Abstract
Significant progress has been made in understanding the pre-symptomatic phase of amyotrophic lateral sclerosis. While much is still unknown, advances in other neurodegenerative diseases offer valuable insights. Indeed, it is increasingly clear that the well-recognized clinical syndromes of Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, spinal muscular atrophy and frontotemporal dementia are also each preceded by a pre-symptomatic or prodromal period of varying duration, during which the underlying disease process unfolds, with associated compensatory changes and loss of inherent system redundancy. Key insights from these diseases highlight opportunities for discovery in amyotrophic lateral sclerosis. The development of biomarkers reflecting amyloid and tau has led to a shift in defining Alzheimer’s disease based on inferred underlying histopathology. Parkinson’s disease is unique among neurodegenerative diseases in the number and diversity of non-genetic biomarkers of pre-symptomatic disease, most notably REM sleep behaviour disorder. Huntington’s disease benefits from an ability to predict the likely timing of clinically manifest disease based on age and CAG-repeat length alongside reliable neuroimaging markers of atrophy. Spinal muscular atrophy clinical trials have highlighted the transformational value of early therapeutic intervention, and studies in frontotemporal dementia illustrate the differential role of biomarkers based on genotype. Similar advances in amyotrophic lateral sclerosis would transform our understanding of key events in pathogenesis, thereby dramatically accelerating progress towards disease prevention. Deciphering the biology of pre-symptomatic amyotrophic lateral sclerosis relies on a clear conceptual framework for defining the earliest stages of disease. Clinically manifest amyotrophic lateral sclerosis may emerge abruptly, especially among those who harbour genetic mutations associated with rapidly progressive amyotrophic lateral sclerosis. However, the disease may also evolve more gradually, revealing a prodromal period of mild motor impairment preceding phenoconversion to clinically manifest disease. Similarly, cognitive and behavioural impairment, when present, may emerge gradually, evolving through a prodromal period of mild cognitive impairment or mild behavioural impairment before progression to amyotrophic lateral sclerosis. Biomarkers are critically important to studying pre-symptomatic amyotrophic lateral sclerosis and essential to efforts to intervene therapeutically before clinically manifest disease emerges. The use of non-genetic biomarkers, however, presents challenges related to counselling, informed consent, communication of results and limited protections afforded by existing legislation. Experiences from pre-symptomatic genetic testing and counselling, and the legal protections against discrimination based on genetic data, may serve as a guide. Building on what we have learned—more broadly from other pre-symptomatic neurodegenerative diseases and specifically from amyotrophic lateral sclerosis gene mutation carriers—we present a road map to early intervention, and perhaps even disease prevention, for all forms of amyotrophic lateral sclerosis.
Keywords: neurodegeneration, amyotrophic lateral sclerosis (ALS), pre-symptomatic, disease prevention
Benatar et al. provide a state-of-the-field summary of what is known about the pre-symptomatic phase of an array of neurodegenerative diseases. They identify important lessons that could transform understanding of the earliest stages of ALS, and thereby accelerate progress towards ALS prevention.
Introduction
Emerging evidence from the study of a host of neurodegenerative diseases has made it abundantly clear that the well-recognized clinical syndromes of Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are preceded by periods of variable duration during which the underlying disease process is active despite the absence of even mild, prodromal symptoms. The same may also be true of all but the most severe type of spinal muscular atrophy (SMA). The impact of this understanding has been profound, revealing a disjunction between the presence of disease at the molecular, cellular and network levels versus its clinical manifestations, the latter being influenced by a variety of adaptive processes permitting functional tolerance despite underlying pathology. Increasing opportunity and ability to study the pre-symptomatic phases of these diseases (Fig. 1) has heightened interest in the possibility that early therapeutic intervention—or even prevention—may offer the best hope for the millions predisposed to these devastating neurodegenerative diseases.
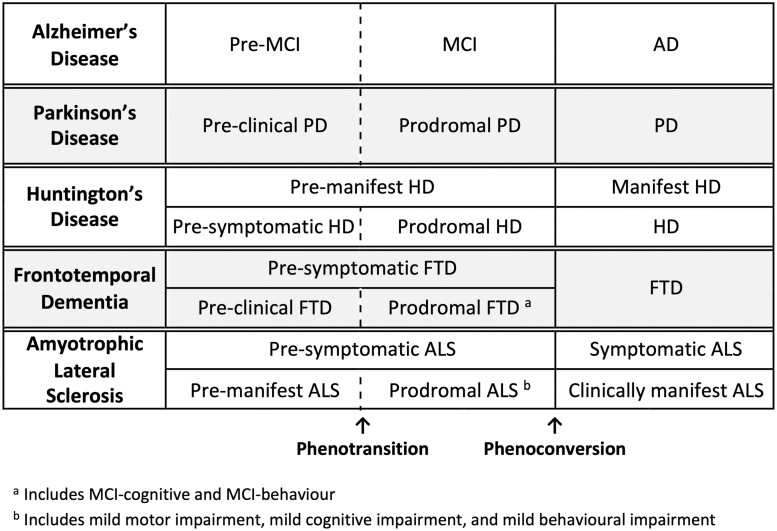
Figure 1.
Terminologies most commonly used in different neurodegenerative diseases. Different fields have used different terms to describe the prodromal phase of disease that precedes clinically overt disease. For Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) and FTD, this period is designated as MCI, prodromal Parkinson’s disease, prodromal Huntington’s disease and prodromal FTD, respectively. In some parlance, prodromal FTD encompasses both MCI-cognition and MCI-behaviour. Similarly, each of these disorders is also characterized by an even earlier stage of asymptomatic disease (pre-MCI, preclinical Parkinson’s disease, pre-symptomatic Huntington’s disease and preclinical FTD, respectively), during which clinical symptoms and signs are absent, but biomarker evidence may be present. Terminology for SMA is less well-defined.
This background served as the impetus for the First International Pre-Symptomatic ALS Workshop (27 January 2020, in Miami, FL; Supplementary material) and on which this paper is based. First, we review the state-of-the-field as well as experiences and lessons shared by attendees who study the pre-symptomatic phases of various neurodegenerative diseases and their relevance for the study of pre-symptomatic ALS. We then summarize our recommendations for the study of motor, cognitive and behavioural manifestations of early ALS; the critical importance of biomarkers to this endeavour; the challenges of genetic and biomarker counselling; the pertinent ethical, legal and social implications; and considerations for the design of early intervention or disease prevention clinical trials.
