PURPOSE
Nivolumab + ipilimumab (nivo + ipi) is highly efficacious but has high toxicity. Standard treatment in advanced melanoma is four doses of nivo + ipi followed by nivo alone. Whether four doses of nivo + ipi are needed is unclear.
METHODS
The Adaptively Dosed ImmunoTherapy Trial (ADAPT-IT) study (NCT03122522) is a multicenter, single-arm phase II clinical trial. Patients received two doses of nivo (1 mg/kg) + ipi (3 mg/kg) followed by a computed tomography scan at week 6. Patients without new lesions or index lesion tumor growth of > 4% had protocol-defined early favorable antitumor effect (FATE) and ceased nivo + ipi, transitioning to nivo monotherapy. Patients without FATE at week 6 received the standard third and fourth doses of nivo + ipi followed by nivo monotherapy. The primary end point was response rate by RECIST 1.1 at week 12. Secondary end points included additional efficacy assessments and safety.
RESULTS
Sixty patients were enrolled; 41 patients (68%) had FATE at week 6 and met criteria for stopping nivo + ipi. Best overall response rates by RECIST at week 12 or any time afterward were 48% (95% CI, 35 to 62) and 58% (95% CI, 45 to 71), respectively. With a median follow-up of 25 months, the estimated 18-month progression-free survival and overall survival are 52% and 80%, respectively. Fifty seven percent of patients had grade 3-5 treatment-related toxicity.
CONCLUSION
The efficacy and toxicity of standard four dose nivo + ipi induction therapy in melanoma is likely driven by the first two doses. An interim computed tomography scan after two doses guided cessation of combination dosing and identified almost all responders. Longer follow-up and further study are needed to fully understand the implications of a shortened induction course of nivo + ipi.
INTRODUCTION
Combination immunotherapy with nivolumab (nivo) + ipilimumab (ipi) is efficacious for many tumor types, including unresectable stage III or IV metastatic melanoma. Standard combination therapy in metastatic melanoma is four doses of nivo 1 mg/kg + ipi 3 mg/kg in an induction phase followed by nivo monotherapy in a maintenance phase.1-3 The four-dose induction phase was based upon prior ipi monotherapy experience in which four doses of induction therapy had become standard.4 Yet, the need for four doses has never been established.
CONTEXT
Key Objective
Nivolumab + ipilimumab (nivo + ipi) is highly effective in melanoma and other tumor types, but no prior studies have addressed the efficacy and toxicity of giving fewer induction doses of treatment. This prospective study investigated whether certain patients with early favorable antitumor effect after two doses of nivo + ipi could be spared the standard third and fourth doses.
Knowledge Generated
The efficacy of stopping nivo + ipi after two doses in patients without early tumor growth appears similar to other trials with the standard four doses of nivo + ipi. Toxicity was not reduced with this approach.
Relevance
The efficacy and toxicity from nivo + ipi appears driven by the first two doses of treatment, raising questions about the need for doses 3 and 4. A computed tomography scan after two doses of nivo + ipi identifies most patients who benefit from treatment and may help guide future treatment plans.
Given the high efficacy and toxicity of nivo 1 mg/kg + ipi 3 mg/kg, studies to identify the optimal dose and schedule are especially relevant. Decreasing the dose of ipi to 1 mg/kg with a higher dose of nivo (3 mg/kg) was shown to reduce toxicity,5 but no prior studies have tested fewer than four combination doses. The efficacy and toxicity of intentionally giving fewer than four doses is of particular interest, given the observation that patients have responded following only one dose,6 and patients who discontinue combination dosing before receiving four doses because of toxicity have similar outcomes to all patients.1,7
With the goal of describing the efficacy and toxicity of intentionally giving fewer doses of nivo + ipi, we conducted the Adaptively Dosed ImmunoTherapy Study (ADAPT-IT). Since this was the first time fewer than four doses of nivo + ipi was being tested, we used an early, interim radiologic response assessment to identify patients who may be the best candidates for discontinuing the nivo + ipi combination after only two doses and moving to nivo monotherapy.
METHODS
Study Participants
Patients were enrolled into this investigator-initiated, phase II study at three medical centers in the United States (Memorial Sloan Kettering Cancer Center, New York, NY; Hartford Healthcare, Hartford, CT; and Lehigh Valley Health Network, Allentown, PA). The first patient began treatment on June 1, 2017. The database lock for this analysis took place on December 31, 2020.
