Background.
In the early months of the coronavirus disease 2019 (COVID-19) pandemic, our center reported a mortality rate of 34% in a cohort of 32 lung transplant recipients with COVID-19 between March and May 2020. Since then, there has been evolving knowledge in prevention and treatments of COVID-19. To evaluate the impact of these changes, we describe the clinical presentation, management, and outcomes of a more recent cohort of lung transplant recipients during the second surge and provide a comparison with our first cohort.
Methods.
We conducted a retrospective cohort study that included all consecutive lung transplant recipients who tested positive for severe acute respiratory syndrome coronavirus 2 between November 2020 and February 28, 2021. We compared baseline demographics and major outcomes between the first- and second-surge cohorts.
Results.
We identified 47 lung transplant recipients (median age, 60; 51% female) who tested positive for severe acute respiratory syndrome coronavirus 2 between November 2020 and February 28, 2021. The current cohort had a higher proportion of patients with mild disease (34% versus 16%) and fewer patients with a history of obesity (4% versus 25%). Sixty-six percent (n = 31) required hospitalization and were treated with remdesivir (90%) and dexamethasone (84%). Among those hospitalized, 77% (n = 24) required supplemental oxygen, and 22% (n = 7) required invasive mechanical ventilation. The overall 90-d mortality decreased from 34% to 17% from the first cohort to the second (adjusted odds ratio, 0.26; 95% confidence interval, 0.08-0.85; P = 0.026).
Conclusions.
Although COVID-19–associated mortality rate in lung transplant recipients at our center has decreased over time, COVID-19 continues to be associated with significant morbidity and mortality.
INTRODUCTION
As of December 11, 2021, there have been over 49 million confirmed cases of coronavirus disease 2019 (COVID-19) in the United States, with almost 800 000 deaths.1 Immunosuppressed state, particularly among solid organ transplant recipients, has emerged as a risk factor for severe disease and poor outcomes.2 Existing literature on COVID-19 among solid organ transplant recipients reports mortality rates between 0% and 39%,3-21 and more specifically between 8% and 39% among lung transplant recipients.4,13-15,18,21
Our center reported on the findings and outcomes of the first 32 consecutive lung transplant recipients with COVID-19 identified between March 19, 2020, and May 29, 2020.4 This early surge in cases was immediately followed by a brief period of no new cases between July and October of 2020. Coinciding with the larger national surge in the fall of 2020, our center once again began to identify new cases among lung transplant recipients in November of 2020.
Reports in the United States at both population and healthcare systems levels have found lower COVID-19–associated mortality over time since the early surge in the spring of 2020.1,22,23 Although it remains uncertain if COVID-19 survival outcomes truly improved over time, there have been objective changes since the early surge, including increase in testing availability, new pharmacological treatments, shifting hospitalized demographics, growing experience, and improved availability of healthcare resources.23-31 Follow-up reports are needed on COVID-19 outcomes among lung transplant recipients who incorporated these changes.
To characterize the trends in COVID-19 outcomes over time at our center, we performed a retrospective analysis describing the disease characteristics, management, complications, and outcomes of a more recent cohort of lung transplant recipients with COVID-19 at our center from November 2020 to February 2021 (“second surge”) and provide a comparison with the first cohort from March and May of 2020 (“first surge”).
MATERIALS AND METHODS
Subjects
This is a retrospective cohort study of all consecutive lung transplant patients followed by our center who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by polymerase chain reaction (PCR) testing between November 1, 2020, and February 28, 2021. Remarkably, there were no new laboratory-confirmed cases of acute infection in our lung transplant recipients between July and October 2020. We followed the same classification that was used in our first case series to facilitate direct comparison between the cohorts. Patients were classified as having mild COVID-19 if they did not require hospitalization; moderate COVID-19 if they required hospitalization; and severe COVID-19 if they were admitted to an intensive care unit or stepdown unit or required nonrebreather mask, high-flow nasal cannula, noninvasive ventilation, or invasive mechanical ventilation at any point during the disease course. Patients who had received at least 1 dose of a COVID-19 vaccine before their positive SARS-CoV-2 test were also included in the study.
Patient demographics, medical history, and baseline medications were obtained from the electronic health record (EHR). The definitions and criteria for baseline comorbidities are included in SDC 1 (SDC, http://links.lww.com/TXD/A404).
Study Design
Throughout the study period, patients who contacted our program to report symptoms that were concerning for COVID-19 or exposure to a confirmed or suspected case of COVID-19 were advised to undergo SARS-CoV-2 testing. Additionally, patients received mandatory SARS-CoV-2 PCR testing on admission to the hospital and within 5 d before scheduled procedures, including pulmonary function tests and bronchoscopies.
Symptoms and vital signs at the time of SARS-CoV-2 testing and at the time of admission to hospital were obtained from the EHR (SDC 2, SDC, http://links.lww.com/TXD/A404).
The patients’ clinical course, treatments, and laboratory values were obtained from the EHR, records provided by the managing teams at outside hospitals, and patients or their family members (SDC 2, SDC, http://links.lww.com/TXD/A404). We also compared baseline characteristics and outcomes between the first-surge and current cohorts. Our study treatment protocol was in accordance with COVID-19 clinical guidelines of our center and included treatment with monoclonal antibodies, dexamethasone, remdesivir, tocilizumab, and additional steroid therapy in conjunction with transplant infectious disease consult service (SDC 3, SDC, http://links.lww.com/TXD/A404).
Patients were followed until death or study end (May 31, 2021) to allow 3 mo of follow-up. This study was approved by the Columbia University Human Research Protection Office and the Institutional Review Board. The authors complied with the ethical standards and the US regulations.
