PURPOSE
Fatigue is common and troublesome among breast cancer survivors; however, limited tools exist to predict its risk.
PATIENTS AND METHODS
Participants with stage I-III breast cancer were prospectively included from CANTO (ClinicalTrials.gov identifier: NCT01993498), collecting longitudinal data at diagnosis (before the initiation of any cancer treatment) and 1 (T1), 2 (T2), and 4 (T3) years after diagnosis. The main outcome was severe global fatigue at T2 (score ≥ 40/100, European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-C30). Analyses at T3 were exploratory. Secondary outcomes included physical, emotional, and cognitive fatigue (EORTC Quality of Life Questionnaire-FA12). Multivariable logistic regression models retained associations with severe fatigue by bootstrapped Augmented Backward Elimination. Validation methods included 10-fold internal cross-validation, overoptimism-corrected area under the receiver operating characteristic curves, and external validation.
RESULTS
Among 5,640, 5,000, and 3,400 patients at T1, T2, and T3, respectively, the prevalence of post-treatment severe global fatigue was 35.6%, 34.0%, and 31.5% in the development cohort. Retained risk factors for severe global fatigue at T2 were severe pretreatment fatigue (adjusted odds ratio v no 3.191 [95% CI, 2.704 to 3.767]); younger age (for 1-year decrement 1.015 [1.009 to 1.022]), higher body mass index (for unit increment 1.025 [1.012 to 1.038]), current smoking behavior (v never 1.552 [1.291 to 1.866]), worse anxiety (v noncase 1.265 [1.073 to 1.492]), insomnia (for unit increment 1.005 [1.003 to 1.007]), and pain at diagnosis (for unit increment 1.014 [1.010 to 1.017]), with an area under the receiver operating characteristic curve of 0.73 (95% CI, 0.72 to 0.75). Receipt of hormonal therapy was a risk factor for severe fatigue at T3 (v no 1.448 [1.165 to 1.799]). Dimension-specific risk factors included body mass index for physical fatigue and emotional distress for emotional and cognitive fatigue.
CONCLUSION
We propose a predictive model to assess fatigue among breast cancer survivors, within a personalized survivorship care framework. This may help clinicians to provide early management interventions or to correct modifiable risk factors and offer more tailored monitoring and education to patients at risk of severe post-treatment fatigue.
INTRODUCTION
Cancer-related fatigue is one of the most distressing and common post-treatment sequelae among survivors of early-stage breast cancer.1-3 More than 30% of patients with breast cancer experience persistent fatigue symptomatology up to 10 years after treatment completion.4-7 Cancer-related fatigue can result in substantial adverse physical, psychosocial, and socioeconomic consequences, having a negative impact on overall quality of life.6 Nevertheless, fatigue is still rarely discussed or proactively managed.8-10 Cancer-related fatigue is multidimensional in its manifestation, involving physical, emotional, and cognitive dimensions, and most likely multifactorial, being determined by multiple patient characteristics and contextual, psychosocial, and behavioral factors, comorbid conditions, and biologic factors including inflammation, disease characteristics, and antineoplastic therapies.3
CONTEXT
Key Objective
This study aimed at identifying patients who have an increased risk of severe and persistent post-treatment fatigue 2 years after diagnosis of early-stage breast cancer.
Knowledge Generated
More than one-in-three patients endured persistent severe post-treatment fatigue. Younger age, higher body mass index, smoking behavior, and concomitant symptom clusters including pretreatment fatigue, anxiety, insomnia, and pain emerged as key risk factors for the development of severe fatigue 2 years after diagnosis. Exploratory models identified receipt of hormonal therapy as an additional risk factor for severe fatigue 4 years after diagnosis.
Relevance
We propose predictive models that may help clinicians to better assess fatigue at diagnosis of breast cancer and provide timely management interventions to those experiencing severe pretreatment fatigue. Our models may also aid the prompt identification of modifiable risk factors and raise awareness to recognize early signs and act timely on worsening symptoms in patients at long-term risk of severe post-treatment fatigue.
Current knowledge of the long-term prevalence, trajectory, and risk factors of breast cancer–related fatigue is still limited,4,6,11 which hampers our ability to capture its complexity and variability and to clearly identify those at risk of severe fatigue to potentially target with effective interventions. The prospective multicenter CANcer TOxicity (CANTO) cohort (ClinicalTrials.gov identifier: NCT01993498) aims at characterizing toxicities of breast cancer, building on an extensive longitudinal collection of clinical, behavioral, tumor, treatment, and patient-reported outcome data.12 In this study, we used CANTO to develop and validate a risk model and to generate a predictive tool for long-term severe fatigue.
PATIENTS AND METHODS
Study Design and Patient Selection
We included CANTO participants with stage I-III breast cancer. CANTO collects data at diagnosis of breast cancer (ie, before the initiation of any cancer treatment) and then at 1 (T1), 2 (T2), and 4 (T3) years after diagnosis (corresponding to approximately 3-6 months, 1 year, and 3 years after primary treatment completion, respectively, including breast surgery, chemotherapy and/or radiation therapy). The CANTO study design (ClinicalTrials.gov identifier: NCT01993498) was previously described.12 All patients provided written informed consent. The study was approved by the ethics committee (ID-RCB:2011-A01095-36,11-039).
Outcome Assessment
The primary outcome of interest was global fatigue, assessed using the multi-item scale of the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C3013-15: Item 10—Did you need to rest? Item 12—Have you felt weak? Item 18—Were you tired? Patients reported fatigue levels on a 4-point Likert scale per item (response values: 1, not at all; 2, a little; 3, quite a bit; and 4, very much). Responses were converted to a 0-100 scale using a standard scoring algorithm,15 as follows:
§ is the difference between maximum and minimum possible values of Raw Score. Most items of the EORTC QLQ-C30, including the three items contributing to the global fatigue scale, are scored 1-4, and therefore, the range equals 3.
