Abstract
The development of innovative single-cell technologies has allowed the high-dimensional transcriptomic and proteomic profiling of individual blood and tissue cells. Recent single-cell studies revealed a new cellular heterogeneity of atherosclerotic plaque tissue and allowed a better understanding of distinct immune functional states in the context of atherosclerosis. In this brief review, we describe how single-cell technologies have shed a new light on the cellular composition of atherosclerotic plaques, and their response to diet perturbations or genetic manipulation in mouse models of atherosclerosis. We discuss how scRNA-seq, CITE-seq, ATAC-seq, and CyTOF platforms have empowered the identification of discrete immune, endothelial, and smooth muscle cells alterations in atherosclerosis progression and regression. Finally, we review how single cell approaches have allowed mapping the cellular and molecular composition of human atherosclerotic plaques and the discovery of new immune alterations in plaques from patients with stroke.
Keywords: single-cell technologies, atherosclerosis, immune cells, non-immune cells, heterogeneity
Introduction
Atherosclerosis arises from the accumulation of oxidized low-density lipoprotein (oxLDL) in the subendothelial space of medium and large size arteries and consequent macrophage accumulation to clear the arterial wall from lipids1, 2. As the disease progresses, macrophage phagocytic function becomes defective, resulting in the progressive accumulation of dead cells and cholesterol crystals in the necrotic core3-8. These processes sustain a persistent and complex inflammatory microenvironment which consists of macrophages, T cells, natural killer (NK) cells, NKT cells, B cells and neutrophils8-14. The intrinsic capacity of immune cells for tissue adaptation creates a highly specialized plaque microenvironment whose nuances and impact on the disease remain to be fully discovered15. The balance between pro-atherogenic or pro-resolving plaque macrophages dictates either plaque progression or repair in mouse models3-5, 16-18, but the drivers of this plasticity remain to be fully elucidated to be exploited therapeutically. Similarly, plaque T cells present a profound diversity that ranges from pro-atherogenic CD4+ T helper (Th)-1 cell to anti-atherogenic Th-2 cells, as well as Th-17 cells and heterogenous subsets of cytotoxic CD8+ T cells with disease-associated functions still not fully understood11, 15, 19, 20. The functional complexity of plaque immune cells is also underscored by the heterogeneity of T regulatory (Treg) cells11. Although Tregs are canonically considered a unique population of anti-atherogenic cells, they are more heterogenous than initially thought comprising subsets with pro-atherogenic functions11. The CANTOS21 and the COLCOT22 trials provided the first in-human evidence of a causative role of inflammation in atherosclerotic cardiovascular disease (CVD), prompting a revived interest in the field of immune mechanisms of human disease. Since then, a deep understanding of the inherent immune cell diversity in the atherosclerotic vasculature has become a research priority, and a prerequisite for the successful development of precise immunotherapies for patients with CVD15. In this review, we discuss how single-cell technologies have revealed a new understanding of the cellular landscape of murine and human atherosclerosis plaques and deeply impacted research directions in the field of vascular biology.
Single-cell technologies uncover new cellular features in atherosclerosis
The rapid evolution of single-cell technologies in the past decade has radically transformed our ability to characterize the cellular heterogeneity of blood and tissue specimens23-25 (Figure 1A). Unlike bulk RNA sequencing—that averages transcriptional expression across cells and cannot discriminate the transcriptional variation of individual cells— single-cell RNA sequencing (scRNA-seq) quantifies the gene expression of individual cells within normal and pathological contexts. Using scRNA-seq, apparently similar cell populations can be deconstructed into subpopulations with distinct transcriptional profiles that reflect diverse metabolic states and unique biological functions (Figure 1B)26-29. The unbiased computational approaches used to analyze scRNA-seq data are ideal to discover new immune cell clusters, including rare ones, and to predict cellular trajectories based on the transcriptional transition of cells along a dynamic biological process29. Using scRNA-seq data is also possible to infer specific ligand–receptor interactions associated with cell development and disease states, a method that can identify key cell–cell communications within heterogeneous tissues30-32. These computational methods have been comprehensively reviewed in33, 34.
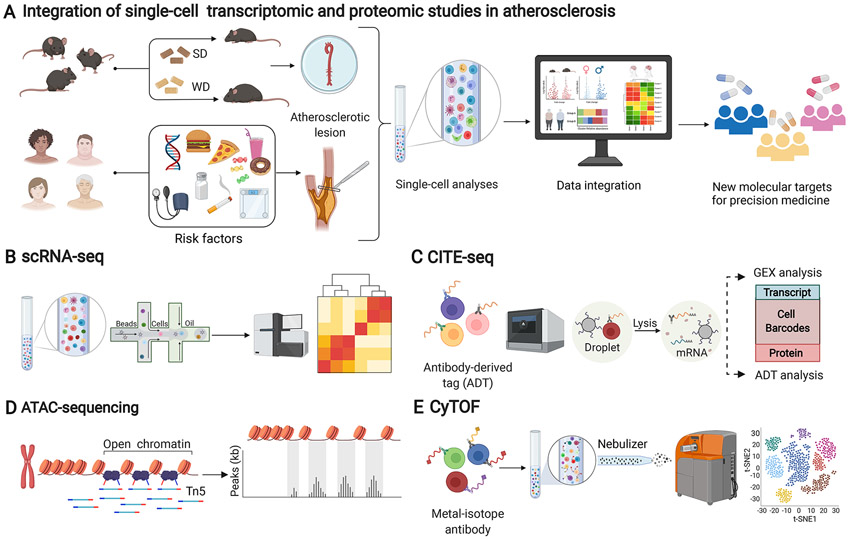
Figure 1. Schematic overview of single-cell transcriptomic and proteomic analysis of experimental and human atherosclerosis to advance precision medicine.