Statistical Analysis
Statistical analysis was performed using Stata/SE version 15.1. Continuous and categorical variables were compared using the t test and 1-way analysis of variance. Survival in each cohort was compared using logistic regression and adjusted for relevant covariates, including age, obesity, bronchiolitis obliterans syndrome (BOS) status, single versus double lung transplant status, and preceding augmented immunosuppressive therapy.
RESULTS
Baseline Characteristics
We identified 47 consecutive lung transplant patients who tested positive for SARS-CoV-2 between November 1, 2020, and February 28, 2021. The median age was 65 y (range, 20–79 y). Patients were 51% female and 68% Caucasian. They had received a single lung transplant (60%) most commonly for interstitial lung disease (60%). The median time from transplant to COVID-19 diagnosis was 4.3 y (range, 20 d–18 y). Thirty percent of patients had BOS stage 1 or greater. The majority (81%) were on triple immunosuppression therapy with a cell-cycle inhibitor, calcineurin inhibitor, and prednisone. Nine patients (18%) were off cell-cycle inhibitors. Less than half (45%) of patients were taking azithromycin for BOS. Within the 3 mo before COVID-19 diagnosis, 34% of patients received immunosuppression augmentation, including 4 patients who received induction therapy for transplantation. Most patients had comorbidities at baseline: hypertension (75%), chronic kidney disease (62%), and diabetes (55%). Only 2 patients (4%) had obesity, though 38% were overweight. Six patients (13%) had Aspergillus infection within the past year. Three patients (6%) had received 1 dose, and 1 patient (2%) had received 2 doses of COVID-19 vaccine before their COVID-19 diagnosis, though none were fully vaccinated as defined by the Centers for Disease Control and Prevention. All 4 had been vaccinated after lung transplantation.
Baseline characteristics of patients with mild, moderate, and severe COVID-19 are reported in Table 1. The demographics were similar between the 3 severity groups, although patients with mild disease tended to be younger. Patients who had prior Aspergillus infection more commonly had severe disease.
TABLE 1.
Baseline characteristics by COVID-19 severity
| Mild (n = 16) | Moderate (n = 18) | Severe (n = 13) | P | |
|---|---|---|---|---|
| Age, median (IQR) | 60 (48–69) | 66 (57–72) | 67 (60–73) | 0.27 |
| Sex, n (%) | 0.15 | |||
| Male | 8 (50) | 6 (33) | 9 (69) | |
| Female | 8 (50) | 12 (67) | 4 (31) | |
| Ethnicity, n (%) | 0.24 | |||
| Caucasian | 13 (81) | 10 (56) | 9 (69) | |
| Hispanic | 2 (13) | 4 (22) | 2 (15) | |
| African American | 1 (6) | 2 (11) | 1 (8) | |
| Asian | 0 (0) | 2 (11) | 1 (8) | |
| Transplant indication, n (%) | 0.16 | |||
| ILD | 7 (44) | 11 (61) | 10 (77) | |
| COPD | 4 (25) | 3 (17) | 2 (15) | |
| Sarcoid | 0 (0) | 2 (11) | 1 (8) | |
| CF and non-CF bronchiectasis | 5 (31) | 1 (6) | 0 (0) | |
| Othera | 0 (0) | 1 (6) | 0 (0) | |
| Transplant type, n (%) | 0.83 | |||
| Single | 8 (50) | 10 (56) | 8 (62) | |
| Double | 8 (50) | 8 (44) | 5 (38) | |
| Years since transplant, median (IQR) | 3.8 (2.9–8.9) | 5.8 (2.5–10.4) | 3 (1.2–8.6) | 0.70 |
| BOS stage, n (%) | 0.36 | |||
| 1 | 0 (0) | 4 (22) | 1 (8) | |
| 2 | 3 (19) | 2 (11) | 0 (0) | |
| 3 | 2 (13) | 1 (6) | 1 (8) | |
| Baseline IS regimen, n (%) | ||||
| Mycophenolate <2000 mg/d | 3 (19) | 6 (33) | 8 (62) | 0.22 |
| Mycophenolate ≥2000 mg/d | 10 (63) | 3 (17) | 3 (23) | |
| Azathioprine <150 mg/d | 1 (6) | 3 (17) | 0 (0) | |
| Azathioprine ≥150 mg/d | 0 (0) | 1 (6) | 0 (0) | |
| No cell-cycle inhibitorb | 2 (13) | 5 (28) | 2 (15) | |
| Tacrolimus | 16 (100) | 17 (94)c | 13 (100) | 1 |
| Sirolimus | 0 (0) | 1 (6)c | 0 (0) | |
| Cyclosporine | 0 (0) | 1 (6) | 0 (0) | |
| Prednisone <10 mg/d | 11 (69) | 11 (61) | 5 (38) | 0.25 |
| Prednisone ≥10 mg/d | 5 (31) | 7 (39) | 8 (62) | |
| Azithromycin for BOS | 7 (44) | 8 (44) | 6 (46) | 0.99 |
| Recent IS augmentation, n (%) | 7 (44)d | 4 (22)e | 5 (38)d,f | 0.40 |
| Induction (basiliximab + solumedrol) | 3 (19) | 0 (0) | 1 (8) | |
| Steroid pulse | 1 (6) | 1 (6) | 3 (23) | |
| Steroid taper | 2 (13) | 1 (6) | 0 (0) | |
| rATG | 2 (13) | 0 (0) | 1 (8) | |
| Immune-modulating (ECP, IVIG) | 0 (0) | 3 (17) | 1 (8) | |
| Received COVID-19 vaccine, n (%) | 1 (6) | 1 (6) | 2 (15) | 0.60 |
| Only first dose | 1 (6) | 0 (0) | 2(15) | |
| Both doses | 0 (0) | 1 (6) | 0 (0) | |
| BMI, mean (IQR) | 24.2 (21.7–27.6) | 24.3 (22.4–26.4) | 24.4 (24–25) | 0.92 |
| Comorbidities, n (%) | ||||
| Hypertension | 10 (63) | 14 (78) | 11 (85) | 0.38 |
| CKD | 6 (38) | 14 (78) | 9 (69) | 0.056 |
| Heart disease | 3 (17) | 4 (22) | 5 (38) | 0.46 |
| Diabetes | 9 (56) | 7 (39) | 10 (77) | 0.11 |
| Overweight (BMI 25–29.9) | 5 (31) | 6 (33) | 7 (54) | 0.41 |
| Obesity (BMI ≥30) | 1 (6) | 1 (6) | 0 (0) | 0.40 |
| Active malignancy | 2 (13) | 3 (17) | 1 (8) | 0.77 |
| Recent Aspergillus infection | 0 (0) | 1 (6) | 5 (38) | 0.003 |
aOther transplant indication includes LAM.