As secondary outcomes, we assessed the physical, emotional, and cognitive dimensions of fatigue, evaluated by EORTC QLQ-FA12.16 This is a multidimensional instrument measuring fatigue to be used in conjunction with the core EORTC QLQ-C30. The questionnaire includes five items for physical fatigue (items 1-5), three for emotional fatigue (items 6-8), and two for cognitive fatigue (items 9-10; Data Supplement, online only). In accordance with the scales of the EORTC QLQ-C30, the FA12 scores are transformed to the range of 0-100, following the same scoring algorithm.
For symptom scales, including fatigue, a higher score represents a higher level of symptomatology and/or problems. All standardized fatigue scores were dichotomized using a threshold of ≥ 40/100,6 indicating clinically relevant fatigue likely affecting patient's daily life and limiting usual activities, therefore requiring dedicated clinicians' attention and prompting supportive care needs.6,17-20
Other Variables of Interest
Candidate predictors were selected on the basis of clinical expertise and previous evidence of association with fatigue3,6 and included clinical features, treatment-related factors, and symptoms (including pretreatment fatigue), defined as in Table 1.21-24
TABLE 1.
Distribution of Cohort Characteristics at 2 Years and 4 Years After Diagnosis, No. (%)
Statistical Analysis
Cohort and outcome description.
Descriptive statistics summarized distribution of predictors and prevalence of severe fatigue at T1, T2, and T3 in the overall cohort.
Model development.
Patients from the 2012 to 2015 enrollment period of the CANTO study were included in the development cohort according to the availability of global fatigue assessments (n = 5,640 at T1, n = 5,000 at T2, and n = 3,400 at T3; complete case; Data Supplement). Potential predictors of severe fatigue were tested in multivariable logistic regression models, using a bootstrapped (No. = 100) Augmented Backward Elimination procedure. Variable selection combines backward elimination on the basis of significance (P < .05) and the change‐in‐estimate criterion, so that nonsignificant variables are retained if their exclusion leads to a relevant change in the parameter estimates of other variables in the model.25 The main prediction analysis focused on the risk of severe global fatigue at year 2 (T2) after diagnosis. Risk assessment at year 4 (T3) was considered exploratory. We evaluated the discrimination ability of the model by C-statistics, calculating the area under the receiver operating characteristic curve (AUC).
Internal validation.
The model was internally validated using 10-fold internal cross-validation and plotting the observed and estimated probability of severe fatigue for each model.26 To estimate how well the model would perform in external data sets, an overoptimism penalty was subtracted from the C-statistic of the final model.27
External validation.
Model performance was externally assessed in a validation cohort from a subsequent CANTO enrollment period that extended until 2017 (n = 2,461 at T1, n = 2,101 at T2, and n = 1,469 at T3; Data Supplement). Models previously fitted in the development cohort, including all predictors retained by Augmented Backward Elimination, were applied to patients in the validation cohort. Predictive performance was evaluated by the C-statistic and visually exploring model calibration.
Fatigue risk prediction.
To obtain a final, parsimonious model, we fit a logistic regression including a set of predictors retained in the development cohort, which were consistent in the validation cohort.