(A) Atherosclerotic tissue from either experimental mouse models of atherosclerosis or patients with atherosclerotic cardiovascular disease is used for single-cell analysis and subsequent data integration across species. Mechanistic studies in mouse experimental models provide comprehensive identification of possible targets to design new therapeutic strategies for precision medicine in patients. (B) scRNAseq detects the transcriptome of individual cells to allow thousands of micro-reactions to occur in parallel to identify transcriptional variations of individual cells. (C) CITE-seq allows the simultaneous analysis of the transcriptome and the proteome of individual cells immunostained with DNA-barcoded antibodies to convert their detection into a sequenceable readout. (D) scATAC-seq identifies chromatin accessible regions by inserting adapters with Tn5 transposase. After sequencing, the reads can then be used to infer genomic regions of increased accessibility and to map regions of transcription factor binding. (E) CyTOF allows a high-dimensional, multi-parametric quantitative protein detection analysis that uses time-of-flight mass spectrometer as a readout. The single-cell suspension is stained with antibodies conjugated with metal isotopes with no spectral overlap. SD: standard laboratory diet; WD: western diet; GEX: gene expression; sc-RNAseq: single cell RNA sequencing; CITEseq: Cellular Indexing of Transcriptomes and Epitopes by Sequencing; sc-ATACseq: Assay for Transposase-Accessible Chromatin sequencing; CyTOF: Cytometry by Time-Of-Flight. The figure was created with Biorender (biorender.com).
The advancement of new scRNA-seq technologies has allowed researchers to fully recapitulate the transcriptional landscape of cells from tissues relevant to CVD, often using limited clinical tissue samples from which the isolation of sufficient amounts of viable cells is a challenge. A comprehensive guide for the design and implementation of sequencing technologies to study atherosclerosis has been comprehensively discussed in15, 35, 36. A possible drawback of scRNA-seq consists in the lack of precise references based on canonical markers for cell annotation based on prior knowledge. To overcome this limitation, in 2017 Stoeckius and colleagues37 introduced a new single-cell multi-omics method called cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq). CITE-seq relies on the use of oligonucleotide-labelled antibodies to allow the simultaneous analysis of surface protein markers and gene expression in a single cell with high-throughput (Figure 1C). The method is compatible with existing scRNA-seq methods and can achieve an unparalleled characterization of immune cell populations based on gene expression through the simultaneous identification of canonical protein markers on the same cell. Additional technological advancements include the development of single-cell transposase-accessible chromatin with sequencing (scATACseq) to provide complementary information to both scRNAseq and CITE-seq38 (Figure 1D). Specifically, scATACseq allows the analysis of open chromatin regions (OCR), regions of the genome that are accessible to the binding of DNA regulatory elements that influence gene expression. scATAC-seq utilizes the enzyme Tn5 transposase to insert sequencing adapters into OCR to study gene expression in these regions. scATAC-seq is an efficient method to identify epigenetic changes of heterogeneous cell phenotypes in response to factors like age, stress, environment, disease or chronic antigen stimulation39. Cytometry by time of flight (CyTOF) is single-cell proteomic quantitative method that allows high dimensional profiling of individual cell from limited heterogeneous samples by using metal isotope detection in discrete peaks, overcoming the limitations of spectral overlap and compensation requirements for fluorochrome signals when using conventional flow cytometry (Figure 1E). CyTOF has allowed deeper understanding of the heterogeneity of cells in blood and tissues both at the molecular and phenotypic levels by using several analytical tools and algorithms to decipher the data in an unbiased manner. Basic methods to effectively analyze CyTOF complex data are described in15, 40.
Single-cell immune cell heterogeneity of atherosclerosis in mice
Although atherosclerosis has been extensively studied for several decades, the cellular diversity of atherosclerotic plaques remains to be fully understood. Recent scRNA-seq studies of apolipoprotein E (Apoe)−/− and low-density lipoprotein receptor (Ldlr)−/− mice, fed either an atherogenic diet or a standard laboratory diet (SD), have provided a first glimpse into the different origin, metabolic and activation states of plaque immune cell populations at different time points during atherogenesis. (Table 1). Using scRNA-seq, Winkels et al.41 characterized aortic leukocytes from Apoe−/− and Ldlr−/− mice. The analysis of the thoracic and abdominal aorta of 8 weeks old Apoe−/− mice fed a western diet (WD) for 12 weeks, showed that the complexity of the immune cell compartment evolved with plaque progression. Specifically, the 5 clusters of leukocytes detected in the aorta from Apoe−/− mice fed a SD progressed into the 11 aortic clusters found in Apoe−/− mice fed a WD. In particular, the frequency of macrophage, Th-17 and CD4/CD8 mixed T cell clusters increased, while Th-2 and monocyte clusters diminished. When compared to the atherosclerotic aorta from Ldlr−/− mice fed either a SD or a WD, plaque immune cell composition was largely comparable, except for a higher content of B cells in Apoe−/− mice and a higher number of macrophages in Ldlr−/− mice. Using a similar experimental design and mouse models, Cochain and colleagues42 performed an unsupervised clustering of CD45+ cells from the aorta of both Ldlr−/− mice fed either a WD or a SD. A focused analysis of the myeloid cell compartment identified tissue resident-like macrophages in mice fed either a SD or a WD. In contrast, inflammatory Il1b+Nrlp3+ macrophages and Trem2hi macrophages were exclusively enriched in the aorta from mice fed a WD. Aortic tissue resident-like macrophages expressed resident (i.e. F13a1 and Lyve1) and M2-like genes (i.e. Folr2, CBR2, and Mrc1), and low levels of transcripts encoding pro-inflammatory cytokines. Trem2hi cells displayed a unique gene signature which included cholesterol metabolism, cholesterol efflux and oxidative phosphorylation functions, resembling a foamy phenotype42. Notably, Trem2hi foamy-like macrophages were described independently by both Kim et al43 and Lin and colleagues44, supporting the robustness of scRNA-seq analysis across studies, despite differences in the experimental design and methods used (Table 1). Cochain et al42 also identified other plaque myeloid clusters including a monocyte cluster expressing Ly6c2, Ccr2 and Csf1r, and a monocyte-derived dendritic cell cluster expressing Cd209a, Cd74, Flt3, and H2-eb1. Interestingly, while all resident, inflammatory and Trem2hi macrophage clusters seen in Ldlr−/− were confirmed in Apoe−/− and Ldlr−/− mice, the overall frequency of macrophages was lower in Apoe−/−42. This discrepancy might be not only related to differences in the genetic mouse model used but also to variations in sex and the type and duration of the diet used (Table 1).
Table 1.