bPatients were off cell-cycle inhibitors for active or history of malignancy, infection (Cryptococcus), treatment with alemtuzumab, or cytopenia.
cOne patient was taking both tacrolimus and sirolimus.
dOne patient received both thymoglobulin and steroid pulse.
eOne patient received both a steroid pulse and ECP.
fOne patient received both induction therapy and a steroid pulse.
BMI, body mass index; BOS, bronchiolitis obliterans syndrome; CF, cystic fibrosis; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; ECP, extracorporeal photopheresis; ILD, interstitial lung disease; IQR, interquartile range; IS, immunosuppression; IVIG, intravenous immunoglobulin; LAM, lymphangioleiomyomatosis; rATG, rabbit antithymocyte globulin.
Clinical Presentation
Thirty-four percent of patients had mild, 38% moderate, and 28% severe COVID-19. Median duration of symptoms before SARS-CoV-2 testing was 3 d (range, 0–14 d). Seven patients (15%) had a positive SARS-CoV-2 test on asymptomatic preprocedural testing. These patients tended to have mild disease, with 2 patients ultimately requiring hospitalization. Three patients (6%) tested positive for SARS-CoV-2 while already admitted for non–COVID-19 diagnoses and had presumed nosocomial COVID-19 infection.
Symptoms reported at time of diagnosis included fever (38%), cough (72%), dyspnea (70%), gastrointestinal symptoms (51%), and altered mental status (15%). Patients who had fever, cough, or dyspnea were more likely to develop moderate or severe disease. Abnormal vital signs among the 31 patients admitted for COVID-19 and those who tested positive for SARS-CoV-2 as inpatient included hypoxemia (55%), tachypnea (65%), and tachycardia (58%). Patients who presented with hypoxemia, tachypnea, or altered mental status were more likely to develop severe disease. Symptoms at the time of diagnosis for all patients and vital sign abnormalities for hospitalized patients are reported in Table 2.
TABLE 2.
Clinical presentation as reported by outpatients and at hospital admission for inpatients by COVID-19 severity
| Mild (n = 16) | Moderate (n = 18) | Severe (n = 13) | P | |
|---|---|---|---|---|
| Duration of symptoms (d) before testing, median (IQR) | 2 (0–7) | 3 (2–6) | 3 (2–3) | 0.72 |
| Detected on routine testing, n (%) | 5 (31) | 1 (6) | 1 (8) | 0.078 |
| Prior COVID-19 vaccination, n (%)a | 1 (6) | 1 (6) | 2 (15) | 0.60 |
| Fever, n (%) | 1 (6) | 13 (72) | 4 (31) | <0.001 |
| Cough, n (%) | 8 (50) | 15 (83) | 11 (85) | 0.048 |
| Dyspnea, n (%) | 4 (25) | 16 (89) | 13 (100) | <0.001 |
| GI symptoms, n (%) | 6 (38) | 12 (67) | 6 (46) | 0.23 |
| Hypoxemia, n (%) | – | 5 (28) | 12 (92) | <0.001 |
| Tachypnea, n (%) | – | 8 (44) | 12 (92) | 0.0048 |
| Tachycardia, n (%) | – | 8 (44) | 10 (77) | 0.07 |
| Hypotension, n (%) | – | 1 (6) | 3 (23) | 0.16 |
| Altered mental status, n (%) | – | 1 (6) | 6 (46) | 0.018 |
aNone of the patients with prior COVID-19 vaccination were fully vaccinated as defined by Centers for Disease Control and Prevention guidelines.
IQR, interquartile range; COVID-19, coronavirus disease 2019; GI, gastrointestinal.
Median values of laboratory results obtained upon admission for moderate and severe COVID-19 patients are shown in Table S1 (SDC, http://links.lww.com/TXD/A404). Higher levels of C-reactive protein and lactate dehydrogenase upon admission were more common in those who developed severe disease.
Most hospitalized patients (68%) had new pulmonary infiltrates on admission, with the majority (57%) having bilateral infiltrates, regardless of transplant type (Table 3). Throughout their disease course, 92% of patients who required supplemental oxygen developed pulmonary infiltrates, and all patients with severe disease exhibited diffuse, bilateral infiltrates on imaging. Additional radiographic data, categorized by COVID-19 severity, are shown in Table 3.
TABLE 3.