Sensitivity analyses.
A sensitivity analysis was conducted including patients who responded to global fatigue assessments at all time points.
Power considerations.
Procedures to calculate the sample size required to obtain a satisfactory outcome prediction were previously published.28 Briefly, with a binary outcome prevalence of 31%-35%, a minimal sample size of 998 patients was needed to minimize overfitting (expected shrinkage of predictor effects 10% or lower) and to ensure precise estimation of key parameters in the prediction model at T1 (including an absolute difference of 0.05 in the model apparent and adjusted R2 value). To achieve the same criteria at T2 and T3, at least 665 and 659 participants were required, respectively.28 To maximize power, model performance was assessed among all patients who had global fatigue assessments available at T1, T2, and T3, respectively.
This study followed the TRIPOD29 Checklist for Prediction Model Development and Validation. Additional methodological details are provided in the Data Supplement.
Statistical analysis was performed using SAS statistical software Version 9.4. Statistical significance was defined with a two-sided P < .05.
RESULTS
Primary Outcome Evaluation: Severe Global Fatigue
Characteristics of the overall population are shown in Table 1 and in the Data Supplement by severe global fatigue.
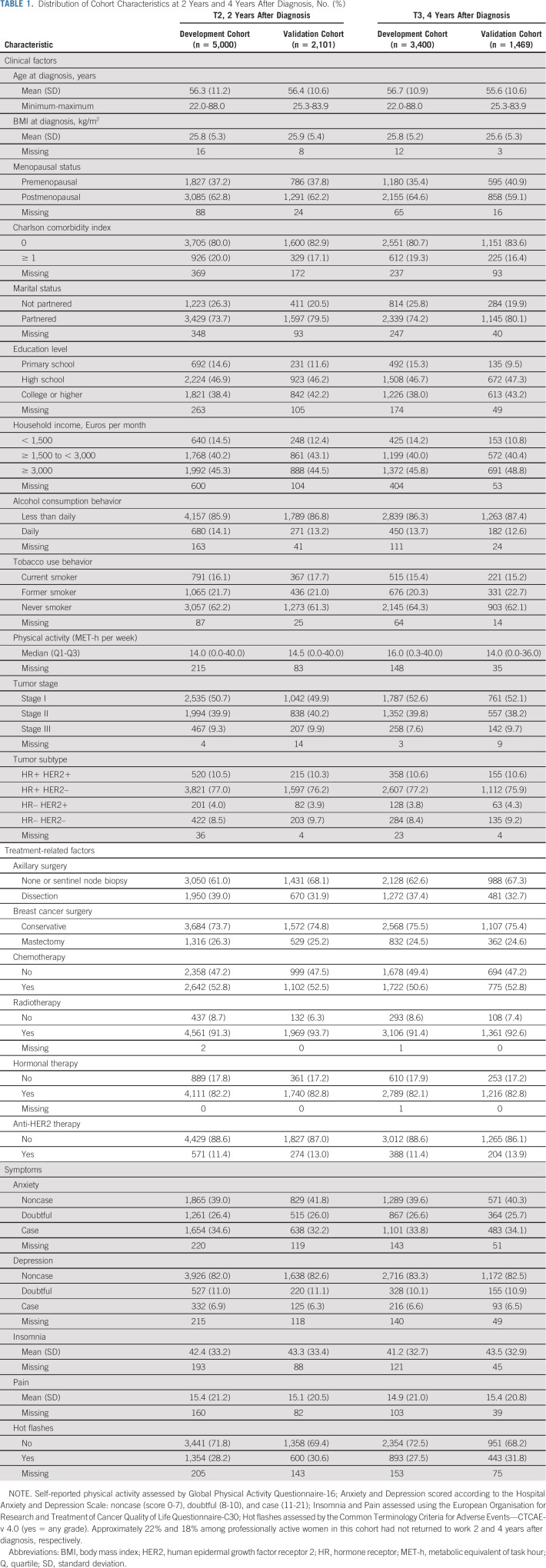
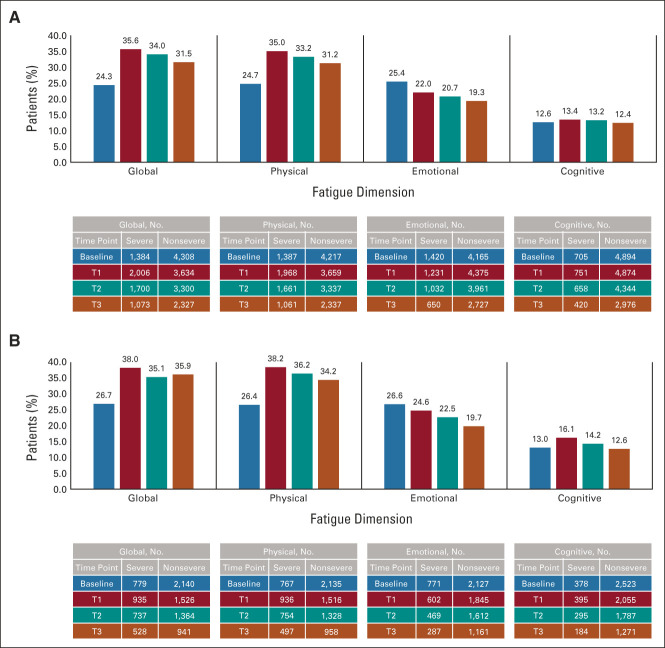
In the development and validation cohorts, respectively, prevalence of severe global fatigue at baseline was 24.3% and 26.7%, reached 35.6% and 38.0% at T1 (ie, closest to primary treatment completion), was substantially unchanged to 34.0% and 35.1% at T2, and remained elevated at 31.5% and 35.9% until T3 (Fig 1).
FIG 1.
Prevalence of severe global fatigue and of severe fatigue by dimension over time in (A) development cohort and (B) validation cohort. Baseline represents breast cancer diagnosis.
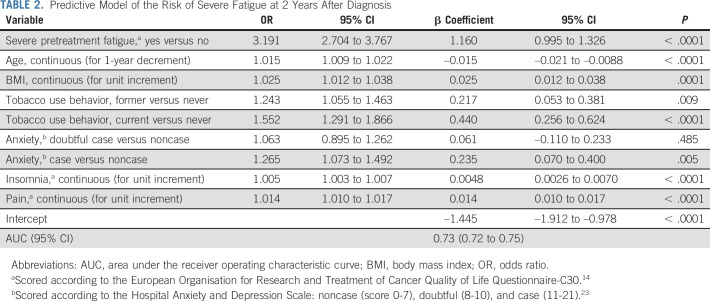
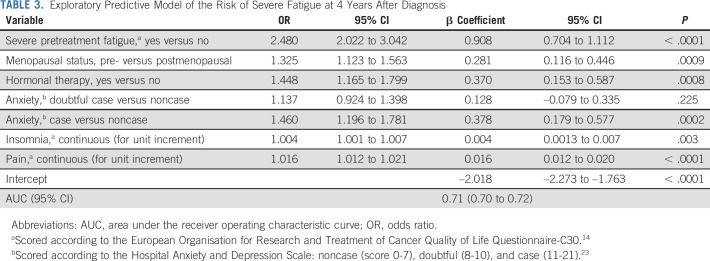
Reporting severe pretreatment fatigue was a consistent predictor of post-treatment fatigue at all time points (Tables 2 and 3; Data Supplement).
TABLE 2.
Predictive Model of the Risk of Severe Fatigue at 2 Years After Diagnosis
TABLE 3.
Exploratory Predictive Model of the Risk of Severe Fatigue at 4 Years After Diagnosis
In the main predictive model for severe global fatigue at T2, six other predictors were consistent in the development and validation models. These included younger age (adjusted odds ratio for 1-year decrement 1.015 [95% CI, 1.009 to 1.022]), higher body mass index (BMI; for unit increment 1.025 [1.012 to 1.038]), current smoking behavior (v never 1.552 [1.291 to 1.866]), and concomitant symptoms at diagnosis such as worse anxiety (v noncase 1.265 [1.073 to 1.492]), insomnia (for unit increment 1.005 [1.003 to 1.007]), and pain (for unit increment 1.014 [1.010 to 1.017]; Table 2; AUC 0.73 [95% CI, 0.72 to 0.75]).
In the exploratory model for fatigue at T3, premenopausal status (v postmenopausal 1.325 [1.123 to 1.563]) and receipt of hormonal therapy (v no 1.448 [1.165 to 1.799]) surfaced as risk factors for severe fatigue (Table 3; AUC 0.71 [95% CI, 0.70 to 0.72]). Of note, 38.6% and 87.4% of premenopausal women age < 40 and 40 years or older, respectively, reported postchemotherapy interruption of menses at T3 (< 5% overall received ovarian function suppression), which was not associated with severe fatigue (P = .914 and P = .515, respectively).