Single cell analysis of normal and atherosclerotic vasculature in mice
| Cell type | Tissue | Experimental model design | Sex | Type of diet | Duration of diet (weeks) |
Single cell technology |
Tissue dissociation methods | Single cell isolation | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| IMMUNE CELLS | Leukocytes | aorta | Ldlr−/−; Apoe−/− | female (Apoe−/−) | WD (0,2% chol, 42% fat- Envigo Teklad, TD 88137); HCD (unspecified composition); SD | 12 w | scRNA-seq; CyTOF | Enzymatic digestion (HBSS with 45 U/mL Collagenase I, 250 U/mL Collagenase XI, 120 U/mL Hyaluronidase, 120 U/mL DNAse I) for 1h at 37° C followed by resting for 1h | FACS sorting of CD45+ cells | 41 |
| Aortic Macrophages | aorta | Ldlr−/−; Apoe−/− | female (Apoe−/−); male (LDLr−/−) | Atherogenic diet (15% milk fat, 1.25% cholesterol; Altromin); WD (0.2% chol, 42% fat- Envigo Teklad, TD 88137); SD | 11-20 w (atherogenic diet); 12 w (WD) | scRNA-seq | Enzymatic digestion (RPMI with 450U/ml Collagenase I, 125U/ml Collagenase XI and 60U/ml hyaluronidase) for 40 minutes at 37°C | FACS sorting of CD45+ cells | 42 | |
| Foamy and non-foamy macrophages | aorta | Apoe−/−(AAV8 injection); Ldlr−/−; Cxcr1GFP; LysMCre; Rosa26tdTomato; D374YhPCSK9transgenic mice | male | WD (0.15% chol,20% fat -TD AIN-76A); SD | 19 w (D374-hPCSK9 mice); 12-25 w (Ldlr−/−); 12-22 w (Apoe−/− regression model) | scRNA-seq | Enzymatic digestion (PBS solution with calcium and magnesium with 90 U/mL DNase I , 675 U/mL collagenase I, 187.5 U/mL collagenase XI, 90 U/mL hyaluronidase) at 37 °C for 70 min with gentle shaking | FACS sorting of CD45+ and SSChiBODIPYhi cells | 43 | |
| CX3CR1+ precursor derived macrophages | aortic arch | Cx3cr1 CreERT2-EYFP; Rosa26tdTomato; Ldlr−/− (AAV-mPCSK9); Regression model switched to SD and ApoB-ASO (50mg/kg twice a week for 2 weeks). | male | WD (Dyets Inc #101977); SD | 18 w | scRNA-seq | Enzymatic digestion (Dulbecco’s PBS with 4 U/ml liberase TH, 0.1 mg/ml DNase I, 60 U/ml hyaluronidase) at 37°C for 60 min | FACS sorting of CD11b+TdTomato+ cells | 44 | |
| Leukocytes | aorta | Apoe−/− | unspecified | WD (cholate free high fat diet: 15% cocoa butter; 0.25% cholesterol; 40.5% sucrose; Special Diet Services) | 12 w | CyTOF | Enzymatic digestion with enzyme cocktail (containing 450 U/mL collagenase type I, 125 U/mL collagenase type XI, 60 U/mL hyaluronidase, and 60 U/mL Dnase) at 37 °C for 50 min | NA | 45 | |
| Aortic intima resident macrophages (MacAIR) | aorta | C57BL/6; Ldlr−/−; Cx3cr1CreERT2; Rosa26LSL-Tomato | unspecified | WD (0,2% chol, 42% fat -Envigo Teklad, TD 88137); Tamoxifen diet (500 mg/kg tamoxifen; Envigo Tecklad, TD 130857); SD | 3 w | scRNA-seq | Enzymatic digestion (PBS solution with calcium and magnesium with 90 U/mL DNase I , 675 U/mL collagenase I, 187.5 U/mL collagenase XI, 90 U/mL hyaluronidase) at 37 °C for 70 min with gentle shaking | FACS sorting of CD45+ cells | 47 | |
| Leukocytes | aortic arch | Ntn1fl/flCx3cr1CreERT2+; Ntn1fl/flCx3cr1WT (AAV-mPCSK9) | male | WD (Dyets Inc #101977); SD | 20 w (WD); 4 w (SD) | scRNA-seq | Enzymatic digestion with 5 mg/ml Liberase, 99 ug/ml hyaluronidase and 58 ug/ml DNase I for 15min at 37C° using the GentleMacs dissociator (Miltenyi). | FACS sorting of CD45+ cells | 55 | |
| Leukocytes | aortic arch | Ldlr−/− mice trated with anti-miR33 | male | WD (Dyets Inc #101977); SD | 14 w | scRNA-seq | Enzymatic and mechanic digestion using liberase, hyaluronidase, DNase I and 1M CaCl2 at 37°C for 15 min using the GentleMacs dissociator (Miltenyi) | FACS sorting of CD45+ cells | 18 | |
| NON-IMMUNE CELLS | VSMC | aortic root and ascending aorta | TgMyh11CreERT2; Tcf21flox/flox;Rosa26Sortm14(CAGtdTomato)Hze; Apoe−/− | male (Myh11CreERt2 transgene is linked to chromosome Y | HFD (0,15% chol, 21% milk fat, Dyets TD 31359001); SD | 8-16 w | scRNA-seq; CITE-seq | Mouse aorta: enzymatic digestion (HBSS with 2 U ml−1Liberase and 2 U ml−1elastase) at 37 °C for 1 h | FACS sorting of Tdt+ and Tdt− cells | 56 |
| VSMC | BCA, ascending and thoracic aorta | Cg-GtRosa26Sortm6(ZsGreen1)Hze; Myh11CreERT2; Ldlr−/−; Apoe−/− | male (Myh11CreERt2 transgene is linked to chromosome Y) | WD (0,2% chol, 42% fat- Envigo Teklad, TD 88137); SD | 8-26 w | sc-RNAseq | Enzymatic digestion (RPMI-1640 with 4 U/mL Liberase TM, 60 U/mL hyaluronidase, and 120 U/mL DNase I) at 37°C for 45 min | FACS sorting of Live/ZsGreen1+ and ZsGreen1− cells | 57 | |
| VSMC; Endothelial cells | BCA | Myh11CreERT2; RosaeYFP;Apoe−/−;Cdh5CreERT2; SMCKlf4; SMCOct4; Myh11DreERT2; Lgals3Cre; RosatdTomato-eGFP | male (Myh11CreERt2 transgene is linked to chromosome Y); female/male (Myh11DreERT2) | WD (0,15% chol, 21% milk fat- Envigo Teklad); SD | 10-18 w | sc-RNAseq | Mouse aorta: enzimatic digestion with 1mL Liberase and 1ug/mL ActinomycinD at 37 °C for 1h | FACS sorting of GFP+, eYFP+and tdT+ cells | 58 | |
| VSMC | aortic arch and descending thoracic aorta | Myh11CreERt2; Rosa26Confetti; Rosa26eYFP; Apoe−/−; Sca1GFP | male (Myh11CreERt2 transgene is linked to chromosome Y) | HCD (0,2% chol, 21% fat- Special Diets Services) | 14-18 w | scRNA-seq | Enzymatic digestion: following endothelial cells manual removal using a cotton bud, vessels were dissociated (DMEM with 1 mg/ml Collagenase Type IV, 1 U/ml porcine pancreatic elastase) for 10 min to allow for separation of the adventitia and medial cell layers and then further digested for 1–2 h | FACS sorting of Confetti+, GFP+and GFP−cells | 59 | |