Radiographic changes secondary to COVID-19 by severity
| Radiographic features | Mild (n = 16) | Moderate (n = 18) | Severe (n = 13) | P |
|---|---|---|---|---|
| New infiltrates on admission, n (%) | – | 12 (67) | 9 (69) | 0.89 |
| Double lung transplant patients, n (%) | 6 (33) | 4 (31) | ||
| Bilateral allograft infiltrates | 2 | 4 | ||
| Single lung transplant patients, n (%) | 6 (33)a | 5 (38) | ||
| Predominant native infiltrates | 1 | 0 | ||
| Predominant allograft infiltrates | 2 | 1 | ||
| Bilateral infiltrates | 2 | 4 | ||
| New infiltrates during disease course, n (%) | 3 (19)b | 13 (72) | 13 (100) | <0.001 |
| Double lung transplant patients, n (%) | 2 (13) | 6 (33) | 5 (38) | |
| Bilateral allograft infiltrates | 1 | 6 | 5 | |
| Single lung transplant patients (%) | 1 (6) | 7 (39)a | 8 (62) | |
| Predominant native infiltrates | 0 | 1 | 0 | |
| Predominant allograft infiltrates | 1 | 2 | 0 | |
| Bilateral infiltrates | 0 | 3 | 8 | |
| Focal infiltrates, n (%) | 2 (13%) | 1 (6%) | 0 (0%) | 0.41 |
| Diffuse infiltrates, n (%) | 1 (6%) | 11 (61%) | 13 (100%) | <0.001 |
| No infiltrates, n (%) | 13 (81%) | 5 (28%) | 0 (0%) | <0.001 |
| Chest CT available, n (%) | 9 (56%) | 15 (83%) | 6 (46%) | |
| GGO alone | 3 | 5 | 3 | 0.77 |
| Consolidation alone | 0 | 2 | 0 | 0.37 |
| Both | 0 | 3 | 3 | 0.032 |
| None | 6 | 5 | 0 | 0.027 |
aMissing laterality information for 1 patient.
bFor patients with mild disease, these data are from chest imaging obtained during follow-up.
COVID-19, coronavirus disease 2019; CT, computed tomography of the chest; GGO, ground glass opacities.
Treatment
Thirty-one patients (66%) required hospitalization for moderate-to-severe COVID-19. Among those admitted, 77% required supplemental oxygen, including mechanical ventilation in 7 patients (23% of the hospitalized cohort). During their hospitalization for COVID-19, 26 patients (84%) were treated with dexamethasone, and 28 (90%) received remdesivir. Twenty-seven hospitalized patients (84%) received broad-spectrum antibiotics. Cell-cycle inhibitors were continued without dose reduction in most patients with mild disease. In contrast, cell-cycle inhibitors were either held or continued at a lower dose in 61% of patients hospitalized with moderate-to-severe disease. Steroid pulse and/or taper was also initiated in 15 patients who were hospitalized (48%). Four patients required renal replacement therapy.
Ten patients (21%) from the entire cohort received monoclonal antibody-based treatment at a median of 7 d within symptom onset. Six of these patients continued to be managed in the outpatient setting, and 2 developed severe COVID-19 (Table 4).
TABLE 4.
Treatments administered in patients with COVID-19 by severity
| Mild (n = 16) | Moderate (n = 18) | Severe (n = 13) | P | |
|---|---|---|---|---|
| Highest oxygen requirement, n (%) | – | <0.001 | ||
| MV | 0 (0) | 7 (54) | ||
| HFNC | 0 (0) | 4 (31) | ||
| NIV | 0 (0) | 0 (0) | ||
| NRB | 0 (0) | 2 (15) | ||
| NC | 11 (61) | 0 (0) | ||
| Dexamethasone, n (%) | – | 13 (72) | 13 (100) | 0.039 |
| Steroid augmentation beside dexamethasone, n (%) | – | 5 (28) | 10 (77) | 0.006 |
| Remdesivir, n (%) | – | 15 (83) | 13 (100) | 0.13 |
| <5 da | 3 | 2 | ||
| 5 d | 12 | 10 | ||
| 10 d | 0 | 1 | ||
| Convalescent plasma, n (%) | – | 3 (17) | 2 (15) | 0.93 |
| Tocilizumab, n (%) | – | 0 (0) | 2 (15) | 0.09 |
| mAb therapy (before admission), n (%) | 6 (38) | 2 (11)b | 2 (15) | 0.15 |
| Bamlanivimab | 6 | 1 | 2 | – |
| Casirivimab/imdevimab | 0 | 1 | 0 | – |
| Days from positive test to mAb therapy, mean (range) | 4.2 (1–10) | 5.5 (5–6) | 4.5 (4–5) | |
| Days from symptoms to mAb therapy, mean (range) | 4.8 (2–7) | 4.5 (2–7) | 7 (7–7) | |
| Days from mAb therapy to admission, mean (range) | – | 6 (1–11) | 2 (0–4) | |
| Cell-cycle inhibitor reduced or held, n (%)c | 1 (6%) | 9 (50%) | 10 (77%) | <0.001 |
| Broad-spectrum antibiotics on admission, n (%) | – | 14 (78%) | 12 (92%) | 0.26 |
| Broad-spectrum antibiotics during disease course, n (%) | – | 13 (72%)d | 13 (100%) | 0.039 |
| Dialysis, n (%) | – | 0 (0%)e | 4 (31%)e | 0.009 |
| Vasopressors, n (%) | – | 0 (0%) | 7 (54%) | <0.001 |
| Enrolled in clinical trial, n (%) | – | 0 (0%) | 4 (31%) | 0.01 |
aFive patients received less than the standard 5-d course because of improvement of oxygenation status to room air or complications including concern for hepatotoxicity or acute kidney injury.
bOne patient received mAb after their index COVID-19 admission and is not included in this total.
cOne patient with mild disease, 5 patients who had moderate disease, and 2 patients who had severe disease were already off cell-cycle inhibitors at baseline and are not included in these totals.
dAntibiotic data could not be determined for 1 patient who was admitted to an outside hospital.
eOne patient in both the moderate and severe groups was already on hemodialysis at baseline and not included in this total.
