauchi Y, Nagase T. Development of an in vitro assay to evaluate contractile function of mesenchymal cells that underwent epithelial‐mesenchymal transition. J Vis Exp. 2016;2016:e53974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang T, Day JH, Su X, et al. Investigating fibroblast‐induced collagen gel contraction using a dynamic microscale platform. Front Bioeng Biotechnol. 2019;7:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fang Q, Schulte NA, Kim H, et al. Effect of budesonide on fibroblast‐mediated collagen gel contraction and degradation. J Inflam Res. 2013;6:25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]


