Abstract
Intra-lot and inter-lot variability in ceftaroline fosamil was detected in the DQS using Fourier transform near-infrared spectrometry. One vial of 18 sampled from Lot0013E06 appeared 9.0 SDs from the center of the rest of the vials. Six lots in the spectral library clustered in two large groups.
Introduction
The University of Kentucky’s (UK) Drug Quality Study (DQS) was established in August of 2019 to engage in consumer-level quality assurance testing for drugs used within UK HealthCare’s pharmacies. DQS currently screens medications, using FT-NIR and Raman spectroscopy, for quality defects indicated by variability in absorbance peak intensities and locations. Following 15 months of continuous monitoring, DQS has assembled a spectral library containing medications typically used in an inpatient care setting. Statistical analyses using DQS’ spectral library can now be performed to identify potential intra-lot and inter-lot variability in medications under review. Using MedWatch, DQS reports its findings in an effort to hold manufacturers accountable for GMP requirements and to improve patient outcomes by exerting positive pressure on the pharmaceutical supply chain. At all levels, DQS staff are committed to achieving service excellence by pursuing compliance with the standards set forth by our patients and broad GxP requirements.
Drug Product
Ceftaroline fosamil is a sterile, semi-synthetic, prodrug antibacterial of the cephalosporin class of beta-lactams. The drug is a prescription medicine used to treat bacterial infections such as community-acquired bacterial pneumonia caused by gram-positive and gram-negative bacteria, and acute skin and skin structure infections caused by gram-positive and gram-negative bacteria, including methicillin-resistant Staphylococcus aureus. It can be used alone or with other medications.
Issues
The DQS team has identified possible quality control issues (intra-lot and inter-lot variability) with ceftaroline fosamil 600 mg, manufactured by Allergan, that may require further investigation.
The lot with intra-lot variability was Lot0013E06 with an expiration date of 06/30/2022. The lots forming the spectral library were Lot0003E06, Lot0004E0, Lot0008E06, Lot0009E06, Lot0010E06, and Lot0017D96.
Methods
FT-NIR (Fourier Transform Near-Infrared) Spectrometry
Using nondestructive analytical techniques, FT-NIR spectra were collected for inventory belonging to Lot0013E06 as part of routine medication quality screening. A representative sample of 18 individual vials were selected for screening from Lot0013E06 and noted to be stored under proper conditions, in their original packaging at ambient room temperature. FT-NIR spectra were collected noninvasively and nondestructively through the bottom of the vials using a Thermo Scientific Antaris II FT-NIR Analyzer (Waltham, MA, USA).
Multiplicative Scatter Correction (MSC)
Multiplicative scatter correction (MSC) is a widely used spectrometric normalization technique. Its purpose is to correct spectra in such a way that they are as close as possible to a reference spectrum, generally the mean of the data set, by changing the scale and the offset of the spectra (Isaksson, 1988).
BEST (Bootstrap Error-Adjusted Single-sample Technique)
The BEST calculates distances in multidimensional, asymmetric, nonparametric central 68% confidence intervals in spectral hyperspace (roughly equivalent to standard deviations)(Dempsey, 1996). The BEST metric can be thought of as a "rubber yardstick" with a nail at the center (the mean). The stretch of the yardstick in one direction is therefore independent of the stretch in the other direction. This independence enables the BEST metric to describe odd shapes in spectral hyperspace (spectral point clusters that are not multivariate normal, such as the calibration spectra of many biological systems). BEST distances can be correlated to sample composition to produce a quantitative calibration, or simply used to identify similar regions in a spectral image. The BEST automatically detects samples and situations unlike any encountered in the original calibration, making it more accurate in chemical investigation than typical regression approaches to near-IR analysis. The BEST produces accurate distances even when the number of calibration samples is less than the number of wavelengths used in calibration, in contrast to other metrics that require matrix factorization. The BEST is much faster to calculate as well (O(n) instead of the O(n3) required by matrix factorization.)