Eligible patients were age 18 years or older with cytologically or histologically confirmed unresectable stage III or IV melanoma with one or more metastases measurable according to RECIST version 1.1. An Eastern Cooperative Oncology Group performance status of 0 or 1 and adequate organ function were required. Exclusion criteria included active autoimmune disease defined as requiring systemic treatment with either corticosteroids (> 10 mg daily of prednisone equivalents) or other immunosuppressive medications within 14 days of study drug administration, active hepatitis B or C infection, and prior treatment with immune checkpoint inhibitors in the unresectable/metastatic setting.
The study was conducted in accordance with Good Clinical Practice Guidelines as defined by the International Conference on Harmonization and the Declaration of Helsinki. Appropriate institutional review boards and ethics committees at each of the participating centers approved the Protocol (online only). Memorial Sloan Kettering Cancer Center maintained the study database. Annual reports to the institutional review board were provided. All participating patients provided written informed consent before enrollment. A data safety monitoring board monitored all aspects of the trial.
Procedures
This study sought to evaluate the efficacy of stopping induction combination nivo + ipi dosing based upon a prespecified, radiologic metric defined in the Protocol for the first time as favorable antitumor effect (FATE). FATE was demonstrated if (1) the sum of a patient's total RECIST measurable tumor burden decreased (RECIST complete response [CR], partial response [PR], or stable disease [SD] with decreasing tumor burden) or (2) the sum did not increase by > 4%. Growth > 4%, as opposed to any increase over baseline, was selected because a > 4% increase has been described to reflect a true change as opposed to measurement variation.8 To avoid premature discontinuation of combination therapy in this first exploration of de-escalating combination immunotherapy, any new lesion per RECIST 1.1 was considered non-FATE. The intent in defining FATE in this way was to resemble clinical practice where clinicians determine whether patients' tumors are increasing, staying the same, or decreasing without applying formal RECIST thresholds in defining progressive (+20% increase) or responding (–30% decrease) disease.
Patients received two doses of nivo 1 mg/kg + ipi 3 mg/kg, every 3 weeks (Appendix Fig A1, online only). A computed tomography (CT) scan was performed at week 6 (± 1-week window), which was intended to occur after two doses of nivo + ipi but was not delayed in instances of a delayed second nivo + ipi dose. If patients had FATE at week 6, patients began maintenance nivo alone (240 mg every 2 weeks or 480 mg every 4 weeks). If patients did not have FATE at week 6, they were eligible to receive two additional nivo + ipi doses until demonstrating FATE or unacceptable toxicity. In the event of RECIST progressive disease, clinicians could also continue treatment if they believed their patients were having clinical benefit. Patients stopped treatment because of toxicity or withdrawal of consent; there was no prespecified duration of maintenance nivo. CT scans were pursued at week 6, 12, and every 12 weeks thereafter if patients had demonstrated FATE or every 6 weeks if patients did not demonstrate FATE. Safety was assessed as per the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Nivo and ipi were manufactured and provided by Bristol Myers Squibb (New York, NY).
Outcomes
The primary outcome of this study was the objective response rate (CR and PR as per RECIST v1.1) at week 12. Secondary outcomes included rate of FATE at week 6; response rate at week 6 (by RECIST v1.1); response rate at week 12 by immune-related RECIST (irRECIST); duration of response; progression-free survival and overall survival (OS); best overall response rate throughout the duration of the follow-up; and safety. Exploratory outcomes included immunologic biomarkers in the peripheral blood, which will be separately reported.
Statistical Analyses
A Simon two-stage design was used whereby a response rate of 43% at week 12 was considered not promising, a 60% response rate was considered promising, and the probabilities of a Type I and Type II error were set at 0.10 and 0.10, respectively. Best overall response rates (inclusive of longer follow-up) from prior studies of nivo + ipi have ranged from 40% to 61%. In the first stage of the design, 37 patients were accrued and then accrual was held. If at least 14 patients had a week-12 RECIST response, accrual would proceed to the target full enrollment of 60 patients. Since the threshold of at least 14 patients with a week-12 response among the first 37 patients was achieved, the study enrolled to completion (N = 60) with the primary optimistic goal to observe 31 patients with week-12 responses.
Differences in demographics and cumulative toxicity profiles between FATE and non-FATE patients were assessed with Fisher's exact test and the Wilcoxon rank sum test, where appropriate. Toxicity rates were provided for all patients and by FATE at week 6. Toxicity patterns were visualized using a heatmap by toxicity system based upon categorization as defined by CTCAE. Cumulative incidence of grade 3-5 adverse events was assessed from time of treatment start until toxicity. Death without toxicity was treated as a competing risk. Response was estimated with 95% exact confidence intervals by RECIST and irRECIST criteria at 6 and 12 weeks, along with best overall response during the duration of the study follow-up.