| Endothelial cells | carotid artery | C57BL/6 (PCL) | male | SD | NA | scRNA-seq; ATAC-seq | Enzymatic digestion (PBS with 0.5% FBS, 125 U/ mL collagenase type XI, 60 U/mL hyaluronidase type 1-s, 60 U/mL DNase I, and 450 U/mL collagenase type I, 600 U ml-1 Type II Collagenase and 60 U ml-1DNase I for 1 hour at 37°C. | NA | 60 | |
| Adventitial cells | adventitial aorta | C57BL/6; Apoe−/− | male | SD | NA | scRNA-seq | Enzymatic digestion of the peeled off Adventitia (HBSS with calcium and magnesium, 2 mg/mL collagenase I, 2 mg/mL dispase II for 30 minutes | FACS sorting of Hoechst+Dead− cells | 61 |
AAV-mPCSK9: adeno-associated virus vector encoding mouse proprotein convertase subtilisin/kexin type 9; ApoB-ASO:antisense oligonucleotide to apolipoprotein B; Apoe: Apolipoprotein E; ATAC-seq: Transposase-Accessible Chromatin with high-throughput sequencing; BCA: brachiocephalic artery; Chol: cholesterol; CyTOF: cytometry by time-of-flight; DMEM: Dulbecco's Modified Eagle Medium; FACS: fluorescence-activated single cell sorting; FBS: Fetal Bovine Serum; HBBS: Hanks' Balanced Salt Solution; HCD: high-cholesterol diet; HFD: high-fat diet; NA: not applicable; PBS: Phosphate-buffered saline; PCL: partial carotid ligation; scRNA-seq: single-cell RNA sequencing; SD: standard laboratory diet; TD: test diet; VSMC: vascular smooth muscle cells; w: weeks; WD: western diet.
Using CyTOF, Cole et al.45 identified the dynamic changes of the myeloid cell compartment in atherosclerotic aortas from Apoe−/− in response to a WD for 12 weeks. Specifically, while plaque monocytes, plasmacytoid dendritic cells (pDC), and a CD11c+ macrophage subset increased with WD, CD206+ CD169+ macrophages and type-2 conventional dendritic cells (cDC2) were significantly reduced, indicating a remarkable plasticity of plaque myeloid cells in response to diet changes.
Using an elegant combination of scRNA-seq analysis and lineage tracing in established mouse models of atherosclerosis, Lin and colleagues44 identified specific dynamic changes of the plaque macrophage compartment during atherosclerosis progression and regression. In particular, four macrophage clusters were enriched in progressing plaques (Dnase1l3hi, RetnlahiEar2hi, IFN signaturehi and Cd74hiMHCIIhi clusters) compared to two clusters enriched in regressing plaques identified as Ebf1hiCd79ahi and HSPhi clusters, likely reflecting pro-resolving inflammatory responses. This approach also revealed that the 3 macrophage clusters described by Cochain et al.42 were derived from CX3CR1+ monocyte precursors and corresponded to Folr2hi and Trem2hi clusters, and a cluster expressing high levels of chemokines. Lin et al. 44 also identified a subpopulation of stem cell-like monocytes enriched in cell cycle genes and showed that stem-like monocyte with proliferative capacity originate macrophages in atherosclerotic tissue. This observation is in agreement with previous studies showing in situ proliferation of plaque macrophages46, 47 and suggest that both cell proliferation and monocyte-derived macrophages contribute to the disease. Kim et al43 used scRNA-seq to compare the transcriptome of plaque foamy macrophages, identified as BODIPYhiSSChi cells, and non-foamy plaque macrophages. Foamy macrophages expressed genes related to cholesterol metabolism and transport pathways, oxidative phosphorylation, lysosome and proteasome, and fewer inflammatory-related genes. In contrast, non-foamy macrophages expressed inflammatory genes involved in cytokines and chemokines pathways, NF-kB, tumor necrosis factor (TNF) and Toll-like receptor (TLR) signaling. Interestingly, a new analysis by Cochain et al48 of published scRNA-seq data42 showed transcriptional overlaps between Trem2hi plaque macrophages and foam cells, suggesting that Trem2hi macrophages corresponds to the foam cells identified by Kim and collegues43.
Although most scRNA-seq studies focused on myeloid cells and more specifically macrophages41-44, alterations of the adaptive immune compartment were also found in Apoe−/− mice fed a WD41. For instance, a pro-inflammatory transcriptional profile in T cells, memory T cells and cytotoxic CD8+ T cells was associated to a reduction of anti-atherogenic Th2 cells in Apoe−/− mice fed a WD but not a SD. These observations were confirmed by CyTOF41. The analysis of CD19+ cells identified three B cell clusters. The first small cluster comprised B1 cells and expressed genes involved in TNF signaling and cell adhesion pathways. The remaining two clusters showed B2 pro-atherogenic profile and secreted IFN-γ and GM-CSF in-vitro41. Overall, these studies highlight the advantages of high-throughput advanced transcriptomic techniques to resolve the immune cell phenotype spectrum during atherosclerosis progression and regression of experimental atherosclerosis in well-validated mouse models, but also call for standardized experimental approaches that will simplify integrated analyses of different datasets.