Principal Components (PCs)
Principal component analysis is the process of computing the principal components of a dataset and using them to execute a change of basis (change of coordinate system) on the data, usually employing only the first few principal components and disregarding the rest (Joliffe, 2016). PCA is used in exploratory data analysis and in constructing predictive models. PCA is commonly utilized for dimensionality reduction by projecting each data point onto only the first few principal components to obtain lower-dimensional data while preserving as much of the original variation in the data as possible. The first principal component is the direction that maximizes the variance of the projected data. The second principal component is the direction of the largest variance orthogonal to the first principal component. Decomposition of the variance typically continues orthogonally in this manner until some residual variance criterion is met. Plots of PC scores help reveal underlying structure in data.
Results and Discussion
Intralot Analysis
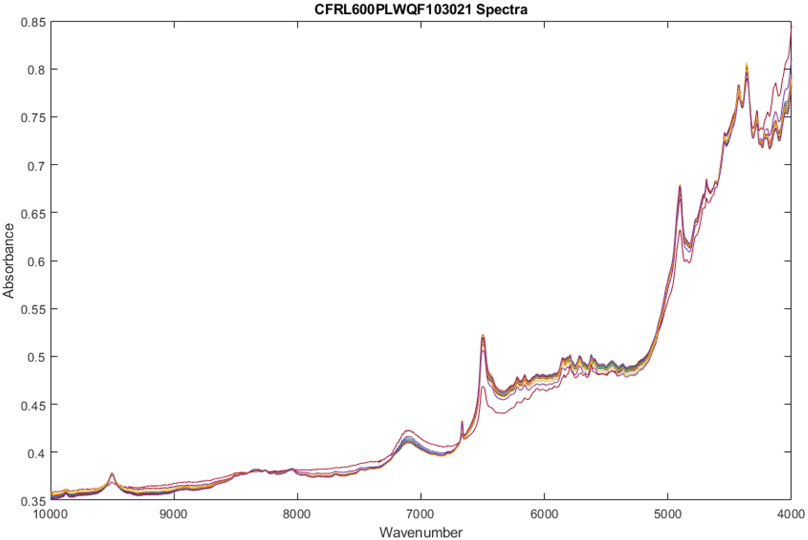
Upon examination of the FT-NIR spectra, absorbance peak location and intensity differences were observed in 1 of 18 vials screened after applying multiplicative signal correction to the spectra. Differences in absorbance peak location were observed at 5700 cm−1, 4850 cm−1, and 4200 cm−1. Variability in absorbance peak intensity is most notably observed at 9500 cm−1, 6650 cm−1, 6500 cm−1, 6200 cm−1, 6150 cm−1, 5850 cm−1, 5800 cm−1, 4700 cm−1, and 4600 cm−1. These findings can be observed directly in Figures 1-3.
Figure 1.
Spectra of 18 vials of Ceftaroline Fosamil from Lot0013E06 after multiplicative scatter correction.
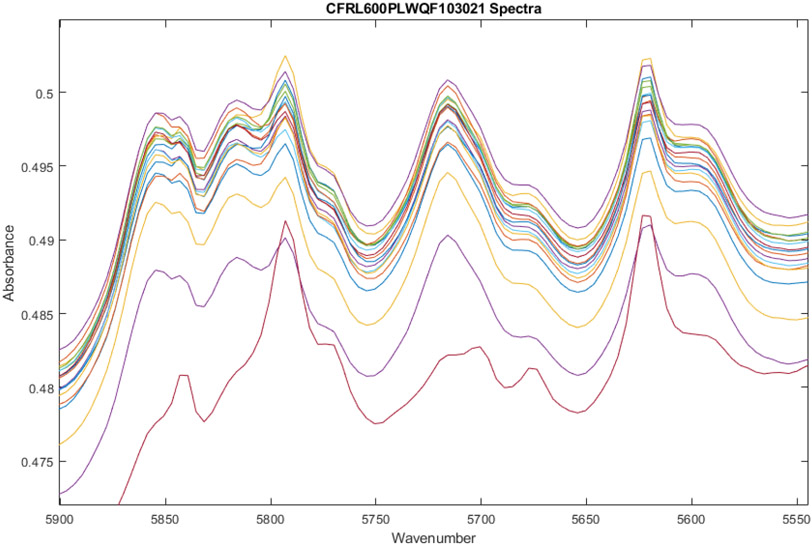
Figure 3.