Pre-symptomatic neurodegenerative diseases
Alzheimer’s disease
Alzheimer’s disease is the most common adult-onset neurodegenerative disorder, with symptoms typically emerging when individuals are in their mid-seventies. The clinical syndrome of Alzheimer’s disease is preceded by a prodromal stage, encompassed under the rubric of mild cognitive impairment (MCI), a syndromic label that does not necessarily imply Alzheimer’s disease as the underlying aetiology. In turn, MCI may be preceded by a phase of disease sometimes referred to as pre-MCI1 or preclinical Alzheimer’s disease. There is also increasing interest in subjective cognitive decline as a risk state preceding MCI.2-5 Most Alzheimer’s disease is sporadic; autosomal dominant gene mutations, in amyloid precursor protein (APP), presenilin-1 (PSEN1), or presenilin-2 (PSEN2), account for <5% of cases. These mutations typically cause earlier onset disease (in the forties and fifties), and penetrance is ∼100%. Susceptibility to late-onset, sporadic Alzheimer’s disease is mediated, at least in part, by apolipoprotein-E (APOE) with three allelic forms: APOE2 (protective), APOE3 (neutral) and APOE4 (increased risk for Alzheimer’s disease). Numerous other susceptibility alleles confer a small but increased risk of developing Alzheimer’s disease.6
In the past, ‘probable Alzheimer’s disease’ or ‘Alzheimer’s disease dementia’ was diagnosed based on the typical clinical syndrome of progressive impairments in two or more specific cognitive domains, including memory, executive, language and visuospatial functions, resulting in impairment in daily function,7 with supporting neuroimaging and CSF biomarkers when available. MCI, in turn, is diagnosed based on objective cognitive impairment in at least one aspect of cognition, along with report of decline by the patient, informant or clinician, in the absence of significant functional impairment.8 Significant work has been done to characterize the early features of mild Alzheimer’s disease including MCI (and even pre-MCI). The rate of progression from cognitively unimpaired to MCI varies between 3% and 6% per year,9 and the rate of progression from MCI to dementia varies from 5% to 20% per year with most studies suggesting a ∼10–15% range.10 Rates of conversion are heavily age-dependent; in the presence of Alzheimer’s disease biomarkers, 3- and 5-year progression rates may be as high as 35% and 85%, respectively.11
Previously, the diagnosis of ‘definite Alzheimer’s disease’ was restricted to those with post-mortem evidence of neuritic plaques and neurofibrillary tangles. More recently, however, there has been a shift in conceptualizing Alzheimer’s disease within an ATN [amyloid (A), tau (T) and neurodegeneration (N)] framework, which defines disease based on A and T, and characterizes progression based on N.12,13 This advance was facilitated by development of biomarkers reflecting the underlying biology of Alzheimer’s disease, initially CSF measures of A and T, but more recently PET ligands that permit in vivo imaging of both A and T. N is determined via MRI measures of brain atrophy or hypometabolism on fluorodeoxyglucose PET, and potentially by elevation of CSF or blood neurofilament light chain (NfL).14 Indeed, plasma NfL is elevated 6.8 estimated years before symptom onset (EYO) of familial Alzheimer’s disease, and trajectories of NfL among mutation carriers diverge from non-mutation carriers ∼16 years before EYO.15 Layered on top of this underlying biological classification is staging by clinical features that, while correlated with ATN state, is independent of the biological framework.16 The ATN framework has thus driven a paradigm shift towards conceptualizing Alzheimer’s disease as a biological, rather than a clinical–pathological, entity.12
Extensive research is underway to develop disease-modifying therapies, and this is an active area of investigation, primarily focusing on A and T as therapeutic targets.17 With the availability of biomarkers for A, T and N, these clinical trials are becoming increasingly sophisticated, using biomarker criteria for entry as well as outcome measures. In fact, the FDA’s accelerated approval of aducanumab, a monoclonal antibody that binds aggregated forms of amyloid-β, was based on a lowering of amyloid, a biomarker of Alzheimer’s disease pathology, with the supposition that it would be ‘reasonably likely to predict’ a clinical benefit. This approval has generated significant controversy since clinical benefit has not yet been demonstrated.18–20 While the initial FDA label included a broad indication for Alzheimer’s disease, it has since narrowed to include patients with MCI or mild dementia due to Alzheimer’s disease, reflecting the clinical trial population.
Recognition of earlier clinical presentations of Alzheimer’s disease (e.g. MCI or subjective cognitive decline) has moved back the clinical detection threshold to allow for earlier intervention in clinical trials. On the assumption that earlier therapeutic targeting of the underlying disease process is more likely to be successful, early detection of clinical features has become paramount. Several trials are now underway involving participants who are amyloid positive but cognitively unimpaired, and these disease-modifying trials will inform the potential prevention of symptomatic Alzheimer’s disease.21–23
Parkinson’s disease
Parkinson’s disease is the second most common neurodegenerative disease, affecting 1–2% of the population over 65 years of age.24 Most Parkinson’s disease is sporadic, but a family history is present in ∼15% of patients. An identifiable genetic cause is found in ∼7%,25 with mutations in LRRK2, GBA, PRKN (parkin), SNCA and PINK1 most commonly implicated.26
There is a striking number and diversity of clinical markers and biomarkers of early Parkinson’s disease, making it unique among neurodegenerative diseases. Olfactory loss, one of the first markers documented to predict Parkinson’s disease, is associated with a ∼5-fold relative risk of Parkinson’s disease/dementia with Lewy bodies.27 Olfactory loss may start as early as 20 years before clinical Parkinson’s disease.28 Constipation, although associated with a lower relative risk (∼2.5), has perhaps an even longer latency,28–30 suggesting it may be a Parkinson’s disease risk factor and a prodromal marker. Other autonomic variables, including urinary and erectile dysfunction, are also associated with Parkinson’s disease, although with a lower relative risk. Laboratory-confirmed neurogenic orthostatic hypotension has very high risk, with up to 10% of affected patients phenoconverting per year.31 Subtle motor impairment is modestly associated with Parkinson’s disease on non-expert examination (relative risk = 1.9), with a stronger association if clinical experts document subtle parkinsonism (relative risk = 8).32 Loss of dopamine transporter on imaging is associated with an 18-fold relative risk of Parkinson’s disease.33 New MRI biomarkers and tissue-based diagnoses (especially skin biopsy and synuclein-seeding assays) have considerable promise. Unlike other neurodegenerative diseases, NfL is less likely to predict Parkinson’s disease, as levels are, at most, only modestly increased. However, NfL could potentially be used in differential diagnosis of prodromal synucleinopathy, as more rapidly progressive neurodegenerative diseases (e.g. multiple system atrophy) have robust increases in NfL.34 Overall, the strongest known predictor of Parkinson’s disease/dementia with Lewy bodies is REM sleep behaviour disorder. Long-term studies show that >80% of patients with REM sleep behaviour disorder eventually develop neurodegenerative synucleinopathy (i.e. Parkinson’s disease, dementia with Lewy bodies or multiple system atrophy).35
These clinical/biomarkers define the presence of a syndrome in which symptoms/signs of disease are evident (prodromal Parkinson’s disease), but insufficient to permit diagnosis of clinical Parkinson’s disease. In addition to this prodromal state, the International Parkinson and Movement Disorders Society (MDS) recognizes a preclinical state, in which neurodegeneration has started, but clinical symptoms/signs have not yet emerged.36 Preclinical stages are not currently definable, because most biomarkers become abnormal only at prodromal stages. As noted previously, however, there are at least 16 prospectively established clinical markers or biomarkers of prodromal disease.29 Because Parkinson’s disease-related neurodegeneration generally starts outside the dopaminergic motor areas (in the olfactory bulb/nucleus, lower brainstem, and peripheral autonomic system), most markers are non-motor. Moreover, the prodromal state can be very long; the average duration is ∼10 years and many patients manifest subtle signs 15–20 years before clinical Parkinson’s disease is diagnosed.36 Most clinical prodromal Parkinson’s disease markers identify all prodromal synucleinopathies, including dementia with Lewy bodies and multiple system atrophy (of note, dementia with Lewy bodies and Parkinson’s disease are no longer considered mutually exclusive conditions by MDS criteria37).
Research criteria for prodromal Parkinson’s disease have been developed by the MDS.29,36 Since no neuroprotective therapy for Parkinson’s disease is currently available, the criteria are mainly for research purposes, especially to help identify candidates for prevention trials of putative neuroprotective treatments. These criteria were designed to solve a particular problem, namely the existence of a broad array of strikingly varied markers with very different predictive strengths. A Bayesian naïve classifier was used to estimate an individual’s prodromal Parkinson’s disease probability. First, the baseline risk of prodromal Parkinson’s disease is identified based on age. Then, diagnostic tests for markers of prodromal Parkinson’s disease are sequentially added; positive tests increase likelihood of disease (by a strength that depends on their predictive value), whereas negative tests decrease likelihood. If the threshold of 80% probability is reached, probable prodromal Parkinson’s disease is diagnosed. These criteria have now been validated in several studies that have found that the positive predictive value is high (i.e. once diagnosis is made, there is a high chance of clinical Parkinson’s disease). Sensitivity, however, depends entirely on the markers assessed; in general, because Parkinson’s disease is relatively uncommon, only very powerful markers (REM sleep behaviour disorder, dopamine transporter imaging, etc.) can increase the probability of prodromal Parkinson’s disease up to the 80% threshold.
Huntington’s disease
Huntington’s disease is a fully penetrant, autosomal dominant neurodegenerative disorder caused by a CAG-trinucleotide repeat expansion in the huntingtin (HTT) gene on chromosome 4.38 Manifest Huntington’s disease is characterized clinically by the triad of motor, cognitive and psychiatric manifestations.39 Predictive genetic testing has made possible the definition of a prodromal stage, during which subtle motor, cognitive and emotional changes evolve before the extrapyramidal motor (and cognitive) signs are of sufficient severity to warrant a clinical diagnosis of Huntington’s disease.40–47 Prodromal Huntington’s disease, in turn, is preceded by a pre-symptomatic period with no signs or symptoms attributable to Huntington’s disease. Together, the pre-symptomatic and prodromal phases comprise pre-manifest Huntington’s disease.