Progression-free survival (PFS) was estimated from the start of treatment until progression or death using RECIST 1.1. Patients alive without progression were censored at last follow-up. OS was estimated from the start of treatment until death. Patients alive at last follow-up were censored. Duration of response among responders was assessed from the time of first response until progression or death. Patients alive and progression-free were censored. Kaplan-Meier methods were used to estimate these clinical outcomes. Patterns of response by FATE were displayed with an event history plot. All analyses were performed with SAS 9.4 TS1M6 (The SAS Institute, Cary, NC).
Role of the Funding Source
The funder of the study, Bristol Myers Squibb, provided nivo and ipi free of charge and provided third-party funding for trial coordination, documentation, and data management. The first author wrote the first draft of the manuscript, which was subsequently reviewed and approved by all authors. Bristol Myers Squibb reviewed the manuscript for medical accuracy before submission but was not involved in study conduct or data analysis. No other writing assistance was provided.
RESULTS
Patients and Treatment
Between May 18, 2017, and February 20, 2019, 60 eligible patients were consented and enrolled. Demographic and baseline characteristics (Table 1) were notable for patients with brain metastases (17%), a high proportion of patients with mucosal melanoma (27%), and some patients (13%) who had received prior systemic therapy (one patient with prior rapidly accelerated fibrosarcoma homolog B [BRAF] and mitogen-activated protein kinase kinase [MEK] inhibition and three patients with prior adjuvant anti-programmed cell death 1 [PD-1] therapy). The median follow-up for survivors was 25 months (range, 5-36 months).
TABLE 1.
Baseline Patient Characteristics and by FATE at Week 6

Ten (17%) patients received one dose of nivo + ipi, 35 (58%) received two doses, six (10%) received three doses, and nine (15%) received four doses. No patients received more than four doses of nivo + ipi. Forty-four (73%) patients received at least one dose of nivo maintenance. The median number of nivo maintenance doses for all patients was three (range, 0-33); the median number of nivo maintenance doses for patients who had FATE was seven (0-33).
Efficacy
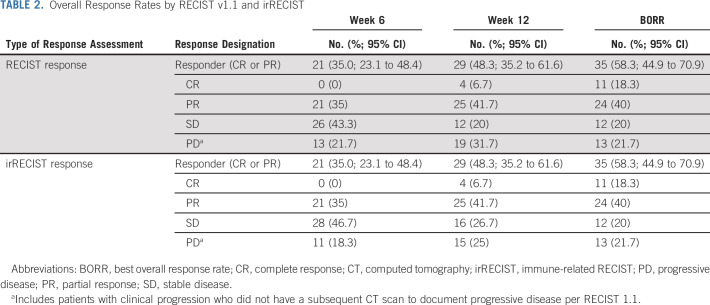
Six weeks after the patients' first dose of nivo + ipi, 41 patients (68%) demonstrated FATE and met trial criteria for stopping nivo + ipi. There were no demographic factors significantly associated with FATE (Table 1). Twenty-one patients (35% [95% CI, 23 to 48]) met RECIST criteria for PR at week 6, which increased to 29 patients (48%; [95% CI, 35 to 62]) at week 12. Thirty-five patients (58%; [95% CI 45 to 71]) had a PR or CR as their best overall response during the duration of the study follow-up (Fig 1, Table 2). Response rates by irRECIST were similar to RECIST (Table 2). Among the 35 patients with a CR or PR by RECIST 1.1, the median duration of response has not yet been reached (Appendix Fig A2, online only), and the probability of remaining in response by 12 months was 79% (95% CI, 61 to 89). Among 21 patients with responses at week 6, six patients had secondary progression by 1 year.
FIG 1.
Patient event histories by non-FATE versus FATE at week 6. For patients who experienced a grade 3-4 toxicity, a purple triangle represents the timing of first grade 3-4 toxicity. Bars are colored by RECIST 1.1 response starting at the week 6 scan and change color if a patient's response changed during the duration of study. Patients who died have a red X at the end of follow-up, and patients who are still alive by the data lock have a black right triangle. The teal circles represent doses of nivo + ipi combination immunotherapy. Three patients in the non-FATE group do not have colored bars to indicate response (two died of treatment-related toxicity and did not have response assessment and one had clinical progression without RECIST response assessment). As demonstrated by three patients in the FATE group with > 2 teal circles, three patients in the FATE group received > 2 doses of nivo + ipi (two patients' treatments were protocol violations and one had concern for clinical progression). CR, complete response; FATE, favorable antitumor effect; IPI, ipilimumab; irTox, immune-related toxicity; nivo + ipi, nivolumab + ipilimumab; PD, progressive disease; PR, partial response; SD, stable disease. The ADAPT-IT study showed that patients with melanoma may only need two doses of nivolumab + ipilimumab.
TABLE 2.