Among treatment-related variables, although the development model retained an association between the receipt of chemotherapy and severe fatigue at year 1 (T1), closest to completion of primary treatment (adjusted odds ratio v no 1.270 [95% CI, 1.099 to 1.466]; Data Supplement), this did not seem to persist in later time-point models. By contrast, hormonal therapy represented a significant correlate of severe global fatigue in development models at T2, approximately 1 year into hormonal therapy, and was confirmed as a significant predictor of fatigue at T3, after a longer course of treatment, approximately 3 years.
Model β coefficients for regression equations are presented in Tables 2 and 3. The Data Supplement shows model calibration plots.
Secondary Outcomes Evaluation: Fatigue Dimensions
Prevalence of severe fatigue dimensions followed similar patterns to that of global fatigue, except for emotional fatigue, which tended to progressively improve (Fig 1). Consistent with global fatigue, there was a close relationship between reporting severe pretreatment fatigue and concomitant pain at diagnosis with each of the three dimensions, and chemotherapy was retained as a risk factor for all dimensions of fatigue at T1 in the development cohort. Dimension-specific predictors at all time points included higher BMI for physical fatigue and emotional distress for emotional and cognitive fatigue (Data Supplement).
Sensitivity Analyses
The impact of treatment-related variables on fatigue was consistent in sensitivity analyses. In particular, receipt of hormonal therapy was a risk factor for longer-term severe fatigue at T3 (data not shown).
DISCUSSION
Fatigue is a very common side effect among patients with breast cancer, but limited tools exist to predict its risk.6 About one-in-three patients in CANTO endured persistent, severe fatigue over time. Using the wealth of information of this cohort, we identified clinicobehavioral risk factors and generated a risk model for severe fatigue 2 years after diagnosis of breast cancer, as well as an exploratory model, to provide further insight into risk of severe fatigue 4 years after diagnosis. Dimension-specific risk factors were identified.
Our study confirms and expands the knowledge about relevant risk factors for severe fatigue in survivors of breast cancer.3,6,11,30-35 Across global and fatigue dimensions, pretreatment fatigue represented the strongest and most consistent predictor. Pretreatment fatigue may set the stage for elevated fatigue even years after treatment completion, because of a disruption in biologic, psychologic, or behavioral mechanisms that exist before treatment onset.3,4,36-39 Younger age, and, accordingly, premenopausal status, also emerged as risk factors, as previously shown.5,36,40 In addition, a vulnerable phenotype was represented by patients with high concomitant symptom burden at diagnosis, experiencing several other frequently reported correlates of fatigue, such as sleep disturbances, pain (which may include chronic neuropathic pain further exacerbated by more extensive surgical procedures),41,42 depression, and anxiety.3,6 Our results also suggest that several correlates are often shared across distinct fatigue dimensions and over time. However, notable was the closer relationship of physical fatigue with increased BMI and that of emotional and cognitive fatigue with psychologic distress and vulnerability, which highlights a need of examining risk factors in view of their possible dimension-specific effects.3,6
There seemed to be variation in the way that treatment-related factors affect fatigue at different stages of survivorship. In the shorter term (T1), we found that the chemotherapy-related impact seems transitory and mostly evident in the aftermath of treatment, in line with previous reports, for example, those focused on cognitive function.43,44 By contrast, a more marked detrimental association between hormonal therapy and fatigue was confirmed after longer exposure (T3). From a broader perspective, these findings support the notion that the impact of hormonal therapy on quality of life does not seem to taper off over time. The Mind Body Study had nicely shown that hormonal therapy exacerbates an array of treatment-associated symptoms, being likely responsible for the failure to resolve some common chemotherapy-related toxicities.45 Analogously, we had previously suggested how hormonal therapy seems to attenuate the recovery of patient-reported functions that typically get better over time, including emotional function and future persectives.7 Data from the present study underscore that some patients receiving hormonal therapy—particularly younger, premenopausal women—may require dedicated attention. This is all the more important in consideration of recently implemented strategies to escalate hormonal therapy by adding ovarian function suppression46 or extending its duration beyond 5 years.47-49
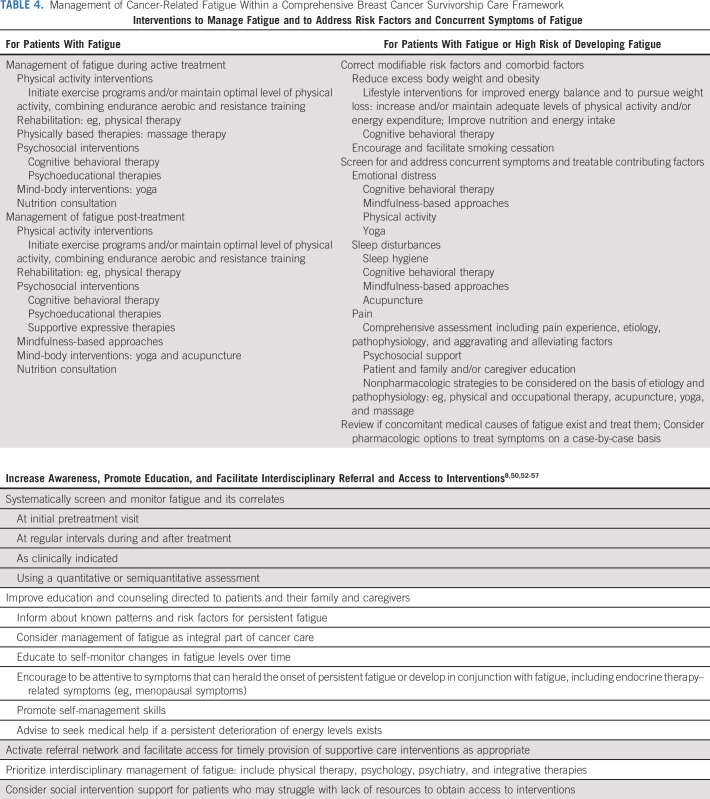
Substantial evidence shows that cancer-related fatigue is underaddressed3,6,8,50 and that the utilization of strategies to manage this symptom may be suboptimal.51 In light of the collective research to date, including the present study, we propose a risk-stratified framework of long-term toxicity management, applied to fatigue (Table 4), and an online tool for fatigue risk calculation.58 We envision a clinical care setting where incoming new patients are systematically screened for fatigue and risk factors at breast cancer diagnosis, before the initiation of any cancer treatment. Some among them would already experience pretreatment fatigue and require the upfront utilization of interventions to treat this symptom.8,50 By contrast, among patients without severe fatigue at diagnosis, a detailed evaluation of factors included in our models would allow a more personalized approach.59-61 The models we propose include several modifiable behavioral risk factors for which meaningful interventions exist as well as concomitant symptom clusters that can be specifically treated, in the context of a comprehensive survivorship care model that addresses multiple dimensions of health and health promotion.62 In addition, among patients who do not report severe pretreatment fatigue, being at risk of long-term post-treatment fatigue may indicate a more attentive assessment. This can help to increase awareness among providers and patients to recognize symptoms that can herald the onset of persistent fatigue, or develop in conjunction with it, including endocrine therapy–related menopausal symptoms. Increased awareness may then trigger earlier management and referral and facilitate patient access to supportive care when most needed.50
TABLE 4.