COVID-19, coronavirus disease 2019; HFNC, high flow nasal cannula; mAb, monoclonal antibody; MV, mechanical ventilation; NC, nasal cannula; NIV, noninvasive ventilation; NRB, nonrebreather.
Clinical Outcomes
The overall 90-d all-cause mortality from our center’s second COVID-19 cohort from November 2020 to February 28, 2021, was 17%. The mortality rate among patients with severe disease was 54%. There was no mortality among patients who did not develop hypoxemia.
Of the 31 patients who required hospitalization, 6 patients (19%), all with severe disease, died during their COVID-19 hospitalization. All 18 hospitalized patients with moderate disease were discharged home post–COVID-19 admission. Two patients, one with severe disease and another with moderate disease, both of whom required supplemental oxygen during admission, were discharged and subsequently died after their index COVID-19 admission.
Seven patients (15%) from the entire cohort required invasive mechanical ventilation with a median of 11 d on the ventilator; 5 of those patients ultimately died. Most of the patients with severe disease (86%) who survived their COVID-19 hospitalization remained alive at the end of the study period with 33% still requiring supplemental oxygen. Most of the patients (63%) with moderate disease who required supplemental oxygen during hospitalization were discharged on room air; only 3 patients with moderate disease remained on supplemental oxygen by the end of the study.
Complications including thromboembolism, transaminitis, neurological events, and coinfections were more common among patients with severe disease (Table 5). One-third of the severe disease cohort required renal replacement therapy. Median values of abnormal laboratory results during disease course are shown in Table S1 (SDC, http://links.lww.com/TXD/A404). Patients with severe disease more commonly had elevated creatinine, aspartate aminotransferase, C-reactive protein, procalcitonin, lactate dehydrogenase, and troponin during hospitalization.
TABLE 5.
Clinical course and complications in patients with moderate and severe COVID-19
| Moderate (n = 18) | Severe (n = 13) | P | |
|---|---|---|---|
| Died, n (%) | 1 (6) | 7 (54) | <0.001 |
| Required mechanical ventilation, n (%) | – | 7 (54) | – |
| Days on ventilator | – | Median, 11 (IQR, 3–33)Mean, 22.9 (range, 3–74) | – |
| Received ECMO support, n (%) | 0 (0) | 0 (0) | – |
| Total LOS (d), median (IQR) | 9 (5–15) | 28 (10–57) | 0.025 |
| Combined ICU/SDU LOS (d) | – | Median, 13 (IQR, 10–36)Mean, 29 (range, 0–106) | – |
| Complications, n | |||
| AKI | 10 (63)a | 11 (85)a | 0.083 |
| New arrhythmia | 2 (13) | 2 (15) | 0.74 |
| VTE/arterial thrombi | 0 (0) | 4 (31) | 0.011 |
| Transaminitis | 6 (38) | 10 (77) | 0.023 |
| Neurological events | 1 (6) | 7 (54) | 0.006 |
| Coinfection | 5 (31) | 9 (69) | 0.031 |
| Respiratoryb | 2 (13) | 5 (38) | 0.092 |
| BSIc | 2 (13) | 2 (15) | 0.78 |
| CMV | 1 (6) | 3 (23) | 0.18 |
| Otherd | 2 (13) | 3 (23) | 0.43 |
aOne patient was already on hemodialysis at baseline and is not included in this total.
bRespiratory infections included rhinovirus, enterovirus, Aspergillus fumigatus, Pseudomonas aeruginosa, Stenotrophomonas, Klebsiella, E coli, and methicillin-resistant Staphylococcus aureus.
cBlood stream infections included Staphylococcus epidermidis, vancomycin-resistant Enterococcus.
dOther coinfections included Epstein-Barr virus; gastrointestinal cultures positive for Clostridium difficile, Yersinia; and urine cultures positive for Pseudomonas aeruginosa, BK virus, and Citrobacter.
AKI, acute kidney injury; BSI, blood stream infection; CMV, cytomegalovirus; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenator; E coli, Escherichia coli; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; SDU, step down unit; VTE, venous thromboembolism.
The average time to first negative SARS-CoV-2 PCR was 56 d in the 34 patients with available retesting data and did not differ greatly among severity groups (Table 6). One patient with severe disease who required readmission intermittently had positive SARS-CoV-2 test results at 115 d after the first positive test.
TABLE 6.
Clinical outcomes by COVID-19 severity
| Outcomes | Mild (n = 16) | Moderate (n = 18) | Severe (n = 13) | P |
|---|---|---|---|---|
| Mortality, n (%)a | 0 (0)a | 1 (6)a | 7 (54)b | <0.001 |
| New O2 requirement on discharge, n (%) | – | 4 (22) | 5 (38) | 0.021 |
| On O2 at follow-up, n (%) | 0 (0) | 3 (17) | 2 (15) | 0.087 |
| Discharged to home, n (%) | – | 18 (100) | 4 (31) | 0.0018 |
| Time until first negative swab (d), median (IQR) | 46 (22–99) (n = 15) | 53 (28–71) (n = 13) | 46 (38–55.5) (n = 6) | 0.66 |
aOne patient was discharged home and died suddenly at home.
bOne patient was readmitted from a subacute rehab facility 8 d after initial discharge for non–COVID-19 pneumonia (PCR testing negative) and CMV viremia.
CMV, cytomegalovirus; COVID-19, coronavirus disease 2019; IQR, interquartile range; PCR, polymerase chain reaction.
Comparison to First-Surge Cohort
Comparisons of the baseline demographics and major outcomes between the first- and second-surge cohorts are shown in Table 7.
TABLE 7.