The clinical diagnosis of manifest Huntington’s disease has traditionally been based on motor signs, with the severity of the extrapyramidal movement disorder quantified using the Unified HD Rating Scale,48 yielding a ‘total motor score’ (range 0–124). The study of pre-manifest CAG-repeat expansion carriers and individuals with a family history of Huntington’s disease, by contrast, has relied on a ‘diagnostic confidence’ scale, with motor abnormalities rated as: 0 = normal (no motor abnormalities); 1 = non-specific motor abnormalities; 2 = motor abnormalities that may be signs of Huntington’s disease (50–89% confidence); 3 = motor abnormalities that are likely signs of Huntington’s disease (90–98% confidence);and 4 = motor abnormalities that are unequivocal signs of Huntington’s disease (≥99% confidence).49 More recently, a Task Force of the MDS proposed that cognitive changes be added as important components of the diagnosis of Huntington’s disease, effectively upgrading some previously ‘pre-symptomatic’ individuals to prodromal, and some ‘prodromal’ individuals to manifest disease.49
The age at which manifest Huntington’s disease is likely to appear may be predicted using the CAG-repeat length. A useful variable for studying natural history is the CAG Age Product (CAP) score, calculated as: age × (CAG − L), where age is current age, CAG is the repeat length, and L is a constant close to the threshold of CAG length for onset of Huntington’s disease. The value chosen for L varies among researchers but is generally close to 30. As is apparent from the formula, the greater the CAG length, the higher the CAP score at any age. The CAP score can be thought of as a measure of cumulative exposure to the effects of the expanded CAG repeat.39,50,51 The higher the CAP score, the closer in time the individual is to phenoconversion to clinically manifest Huntington’s disease.52 Genetic modifiers also contribute to the variance in age of onset and rate of progression.53
With remarkable consistency in single-54 and multi-centre studies such as PREDICT-HD55 and TRACK-HD,56 structural MRI shows that atrophy of the striatum and other brain regions begins at least 15 years before emergence of clinically manifest Huntington’s disease, at a CAP score of ∼200. The progressive atrophy of these regions is remarkably steady, reaching ∼40–50% at the time of motor onset. The size of the striatum in the pre-manifest period can predict time to motor onset even after accounting for CAG-repeat length.57
CSF and blood biomarkers have also proven strikingly effective in tracking natural history and response to therapeutics in Huntington’s disease. Mutant HTT (mHTT), derived from dying neurons58 and present at femtomolar concentrations in CSF, can be measured with high sensitivity and accuracy.58–60 It is elevated in Huntington’s disease, both in the pre-manifest and manifest stages, is associated with clinical decline, and correlates with markers of neuronal degeneration. Quantification of CSF mHTT has also provided essential evidence of target engagement in the first trial of a HTT-lowering therapy.61
NfL is also increased in Huntington’s disease CSF,62–64 but has a different trajectory; and CSF levels appear to have a more powerful predictive effect on future disease status.65 Remarkably, blood NfL levels increase with progression, including in pre-manifest individuals over 10 years from predicted onset, and are also associated with clinical progression, brain atrophy and emergence of clinical disease.62 Recently, it was demonstrated that alterations of specific peptide neuromodulators in CSF may provide the first markers of involvement of striatal medium spiny neurons that are preferentially involved in early Huntington’s disease, thus further facilitating studies of natural history and possibly experimental therapeutics.66,67
Antisense oligonucleotide-based approaches have been developed to target HTT mRNA via both non-allele-specific (Tominersen/Roche) and mutant allele-specific (Wave Life Sciences) approaches. Promising phase 1/2a results61 prompted a phase 3 trial (GENERATION HD1), but this was unfortunately halted due to worsening clinical outcomes in the treated groups, raising the question of whether the poor outcomes were due to knockdown of wild-type HTT or off-target effects. Two other antisense oligonucleotide trials by Wave have also been halted, though a third, using a different backbone, continues, as do several other therapeutic strategies. Despite these setbacks, there is increasingly interest in the possibility of early intervention studies in the pre-manifest population.
Spinal muscular atrophy
SMA is an autosomal recessive neuromuscular disease characterized by progressive degeneration of motor neurons in the anterior horn of the spinal cord and brainstem, resulting in muscle weakness and atrophy.68 In classic SMA, homozygous deletions or compound heterozygous mutations in the SMN1 gene on chromosome 5q13 prevent production of full-length functional survival of motor neuron (SMN) protein, necessary for motor neuron survival and function.69 Disease onset and rate of progression are roughly inversely correlated with the number of copies of the SMN2 gene, a paralogous gene that produces ∼10% functional protein/copy.70
SMA manifests as a continuum of phenotypic severity which has been historically classified, based on age at symptom onset and highest attainment of function, with five subtypes ranging from the most severe phenotype with prenatal onset (type 0) to the mildest phenotype with adult onset (type 4). Most individuals with SMA type 1, representing about 50–60% of incident cases, have a symptom-free period after birth,71 followed by an abrupt decrease in motor function, which eventually progresses to general hypotonia and quadriparesis over several weeks.72 SMA may be characterized by a period of mild prodromal symptoms prior to clinically definite disease.73,74 This ‘pauci-symptomatic’ phase, characterized by mild hypotonia, blunted infantile motor responses, reduced to absent deep tendon reflexes, or reduced compound muscle action potential (CMAP) may be identified in some patients by the neurologist, prior to any gross abnormality being noted by parents or even primary care physician. While there is no consensus around a formal definition of the clinical onset of disease (i.e. phenoconversion) of SMA among SMN1 deletion carriers, clinical onset may operationally be described as the age at which the first clear signs of weakness (delayed motor development, loss of motor function, etc.) are reported/identified by parents or physicians.75 Trials in pre-symptomatic SMA patients have required an absence of definitive clinical signs or symptoms in a baby with a homozygous deletion of SMN1, and 2 or 3 copies of SMN2. Eligibility in two of these trials, however, also required a minimal ulnar CMAP.
While not a definitive prognostic biomarker in isolation, SMN2 copy number can inform anticipated age of onset and phenotypic severity. This biomarker and the well-characterized penetrance and natural history of SMA enabled initiation of clinical studies in pre-symptomatic infants with homozygous SMN1 deletions who were identified by newborn screening or positive family history.76–78 Moreover, data from the nusinersen studies in pre-symptomatic patients have demonstrated the potential utility of the phosphorylated neurofilament heavy chain (pNfH) level as a prognostic biomarker; in pre-symptomatic infants in the NURTURE study, baseline plasma and CSF pNfH levels were meaningfully elevated, particularly in those with two copies of SMN2, who are predicted to have a more severe phenotype.76,79
With recent advances in therapeutic approaches for the treatment of SMA, the importance of early intervention has become clear. Within the symptomatic population, the strongest predictors of treatment response have been age at treatment initiation and disease duration.72,80,81 Interventional studies in pre-symptomatic individuals have demonstrated efficacy far beyond what has been observed from the same treatment in post-symptomatic individuals.76,80,82,83 These data may inform other neurodegenerative diseases in which a pre-symptomatic or prodromal phase can be identified to trigger early intervention.
Frontotemporal dementia
Frontotemporal lobar degeneration (FTLD) is the overarching term for a group of disorders characterized by CNS accumulation of toxic protein aggregates, most commonly composed of either microtubule associated protein tau (abbreviated tau) or transactive response DNA-binding protein of ∼43kD (TDP-43, encoded by TARDBP).84,85 The clinical, pathological, and genetic aspects of FTLD, and the associated nomenclature, are complex. The sporadic FTLD syndromes (s-FTLD) include behavioural variant FTD (bvFTD), FTD plus ALS (FTD-ALS), the semantic variant of primary progressive aphasia (svPPA), the non-fluent/agrammatic variant of PPA (nfPPA), corticobasal syndrome (CBS) and the classic progressive supranuclear palsy syndrome (PSP-RS), also called Richardson’s syndrome.86 The clinical syndromes of bvFTD, svPPA and nfPPA are commonly referred to by the collective term ‘FTD’. Moreover, mutations in genes encoding microtubule associated protein tau (MAPT), progranulin (GRN), or chromosome 9 open reading frame 72 (C9orf72)86,87 cause a dominantly inherited form of familial FTLD (f-FTLD).86,87 These mutations (found in >20% of all FTLD patients) account for >50% of f-FTLD,86,87 providing a unique opportunity to study the pre-symptomatic and prodromal stages of disease among unaffected mutation carriers. Age of onset varies significantly in f-FTLD, ranging from ∼30–80 years even within the same family, and there are no reliable algorithms for predicting age of onset, except among MAPT mutation carriers.86,87 Such models would be critical to guide participant selection for clinical trials using symptom onset as an outcome.