Overall Response Rates by RECIST v1.1 and irRECIST
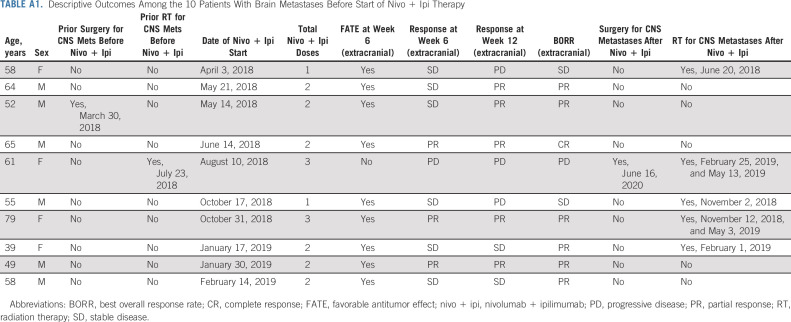
Median PFS has not been reached; 12- and 18-month PFS estimates were 53% and 52%, respectively (Fig 2A). Median OS has not been reached; 12- and 18-month OS estimates were 81% and 80%, respectively (Fig 2B). Among the 41 patients with FATE at week 6 who met criteria for early cessation of nivo + ipi, the median PFS has not been reached with 12-month (measured from the start of week 6 FATE) PFS and OS estimates of 68% and 85%, respectively (Appendix Fig A3, online only). Although formal intracranial response assessments were not part of the Protocol, among the 10 patients with brain metastases before the start of nivo + ipi, five ultimately underwent radiotherapy for intracranial lesions, and one of these five subsequently also required surgical resection of a progressive lesion (Appendix Table A1, online only). Five patients, all in the FATE group, did not require locoregional treatment of brain metastases after starting nivo + ipi.
FIG 2.
Kaplan-Meier curves for PFS (A) and OS (B) with blue shading reflecting the 95% CI. Vertical dotted lines with respective values reflect the estimated PFS/OS at 6, 12, and 18 months. OS, overall survival; PFS, progression-free survival.
Among the 41 patients with FATE at week 6, the best overall response (Fig 1) was CR in 10; PR in 23; and SD in eight for a response rate of 81% (95% CI, 65 to 91). Three patients with FATE had > 2 doses of nivo + ipi. Additional nivo + ipi dosing in these patients did not obviously improve their response outcomes.
For the 19 patients who did not have FATE at week 6, the best overall response was progressive disease in 13; SD in four; PR in one; and CR in one for a response rate of 11% (95% CI, 1 to 33). Following two doses of nivo + ipi at week 6, the patient who ultimately had a PR was considered non-FATE (RECIST and irRECIST SD) because of one increasing axillary lymph node, despite all other tumor lesions decreasing. Since this patient did not have FATE, the patient received doses 3 and 4 of nivo + ipi. By 12 weeks and before nivo maintenance, this patient's axillary lymph node had already started to decrease substantially, and by 6 months while on nivo maintenance, the patient met criteria for a PR. The other patient had shrinkage of one lymph node but transient increase in one splenic metastasis (RECIST and irRECIST SD) at week 6 after two doses of nivo + ipi. This patient then had toxicity and did not receive further nivo + ipi; ultimately, the patient started nivo maintenance and had a PR at 6 months and a CR by approximately 2.5 years.
Safety
All patients (100%) had treatment-related adverse events (Table 3; Appendix Table A2, online only). The rate of grade 3-5 treatment-related adverse events was 57%. There was no difference in the rate of grade 3-5 treatment-related adverse events in the patients with FATE (56%) at week 6 compared with patients without FATE (58%). The rate of grade 3-5 laboratory toxicity (termed Investigations per CTCAE), such as alanine aminotransferase and aspartate aminotransferase, was higher for the non-FATE patients (47%, 9 of 19) compared with the FATE patients (10 of 41, 25%). No other obvious differences were present in the spectrum, pattern, or severity of toxicities among patients who demonstrated FATE versus those who did not (Appendix Fig A4, online only). There also did not appear to be a pattern in the time of onset of first grade 3-5 toxicity by FATE (Fig 1). Fifty-one patients (85%) received systemic immunosuppression to manage adverse events, most commonly prednisone (≥ 10 mg per day), which was used in 44 (73%) of patients. The cumulative incidence of grade 3-5 adverse events increased steadily during the first 3 months but then plateaued after approximately 6 months (Appendix Fig A5, online only). Among 31 patients who had FATE at week 6 and received two doses of nivo + ipi, 17 (55%) had grade 3-5 treatment-related adverse events.
TABLE 3.