Comparison between first and second cohorts
| First-surge cohort (n = 32) | Second-surge cohort (n = 47) | P | |
|---|---|---|---|
| Age, y, median (IQR) | 65 (51–69) | 65 (57–72) | 0.52 |
| Sex, n (%) | |||
| Female | 16 (50) | 24 (51) | 0.93 |
| Ethnicity, n (%) | 0.45 | ||
| Caucasian | 16 (50) | 32 (68) | |
| Hispanic | 9 (28) | 8 (17) | |
| African American | 7 (22) | 4 (8) | |
| Asian | 0 (0) | 3 (6) | |
| Transplant indication, n (%) | 0.11 | ||
| ILD | 15 (47) | 28 (59) | |
| Transplant type, n (%) | 0.85 | ||
| Single | 17 (53) | 26 (55) | |
| Years since transplant, median (IQR) | 5.6 (2–8.6) | 4.3 (2–9.7) | 0.77 |
| BOS stage, n (%) | 0.40 | ||
| 1 | 4 (13) | 5 (11) | |
| 2 | 2 (6) | 5 (11) | |
| 3 | 1 (3) | 4 (9) | |
| Baseline IS regimen, n (%) | |||
| Mycophenolate ≥2000 mg/d | 13 (41) | 16 (34) | 0.10 |
| Azathioprine ≥150 mg/d | 2 (6) | 1 (2) | |
| No cell-cycle inhibitor | 1 (3) | 9 (19) | |
| Tacrolimus | 24 (75) | 46 (98) | 0.004 |
| Prednisone ≥10 mg/d | 7 (22) | 20 (43) | 0.06 |
| Azithromycin for BOS | 17 (53) | 21 (45) | 0.47 |
| Recent IS augmentation, n (%) | 8 (25)a | 16 (34)b | 0.40 |
| Comorbidities, n (%) | |||
| Hypertension | 18 (56) | 35 (74) | 0.09 |
| CKD | 21 (65) | 29 (62) | 0.82 |
| Heart disease | 6 (19) | 12 (26) | 0.49 |
| Diabetes | 14 (44) | 26 (55) | 0.32 |
| Obesity (BMI ≥30) | 8 (25) | 2 (4) | 0.018 |
| Active malignancy | 1 (3) | 6 (13) | 0.14 |
| Duration of symptoms (d) before testing, median (IQR) | 4 (1.75–7.25) | 3 (1.75–6) | 0.11 |
| Disease severity, n (%) | |||
| Mild disease | 5 (16) | 16 (34) | 0.070 |
| Moderate disease | 14 (44) | 18 (38) | 0.633 |
| Severe disease | 13 (41) | 13 (28) | 0.234 |
| Hospitalization, n (%) | 27 (84) | 31 (66) | 0.074 |
| Mechanical ventilation, n (%) | 10 (31%) | 7 (15) | 0.084 |
| Mortality, n (%) | 11 (34) | 8 (17) | 0.078 |
aThree percent received induction therapy (basiliximab + solumedrol), 3% received steroid pulse, 25% received steroid taper, and 8% received rATG.
bEight percent received induction therapy (basiliximab + solumedrol), 11% received steroid pulse, 6% received steroid taper, and 8% received rATG.
BMI, body mass index; BOS, bronchiolitis obliterans syndrome; CKD, chronic kidney disease; ILD, interstitial lung disease; IQR, interquartile range; IS, immunosuppression.
Under crisis standards of care in the spring of 2020, where testing was severely limited, PCR testing at our center was almost exclusively performed on symptomatic patients, mostly in those presenting to the emergency department. Asymptomatic and preprocedural screening were not available at the time. As testing became more widely available over the course of the pandemic, the demographics of COVID-19 patients from the second surge at our center evolved to include higher proportion of patients with milder disease (16% versus 34%; P = 0.70) and lower proportion of severe disease (28% versus 41%; P = 0.234).4 In fact, 15% of patients from the current cohort were asymptomatic at the time of positive preprocedure PCR testing. The current cohort also contained a lower percentage of hospitalized (66% versus 84%; P = 0.07) and mechanically ventilated (15% versus 31%; P = 0.08) patients.4 The duration of reported symptoms before SARS-CoV-2 testing tended to be shorter in the current cohort (3 versus 4 d; P = 0.11).4 One major difference in patient baseline characteristics between the 2 cohorts was the lower proportion of patients with obesity in the second-surge cohort (4% versus 25%; P = 0.02).4 Another difference was the higher proportion of patients on tacrolimus at baseline in the current cohort (98%, versus 75%; P = 0.004). Otherwise, patient baseline characteristics were similar between the 2 groups.
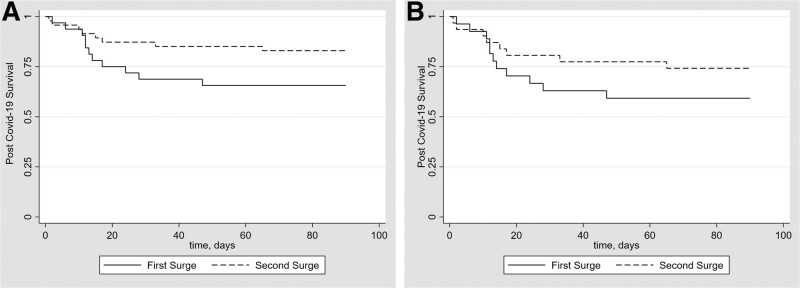
Major differences in pharmacological treatments reflect standard of care from each time period. Most of the patients in the first-surge cohort received hydroxychloroquine (84%) and azithromycin (75%), whereas none of the patients from the current cohort received either of these treatments. Additionally, whereas less than half of patients in the first-surge cohort (44%) received steroid augmentation, 84% of the current cohort with hypoxemia received corticosteroids equivalent in dose of dexamethasone 6 mg/d or higher per updated treatment guidelines. There was a trend toward reduced mortality in the second-surge cohort in both unadjusted and adjusted analyses (17% versus 34%; adjusted odds ratio, 0.26; 95% confidence interval, 0.08-0.85; P = 0.026; Figure 1A). This trend toward reduced mortality in the second-surge cohort was also observed in both the unadjusted and adjusted analyses of patients with moderate-to-severe COVID-19 (26% versus 41%; adjusted odds ratio, 0.28; 95% confidence interval, 0.79-1.03; P = 0.056; Figure 1B).