While the presence of an overt FTLD phenotype can be defined based on established criteria,88,89 defining the initial onset of symptoms in f-FTLD is significantly more difficult. This is especially so for bvFTD, the most common phenotype, in which an initially subtle change in behaviour, personality or comportment is common. Determining which symptoms are manifestations of a neurodegenerative process (rather than part of the normal spectrum or a primary psychiatric disorder) is challenging. Furthermore, loss of insight, an inherent aspect of bvFTD, complicates reliance on patient self-report and necessitates information from a knowledgeable informant. Similarly, a change in language functioning—particularly word-finding difficulty—is a common complaint among individuals in general and often increases with age; differentiating anomia due to evolving primary progressive aphasia from age-related anomia may be difficult. And while memory impairment is relatively uncommon in early sporadic bvFTD, it is more common in familial bvFTD. These issues underscore the need for a broadly defined prodromal stage of evolving f-FTLD, which has led some to apply the Alzheimer’s disease concept of MCI90 to f-FTLD,91,92 with the terms MCI-cognitive and MCI-behaviour used by some in the FTLD community to describe the period of uncertainty where cognitive and behavioural manifestations representing a departure from normal are present, but not yet of sufficient severity to warrant designation of dementia. Importantly, each term (MCI-cognitive and MCI-behaviour) requires that clinical manifestations represent a change from a premorbid state and previous level of functioning.
The concept of MCI-behaviour (or simply mild behavioural impairment, MBI93) has been built on the foundation of the international consensus criteria for bvFTD and is purposefully loosely defined.91 Specifically, while possible bvFTD requires three or more abnormal behaviours (disinhibition; apathy/inertia; loss of sympathy/empathy; perseverative, stereotyped or compulsive/ritualistic behaviour; hyperorality and dietary changes), MCI-behaviour requires only either the presence of one of these behaviours, or the emergence of delusions or hallucinations or other odd behaviours. Therefore, this definition of MCI-behaviour is intended to be sensitive but not necessarily specific to evolving FTLD. Operationally, the emergence of MCI-behaviour has been determined based on a change (rating > 0) in the Behavioural/Personality/Comportment domain of the Clinical Dementia Rating Dementia Staging Instrument plus National Alzheimer Coordinating Center (NACC) FTLD Module Behaviour and Language Domains, which is commonly abbreviated to CDR® plus NACC FTLD (additional details are provided in the Supplementary material). This is still an emerging field without consensus on terminology to describe prodromal FTLD.
There is growing evidence for the utility of biomarkers in FTLD, particularly based on data from the GENFI94 and ARTFL/LEFFTDS/ALLFTD (‘ALLFTD’) cohorts.86,91 Of note, the symptomatic onset of disease is defined differently in these two cohort studies: whereas GENFI considers it to be the time of diagnosis of overt FTLD (e.g. diagnosis of bvFTD, PPA, or similar phenotype), ALLFTD considers it to be the time of CDR® plus NACC FTLD score >0 and/or the diagnosis of MCI or an overt FTLD syndrome. Plasma and CSF NfL, and phosphorylated tau isoforms in plasma, are the most promising fluid biomarkers reported to date. NfL is elevated in s-FTLD and most of the f-FTLD syndromes95; importantly, longitudinal measurements from GENFI showed that increasing levels of NfL could identify MAPT, GRN and C9orf72 mutation carriers approaching symptom onset.96 A recent cross-sectional analysis from ALLFTD showed that plasma pTau181 levels were increased in pre-symptomatic (FTLD-CDR score = 0) MAPT mutation carriers with AD-like mixed 3R/4R tau pathology compared to controls.95 Among the many MRI measures evaluated in cross-sectional and longitudinal studies in FTLD,96,97–103 volumetric MRI has shown the most promise in characterizing changes during the pre-symptomatic phase of f-FTLD (FTLD-CDR score = 0), with the topography of changes depending on the mutated gene.94,98,99,101 Initial enthusiasm for use of tau PET ligands has faded, however, since none so far are adequately sensitive or specific for FTLD-associated tau fibrils;104 current tau PET tracers do not seem to differentiate tau versus TDP-43 proteinopathies. Similarly, these PET tracers are not considered useful in s-FTLD associated with tau pathology (e.g. PSP, CBD, bvFTD-tau, PPA-tau) or in f-FTLD associated with MAPT mutations (except in rare instances).105
Lessons for amyotrophic lateral sclerosis
ALS is a disorder characterized principally by degeneration of upper and lower motor neurons, as well as frontotemporal systems to a variable extent. While the cause of disease remains largely unknown, 10–20% have a clear genetic aetiology. An intronic C9orf72 hexanucleotide repeat expansion and missense mutations in SOD1 are the most common genetic causes, with a variety of other genes occasionally implicated. Asymptomatic carriers of mutations in these genes comprise the only population known to be at high risk for ALS, and in whom a study of pre-symptomatic disease may realistically be considered.
Biomarkers are critically important for studying pre-symptomatic disease. For Alzheimer’s disease, biofluid and imaging biomarkers capture the underlying biology of amyloid and tau pathology. For Huntington’s disease, biofluid and imaging biomarkers partially reflect disease biology (e.g. CSF mHTT), and partially reflect the resulting neurodegeneration (e.g. neurofilament release and striatal atrophy). In ALS, available biomarkers overwhelmingly reflect neurodegeneration (e.g. neurofilament, p75ECD) or neuroinflammation (e.g. chitinases), which may simply represent a reaction to neurodegeneration. Nuclear clearance and cytoplasmic aggregation of TDP-43, however, is the neuropathological hallmark of all ALS (except for ∼3% of cases associated with SOD1 and FUS mutations). Biomarkers reflective of this core biology are urgently needed. These would permit a shift away from conceptualizing ALS as a clinical syndrome and empower the study of ALS as one or more biological entities, in the same way that the identification of CSF and PET-imaging markers of amyloid and tau permitted for Alzheimer’s disease. In turn, these biomarkers might be used to enrol subgroups of patients into different clinical trials where the investigational agent targets the relevant underlying biology. Such biomarkers would also facilitate clinical trials that aim to prevent the emergence of clinical disease, akin to how amyloid and tau biomarkers are being used in the field of Alzheimer’s disease.
The study of pre-symptomatic disease for any neurodegenerative disorder benefits enormously from knowledge of penetrance as well as the ability to predict the timing with which clinically manifest disease is likely to emerge. The Huntington’s disease community is most advanced in this regard, with the CAP score providing information on how close someone is to phenoconversion. Similarly, in SMA, SMN2 copy number and the experience of affected siblings (when available) may be useful in predicting the timing of symptom onset and disease course.76 By contrast, age at onset of parents (or other family members) does not reliably predict FTD age of onset (except among MAPT mutation carriers)87 or of ALS; neither does C9orf72 repeat expansion length.106 Discovery of biomarkers that predict age of onset in ALS and FTD could transform the study of the pre-symptomatic stage of these diseases.
Pre-symptomatic Parkinson’s disease may yield the greatest insights into the pre-symptomatic stage of sporadic ALS. Currently, because the incidences of both ALS and Parkinson’s disease are low (although that of Parkinson’s disease is significantly higher), both fields share the same challenge that pre-symptomatic and prodromal disease can only be studied in those at significantly elevated risk of disease; but ALS lacks the Parkinson’s disease field’s advantage of diverse and highly predictive markers. While in ALS there is currently no clinical prodromal marker to mimic the Parkinson’s disease approach, analogous probability-estimation mathematical models might be considered if/when markers in ALS are discovered; these might be used, for example, to identify, study and even treat those at risk for sporadic ALS. Moreover, carriers of ALS-associated genetic mutations resemble individuals with prodromal synucleinopathies in that they are at risk of either motor or cognitive-predominant degeneration. ALS prevention trials in gene mutation carriers should, therefore, consider end points focused on both motor and extra-motor syndromes.
In SMA, pre-symptomatic trials have proceeded despite the absence of a uniformly accepted definition of pre-symptomatic disease. Eligibility criteria and definitions indicative of symptom onset have, therefore, varied across studies. These differences confound cross-study interpretation and limit translatability into clinical practice, highlighting the importance of establishing these disease state definitions prior to conducting similar trials in carriers of ALS-associated gene mutations.