Management of Cancer-Related Fatigue Within a Comprehensive Breast Cancer Survivorship Care Framework
Finally, our study offers inspiration for future research in the field, including indications to design meaningful interventional trials. We highlight priorities such as a need to better assess relevant thresholds discriminating between low versus high predicted risk, to validate effectiveness of preventive interventions, to define optimal frequency of fatigue assessments, and to bridge risk stratification with patient activation toward symptom monitoring and acquisition of self-management skills. Acceptability and interpretability of the model should also be qualitatively explored. Future efforts could also be directed at developing adaptive models that provide a dynamic risk assessment. eHealth might serve well this purpose. Digital tools were used to follow-up patient-reported symptoms, and they were effective in reducing symptom burden and improving health-related quality of life, particularly during or shortly after treatment.63-67 Nevertheless, although eHealth may potentially facilitate the sustainability of long-term cancer survivorship care, fully automated behavioral intervention technologies for symptom monitoring, real-time feedback, and personalized overview of supportive care options have not consistently improved knowledge, skills, and confidence for self-management among cancer survivors.68
The translation of risk prediction into delivery of innovation also comes with several challenges. Model use should integrate clinical judgment to aid decision process, and considerations should be given to issues related to risk communication and incorporation into existing workflows. Social determinants of health should be identified as they may generate disparities in access to interventions and resource utilization, elevating barriers among strata with lower level of health and digital literacy and reduced activation.69 Notably, 22% and 18% among professionally active women in this cohort had not returned to work 2 and 4 years after diagnosis, respectively, consistent with previous findings.70,71 The subsequent potential impact on the ability to meet more intense financial demands and on the resources to deal with survivorship-related struggles calls for a need of integrating a social intervention plan into survivorship care models.
Some limitations must be acknowledged. Our models were specifically developed and validated to fit CANTO data and might not be fully generalizable to all cancer populations. CANTO was designed to assess evolution of chronic toxicities among survivors without evidence of active disease, which might influence the trajectory of symptoms. We acknowledge potential for bias because of study termination for patients who experience disease recurrence. Furthermore, patients not providing fatigue assessments at later time points may partly overlap with those at risk of developing severe fatigue (ie, prone to unhealthy behaviors, with lower income, more symptomatic at diagnosis). Reduced retention may further limit generalizability, and therefore, exploratory models at T3 are provided with the caveat of interpreting their outputs with caution.
Strengths include a large cohort size, a prospective and longitudinal design following patients from diagnosis into treatment completion through the long-term survivorship, and evaluation of distinct, nuanced dimensions of fatigue. Models were internally and externally validated, demonstrating transportability and accurate predictions among patients drawn from a different, although related population.29,72-74 Performance was globally satisfactory,60,61 and models performed similarly well when validated in external patients, providing acceptable discrimination (AUC = 0.70-0.80).75 Consistency with previous literature and plausibility of the underlying risk mechanisms and processes suggest that our models are clinically sound rather than solely relying on statistical selection methods. In addition, our models allow identification of modifiable risk factors and treatable correlate symptoms, and this was suggested to be a key feature to prioritize rather than simply pursuing maximization of model precision.26,76
In conclusion, we assessed the long-term prevalence and risk factors for severe fatigue up to four years after breast cancer diagnosis. We then propose predictive models that may help clinicians to better assess fatigue at diagnosis and provide timely management interventions to those experiencing severe pretreatment fatigue. Our models may also aid the prompt identification of modifiable risk factors and raise awareness to recognize early signs and worsening symptoms in patients at long-term risk of severe post-treatment fatigue. This framework may be extended to other prevalent toxicities in survivorship care, building on the integration of patient-reported outcomes in clinical practice and the increasing accessibility to digital symptom management solutions.
Better understanding of mechanisms of fatigue, including its underlying biologic underpinnings, and testing of screening and prevention algorithms in clinical care settings are needed to implement efficient risk-stratified management interventions for cancer-related fatigue.
ACKNOWLEDGMENT
We thank Yuki Takahashi for her help with manuscript drafting.