Cumulative Treatment-Related Adverse Eventsa
In the overall study, there were three deaths attributed to treatment. Two patients who each received one dose of nivo + ipi died from myocarditis. One patient who had secondary adrenal insufficiency and a new deep vein thrombosis, who had recently been started on anticoagulation, died suddenly at home; no autopsy was performed, and this death was conservatively considered related to treatment, given the possibility that adrenal insufficiency may have contributed.
DISCUSSION
The results of this study indicate that the efficacy and toxicity of standard nivo 1 mg/kg + ipi 3 mg/kg combination immunotherapy (four doses of combination) is largely driven by the first two doses of treatment. Although additional follow-up is needed to fully assess response durability, treatment-free survival, and longer-term OS, these early findings inform clinical practice as they provide reassuring information to clinicians that four doses of nivo + ipi may not be necessary. In clinical practice, these findings may be especially relevant for patients with evidence of early efficacy who may be at high risk for new or worsening toxicity with ongoing combination dosing.
To our knowledge, this is the first study of intentionally giving a maximum of two doses of nivo + ipi in patients with unresectable stage III or IV melanoma. Other studies, in different clinical contexts such as resectable stage III melanoma, have similarly shown high efficacy of only two doses of nivo + ipi. In two neoadjuvant studies, two doses of nivo + ipi led to radiographic responses in 57%-63% of patients.9,10
Among the 19 patients who did not have FATE at week 6, two (11%) ultimately responded. Both patients had evidence of partial antitumor efficacy after two doses of nivo + ipi at week 6 with only one lesion increasing while all other lesions were decreasing. No patients with an increase in multiple tumor lesions during nivo + ipi ultimately responded. To our knowledge, these are the first data on the outcomes of patients with early primary progression of all lesions during nivo + ipi from a prospective trial in melanoma and could be used to define patient populations for future clinical trials testing earlier incorporation of new agents in nivo + ipi pretreated melanoma. For example, if all tumor lesions are increasing after two doses of nivo + ipi, future trials could explore whether escalating therapy with the addition of an agent onto ongoing nivo + ipi improves outcomes.
Shortening the induction course of nivo + ipi to two doses on the basis of the proposed FATE metric did not reduce the rate of grade 3-4 treatment-related adverse events nor lead to any measurable reduction in the use of immunosuppression or treatment-related fatality compared with prior studies.1,11 These unexpected findings suggest that not only efficacy, but also toxicity of combination nivo + ipi are driven by the first two doses of treatment. Prior standard-dose nivo (1 mg/kg) + ipi (3 mg/kg) studies show that many patients are unable to receive four doses, because of toxicity in most cases; in the pivotal Checkmate 067 study, 57% received all four doses, and 29% received one or two combination doses. As expected by our study design, patients in our trial received fewer doses of combination therapy with 75% of patients receiving only one or two doses. Since our study was the first to test a shortened combination induction course, we felt it was more appropriate in this initial exploration to select patients for fewer nivo + ipi doses only if they had a stable or improving tumor burden (Protocol defined FATE) as opposed to giving all patients only two doses or randomly assigning into differential dose cohorts.
Despite the best overall response rate (58%) approximating that of prior randomized large phase trials of nivo 1 mg/kg + ipi 3 mg/kg (51%-61%),5,12,13 our week-12 response rate of 48% was below our prespecified optimistic promising week-12 response rate of 60%. Since the week-12 response rate to standard nivo + ipi in these prior studies has not been previously reported, it remains possible that our week-12 response rate is in line with prior studies of standard nivo + ipi. We chose response rate at week 12 for the primary efficacy end point to rapidly ensure that our dose-sparing approach was not obviously compromising efficacy.
We acknowledge several study limitations. A blinded, independent central radiographic review may have standardized response assessments beyond the investigator-assessed responses that were used. Furthermore, pharmacokinetic data are not available to more fully understand effects of shortening the number of nivo + ipi doses. Analyses of pharmacodynamic changes in the peripheral blood within this trial and others are ongoing and ultimately may provide additional insights. An additional study is needed for patients with brain metastases to determine the optimal dosing of nivo + ipi to prevent the need for locoregional treatment modalities. The novel early interim efficacy, radiographic assessment used in this study, FATE, has not previously been explored. The FATE criteria were specified a priori in this study to resemble clinical practice as much as possible where clinicians use CT scan findings to simply determine whether patients' tumors are growing (non-FATE in this study) or not growing/shrinking (FATE). Although patient selection could always influence results since each center has inherent biases in how to treat patients with nivo + ipi, we feel that involving three centers in this study at least partially reduced potential inherent biases regarding patient selection. There were patients who had secondary progression after initial response, including among patients with response at week 6, but given rates of secondary progression after standard-dose nivo + ipi,13 whether the number of nivo + ipi doses affects response durability remains unclear.