FIGURE 1.
A, Kaplan-Meier Plot of the probability of survival from COVID-19 diagnosis to day 90 in lung transplant recipients with COVID-19 from the first and second surges. There was a trend toward reduced 90-d mortality in the second-surge cohort in both unadjusted and adjusted analyses (17% vs 34%; adjusted OR, 0.26; 95% CI, 0.08-0.85; P = 0.026). B, Kaplan-Meier Plot of the probability of survival from COVID-19 diagnosis to day 90 in lung transplant recipients with moderate-to-severe COVID-19 from the first and second surges. There was a trend toward reduced 90-d mortality in the second-surge cohort of patients with moderate-to-severe COVID-19 in unadjusted and adjusted analyses (26% vs 41%; adjusted OR, 0.28; 95% CI, 0.79-1.03; P = 0.056). CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio.
DISCUSSION
Notably, after the first surge in the spring of 2020 at our center, there were no new acute COVID-19 cases between July and October 2020. This initial brief pause in new cases among our center’s lung transplant recipients coincided with local and national trends and may have reflected improved adherence to social distancing and self-isolation practices. We then identified 47 consecutive lung transplant recipients with positive SARS-CoV-2 between November 1, 2020, and February 28, 2021, and described their clinical presentations, management, and outcomes in this report. It is worthwhile noting that our study period preceded the rise of Delta strain (B.1.617.2), which is currently the predominant variant in the United States and is associated with higher rates of transmission and severe disease.1
In this second-surge cohort, we report an overall 90-d mortality rate of 17% in lung transplant recipients with COVID-19. As demonstrated in prior studies, severe COVID-19 with hypoxemia requiring supplemental oxygen, particularly among those requiring mechanical ventilation, was associated with increased mortality.13,14,18 On the contrary, the subset of patients who did not develop hypoxemia had favorable outcomes with no mortality observed. As for the patients initially requiring low-level supplemental oxygen support, overall mortality was still quite low with no inpatient mortality and 1 patient who died after discharge. Our findings suggest that the development of hypoxemia requiring supplemental oxygenation is an important prognostic marker of poor outcomes in lung transplant recipients.
Our findings also suggest a trend toward improved mortality in the second-surge COVID-19 cohort at our center. We feel that it is imperative to provide an updated mortality outcome for this population from a more recent study period that incorporated current evidence-based treatments. Without the extraordinary healthcare system capacity constraints and limited testing availability experienced in the early months of the pandemic, the lower mortality rate in our current report likely represents a more accurate estimate of COVID-19–associated mortality in this population.
We acknowledge that there are many inherent differences in characteristics between 2 study cohorts of our center in this comparative analysis. The challenges uniquely present at the onset of the pandemic—including COVID-19 being a novel disease with limited prior knowledge and experience in additional to scarcity of healthcare resources inherently—resulted in the higher illness severity of the first-surge cohort. Over the course of the pandemic, COVID-19 demographics of our center evolved to include more patients with milder disease and fewer patients with severe disease. To attenuate the potential bias toward improved outcome from the higher number of mild COVID-19 patients in this cohort, we performed a logistic regression on only moderate-to-severe COVID-19 patients from both cohorts. The trend toward improved mortality in the second-surge cohort with higher severity of illness persisted. We suspect that earlier and improved access to healthcare resources and updated COVID-19 treatments may have contributed to improved mortality in this population.
To our knowledge, we provide the first report on prolonged SARS-CoV-2 viral shedding in lung transplant recipients. Similar findings were described in kidney transplant recipients with 1 study reporting a kidney transplant recipient with a persistently positive nasal SARS-CoV-2 PCR at >66 d from symptom onset despite clinical recovery,32 and another reported 25% of its kidney transplant recipient cohort with persistently positive respiratory PCR at day 30 after symptom onset.33 Whether prolonged respiratory viral shedding also signifies a prolonged viral transmission window in lung transplant recipients with COVID-19 remains unknown. Furthermore, the clinical correlation of a prolonged positive respiratory tract viral PCR to quantitative viral load, cycle threshold, and viral cell culture in this immunocompromised population remains to be studied.
Our study has many strengths and provides the largest single-center cohort on the impact of COVID-19 in lung transplant recipients beyond the early months of the pandemic. The therapeutic regimen and management practices used in our study incorporated the most current evidence-based guidelines. Given limited data to guide management of lung transplant recipients with COVID-19, our protocol represents relatively large experience of our center and was associated with reasonable outcomes compared with the general population and prior cohorts. Our report also contains the largest number of lung transplant recipients with COVID-19 who were effectively managed in an ambulatory setting.
This retrospective cohort study is limited by its small sample size. There may have been patients with mild symptoms who were not tested and those who tested positive but did not self-report to our center. There was also likely a lead-time bias when comparing outcomes between the 2 cohorts with earlier diagnosis (including through preprocedural testing) and earlier presentation after symptom onset in the second-surge cohort. Finally, long-term functional and survival outcomes are not yet available for reporting.