Zoom-in on spectra of 18 vials of Ceftaroline Fosamil from Lot0013E06 after multiplicative scatter correction. Some peaks have grown in vial 7 (the lowest red line), most notably at 5625, 5675, 5700, and 5790 cm-1. Other peaks have diminished, such as 5720 and 5860 cm−1.
The observation of variability in absorbance peak intensity and absorbance peak location differences may indicate the presence of product impurities and/or degradation products. Statistical analysis of the collected FT-NIR spectra indicated that the single vial that produced the spectra containing variability in absorbance peak intensity and absorbance peak location differences suggested intra-lot variability. The one outlier vial had a calculated distance of 9.0 multidimensional standard deviations (SDs) from the center of the 17 other vials with the same lot number. Using a group membership range between 0 and 3 SDs, a standard deviation of 9.0 signals a potential quality control issue in Lot0013E06.
Inter-Lot Analysis
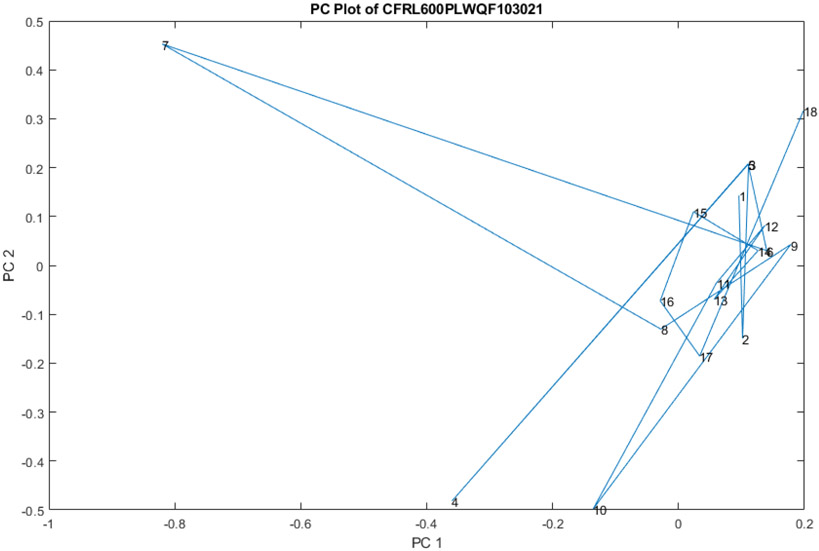
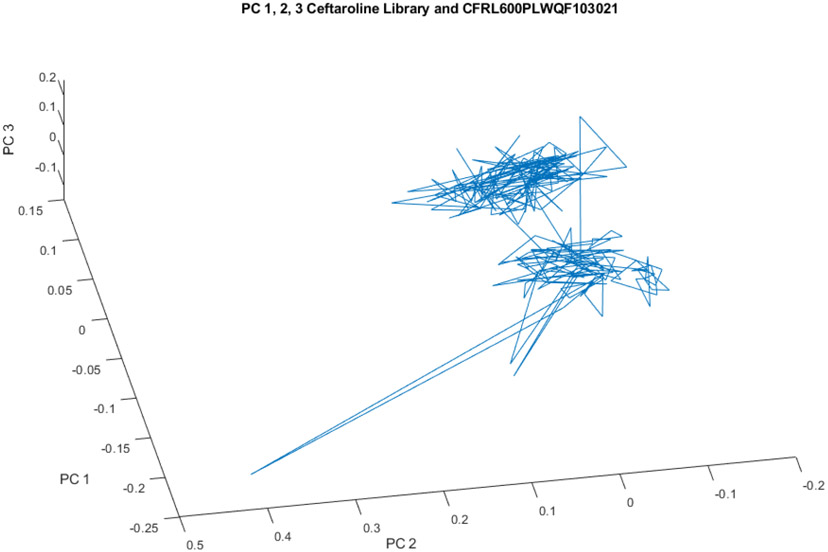
In addition, upon comparing Lot0013E06 to other lots collected for our spectral library, it was discovered that there is inter-lot variability as well as intra-lot variability. Our library of 216 ceftaroline fosamil 600 mg spectra from 6 other lots falls into 2 (or 3) groups (see principal component plot). It is not clear which of these groups represent the approved product, or whether they all are the approved product. The presence of intra-lot and inter-lot variability may warrant further investigation of the product, ceftaroline fosamil 600 mg, and the manufacturing process controls of its producer.