Of all the neurodegenerative disorders discussed before, ALS and FTD have the most in common given their overlapping genetic risk, pathology and clinical manifestation. In addition, those with FTD are at risk for developing ALS, and vice versa. Strategies for studying and defining prodromal cognitive/behavioural syndromes in individuals at genetic risk for FTD, therefore, have direct implications for ALS, especially for genetic factors that predispose to both diseases. It is also likely that progress in uncovering biomarkers of pre-symptomatic FTD, especially C9orf72 mutation carriers, will be immediately relevant to ALS.
Pre-symptomatic amyotrophic lateral sclerosis
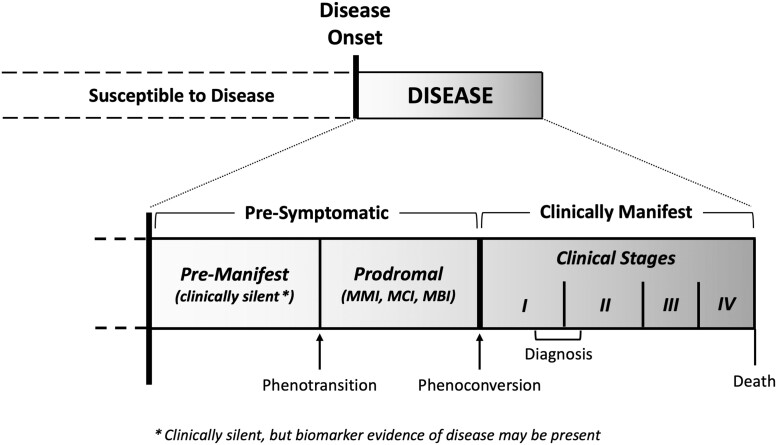
The existing conceptual framework for ALS recognizes two phases of disease: pre-symptomatic and symptomatic.107 The symptomatic phase represents the clinical syndrome of ALS that is readily recognized based on progressive weakness with upper and lower motor neuron signs in the same body region.108 And as described next, we have found the pre-symptomatic phase to comprise a pre-manifest (or clinically silent) stage and, at least in some individuals, a prodromal stage characterized by mild motor, cognitive or behavioural abnormalities (Fig. 2). Importantly, we draw a distinction between the underlying disease versus what is observable and can be operationally defined.
Figure 2.
Conceptual framework for studying pre-symptomatic ALS. The natural history of ALS, as a biological entity, includes a pre-manifest (i.e. clinically silent) stage that is typically not observable except when disease-related biomarker abnormalities are detected. These biomarker abnormalities, if present, serve as the first (and only) indication that the disease process has begun. The pre-manifest stage may be followed by a prodromal stage that is characterized by mild motor, cognitive or behavioural impairment (MMI, MCI or MBI, respectively); the prodromal stage is most likely to be observed in individuals with more slowly progressing disease. In turn, this prodromal clinical stage gives way to clinically manifest ALS. The term phenotransition describes the transition from the pre-manifest to the prodromal stage, and the term phenoconversion describes the transition to clinically manifest ALS. The shaded gradient reflects the fact that these periods exist along a continuum. Note that the figure is not drawn to scale, as the relative duration of each period is largely unknown and may vary between individuals.
Motor manifestations
Minimal assessments required when studying pre-symptomatic amyotrophic lateral sclerosis
To be confident that an ALS gene mutation carrier does not have clinically manifest disease, one needs evidence for the absence of motor neuron dysfunction (or evidence that only minor abnormalities that do not amount to clinically manifest disease, are present). This requires a careful history, combined with detailed neuromuscular examination by an ALS expert and a comprehensive EMG (sampling at least three to four muscles innervated by different peripheral nerve and nerve roots bilaterally in the cervical and lumbosacral regions; at least one bulbar muscle; and the thoracic paraspinal muscles at four levels).109,110
Pre-manifest disease
The pre-manifest (clinically silent) stage begins at disease onset, which is currently undefinable. We rely, therefore, on biomarker abnormalities (e.g. increased neurofilament above an accepted normal range) as evidence that disease has already begun. In addition, the designation of pre-manifest requires evidence for the absence of relevant motor symptoms, examination findings indicative of motor neuron dysfunction or ongoing denervation changes on EMG. An important caveat is that minor clinical or EMG changes due to another cause (e.g. carpal tunnel syndrome, cervical/lumbar spine disease) may be permitted, with clinical judgement (incorporating results of relevant investigations, if needed) being essential in attributing these to the confounder. While mild chronic reinnervation changes are frequently encountered and are likely attributable to some other underlying disorder, ongoing denervation changes are rare. Thus, if clinical judgement is that minor observed abnormalities are due to something other than ALS, then an ALS gene mutation carrier may still be deemed pre-manifest.
Prodromal mild motor impairment
Clinically manifest ALS is preceded, at least in some patients, by a prodromal stage that is characterized by non-specific symptoms (e.g. muscle cramps, reduced exercise tolerance), signs (e.g. fasciculation, isolated loss of ankle reflexes, diffuse hyperreflexia) or EMG abnormalities (e.g. positive sharp waves in a single limb muscle or thoracic paraspinal muscles) in the absence of progressive muscle weakness. Importantly, to meet the criteria for prodromal disease, these findings—which represent a departure from the spectrum of healthy physiology—should be insufficient to permit an experienced neurologist to declare the unequivocal emergence of clinically manifest ALS and should not obviously be attributable to another cause.
By analogy to the recognized clinical syndromes of MCI and MBI in other neurodegenerative diseases, we have proposed the term mild motor impairment (MMI) to describe this prodromal period. Of note, prodromal manifestations are still considered ‘pre-symptomatic’ since they are insufficient to permit determination that clinically manifest disease has emerged. We suggest the term phenotransition to describe the initial appearance of mild impairment and the shift from the pre-manifest to the prodromal stage of pre-symptomatic disease. The rationale for this term is multifold: it differentiates the emergence of MMI from clear clinical evidence of ALS; captures the essential observation that MMI has an overt phenotype; and embodies the notion that the individual is entering a transitional stage (e.g. MMI). As discussed next, this same framework is also applicable to the early changes in cognition/behaviour in prodromal FTD and ALS. The timing of onset of these prodromal manifestations may be difficult to define, either because they are insidious or because they are observed post hoc by the examiner rather than reported real-time by the subject. As such, operationally, it is often only feasible to declare that phenotransition has occurred than to define when it occurred.
Phenoconversion to amyotrophic lateral sclerosis
Phenoconversion, the transition between pre-symptomatic and symptomatic phases of disease, may emerge from the prodromal stage or, in the absence of a prodrome, directly from the pre-manifest state. Operationally, phenoconversion is defined by the emergence of symptoms or objective motor (clinical or EMG) signs that a trained evaluator would reasonably interpret as unequivocal evidence of clinically manifest ALS. Sudden onset of focal weakness, arising from a background of normality, can easily be identified as evidence of phenoconversion, especially with subsequent confirmation of motor neuron dysfunction based on clinical and EMG examinations soon after symptom onset. In such instances, there may be no apparent prodromal period of MMI, and the timing of phenoconversion may be reliably determined. By contrast, when non-specific symptoms emerge gradually, and clinical or EMG findings accrue over time, the determination of phenoconversion may be based on the totality of evidence accumulated to date. Under such circumstances, it is often only feasible to declare that phenoconversion has occurred than to define when it occurred (with attendant implications for use of phenoconversion as a clinical trial end point). Moreover, MMI as a prodromal stage, should be differentiated from the uncertainty that exists when mild motor findings are the manifestation of some co-existing/confounding illness.
Cognitive/behavioural manifestations before phenoconversion to amyotrophic lateral sclerosis
Frontotemporal spectrum dysfunction in amyotrophic lateral sclerosis
Carriers of certain genetic mutations (e.g. C9orf72, VCP, FUS, TARDBP) may develop ALS, FTD or both. Those who phenoconvert to clinically manifest FTD likely pass through a pre-manifest stage as well as a prodromal stage during which there are disturbances of cognition (including language) or behaviour that represent a departure from normal, but which are of insufficient severity to warrant a diagnosis of FTD. Although criteria describing cognitive and behavioural impairment in those with ALS exist (ALSci/ALSbi),111,150 a method for characterizing these neuropsychological features in gene mutation carriers without ALS is currently lacking. Borrowing from the Alzheimer’s disease literature, we suggest the term MCI to also describe the early cognitive changes that may precede FTD, emphasizing that while these deficits occur most frequently in the executive and language domains, other domains might also be affected and should be addressed through formal testing. By analogy to MCI, we recommend the term MBI to reflect the emergence of behaviours (e.g. apathy, disinhibition) that reflect a departure from normal, but which do not warrant a diagnosis of FTD. In both cases, disturbances represent a clear change from prior level of functioning. Individuals meeting criteria for MCI and MBI would receive both classifications. While conceptually simple, operationally defining MCI and MBI in the context of pre-symptomatic ALS is challenging. Here we provide broad recommendations for making these assessments and for determining the emergence of MCI or MBI. Of note is the difference between ALSci/ALSbi and MCI/MBI: their designations apply to, respectively, those who have and those who have not developed clinically manifest ALS.