Barbara Pistilli
Consulting or Advisory Role: Puma Biotechnology, Pierre Fabre, Novartis, Myriad Genetics, AstraZeneca, Daiichi Sankyo/UCB Japan
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst), Merus (Inst), Daiichi-Sankyo (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, MSD Oncology, Novartis, Pierre Fabre
Suzette Delaloge
Consulting or Advisory Role: AstraZeneca (Inst), Pierre Fabre (Inst)
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Puma Biotechnology (Inst), Lilly (Inst), Novartis (Inst), Sanofi (Inst), Exact Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Roche
Nancy U. Lin
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, California Institute for Regenerative Medicine (CIRM), Denali Therapeutics, AstraZeneca, Prelude Therapeutics
Research Funding: Genentech (Inst), Pfizer (Inst), Seattle Genetics (Inst), Merck (Inst), Zion (Inst)
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Patricia A. Ganz
Leadership: Intrinsic LifeSciences (I)
Stock and Other Ownership Interests: Xenon Pharma (I), Intrinsic LifeSciences (I), Silarus Therapeutics (I), Teva, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: InformedDNA, Vifor Pharma (I), Ambys Medicines (I), Global Blood Therapeutics (I), GlaxoSmithKline (I), Ionis Pharmaceuticals (I), Akebia Therapeutics (I), Protagonist Therapeutics (I), Regeneron (I), Sierra Oncology (I), Rockwell Medical Technologies Inc (I), Astellas Pharma (I), Gossamer Bio (I), American Regent (I), Disc Medicine (I), Blue Note Therapeutics, Grail
Research Funding: Blue Note Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease, Up-to-Date royalties for section editor on survivorship (I)
Travel, Accommodations, Expenses: Intrinsic LifeSciences (I)
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for coauthoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Fabrice André
Stock and Other Ownership Interests: Pegacsy
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Roche (Inst), Daiichi (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Stefan Michiels
Consulting or Advisory Role: IDDI, Sensorion, Biophytis, Servier, Yuhan, Amaris Consulting, Roche
Ines Vaz-Luis
Honoraria: AstraZeneca (Inst), Amgen (Inst), Pfizer (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the American Society of Clinical Oncology annual meeting 2021 during the Poster Discussion Session on June 4, 2021 (abstr 12022). A.D.M. received the Conquer Cancer, the ASCO Foundation Pain and Symptom Management Special Merit Award for the present work. This Merit Award recognizes the highest-ranking abstract in the Pain and Symptom Management category as determined by the Scientific Program Committee.
SUPPORT
Supported by a Career Pathway Grant in Symptom Management from Conquer Cancer, the ASCO Foundation and Rising Tide Foundation for Clinical Cancer Research to A.D.M.; a Career Catalyst Research grant from Susan G. Komen (Grant No. CCR17483507) to I.V.-L.; and grants from Odyssea and Foundation Gustave Roussy. This work was also supported by the French Government under the Investment for the Future program managed by the National Research Agency (ANR), Grant No. ANR-10-COHO-0004 (CANTO), and by the Prism project, funded by the ANR, Grant No. ANR-18-IBHU-0002.
AUTHOR CONTRIBUTIONS
Conception and design: Antonio Di Meglio, Barbara Pistilli, Suzette Delaloge, Patricia A. Ganz, Ines Vaz-Luis
Financial support: Antonio Di Meglio, Fabrice Andre, Ines Vaz-Luis
Administrative support: Anne-Laure Martin
Provision of study materials or patients: Sibille Everhard, Anne-Laure Martin, Carole Tarpin, Christelle Levy, Olivier Rigal, Suzette Delaloge
Collection and assembly of data: Antonio Di Meglio, Julie Havas, Barbara Pistilli, Sibille Everhard, Anne-Laure Martin, Carole Tarpin, Laurence Vanlemmens, Christelle Levy, Olivier Rigal, Suzette Delaloge, Ines Vaz-Luis
Data analysis and interpretation: Antonio Di Meglio, Julie Havas, Davide Soldato, Daniele Presti, Elise Martin, Barbara Pistilli, Gwenn Menvielle, Agnes Dumas, Cecile Charles, Charles Coutant, Carole Tarpin, Suzette Delaloge, Nancy U. Lin, Patricia A. Ganz, Ann H. Partridge, Fabrice André, Stefan Michiels, Ines Vaz-Luis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Development and Validation of a Predictive Model of Severe Fatigue After Breast Cancer Diagnosis: Toward a Personalized Framework in Survivorship Care
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Barbara Pistilli
Consulting or Advisory Role: Puma Biotechnology, Pierre Fabre, Novartis, Myriad Genetics, AstraZeneca, Daiichi Sankyo/UCB Japan
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst), Merus (Inst), Daiichi-Sankyo (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, MSD Oncology, Novartis, Pierre Fabre
Suzette Delaloge
Consulting or Advisory Role: AstraZeneca (Inst), Pierre Fabre (Inst)
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Puma Biotechnology (Inst), Lilly (Inst), Novartis (Inst), Sanofi (Inst), Exact Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Roche
Nancy U. Lin
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, California Institute for Regenerative Medicine (CIRM), Denali Therapeutics, AstraZeneca, Prelude Therapeutics
Research Funding: Genentech (Inst), Pfizer (Inst), Seattle Genetics (Inst), Merck (Inst), Zion (Inst)
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Patricia A. Ganz
Leadership: Intrinsic LifeSciences (I)
Stock and Other Ownership Interests: Xenon Pharma (I), Intrinsic LifeSciences (I), Silarus Therapeutics (I), Teva, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: InformedDNA, Vifor Pharma (I), Ambys Medicines (I), Global Blood Therapeutics (I), GlaxoSmithKline (I), Ionis Pharmaceuticals (I), Akebia Therapeutics (I), Protagonist Therapeutics (I), Regeneron (I), Sierra Oncology (I), Rockwell Medical Technologies Inc (I), Astellas Pharma (I), Gossamer Bio (I), American Regent (I), Disc Medicine (I), Blue Note Therapeutics, Grail
Research Funding: Blue Note Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease, Up-to-Date royalties for section editor on survivorship (I)
Travel, Accommodations, Expenses: Intrinsic LifeSciences (I)
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for coauthoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Fabrice André
Stock and Other Ownership Interests: Pegacsy
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Roche (Inst), Daiichi (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Stefan Michiels
Consulting or Advisory Role: IDDI, Sensorion, Biophytis, Servier, Yuhan, Amaris Consulting, Roche
Ines Vaz-Luis
Honoraria: AstraZeneca (Inst), Amgen (Inst), Pfizer (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Runowicz CD, Leach CR, Henry NL, et al. : American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin 66:43-73, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine and National Research Council : From Cancer Patient to Cancer Survivor—Lost in Transition. Washington, DC, National Academies Press, 2006. 10.17226/11613 [DOI] [Google Scholar]