A large integrative analysis of a total of 15,288 cells from nine murine scRNA-seq datasets49 has allowed the identification of subsets of cells that were not detected in individual studies. This approach identified 17 cell clusters that comprised all major immune cells and confirmed that macrophages are highly abundant in the atherosclerotic aorta of mice. Macrophage clusters included resident-like macrophages expressing Pf4, a canonical marker of megakaryocytes and platelets, Trem2+ foamy macrophages, inflammatory macrophages, IFN-inducible macrophages and cavity macrophages, resembling peritoneal and other serous cavity macrophages. In this meta-analysis a substantial number of plaque foam cells were predicted to be of vascular smooth muscle (VSMC) origin, a result in line with previous experimental findings that lipid-rich macrophage cells in atherosclerotic plaques derive from VSMCs50-54. Interestingly, using scRNA-seq Schlegel et al. showed that Cx3cr1- macrophage expressed high levels of VSMC markers and genes involved in membrane re-organization and cell differentiation, suggesting their differentiation into myofibroblast55, data consistent with a bi-directional plasticity between VSMC and macrophages in the plaque microenvironment. Overall the funidings from scRNA-seq studies show that cell infiltrating atherosclerotic plaques present a high degree of plasticity that goes beyond the canonical boundaries between cells of hematopoietic and non-hematopoietic origin. Future scRNAseq studies, specifically designed to include both plaque immune and non-immune cells and using standardized methods for single-cell analysis including dissociation methods, are needed to predict specific trajectories of differentiation and robustly pinpoint candidate genes involved.
Single-cell heterogeneity of non-immune cells in murine atherosclerosis
A growing number of single-cell studies have investigated the non-immune cell compartment of normal and atherosclerotic vessels in mice to understand their contribution to the disease (Table 1).
The importance of non-immune cell plasticity in atherosclerosis has emerged from the observation that foam cells of non-hematopoietic origin derive from VSMCs49-54. Indeed, combining VSMCs lineage tracing and scRNA-seq analysis of the aorta from Apoe−/− mice, Wirka et al56 could not replicate these observations, but showed that VSMCs differentiate into fibroblast-like cells, a process partially dependent of TCF21 expression. The discrepancy with previous data suggests that distinct microenvironmental cues drive discrete cell differentiations and call for more investigation to fully dissect the complexity of these cellular processes. According to this possibility, by integrating cell-specific mapping and scRNAseq, Pan et al.57 identified a transitional, multipotent VSMC-derived cell type called “SEM”. SEM can differentiate into multiple cell types during atherogenesis, including macrophage-like, fibrochondrocyte-like cells capable to revert to a VSMC phenotype. This VSMC transition was driven by retinoic acid signaling dysregulation in both mouse and human atherosclerosis. Other scRNA-seq studies identified the stem cell pluripotency genes KFL4 and OCT4 as key regulators of VSMC transition to multiple phenotypes during atherosclerosis progression58. A rare subset of stem cell antigen 1+ (Sca1+) VSMCs was also identified in healthy mouse vessels59. These cells presented a downregulation of canonical contractile genes (e.g. Myh11, Acta2, Tagln, and Cnn1) that was associated with an increased expression of genes associated with responses to inflammation and growth factors. Sca1+ VSMC were also present in the core but not the cap of plaques from Apoe−/− mice.
scRNA-seq and scATAC-seq have also identified the specific transcriptional changes of endothelial cell (EC) reprogramming during atherogenesis60. In a model of partial carotid ligation, ECs exposed to chronic disturbed flow expressed genes involved in pro-atherogenic pathways such as inflammation, apoptosis, vascular development and endothelial-to-mesenchymal transition. ECs also acquired an immune cell–like phenotype, suggesting that the potential for a broad immune reprogramming of ECs under disturbed flow conditions.
While most scRNA-seq studies on non-immune cells analyzed the whole arterial wall, Gu et al.61 characterized cells in the aortic adventitia from C57BL/6J and Apoe−/− mice fed a SD. Among the six transcriptionally distinct clusters of non-immune cells, mesenchymal cells were the predominant non-immune population in the adventitia that comprised 4 distinct clusters, with similar enrichment between C57BL/6J and Apoe−/− mice. Mesenchymal cluster I (Mesen I) was the largest and expressed genes involved in structural organization, Mesen II was a Ccl2hi stem/progenitor-like cell population with pro-inflammatory and chemo-attractant capacity, Mesen III had a lipid metabolism involvement and Mesen IV displayed ossification properties, chondrocyte and heart development transcriptomic profiles. The remaining two clusters included adventitial ECs, and VSMCs. A rare subset of adventitial neuron-like cells that expressed Rbfox (neuronal nuclei) and Ache (acetylcholinesterase) was also identified.
Overall these studies have advanced our understanding of non-immune cell heterogeneity and plasticity in the healthy and atherosclerotic vasculature and provided new knowledge that will help advance the development of new therapeutic avenues for atherosclerosis.
Uncovering the single-cell transcriptional immune variations in human atherosclerosis
Single-cell studies of immune cells in human atherosclerotic lesions are limited to a few studies31, 32. In agreement with murine reports, both studies identified T cells and macrophages as the most abundant populations within the diseased vasculature.
Our group used scRNA-seq and CITE-seq to perform a single-cell immune cell mapping of a total of 7,169 CD45+ cells from 6 human atherosclerotic plaques to complement a comprehensive CyTOF mass cytometry analysis of >90,000 CD45+ cells from human carotid plaques of 46 patients31. Using this multi-omics approach, we uncovered new innate and adaptive immune cell-to-cell variations associated with clinical cerebrovascular symptoms. Overall, different single-cell technologies applied to independent cohorts consistently revealed that T cells were a dominant plaque cell type, expressed tissue residence markers and exhibited a more activated and differentiated phenotype in carotid plaques vs. blood from the same patient. Symptomatic patients with stroke presented an enrichment of a subset of effector memory CD4+ T cells in disrupted plaques that presented specific transcriptional alterations of T cell migration, activation and differentiation signaling. CD4+ T cells from asymptomatic patients with no stroke exclusively upregulated genes involved in the pro-inflammatory IL1 and IL6 signaling pathways, suggesting that anti-inflammatory therapies targeting these pathways might not be equally efficient in patients at different stages of the disease. scRNA-seq of plaque CD8+ T cells showed a similar activation in both patients with and without stroke, and partially overlapped the CD4+ T cell clusters. However, plaque CD8+ T cells from stroke patients co-expressed gene signatures consistent with T cell activation and an exhaustion-like reprogramming, possibly caused by chronic non-resolving inflammation, that remain to be explored mechanistically. Plaque macrophage transcriptional alterations were reminiscent of the macrophage heterogeneity found in experimental atherosclerosis41-45, 49 and included activated and inflammatory clusters of cells and foam cells. In patients with stroke, macrophages expressed sets of genes such as CCL5 and genes encoding granzymes that have been implicated with plaque instability20. These patients also presented a pathogenic subset of macrophages expressing genes related to iron metabolism31 that was previously described by Guo and colleagues62.