Although most nivo + ipi side effects occur after the second dose of nivo + ipi,14 10 patents in our study had to stop nivo + ipi after receiving only one dose. Among these 10 patients, four had a RECIST response (1CR and 3 PR). These responses, in combination with other published cases of responses after one dose,6 raise the currently unanswered question of the efficacy and side effects of a single dose of nivo 1 mg/kg + ipi 3 mg/kg, which we are planning to address in an upcoming neoadjuvant clinical trial for patients with surgically resectable high-risk stage III melanoma.
It would be ideal if a baseline demographic feature were able to determine which patients are the best candidates for a shortened induction nivo + ipi course. In an exploratory post hoc analysis, we were not able to identify baseline demographic factors that were associated with FATE. We chose to focus our treatment modification strategy using an on-treatment event (week-6 scan), in part, given other data that suggest on-treatment immunologic biomarkers are better predictors of longer-term outcomes than baseline biomarkers.15,16
In summary, we completed the first study of adaptively dosed combination immunotherapy where treatment was modified on the basis of the results of an early, interim CT scan. A CT scan after two doses of nivo + ipi may help guide future treatment plans, but the impact of treatment modification on response durability and longer-term OS requires additional follow-up. Larger, randomized studies are also needed to confirm these findings.
ACKNOWLEDGMENT
The authors would like to thank Tiffany Brown, Brooke Freeman, Olivia Gibson, Amanda Hill, David Katzman, Sirinya O'Shea, Erica Payson, Nana Prempeh-Kete Ku, Julian Mendoza-Robles, and Lanier Tanner for their assistance in patient care and clinical trial data curation.
APPENDIX
FIG A1.
Study schema. aFollowing week 12, patients received maintenance nivo but were eligible for additional nivo + ipi if they had tumor growth. Nivo + ipi, nivolumab + ipilimumab.
FIG A2.

Duration of response among all patients with a RECIST response. Blue shading reflects the 95% CI. Vertical dotted lines with respective values reflect the estimated rates of ongoing response at 6 and 12 months following initial response.
FIG A3.
PFS (A) and OS (B) among patients with FATE at week 6. Blue shading reflects the 95% CI. Vertical dotted lines with respective values reflect the estimated rates at 12 and 24 months from the week-6 CT scan. CT, computed tomography; FATE, favorable antitumor effect; OS, overall survival; PFS, progression-free survival.
FIG A4.

Each column represents a patient broken down by FATE versus non-FATE at week 6. Rows represent treatment-related side-effect categories as per CTCAE with colors reflecting CTCAE grade. CTCAE, Common Terminology Criteria for Adverse Events; FATE, favorable antitumor effect.
FIG A5.

Cumulative incidence of any grade 3-5 treatment-related adverse events. IRTox, immune-related toxicity.
TABLE A1.
Descriptive Outcomes Among the 10 Patients With Brain Metastases Before Start of Nivo + Ipi Therapy
TABLE A2.
Treatment-Related Adverse Events Affecting ≥ 5% of Patients and All Grade ≥ 3 Events
Michael A. Postow
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Array BioPharma, NewLink Genetics, Incyte, Merck, Eisai, Pfizer
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Array BioPharma (Inst), Infinity Pharmaceuticals (Inst), Regenix (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst)
Debra A. Goldman
Employment: Regeneron
Stock and Other Ownership Interests: Johnson & Johnson
Alexander N. Shoushtari
Consulting or Advisory Role: Immunocore, Bristol Myers Squibb
Researching Funding: Immunocore, Bristol Myers Squibb, Xcovery, Polaris, Novartis (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Allison Betof Warner
Honoraria: LG Chem
Consulting or Advisory Role: Nanbiotix, Iovance Biotherapeutics, Novartis, Shanghai Jo’Ann Medical Technology, BluePath Solutions, Pfizer
Research Funding: Leap Therapeutics
Margaret K. Callahan
Employment: Bristol-Myers Squibb (I), Celgene (I), Kleo Pharmaceuticals (I), Bristol-Myers Squibb (I)
Consulting or Advisory Role: AstraZeneca, Moderna Therapeutics, Merck, Immunocore
Researching Funding: Bristol Myers Squibb (Inst)
Other Relationship: Clinical Care Options, Potomac Center for Medical Education
Omar Eton
Speakers’ Bureau: Merck
Researching Funding: Bristol Myers Squibb (Inst)