Prodromal mild cognitive impairment
Our conceptual framework for characterizing MCI differs somewhat from that used by the FTD community, which relies heavily on the clinical judgement of a behavioural neurologist, combined with subjective informant report and results of neuropsychological testing. By contrast, in the study of pre-symptomatic ALS, we rely on formal objective neuropsychological assessment, conducted or supervised by a qualified neuropsychologist, as the principal means for assessing cognition. This approach is informed by the practical consideration that ALS neurologists may feel less comfortable than cognitive/behavioural neurologists in relying on clinical judgements of MCI. In addition, while subjective cognitive complaints may manifest in the prodromal stage, such symptoms may also be non-specific and of uncertain significance. Moreover, subjective cognitive complaints may be absent despite clinically significant cognitive decline due to limited awareness. We have, therefore, elected to classify those with only subjective cognitive symptoms as ‘uncertain’, and those with deficits on neuropsychological testing (if representing a change from premorbid function) or where clinically meaningful decline on neuropsychological testing is detected (even in the absence of impairment) among those with high premorbid functioning, as MCI.
Formal neuropsychological assessment should include comprehensive testing that: (i) assesses all major cognitive domains (executive, language, memory and visuospatial); (ii) includes an adequate number of tests per domain, depending on the domain’s complexity; and (iii) uses standardized measures with appropriate age, education, sex and race/ethnicity-adjusted normative data whenever possible. Our approach (Table 1) to such an assessment conforms to these principles and builds on experience from the Pre-Symptomatic Familial ALS (Pre-fALS) study.133 We recommend including (but not solely relying on) a standardized brief multi-domain assessment such as the Edinburgh Cognitive and Behavioural ALS Screen (ECAS)136–138 or similar battery139–141 that is sensitive to impairment in ALS and FTD and for which established norms exist, have alternate versions and published reliable change indices to enable continued longitudinal assessment even after phenoconversion to ALS or FTD.
Table 1.
Neuropsychological assessment for MCI in pre-symptomatic ALS
| Cognitive domain | Cognitive processes | Neuropsychological testsa | Recommended number of tests |
|---|---|---|---|
| Multi-domain | Multiple | Edinburgh Cognitive and Behavioural ALS Screen (ECAS)136,b | One test |
| Executive | Concept formation | Three tests of different cognitive processes | |
| Set-shifting | Trail Making Testb,c,d,e | ||
| Inhibition | Stroop Testb,c,f,g | ||
| Social cognition | Reading the Mind in the Eyes116,b | ||
| Fluencyh | Executive and language functioning | FAS letter fluencyb,c | One test |
| Language | Naming | Boston Naming Test115,b,i | Two non-overlapping tests |
| Comprehension | Token Test subtest from the Multilingual Aphasia Examination117 | ||
| Semantic processing | Semantic fluencyb,c,j | ||
| Visuospatial | Spatial perception | Judgement of Line Orientation118,b | Two tests |
| Object perception | Object Decision subtest from the Visual Object and Space Perception Battery119,b | ||
| Memory | Visual and verbal | Visual Reproduction subtest from the Wechsler Memory Scale–IV120,b,k | Two tests |
| Immediate, delayed and recognition | California Auditory Verbal Learning Test121,b |
aIncludes examples of tests that may be used to assess cognition in ALS gene carriers. Alternative tests are available and should be selected depending on research study requirements.
Currently used for longitudinal neuropsychological assessment in the ongoing Pre-Symptomatic Familial ALS (Pre-fALS) study.133
Timed tests may limit their continued utility following motor dysfunction onset.
Comparison between Parts A and B would allow continued testing following motor dysfunction onset.
Part A assesses processing speed.
Comparison between interference and control conditions would allow continued testing following motor dysfunction onset.
May be affected by colour-blindness.
Fluency assesses both executive and language functioning and does not represent a stand-alone domain. We include it separately due to its sensitivity to cognitive deficits in ALS and FTD.122,123,125
May be substituted with the Multilingual Naming Test (MINT)124; however, no alternate forms are available for longitudinal assessment.
Performance on this task may contribute to the criteria for impairment in fluency.
May be substituted with the Benson Complex Figure task126; however, the recognition component of this test is very simple.
Evidence that the current level of cognitive functioning represents a decline from a previous level is essential. This may be determined based on any of three metrics. The first is demonstration of longitudinal decline on serial neuropsychological assessment. This requires selecting tests for which a clinically meaningful change can be defined (e.g. a reliable change index,142 standardized regression-based formula143 or standard deviation index144) consideration of practice effects, which may mask decline; and recognition and control of confounding factors. Alternatively, a decline from an estimate of prior cognitive functioning, assessed at baseline, using standardized tests (e.g. North American Adult Reading Test145 or Test of Premorbid Functioning146,147) or demographic-based methods (e.g. Barona Index148) may be used. Where available, differences between estimated premorbid IQ and performance on neuropsychological tests may be examined using established prediction equations. Alternatively, both scores may be standardized with a difference of two standard deviations representing a clear meaningful decline.149 Finally, in the absence of objective measures of decline, one might rely on evidence of change based on other sources of information (e.g. participant interview, self- or informant-reported cognitive decline, or measures such as the CDR® plus NACC FTLD). However, information from these sources is challenging to operationalize as interpretation requires clinical judgement to determine the impact of confounding factors (e.g. poor insight of the test subject, uncertain informant reliability) and this, in turn, is difficult to standardize across assessors and research centres.
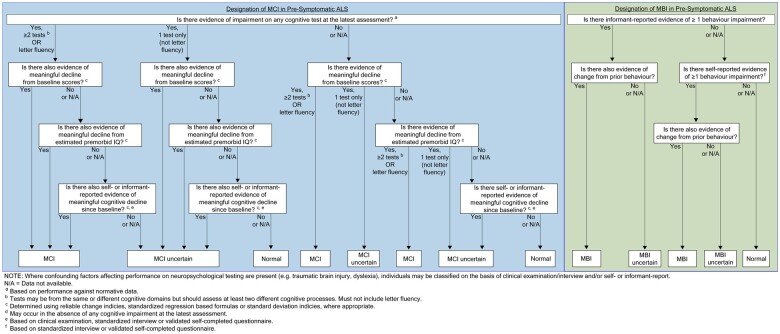
We suggest that MCI in the context of ALS mutation carriers may be defined based on evidence of impairment with meaningful decline on at least two tests assessing two or more different cognitive processes, or a single measure of letter fluency due to its sensitivity in detecting cognitive impairment in ALS150 (Fig. 3). Those with high premorbid functioning may be classified based on the same criteria even in the absence of impairment.
Figure 3.
Decision-tree for the classification of MCI and MBI in pre-symptomatic ALS. An approach to determining the presence of MCI and MBI, based on the results of formal neuropsychological testing and an interview with a reliable informant. This decision-tree emphasizes the need to document changes in cognition and behaviour, and incorporates a hierarchical approach to weighing data from different sources. These guidelines also distinguish mild impairment from instances in which there is uncertainty about cognitive or behavioural impairment.
Prodromal mild behavioural impairment
The principal means of assessing behaviour is an interview with a reliable informant (e.g. ECAS behaviour interview,136 the Frontal Behavioural Inventory151) Since such assessments specifically probe changes in behaviour compared to a previous time point, documenting ‘change’ is simpler than for cognition. If an interview is not possible, the informant may provide information about behavioural changes using a validated self-completed form (e.g. Cambridge Behaviour Inventory–Revised,152 Beaumont Behavioural Inventory,153 Frontal Lobes Systems Behaviour Scale154). Other supportive methods of data collection or sources of information may be used, including participant self-report of changes in behaviour, noting that lack of insight is common. Observer report of behaviour during clinical/research encounters may also be used, noting the limited scope and sensitivity of such observations, as well as the inability to determine whether observed behaviours represent a change. Behaviours of interest include apathy, disinhibition, loss of sympathy/empathy, ritualistic/compulsive behaviours (perseveration) and hyperorality, but not depression and anxiety.91 As assessment of behavioural impairment typically relies on subjective measures, more emphasis on clinical judgement is required (e.g. to determine the reliability of an informant or influence of confounding variables), and this may best be accomplished through a formal multi-disciplinary consensus meeting. In the absence of published literature to inform the relative utility of these different approaches to gathering information about behavioural impairment, we suggest defining MBI based on evidence of changes in one or more behaviours on a standardized interview or a validated self-completed questionnaire, completed by a reliable informant or the participant (Fig. 3).