In summary, COVID-19 in lung transplant recipients is associated with lower but still significant mortality in the second surge of the pandemic. Further studies will be required to assess longer-term outcomes, including mortality, functional status, and graft function. Additionally, the impact of COVID-19 vaccination and the rise of Delta, Omicron, and other variants on disease frequency, severity, and mortality in lung transplant patients will have to be studied.
ACKNOWLEDGMENTS
The authors are grateful for the tireless dedication of all the members of our Lung Transplant Program particularly during the last year of extreme stress caused by the pandemic.
Supplementary Material
Footnotes
K.L. and J.H. contributed equally to this work.
K.L., J.H., L.B., H.R., and S.M.A. contributed to conception, design, acquisition, analysis, interpretation, drafting, revising, and final approval. L.S., H.S.G., M.P., J.S., M.C., M.N., G.R., P.L., B.P.S., J.R.S., and F.D. participated in conception, interpretation of the data, revising the work, and final approval.
The authors declare no funding or conflicts of interest.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Centers for Disease Control and Prevention. COVID data tracker. Available at https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases. Accessed December 11, 2021.
- 2.Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2021;72:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aversa M, Benvenuto L, Anderson M, et al. ; From the Columbia University Lung Transplant Program. COVID-19 in lung transplant recipients: a single center case series from New York City. Am J Transplant. 2020;20:3072–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhry ZS, Williams JD, Vahia A, et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a cohort study. Am J Transplant. 2020;20:3051–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. ; Spanish Group for the Study of COVID-19 in Transplant Recipients. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristelli MP, Viana LA, Dantas MTC, et al. The full spectrum of COVID-19 development and recovery among kidney transplant recipients. Transplantation. 2021;105:1433–1444. [DOI] [PubMed] [Google Scholar]
- 8.Fung M, Chiu CY, DeVoe C, et al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: a case series from the United States. Am J Transplant. 2020;20:3225–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadi YB, Naqvi SFZ, Kupec JT, et al. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105:1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoek RAS, Manintveld OC, Betjes MGH, et al. ; Rotterdam Transplant Group. COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int. 2020;33:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karruli A, Spiezia S, Boccia F, et al. Effect of immunosuppression maintenance in solid organ transplant recipients with COVID-19: systematic review and meta-analysis. Transpl Infect Dis. 2021;23:e13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kates OS, Haydel BM, Florman SS, et al. ; UW COVID-19 SOT Study Team. Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis. 2021;73:e4090–e4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messika J, Eloy P, Roux A, et al. ; French Group of Lung Transplantation. COVID-19 in lung transplant recipients. Transplantation. 2021;105:177–186. [DOI] [PubMed] [Google Scholar]
- 14.Mohanka MR, Mahan LD, Joerns J, et al. Clinical characteristics, management practices, and outcomes among lung transplant patients with COVID-19. J Heart Lung Transplant. 2021;40:936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers CN, Scott JH, Criner GJ, et al. ; Temple University COVID-19 Research Group. COVID-19 in lung transplant recipients. Transpl Infect Dis. 2020;22:e13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira MR, Arcasoy S, Farr MA, et al. Outcomes of COVID-19 in solid organ transplant recipients: a matched cohort study. Transpl Infect Dis. 2021;23:e13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2021;35:100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saez-Giménez B, Berastegui C, Barrecheguren M, et al. COVID-19 in lung transplant recipients: a multicenter study. Am J Transplant. 2021;21:1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma P, Chen V, Fung CM, et al. COVID-19 outcomes among solid organ transplant recipients: a case-control study. Transplantation. 2021;105:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi SG, Rogers AW, Saharia A, et al. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020;104:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Permpalung N, Bazemore K, Chiang TP, et al. Impact of COVID-19 on lung allograft and clinical outcomes in lung transplant recipients: a case-control study. Transplantation. 2021;105:2072–2079. [DOI] [PubMed] [Google Scholar]
- 22.Anesi GL, Jablonski J, Harhay MO, et al. Characteristics, outcomes, and trends of patients with COVID-19-related critical illness at a learning health system in the United States. Ann Intern Med. 2021;174:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16:90–92. [DOI] [PubMed] [Google Scholar]
- 24.Elavarasi A, Prasad M, Seth T, et al. Chloroquine and hydroxychloroquine for the treatment of COVID-19: a systematic review and meta-analysis. J Gen Intern Med. 2020;35:3308–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin YH, Zhan QY, Peng ZY, et al. ; Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM); Chinese Research Hospital Association (CRHA). Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: an evidence-based clinical practice guideline (updated version). Mil Med Res. 2020;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadri SS, Sun J, Lawandi A, et al. Association between caseload surge and COVID-19 survival in 558 U.S. hospitals, March to August 2020. Ann Intern Med. 2021;174:1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asch DA, Sheils NE, Islam MN, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auld SC, Caridi-Scheible M, Robichaux C, et al. ; Emory COVID-19 Quality and Clinical Research Collaborative. Declines in mortality over time for critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48:e1382–e1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledford H. Why do COVID death rates seem to be falling? Nature. 2020;587:190–192. [DOI] [PubMed] [Google Scholar]
- 30.Acosta AM, Mathis AL, Budnitz DS, et al. COVID-19 investigational treatments in use among hospitalized patients identified through the US coronavirus disease 2019-associated hospitalization surveillance network, March 1-June 30, 2020. Open Forum Infect Dis. 2020;7:ofaa528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doidge JC, Gould DW, Ferrando-Vivas P, et al. Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med. 2021;203:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silvano J, Ferreira F, Bustorff M, et al. Viral clearance and serological response to SARS-CoV-2 in kidney transplant recipients. Transplant Proc. 2021;53:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benotmane I, Gautier-Vargas G, Wendling MJ, et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3162–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.