Suresh G. Nair
Stock and Other Ownership Interests: Moderna Therapeutics, Novavax, Biotech, Gilead Sciencs
Research Funding: Bristol-Myers Squibb, Merck, Nektar (Inst)
Katherine S. Panageas
Stock and Other Ownership Interests: AstraZeneca, Pfizer, Sunesis Pharmaceuticals
Jedd D. Wolchok
Stock and Other Ownership Interests: Tizona Therapeutics, Inc, Adaptive Biotechnologies, Imvaq Therapeutics, Beigene, Linnaeus Therapeutics, Arsenal IO, Geogiamune, LLC, Maverick Therapeutics, Apricity Therapeutics, Trieza Therapeutics
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Sellas Life Sciences, Lilly, Tizona Therapeutics, Inc, Amgen, Chugai Pharma, Adaptive Biotechnologies, Ascentage Pharma, PsiOxus Therapeutics, F-Start Biotechnology, Surface Biotechnology, Apricity Therapeutics, PsiOxus Therapeutics, F-Star Biotechnology, Surface Oncology, Apricity Therapeutics, PsiOxus Therapeutics, Astellas Pharma, Recepta Biopharma, Arsenal IO, Boehringer Ingelheim, AstraZeneca, Daiichi Sankyo,Inc, Dragonfly Therapeutics, Geogiamune, Kyowa Kirin Pharmaceutical, Maverick Therapeutics, Werewolf Therapeutics, Trishula Therapeutics, Idera, Imvq Therapeutics, Biscara Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Merck Sharp & Dohme (Inst)
Patents, Royalties, Other Intellectual Property: I am a co-inventor on an issued patent for DNA vaccines for treatment for cancer in companion animals, I am a co-inventor on a patent for use of oncolytic Newcaste Disease virus, I am a co-inventor and receive royalties for a blood test for monitoring myeloid derived suppressor cells. I am co-inventor and receive royalties for a petent for immune modulating antibododies. I am a co-inventor on a patent for CAR+ T cells targeting differentiation antigens as means to treat cancer. I am a co-inventor on a patent for Anti-CD40 agonist mAb fused to Monophosphory Lipid A (MPL) for cancer therapy, Alphavirus Replicon Particles Expressing TRP2, Engineered Vaccinia Viruses for Cancer Immunotherapy, Recombinant Poxviruses for Cancer Immunotherapy
Paul B. Chapman
Consulting or Advisory Role: Merck, Pfizer, Black Diamond Therapeutics
Research Funding: Pfizer, NCI (Inst), Genentech
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part as an oral abstract at the ASCO Virtual Annual Meeting, May 29-31, 2020.
SUPPORT
Supported by Cancer Center Support Grant P30 CA08748 from the National Institutes of Health/National Cancer Institute; Ludwig Collaborative and Swim Across America Laboratory, and Parker Institute for Cancer Immunotherapy.
CLINICAL TRIAL INFORMATION
NCT03122522 (ADAPT-IT)
AUTHOR CONTRIBUTIONS
Conception and design: Michael A. Postow, Allison Betof Warner, Katherine S. Panageas, Jedd D. Wolchok, Paul B. Chapman
Administrative support: Vladislav Raber
Provision of study materials or patients: Michael A. Postow, Alexander N. Shoushtari, Allison Betof Warner, Margaret K. Callahan, Parisa Momtaz, Omar Eton, Suresh G. Nair, Jedd D. Wolchok, Paul B. Chapman
Collection and assembly of data: Michael A. Postow, Ellesa Naito, Marina K. Cugliari, Vladislav Raber, Omar Eton, Suresh G. Nair, Jedd D. Wolchok
Data analysis and interpretation: Michael A. Postow, Debra A. Goldman, Alexander N. Shoushtari, Allison Betof Warner, Margaret K. Callahan, Parisa Momtaz, James W. Smithy, Katherine S. Panageas, Jedd D. Wolchok, Paul B. Chapman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adaptive Dosing of Nivolumab + Ipilimumab Immunotherapy Based upon Early, Interim Radiographic Assessment in Advanced Melanoma (The ADAPT-IT Study)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael A. Postow
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Array BioPharma, NewLink Genetics, Incyte, Merck, Eisai, Pfizer
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Array BioPharma (Inst), Infinity Pharmaceuticals (Inst), Regenix (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst)
Debra A. Goldman
Employment: Regeneron
Stock and Other Ownership Interests: Johnson & Johnson
Alexander N. Shoushtari
Consulting or Advisory Role: Immunocore, Bristol Myers Squibb
Researching Funding: Immunocore, Bristol Myers Squibb, Xcovery, Polaris, Novartis (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Allison Betof Warner
Honoraria: LG Chem
Consulting or Advisory Role: Nanbiotix, Iovance Biotherapeutics, Novartis, Shanghai Jo’Ann Medical Technology, BluePath Solutions, Pfizer
Research Funding: Leap Therapeutics
Margaret K. Callahan