In a second study, Depuydt at al32 combined scRNA-seq and scATAC-seq to analyze immune and non-immune human plaque cells. The scRNA-seq analysis of 3,282 cells from 18 patients identified a total of 14 clusters: 11 were immune cell clusters, comprising mast cells, B cells, myeloid cells and T cells, and 3 were non-immune cell cluster, that included 2 clusters of ECs and one cluster of VSMCs. In this analysis, T cells represented >50% and myeloid cells represented 18.5% of all immune and non-immune plaque cells. The dominance of T cells was confirmed by the histological analysis of the same tissues, and it was consistent with our previous observations by CyTOF, scRNA-seq and CITE-seq in independent cohorts31. Sub-clustering of CD4+ T cells identified 5 distinct subsets reflecting distinct differentiation states. Two of these clusters presented a cytotoxic gene expression profile with high expression of GZMA, GZMK, and PRF1, and lower levels of GMZB suggesting that cytotoxic CD4+ T cells are present in the disease vasculature. Other cell clusters included CD4+ T cells showing naïve and central memory gene expression signatures. A similar approach revealed 3 CD8+ T cell clusters which presented similar differentiation states to those seen in CD4+ T cells. The first cluster was identified as including effector-memory cells expressing CD69, a marker of tissue residency and early activation of T-cell receptor and cytotoxic capacity. The second cluster, was identified as terminally differentiated cytotoxic CD8+ T cells with cytotoxic profile and lack of CD69 expression. Lastly, a central memory T cell-like cluster was identified. Using CyTOF and scRNAseq, we described a similar heterogeneity of plaque CD4+ and CD8+ T cells, comprising activated, cytotoxic and resting cells31. In our study we also found that resting T cells were enriched in blood and less represented in plaque from the same patient, indicating a spcialized adaptive immune response in the plaque microenvironment31. Depuydt and colleagues32 also identified 2 clusters of inflammatory macrophages enriched in classical pro-inflammatory and immune pathways, with increased inflammasome activation and expressing TNF and Toll-like receptors. A third cluster of macrophages expressed genes found in foam cells and pro-fibrotic markers. This cluster also co-expressed the smooth muscle cell marker ACTA2 and the macrophage markers LGALS3 and CD68, suggesting that myeloid cells may gain vascular smooth muscle cell characteristics and others might be transitioning to foam cells. This observation is in line with previous studies showing evidence of high plasticity between these cell subpopulations50-54, 63. Depuydt et al.32 completed their analysis of plaque immune cells using scATAC-seq to identify specific transcriptional regulators of the T cell and macrophage clusters identified in human atherosclerotic plaques. This approach identified specific open chromatin at the promoter region of IL12A (particularly My.3 cluster) and pro-inflammatory macrophages (My.1 cluster) with enrichment of pro-inflammatory transcription factor motifs and activated Th1-like T cells with open chromatin in the IFNG and TNF loci. The cytotoxic function of plaque CD4+ and CD8+ T cells clusters were further confirmed by the open chromatin of GZMB and GZMH loci. scATACseq data also informed on cell-cell communications suggesting that active cytokine production and signaling such as IL-12-IFNγ axis and secretion of cytotoxic proteins by activated effector T cells is taking place in human atherosclerotic plaques.
Perspectives for the use of single-cell technologies for precision medicine in atherosclerosis.
Collectively, single-cell studies of atherosclerosis provide a first step toward the ultimate goal of building a comprehensive single-cell atlas of human and experimental atherosclerosis that will help the development of new precision medicine in atherosclerosis. Existing single-cell studies have started to reveal a greater tissue specialization and plasticity of plaque cells than previously appreciated. By allowing high-dimensional and in-depth analyses of cells from small and limited clinical samples, single-cell studies of human atherosclerosis are especially powerful in resolving the complex cellular architecture of human disease. The natural course of atherosclerosis development in human and mouse models of the disease presents intrinsic differences. Human plaques develop over decades under the multifactional influence of high cholesterol levels, cardiometabolic and environmental risk factors acting on heterogenous genetic backgrounds that cannot be fully recapitulated in mice15. Yet mouse models of atherosclerosis allow pharmacological and genetic manipulation of the disease with molecular and cellular specificity to mechanistically study the effect of targeting genes and pathways on plaque progression and regression64. This approach has been successfully adopted in sc studies designed to test the effect of immune targets to achieve plaque regression. Using scRNA-seq in a mouse model of atherosclerosis with cell-specific, tamoxifen-induced deletion of netrin-1 (Ntn 1) in monocytes and macrophages, Schlegel et al55 showed that Ntn 1 deletion resulted in the expression of genes involved in cell motility and migration in monocytes, and genes implicated in tissue repair in macrophage which resulted in plaque regression. In a separate scRNAseq study, the targeted inhibition of microRNA33 (miR33) using anti-sense oligonucleotides was tested in atherosclerotic mice to study transcriptional changes in the immune cell landscape that were associated to plaque regression18. Specifically, anti-miR33 treatment provoked a pro-resolving macrophage differentiation that was associated to an expansion of Tregs. A concordance analysis with single-cell data from human plaques from patients with and without stroke showed that miR-33 target genes repression correlated with plaque progression in mice and cerebrovascular events in patients, suggesting that targeting miR33 may have beneficial effects on plaque stabilization.
Conclusions
The rapid evolution of single-cell transcriptomics and proteomics techniques has provided new capabilities to resolve the remarkable complexity of atherosclerosis disease. Overall, single-cell technologies are ideal to map the cellular and molecular composition of atherosclerotic tissue and have already provided a first atlas of the immune alterations associated with clinical complications in humans. In the era of personalized medicine, there is an urgent need to establish a causal relationship between plaque heterogeneity and disease progression. Specifically, scRNA-seq may be key in understanding the specific effects of cardiovascular risk factors such as sex, age, smoking, diabetes on disease progression, defective regression in response to lipid lowering drugs and the cellular mechanisms driving clinical cardiovascular outcomes. A systematic integrated cellular mapping of experimental and human atherosclerosis, based on standardized methods across studies, will improve the precise selection of relevant mouse models for the mechanistic validation of the identified targets and signaling pathways in humans and offer opportunities to test new candidate immunotherapies before moving into clinical stage of development.