- 3.Bower JE: Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11:597-609, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower JE, Ganz PA, Desmond KA, et al. : Fatigue in long-term breast carcinoma survivors. Cancer 106:751-758, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bower JE, Ganz PA, Desmond KA, et al. : Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 18:743-753, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Abrahams HJG, Gielissen MFM, Schmits IC, et al. : Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12 327 breast cancer survivors. Ann Oncol 27:965-974, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Ferreira AR, Di Meglio A, Pistilli B, et al. : Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: A prospective patient-reported outcomes analysis. Ann Oncol 30:1784-1795, 2019 [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Cancer-Related Fatigue (Version 1.2021). https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf [Google Scholar]
- 9.Koornstra RHT, Peters M, Donofrio S, et al. : Management of fatigue in patients with cancer—A practical overview. Cancer Treat Rev 40:791-799, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Mitchell SA: Cancer-related fatigue: State of the science. PM R 2:364-383, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Bower JE, Wiley J, Petersen L, et al. : Fatigue after breast cancer treatment: Biobehavioral predictors of fatigue trajectories. Health Psychol 37:1025-1034, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaz-Luis I, Cottu P, Mesleard C, et al. : UNICANCER: French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO). ESMO Open 4:e000562, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sprangers MA, Cull A, Bjordal K, et al. : The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: Guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Qual Life Res 2:287-295, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Aaronson NK, Ahmedzai S, Bergman B, et al. : The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Fayers P, Aaronson NK, Bjordal K, et al. : EORTC QLQ-C30 Scoring Manual. European Organisation for Research and Treatment of Cancer, 2001. https://abdn.pure.elsevier.com/en/publications/eortc-qlq-c30-scoring-manual-3rd-edition [Google Scholar]
- 16.Weis J, Tomaszewski KA, Hammerlid E, et al. : International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ-FA12). J Natl Cancer Inst 109, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Storey DJ, Waters RA, Hibberd CJ, et al. : Clinically relevant fatigue in cancer outpatients: The Edinburgh Cancer Centre symptom study. Ann Oncol 18:1861-1869, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Karakoyun-Celik O, Gorken I, Sahin S, et al. : Depression and anxiety levels in woman under follow-up for breast cancer: Relationship to coping with cancer and quality of life. Med Oncol 27:108-113, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Reidunsdatter RJ, Albrektsen G, Hjermstad MJ, et al. : One-year course of fatigue after post-operative radiotherapy in Norwegian breast cancer patients—Comparison to general population. Acta Oncol 52:239-248, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Giesinger JM, Loth FLC, Aaronson NK, et al. : Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J Clin Epidemiol 118:1-8, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Chang LC, Sangha O, et al. : Can comorbidity be measured by questionnaire rather than medical record review? Med Care 34:73-84, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Global Physical Activity Questionnaire (GPAQ) Analysis Guide. https://www.who.int/ncds/surveillance/steps/resources/GPAQ_Analysis_Guide.pdf [Google Scholar]
- 23.Zigmond AS, Snaith RP: The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361-370, 1983 [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute : Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf [Google Scholar]
- 25.Dunkler D, Plischke M, Leffondré K, et al. : Augmented backward elimination: A pragmatic and purposeful way to develop statistical models. PLoS One 9:e113677, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrell FE: Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY, Springer Verlag, 2001
- 27.Harrell FE, Lee KL, Mark DB: Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361-387, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Riley RD, Ensor J, Snell KIE, et al. : Calculating the sample size required for developing a clinical prediction model. BMJ 368:m441, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Moons KGM, Altman DG, Reitsma JB, et al. : Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): Explanation and elaboration. Ann Intern Med 162:W1-W73, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Bødtcher H, Bidstrup PE, Andersen I, et al. : Fatigue trajectories during the first 8 months after breast cancer diagnosis. Qual Life Res 24:2671-2679, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Donovan KA, Small BJ, Andrykowski MA, et al. : Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol 26:464-472, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt ME, Chang-Claude J, Seibold P, et al. : Determinants of long-term fatigue in breast cancer survivors: Results of a prospective patient cohort study. Psychooncology 24:40-46, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Kober KM, Smoot B, Paul SM, et al. : Polymorphisms in cytokine genes are associated with higher levels of fatigue and lower levels of energy in women after breast cancer surgery. J Pain Symptom Manage 52:695-708.e4, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao H, Bao T, Shen X, et al. : Prevalence and risk factors for fatigue among breast cancer survivors on aromatase inhibitors. Eur J Cancer 101:47-54, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Identification of distinct fatigue trajectories in patients with breast cancer undergoing adjuvant chemotherapy|Profiles RNS. https://pubmed.ncbi.nlm.nih.gov/25876159/ [DOI] [PMC free article] [PubMed]
- 36.Bower JE, Asher A, Garet D, et al. : Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer 125:633-641, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein D, Bennett BK, Webber K, et al. : Cancer-related fatigue in women with breast cancer: Outcomes of a 5-year prospective cohort study. J Clin Oncol 30:1805-1812, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Geinitz H, Zimmermann FB, Thamm R, et al. : Fatigue in patients with adjuvant radiation therapy for breast cancer: Long-term follow-up. J Cancer Res Clin Oncol 130:327-333, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collado-Hidalgo A, Bower JE, Ganz PA, et al. : Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 12:2759-2766, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Andrykowski MA, Schmidt JE, Salsman JM, et al. : Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Oncol 23:6613-6622, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira S, Fontes F, Sonin T, et al. : Neuropathic pain after breast cancer treatment: Characterization and risk factors. J Pain Symptom Manage 54:877-888, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Fontes