Employment: Bristol-Myers Squibb (I), Celgene (I), Kleo Pharmaceuticals (I), Bristol-Myers Squibb (I)
Consulting or Advisory Role: AstraZeneca, Moderna Therapeutics, Merck, Immunocore
Researching Funding: Bristol Myers Squibb (Inst)
Other Relationship: Clinical Care Options, Potomac Center for Medical Education
Omar Eton
Speakers’ Bureau: Merck
Researching Funding: Bristol Myers Squibb (Inst)
Suresh G. Nair
Stock and Other Ownership Interests: Moderna Therapeutics, Novavax, Biotech, Gilead Sciencs
Research Funding: Bristol-Myers Squibb, Merck, Nektar (Inst)
Katherine S. Panageas
Stock and Other Ownership Interests: AstraZeneca, Pfizer, Sunesis Pharmaceuticals
Jedd D. Wolchok
Stock and Other Ownership Interests: Tizona Therapeutics, Inc, Adaptive Biotechnologies, Imvaq Therapeutics, Beigene, Linnaeus Therapeutics, Arsenal IO, Geogiamune, LLC, Maverick Therapeutics, Apricity Therapeutics, Trieza Therapeutics
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Sellas Life Sciences, Lilly, Tizona Therapeutics, Inc, Amgen, Chugai Pharma, Adaptive Biotechnologies, Ascentage Pharma, PsiOxus Therapeutics, F-Start Biotechnology, Surface Biotechnology, Apricity Therapeutics, PsiOxus Therapeutics, F-Star Biotechnology, Surface Oncology, Apricity Therapeutics, PsiOxus Therapeutics, Astellas Pharma, Recepta Biopharma, Arsenal IO, Boehringer Ingelheim, AstraZeneca, Daiichi Sankyo,Inc, Dragonfly Therapeutics, Geogiamune, Kyowa Kirin Pharmaceutical, Maverick Therapeutics, Werewolf Therapeutics, Trishula Therapeutics, Idera, Imvq Therapeutics, Biscara Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Merck Sharp & Dohme (Inst)
Patents, Royalties, Other Intellectual Property: I am a co-inventor on an issued patent for DNA vaccines for treatment for cancer in companion animals, I am a co-inventor on a patent for use of oncolytic Newcaste Disease virus, I am a co-inventor and receive royalties for a blood test for monitoring myeloid derived suppressor cells. I am co-inventor and receive royalties for a petent for immune modulating antibododies. I am a co-inventor on a patent for CAR+ T cells targeting differentiation antigens as means to treat cancer. I am a co-inventor on a patent for Anti-CD40 agonist mAb fused to Monophosphory Lipid A (MPL) for cancer therapy, Alphavirus Replicon Particles Expressing TRP2, Engineered Vaccinia Viruses for Cancer Immunotherapy, Recombinant Poxviruses for Cancer Immunotherapy
Paul B. Chapman
Consulting or Advisory Role: Merck, Pfizer, Black Diamond Therapeutics
Research Funding: Pfizer, NCI (Inst), Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 2. Zimmer L, Livingstone E, Hassel JC, et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;395:1558–1568. doi: 10.1016/S0140-6736(20)30417-7. [DOI] [PubMed] [Google Scholar]
- 3. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lebbé C, Meyer N, Mortier L, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: Results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol. 2019;37:867–875. doi: 10.1200/JCO.18.01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chapman PB, D'Angelo SP, Wolchok JD. Rapid eradication of a bulky melanoma mass with one dose of immunotherapy. N Engl J Med. 2015;372:2073–2074. doi: 10.1056/NEJMc1501894. [DOI] [PubMed] [Google Scholar]
- 7. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807–3814. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McErlean A, Panicek DM, Zabor EC, et al. Intra- and interobserver variability in CT measurements in oncology. Radiology. 2013;269:451–459. doi: 10.1148/radiology.13122665. [DOI] [PubMed] [Google Scholar]
- 9. Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20:948–960. doi: 10.1016/S1470-2045(19)30151-2. [DOI] [PubMed] [Google Scholar]
- 10. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24:1655–1661. doi: 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]
- 11. Shoushtari AN, Friedman CF, Navid-Azarbaijani P, et al. Measuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanoma. JAMA Oncol. 2018;4:98–101. doi: 10.1001/jamaoncol.2017.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sznol M, Ferrucci PF, Hogg D, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol. 2017;35:3815–3822. doi: 10.1200/JCO.2016.72.1167. [DOI] [PubMed] [Google Scholar]
- 15. Chen PL, Roh W, Reuben A, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24:1649–1654. doi: 10.1038/s41591-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]