Highlights.
Single cell technologies have become powerful tools to perform global and unbiased analysis of the cellular composition of normal and diseased tissues.
Single-cell studies of experimental atherosclerosis in mice have revealed a new heterogeneity of the immune and non-immune cell compartments and showed a remarkable cell adaptation and plasticity in the plaque microenvironment.
In depth single-cell studies of limited human plaque specimens have advanced our understanding of the specific cellular alterations in advanced human atherosclerotic plaques.
The adoption of single-cell technologies to larger cohort of well-characterized patients with atherosclerotic cardiovascular disease will help dissect the specific effect of cardiovascular risk factors on disease progression, defective regression and the cellular mechanisms driving clinical cardiovascular outcomes.
Source of funding
Dr. Giannarelli acknowledges research support from the NIH-NHLBI (R01 HL153712-01), AHA (20SFRN35210252) and CZI (NFL-2020-218415).
Abbreviations
- Apo E
apolipoprotein E
- CITE-seq
cellular indexing of transcriptomes and epitopes by sequencing
- CyTOF
cytometry by time-of-flight
- CV
cerebrovascular
- CVD
cardiovascular disease
- EC
endothelial cells
- IL
interleukin
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- NK
natural killer
- ox-LDL
oxidized low-density lipoproteins
- scATAC-seq
single-cell Assay for Transposase-Accessible Chromatin using sequencing
- scRNA-seq
single-cell RNA sequencing
- SD
standard laboratory diet
- Th
T-helper type
- TNF
tumor necrosis factor
- TLR
Toll-like receptor
- Treg
T regulatory cells
- TREM
triggering receptor expressed in myeloid cells
- VSMC
vascular smooth muscle cells
- WD
western diet
Footnotes
Disclosure
The authors declare no competing interests.
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virmani R, Kolodgie FD, Burke AP, Farb A and Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. [DOI] [PubMed] [Google Scholar]
- 3.Koelwyn GJ, Corr EM, Erbay E and Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore KJ, Sheedy FJ and Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore KJ and Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi W, Wang X, Wang NJ, McBride WH and Lusis AJ. Effect of macrophage-derived apolipoprotein E on established atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:2261–2266. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I and Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res. 2016;118:653–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabas I and Lichtman AH. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity. 2017;47:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silvestre-Roig C, Braster Q, Ortega-Gomez A and Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17:327–340. [DOI] [PubMed] [Google Scholar]
- 10.Wolf D and Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saigusa R, Winkels H and Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17:387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Getz GS and Reardon CA. Natural killer T cells in atherosclerosis. Nat Rev Cardiol. 2017;14:304–314. [DOI] [PubMed] [Google Scholar]
- 13.Nour-Eldine W, Joffre J, Zibara K, Esposito B, Giraud A, Zeboudj L, Vilar J, Terada M, Bruneval P, Vivier E, Ait-Oufella H, Mallat Z, Ugolini S and Tedgui A. Genetic Depletion or Hyperresponsiveness of Natural Killer Cells Do Not Affect Atherosclerosis Development. Circ Res. 2018;122:47–57. [DOI] [PubMed] [Google Scholar]
- 14.Winkels H and Ley K. Natural Killer Cells at Ease: Atherosclerosis Is Not Affected by Genetic Depletion or Hyperactivation of Natural Killer Cells. Circ Res. 2018;122:6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez DM and Giannarelli C. Immune cell profiling in atherosclerosis: role in research and precision medicine. Nat Rev Cardiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma M, Schlegel MP, Afonso MS, Brown EJ, Rahman K, Weinstock A, Sansbury BE, Corr EM, van Solingen C, Koelwyn GJ, Shanley LC, Beckett L, Peled D, Lafaille JJ, Spite M, et al. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ Res. 2020;127:335–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstock A, Rahman K, Yaacov O, Nishi H, Menon P, Nikain CA, Garabedian ML, Pena S, Akbar N, Sansbury BE, Heffron SP, Liu J, Marecki G, Fernandez D, Brown EJ, et al. Wnt signaling enhances macrophage responses to IL-4 and promotes resolution of atherosclerosis. Elife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afonso MS, Sharma M, Schlegel M, van Solingen C, Koelwyn GJ, Shanley LC, Beckett L, Peled D, Rahman K, Giannarelli C, Li H, Brown EJ, Khodadadi-Jamayran A, Fisher EA and Moore KJ. miR-33 Silencing Reprograms the Immune Cell Landscape in Atherosclerotic Plaques. Circ Res. 2021;128:1122–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkels H and Wolf D. Heterogeneity of T Cells in Atherosclerosis Defined by Single-Cell RNA-Sequencing and Cytometry by Time of Flight. Arterioscler Thromb Vasc Biol. 2021;41:549–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, Khan NS, Wong CK, Shamailova R, Hill CA, Wang Z, Remark R, Li JR, Pina C, Faries C, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25:1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 22.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, Lopez-Sendon J, Ostadal P, Koenig W, Angoulvant D, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 23.Choi YH and Kim JK. Dissecting Cellular Heterogeneity Using Single-Cell RNA Sequencing. Mol Cells. 2019;42:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.See P, Lum J, Chen J and Ginhoux F. A Single-Cell Sequencing Guide for Immunologists. Frontiers in Immunology. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen TK and Baryawno N. Introduction to Single-Cell RNA Sequencing. Current Protocols in Molecular Biology. 2018;122:e57. [DOI] [PubMed] [Google Scholar]
- 26.Papalexi E and Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol. 2018;18:35–45. [DOI] [PubMed] [Google Scholar]
- 27.Upadhaya S, Sawai CM, Papalexi E, Rashidfarrokhi A, Jang G, Chattopadhyay P, Satija R and Reizis B. Kinetics of adult hematopoietic stem cell differentiation in vivo. J Exp Med. 2018;215:2815–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng S, Papalexi E, Butler A, Stephenson W and Satija R. Molecular transitions in early progenitors during human cord blood hematopoiesis. Mol Syst Biol. 2018;14:e8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stubbington MJT, Rozenblatt-Rosen O, Regev A and Teichmann SA. Single-cell transcriptomics to explore the immune system in health and disease. Science. 2017;358:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armingol E, Officer A, Harismendy O and Lewis NE. Deciphering cell-cell interactions and communication from gene expression. Nat Rev Genet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir E-aD, Amadori L, Khan NS, Wong CK, Shamailova R, Hill CA, Wang Z, Remark R, Li JR, Pina C, Faries C, et al. Single-cell immune landscape of human atherosclerotic plaques. Nature Medicine. 2019;25:1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Depuydt MA, Prange KH, Slenders L, Ord T, Elbersen D, Boltjes A, de Jager SC, Asselbergs FW, de Borst GJ, Aavik E, Lonnberg T, Lutgens E, Glass CK, den Ruijter HM, Kaikkonen MU, et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zappia L, Phipson B and Oshlack A. Exploring the single-cell RNA-seq analysis landscape with the scRNA-tools database. PLoS Comput Biol. 2018;14:e1006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrews TS, Kiselev VY, McCarthy D and Hemberg M. Tutorial: guidelines for the computational analysis of single-cell RNA sequencing data. Nat Protoc. 2021;16:1–9. [DOI] [PubMed] [Google Scholar]