F, Pereira S, Costa AR, et al. : The impact of breast cancer treatments on sleep quality 1 year after cancer diagnosis. Support Care Cancer 25:3529-3536, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Joly F, Giffard B, Rigal O, et al. : Impact of cancer and its treatments on cognitive function: Advances in research from the paris International Cognition and Cancer Task Force Symposium and Update since 2012. J Pain Symptom Manage 50:830-841, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Lange M, Joly F: How to identify and manage cognitive dysfunction after breast cancer treatment. J Oncol Pract 13:784-790, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Ganz PA, Petersen L, Bower JE, et al. : Impact of adjuvant endocrine therapy on quality of life and symptoms: Observational data over 12 months from the mind-body study. J Clin Oncol 34:816-824, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribi K, Luo W, Bernhard J, et al. : Adjuvant tamoxifen plus ovarian function suppression versus tamoxifen alone in premenopausal women with early breast cancer: Patient-reported outcomes in the suppression of ovarian function trial. J Clin Oncol 34:1601-1610, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies C, Pan H, Godwin J, et al. : Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805-816, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray RG, Rea D, Handley K, et al. : aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 31, 2013. (18 suppl; abstr 5) [Google Scholar]
- 49.Del Mastro L, Mansutti M, Bisagni G, et al. : Extended therapy with letrozole as adjuvant treatment of postmenopausal patients with early-stage breast cancer: A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 22:1458-1467, 2021 [DOI] [PubMed] [Google Scholar]
- 50.Bower JE, Bak K, Berger A, et al. : Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol 32:1840-1850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Use of physical activity (PA) and supportive care (SC) among patients (pts) with early breast cancer (BC) reporting cancer-related fatigue (CRF)|OncologyPRO. https://oncologypro.esmo.org/meeting-resources/esmo-breast-cancer-virtual-meeting-2020/use-of-physical-activity-pa-and-supportive-care-sc-among-patients-pts-with-early-breast-cancer-bc-reporting-cancer-related-fatigue-crf
- 52.National Comprehensive Cancer Network. Adult Cancer Pain (Version 2.2021). https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf [Google Scholar]
- 53.National Comprehensive Cancer Network. Distress Management (Version 1.2022). https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf [Google Scholar]
- 54.Franzoi MA, Agostinetto E, Perachino M, et al. : Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol 22:e303-e313, 2021 [DOI] [PubMed] [Google Scholar]
- 55.Ligibel JA, Basen-Engquist K, Bea JW, et al. : Weight management and physical activity for breast cancer prevention and control. Am Soc Clin Oncol Ed Book 39:e22-e33, 2019 [DOI] [PubMed] [Google Scholar]
- 56.Demark-Wahnefried W, Schmitz KH, Alfano CM, et al. : Weight management and physical activity throughout the cancer care continuum. CA Cancer J Clin 68:64-89, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Land SR, Toll BA, Moinpour CM, et al. : Research priorities, measures, and recommendations for assessment of tobacco use in clinical cancer research. Clin Cancer Res 22:1907-1913, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Online risk calculator. https://www.gustaveroussy.fr/fr/interval-breast-cancer-related-fatigue-calculator
- 59.Coulter A, Entwistle VA, Eccles A, et al. : Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev. 2015;2017:CD010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osterman CK, Sanoff HK, Wood WA, et al. : Predictive modeling for adverse Events and risk stratification programs for people receiving cancer treatment. JCO Oncol Pract 10.1200/op.21.00198[epub ahead of print on September 1, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong AS, Handley NR: From risk prediction to delivery innovation: Envisioning the path to personalized cancer care delivery. JCO Oncol Pract 10.1200/OP.21.00581[epub ahead of print on October 12, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Comprehensive Cancer Network. Survivorship (Version 3.2021). https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf [Google Scholar]
- 63.Basch E, Dueck AC, Rogak LJ, et al. : Feasibility assessment of patient reporting of symptomatic adverse Events in multicenter cancer clinical trials. JAMA Oncol 3:1043-1050, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Velikova G, Booth L, Smith AB, et al. : Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. J Clin Oncol 22:714-724, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Absolom K, Warrington L, Hudson E, et al. : Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol 39:734-747, 2021 [DOI] [PubMed] [Google Scholar]
- 66.Mir O, Ferrua M, Fourcade A, et al. : Intervention combining nurse navigators (NNs) and a mobile application versus standard of care (SOC) in cancer patients (pts) treated with oral anticancer agents (OAA): Results of CapRI, a single-center, randomized phase III trial. J Clin Oncol 38, 2020. (15 suppl; abstr 2000) [Google Scholar]
- 67.Kennedy F, Absolom K, Clayton B, et al. : Electronic patient reporting of adverse events and quality of life: A prospective feasibility study in general oncology. JCO Oncol Pract 17:e386-e396, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Hout A, van Uden-Kraan CF, Holtmaat K, et al. : Role of eHealth application oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: A randomised, controlled trial. Lancet Oncol 21:80-94, 2020 [DOI] [PubMed] [Google Scholar]
- 69.Implementation-quick guide module 1: PAM®. https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/supporting-people-manage-health-patient-activation-may14.pdf
- 70.Dumas A, Vaz Luis I, Bovagnet T, et al. : Impact of breast cancer treatment on employment: Results of a multicenter prospective cohort study (CANTO). J Clin Oncol 38:734-743, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Meglio A, Menvielle G, Dumas A, et al. : Body weight and return to work among survivors of early-stage breast cancer. ESMO Open 5:e000908, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steyerberg EW, Vergouwe Y: Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur Heart J 35:1925-1931, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Justice AAC, Covinsky KE, Berlin JJA: Assessing the generalizability of prognostic information. Ann Intern Med 130:515-524, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Collins GS, Altman DG: Predicting the 10 year risk of cardiovascular disease in the United Kingdom: Independent and external validation of an updated version of QRISK2. BMJ 344:e4181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hosmer DW, Lemeshow S, Sturdivant RX: Applied Logistic Regression (ed 3). Wiley, 2013 [Google Scholar]
- 76.Steyerberg EW: Clinical Prediction Models: Application of Prediction Models. Springer Sci1Bus Medi, 2009 [Google Scholar]