Differentiating ‘mild’ from ‘uncertain’
In recognizing MCI and MBI as prodromal states that may not always progress to FTD, it is essential to differentiate mild impairment from uncertain cases. Uncertainty may arise when: (i) it is unclear whether impairment represents a change from a previous level of functioning (e.g. cognitive assessment at only a single time point is available); (ii) impairment and/or decline is evident on only a single cognitive test (excluding letter fluency); or (iii) it is unclear whether observed deficits or behaviours might be attributable to confounding factors (e.g. depression). For MCI, uncertainty may also arise when evidence of cognitive decline is obtained solely from subjective and/or informant reports. For MBI, uncertainly may arise when behaviours represent an ‘extreme’ example of a longstanding personality style, or a single instance of abnormal behaviour that might be attributed to a specific set of circumstances. Cases with no or minimal evidence of impairment or decline are considered normal.
Other considerations
Pre-symptomatic amyotrophic lateral sclerosis biomarkers
Since pre-symptomatic ALS is, by definition, characterized by an absence or paucity of clinical manifestations (pre-manifest and prodromal stages, respectively), biomarkers are essential tools for studying this phase of disease. The first emergence of biomarker abnormalities, for example, may serve to characterize that pre-symptomatic disease has begun, and longitudinal changes in these biomarkers may serve as critically important predictors of when clinically manifest disease might appear. In individuals at genetic risk for ALS, neurofilaments have emerged (so far) as the most promising biomarkers of impending phenoconversion to clinically manifest ALS, based on the: (i) ease with which neurofilaments can be measured in serum/plasma; (ii) technical maturity of available assays with high sensitivity and reproducibility across technical replicates; and (iii) evidence of pre-symptomatic changes in concentration before emergence of clinical manifestations of disease.155,156 Based on the currently available assays, NfL appears superior to pNfH: Among gene mutation carriers who progress to clinically manifest disease, NfL (but not always pNfH) levels are elevated above a normative threshold before phenoconversion.155,156
Amyotrophic lateral sclerosis prevention trials
A clinical trial that tests whether an experimental therapeutic prevents (or delays) the emergence of clinically manifest ALS might use a change in biomarker as the primary outcome measure. In the absence of validated surrogate markers, the most appropriate primary outcome measure would have to be clinical (e.g. phenoconversion), analysed either as time-to-phenoconversion or the frequency of occurrence of phenoconversion within a defined time period. Regardless of the primary outcome measure, due to the overall low annual phenoconversion rate even among ALS gene mutation carriers, it will be necessary to enrich the trial cohort. Since the power of a study with phenoconversion as a central component of the primary outcome depends on the number of events, eligibility criteria should enrich for individuals most likely to phenoconvert within the period of follow-up. Some eligibility criteria to consider include genotype, neurofilament concentration and age. Enrichment strategies, of course, may complicate interpretation and generalizability of trial results to segments of the population that were excluded from the trial. For example, assumptions about phenoconversion rates and the temporal course of rise in serum NfL probably differ between those with SOD1 mutations associated with rapidly versus slowly progressing disease.156
Since disease prevention trials are challenging to implement, adaptive elements of study design should be considered. Examples include seamless phase II/III and group sequential designs that allow dropping of a treatment arm during the trial if probably futile, and sample size re-estimation based on interim data. Such designs require extensive planning and organization to overcome logistical and procedural challenges. Given the challenge of identifying sufficient number of people with pre-symptomatic ALS, particularly if the trial has an enrichment design that enrols only a subset of this population, it might be useful to incorporate information from natural history studies to supplement the randomized placebo group using a Bayesian or frequentist approach, even though this is not without its own set of challenges.157–159 This could reduce sample size requirements but would require carefully documented natural history data from people who would meet eligibility criteria for the trial.127
In addition, the broad geographic area over which study participants are likely to be distributed provides incentive to incorporate remote assessments, to the extent that these can be done rigorously. This approach is especially important in the context of the current COVID-19 global health crisis. Fortunately, serum and plasma are easily collected in the home setting through a remote phlebotomy service, and analytes such as NfL are robust to pre-analytic factors that might be impacted by remote collection.
Genetic and biomarker counselling
Published recommendations for genetic testing and counselling for pre-symptomatic ALS are based on experience from the Pre-fALS study.128 In addition to people already known to carry an ALS gene mutation, the Pre-fALS study enrols participants into disclosure and non-disclosure groups, based on participant choice whether to learn results of genetic testing, with pre-decision counselling offered to those with uncertain preference. By contrast, a disease prevention trial would almost certainly only enrol individuals with a confirmed and disclosed genetic mutation. Pre-decision counselling during trial screening would help potential participants decide whether to opt for disclosure and proceed with pre-test counselling. This additional counselling step may be especially important given the potential for pressure (e.g. from family members) to participate in a trial. Counselling should include discussion of legal considerations in the context of how genetic results will be handled in the medical record, as well as regional safeguards that might exist to protect such information. Within the USA, individual state laws vary as to the legal protections provided, including privacy and antidiscrimination legislation. Additionally, studies conducted outside the USA should consider the legal and ethical norms specific to that geographic area.
Insights from genetic counselling may also inform best practices for disclosure of biomarker results, especially if the biomarker is used to determine trial eligibility. Communicating risk based on biomarker results, such as plasma neurofilament levels, is incrementally more complex than sharing genetic test results given: (i) the (current) greater uncertainty about the clinical implications of these biomarker data; and (ii) that, unlike genetic results, which are largely static (an individual either carries, or does not, a pathogenic mutation, although recognizing that a variant of uncertain significance may be ‘upgraded’ to pathogenic as more information emerges), biomarker results are likely to change over time. Biomarker counselling, therefore, may need to be repeated, especially when new results emerge. Similar to best practices in genetic counselling, consent should be fully informed and free of coercion, and may need to be revisited before disclosure of new biomarker data. The advantages, disadvantages and implications of learning the results should be fully explained and reinforced in writing. Psychosocial readiness to undergo genetic testing and receive results must be adequately appraised, and an infrastructure is necessary to support and manage the potential psychosocial impact of learning biomarker results (normal or abnormal).
Ethical, legal and social considerations
We have previously highlighted the importance of an array of ethical, legal and social issues that arise from studying a population at genetic risk for ALS: the evaluation of psychosocial readiness to undergo genetic testing; the personal and family implications of learning genetic status; the potential repercussions for employment, health and disability insurance; the importance of strict separation between research and medical records to minimize the potential for discrimination and a commitment to communicate a diagnosis of ALS if clinically manifest disease emerges, thereby respecting the participants’ right-to-know and permitting the initiation of early treatment or participation in therapeutic trials.128,129,133
Historically, individuals at elevated genetic risk for ALS were thought of as either having or not having clinically manifest ALS. The recognition of prodromal disease (MMI) as an intermediate clinical syndrome, however, poses new ethical challenges—specifically, what to communicate when MMI is diagnosed and how to convey this information, while balancing the individual’s autonomy and their need to make optimal healthcare decisions on the one hand, with the potential to precipitate stress, anxiety, depression and possible suicidal ideation on the other hand. In the context of a research study, the informed consent should explicitly state whether the emergence of MMI or phenoconversion to ALS will be communicated. Moreover, in both research and clinical settings, this communication should always be done in the context of counselling that includes discussion of both the uncertainties around the implication of an MMI diagnosis and the probability of phenoconversion.