- 35.Williams JW, Winkels H, Durant CP, Zaitsev K, Ghosheh Y and Ley K. Single Cell RNA Sequencing in Atherosclerosis Research. Circ Res. 2020;126:1112–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill CA, Fernandez DM and Giannarelli C. Single cell analyses to understand the immune continuum in atherosclerosis. Atherosclerosis. 2021;330:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, Satija R and Smibert P. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017;14:865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Miragaia RJ, Natarajan KN and Teichmann SA. A rapid and robust method for single cell chromatin accessibility profiling. Nat Commun. 2018;9:5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Placek K, Schultze JL and Aschenbrenner AC. Epigenetic reprogramming of immune cells in injury, repair, and resolution. J Clin Invest. 2019;129:2994–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimball AK, Oko LM, Bullock BL, Nemenoff RA, van Dyk LF and Clambey ET. A Beginner's Guide to Analyzing and Visualizing Mass Cytometry Data. J Immunol. 2018;200:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, Zernecke A, Pramod AB, Ghosh AK, Anto Michel N, Hoppe N, et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res. 2018;122:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE and Zernecke A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res. 2018;122:1661–1674. [DOI] [PubMed] [Google Scholar]
- 43.Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, Jang MY, Seok Jang H, Yun TJ, Lee SH, Yoon WK, Prat A, Seidah NG, Choi J, Lee SP, et al. Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ Res. 2018;123:1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin JD, Nishi H, Poles J, Niu X, McCauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, Ramsey SA, Fisher EA and Loke P. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole JE, Park I, Ahern DJ, Kassiteridi C, Danso Abeam D, Goddard ME, Green P, Maffia P and Monaco C. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc Res. 2018;114:1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams JW, Zaitsev K, Kim KW, Ivanov S, Saunders BT, Schrank PR, Kim K, Elvington A, Kim SH, Tucker CG, Wohltmann M, Fife BT, Epelman S, Artyomov MN, Lavine KJ, et al. Limited proliferation capacity of aortic intima resident macrophages requires monocyte recruitment for atherosclerotic plaque progression. Nat Immunol. 2020;21:1194–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cochain C, Saliba AE and Zernecke A. Letter by Cochain et al Regarding Article, "Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models". Circ Res. 2018;123:e48–e49. [DOI] [PubMed] [Google Scholar]
- 49.Zernecke A, Winkels H, Cochain C, Williams JW, Wolf D, Soehnlein O, Robbins CS, Monaco C, Park I, McNamara CA, Binder CJ, Cybulsky MI, Scipione CA, Hedrick CC, Galkina EV, et al. Meta-Analysis of Leukocyte Diversity in Atherosclerotic Mouse Aortas. Circ Res. 2020;127:402–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allahverdian S, Chehroudi AC, McManus BM, Abraham T and Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Dubland JA, Allahverdian S, Asonye E, Sahin B, Jaw JE, Sin DD, Seidman MA, Leeper NJ and Francis GA. Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ and Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rong JX, Shapiro M, Trogan E and Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A. 2003;100:13531–13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM and Fisher EA. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 2015;35:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlegel M, Sharma M, Brown EJ, Newman AAC, Cyr Y, Afonso MS, Corr EM, Koelwyn GJ, van Solingen C, Guzman J, Farhat R, Nikain CA, Shanley LC, Peled D, Schmidt AM, et al. Silencing Myeloid Netrin-1 Induces Inflammation Resolution and Plaque Regression. Circ Res. 2021;129:530–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK, Fong R, Woo YJ, Liu B, Montgomery SB, Wu JC, et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med. 2019;25:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan H, Xue C, Auerbach BJ, Fan J, Bashore AC, Cui J, Yang DY, Trignano SB, Liu W, Shi J, Ihuegbu CO, Bush EC, Worley J, Vlahos L, Laise P, et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation. 2020;142:2060–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alencar GF, Owsiany KM, Karnewar S, Sukhavasi K, Mocci G, Nguyen AT, Williams CM, Shamsuzzaman S, Mokry M, Henderson CA, Haskins R, Baylis RA, Finn AV, McNamara CA, Zunder ER, et al. Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis. Circulation. 2020;142:2045–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dobnikar L, Taylor AL, Chappell J, Oldach P, Harman JL, Oerton E, Dzierzak E, Bennett MR, Spivakov M and Jorgensen HF. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat Commun. 2018;9:4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andueza A, Kumar S, Kim J, Kang DW, Mumme HL, Perez JI, Villa-Roel N and Jo H. Endothelial Reprogramming by Disturbed Flow Revealed by Single-Cell RNA and Chromatin Accessibility Study. Cell Rep. 2020;33:108491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu W, Ni Z, Tan YQ, Deng J, Zhang SJ, Lv ZC, Wang XJ, Chen T, Zhang Z, Hu Y, Jing ZC and Xu Q. Adventitial Cell Atlas of wt (Wild Type) and ApoE (Apolipoprotein E)-Deficient Mice Defined by Single-Cell RNA Sequencing. Arterioscler Thromb Vasc Biol. 2019;39:1055–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo L, Akahori H, Harari E, Smith SL, Polavarapu R, Karmali V, Otsuka F, Gannon RL, Braumann RE, Dickinson MH, Gupta A, Jenkins AL, Lipinski MJ, Kim J, Chhour P, et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest. 2018;128:1106–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR and Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16:727–744. [DOI] [PubMed] [Google Scholar]
- 64.von Scheidt M, Zhao Y, Kurt Z, Pan C, Zeng L, Yang X, Schunkert H and Lusis AJ. Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metab. 2017;25:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]