Conclusions
Statistically significant differences appear between vials in the same lot, and between different lots of the same product. Additional destructive testing should be conducted to determine whether these lots represent acceptable drug product.
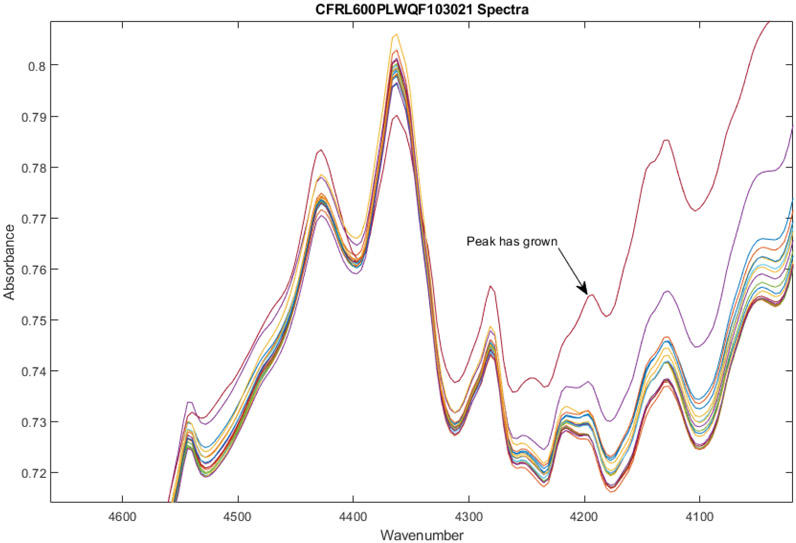
Figure 2.
Zoom-in on spectra of 18 vials of Ceftaroline Fosamil from Lot0013E06 after multiplicative scatter correction. Some peaks have grown in vial 7 (shown in red), most notably at 4190 cm−1.
Figure 4.
Vial 7 appears 9.0 BEST multidimensional SDs from the estimated center of Lot0013E06.
Figure 5.
Spectra of the 216 spectral library vials cluster in 2, or perhaps 3, regions in hyperspace.
Acknowledgements
The project described was supported in part by NSF ACI-1053575 allocation number BIO170011 and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Dempsey RJ, Davis DG, Buice RG Jr, & Lodder RA (1996). Biological and medical applications of near-infrared spectrometry. Applied Spectroscopy, 50(2), 18A–34A. [Google Scholar]
- Isaksson T, & Næs T, (1988). The effect of multiplicative scatter correction (MSC) and linearity improvement in NIR spectroscopy. Applied Spectroscopy, 42(7), 1273–1284. [Google Scholar]
- Jolliffe IT, & Cadima J, (2016). Principal component analysis: a review and recent developments. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 374(2065), 20150202. [DOI] [PMC free article] [PubMed] [Google Scholar]