The recognition of MMI as a clinical entity and the emergence of non-genetic biomarkers of pre-symptomatic ALS and other neurodegenerative disorders pose unique challenges relevant to potential employment and insurance discrimination. These risks are heightened where prodromal status or biomarker results are documented in the medical record or communicated to study participants, who may intentionally or unintentionally disclose this information to employers or insurers. In the USA, federal laws provide some, but inadequate, protection (i.e. the Genetic Information Nondiscrimination Act (GINA),130 the Americans with Disabilities Act, and the Affordable Care Act]. The effectiveness of protections against discrimination based on biomarker status depends on definitions of disease and functional impairment (Table 2). For example, GINA affords protections in the context of genetic risk for disease, but only so long as disease has not yet manifested.131 GINA does not define ‘disease manifestation’, leaving room for interpretation about non-genetic biomarker evidence of disease or a prodromal state such as MMI. Similarly, the Americans with Disabilities Act prohibits discriminatory employment decisions based on a disability [42 U.S.C.A §12112(a)]. While arguments might be ventured that a prodromal state or preclinical markers of neurodegenerative disorders should explicitly be labelled as a disability for purposes of the Americans with Disabilities Act, the conservative approach would be to assume that protections under the Americans with Disabilities Act do not currently apply to prodromal disease or biomarker status. Finally, the Affordable Care Act provides protection against unfair underwriting practices for health insurance based on ‘pre-existing conditions’, but not for life and long-term care insurance. Therefore, if life or long-term care insurers are informed of an individual’s prodromal or biomarker status—either through requests for medical records or disclosure by the individual—they may be permitted to use the information as grounds for denying a policy application or for charging prohibitively high premiums. Use of a certificate of confidentiality in research studies can keep prodromal disease, as well as genetic and biomarker information outside of the medical record. It is unclear from a legal perspective, however, whether someone who has learned of their prodromal, genetic or biomarker status exclusively through research participation that employs a certificate of confidentiality has a requirement to disclose such information to an underwriter. The legal uncertainty in this area is likely reflected outside the USA as well. It is, therefore, essential that researchers and clinicians evaluate the potential benefits and risks associated with disclosure and establish practices that apply consistently within a study or clinical setting. Additionally, the potential discriminatory risks are a critical component of genetic and biomarker testing and should be discussed before testing is offered.
Table 2.
Protections afforded by the Genetic Information Nondiscrimination Act, Affordable Care Act and Americans with Disabilities Act in the USA
| Applicable party | Context | Information category | GINA | ACA | ADA |
|---|---|---|---|---|---|
| Employers | Employment | Genetic status | Yesa | N/A | N/A |
| Biomarker status | Nob | N/A | Maybec | ||
| Functional status | N/A | N/A | Yes | ||
| Insurers | Health insurance | Genetic status | Yes | N/A | N/A |
| Biomarker status | Nob | No | N/A | ||
| Functional status | N/A | Yes | N/A | ||
| Long-term care insurance | Genetic status | No | No | N/A | |
| Biomarker status | No | No | N/A | ||
| Functional status | N/A | No | N/A | ||
| Life insurance | Genetic status | No | N/A | N/A | |
| Biomarker status | No | N/A | N/A | ||
| Functional status | N/A | N/A | N/A |
ACA = Affordable Care Act; ADA = Americans with Disabilities Act; GINA = Genetic Information Nondiscrimination Act; N/A = designated legislation is not applicable/relevant; No = protection not afforded; Yes = protection afforded.
The protections afforded by GINA apply only so long as disease has not yet become manifest. If a biomarker is taken to imply evidence of disease, then GINA no longer applies.
The protections afforded by GINA relate to genetic risk for disease, but do not cover non-genetic biomarkers that indicate risk of disease.
If the biomarker evidence of disease is (i) ‘regarded as’ a disability; or (ii) is taken to represent impairment of a ‘major bodily function’, then the Americans with Disabilities Act might provide protection (but this argument is legally unproven).
As research advancements improve our ability to identify those at risk for overt disease before the emergence of clinically manifest disease, researchers and clinicians will face an increasing number of ethical challenges in the context of consent procedures, documenting results in research and medical records, and disclosing results to individuals. Further work is needed to incorporate, into the informed consent process, a discussion of the potential social and legal consequences in advance of testing. Additionally, research and education are needed to prepare clinicians and researchers for how to disclose results and to communicate risk to patients and research participants.132
Conclusion
The road to ALS prevention began with the meticulous study of pre-symptomatic disease in individuals at genetic risk for ALS.107,128,129,133,155,156 The emergent natural history and biomarker data have been critical to the design and implementation of the first pre-symptomatic ALS trial.134 The focus of this trial on asymptomatic carriers of highly penetrant SOD1 mutations associated with rapidly progressive disease is due to two key factors. First, phenoconversion to clinically manifest ALS is typically abrupt, and we have observed the largest number of phenoconversion events in this subgroup of the Pre-fALS cohort. Second, understanding of the temporal course of blood-based NfL levels during the pre-symptomatic phase of disease, and of the predictive value of a rise in NfL for imminent phenoconversion, is most advanced in this subgroup. In addition, intrathecally administered SOD1 antisense oligonucleotide is ready for investigation in this population given the emerging evidence for its safety and potential efficacy in the symptomatic SOD1 population.135
In addition to advancing the understanding of pre-symptomatic ALS and phenoconversion among this subset of SOD1 mutation carriers, we have also shed light on the pre-symptomatic stage of disease among other mutation carriers. Notably, we observed the presence of a prodromal period of mild motor, cognitive or behavioural impairment that precedes phenoconversion to clinically manifest disease. While the more gradual evolution of disease in these populations poses challenges for operationally defining phenoconversion, recognition of this prodromal stage is vital to shaping and refining our thinking about how pre-symptomatic disease evolves into clinically manifest disease—which will, importantly, inform future early therapeutic intervention (and disease prevention) efforts.
While the study of gene mutation carriers offers the most proximate opportunity to potentially prevent the clinical onset of genetic ALS, the long-term goal is to prevent all forms of ALS. To empower the study of pre-symptomatic disease in populations at risk for developing sporadic ALS, however, we will need to first identify non-genetic risk factors and expand the repertoire of available biomarkers. Although the study of neurofilaments in mutation carriers has provided a first glimpse into pre-symptomatic ALS and will probably be informative for the non-genetic form of the disease, additional biomarker discovery, including those reflective of underlying TDP-43 pathology or markers reflecting broader compensatory mechanisms, will be key. Moreover, the discovery of prodromal clinical markers that predict the future emergence of clinical ALS, akin to progress made in Parkinson’s disease, could be transformative in facilitating the study of pre-symptomatic sporadic ALS and the prevention of its clinical onset.
The challenges ahead are significant. We can, however, forge a path forwards by building on what we have learned through the study of other neurodegenerative diseases, as well as the study of individuals at genetic risk for ALS. This, we believe, is the road map to early intervention for—perhaps even prevention of—all forms of ALS.
Supplementary Material
Acknowledgements
We are grateful for the very thoughtful and helpful comments received from reviewers of this paper. In addition, we are indebted to the participants in the Pre-fALS study—it is through their longstanding and ongoing commitment in this research endeavour that we have been able to gain insight into the earliest manifestations of ALS and to forge a path towards ALS prevention.
Funding
The First International Pre-Symptomatic ALS Workshop was supported through unrestricted grants from The ALS Association, The Muscular Dystrophy Association, The Motor Neurone Disease Association, The Association for Frontotemporal Degeneration, Biogen and AveXis. M.B. and J.W. also receive partial support for the Workshop from the National Institutes of Health (NIH) through R01 NS105479. C.M. is supported as a CReATe Scholar through funding from the National Institutes of Health (U54 NS092091).
Competing interests
M.B. reports grants from The ALS Association, Muscular Dystrophy Association, Motor Neuron Disease Association, Association for Frontotemporal Degeneration, Biogen and AveXis to support the First International Pre-Symptomatic ALS Workshop; as well as personal fees from Biogen outside the submitted work. The University of Miami has licensed intellectual property to Biogen to support design of the ATLAS study. C.M. reports grants from the CReATe Consortium and the ALS Association. R.B.P. reports grants and personal fees from Fonds de la Recherche en Sante, the Canadian Institute of Health Research, Parkinson Canada, the Weston-Garfield Foundation, the Michael J. Fox Foundation, the Webster Foundation and personal fees from Takeda, Roche/Prothena, Teva Neurosciences, Novartis, Biogen, Boehringer Ingelheim, Theranexus, Merck, Abbvie, Jannsen, Curasen and Inception Sciences outside the submitted work. B.F. B. reports funding for clinical trials sponsored by Alector and EIP Pharma. He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr Lewy Body Dementia Program and the Little Family Foundation. R.P. reports grant funding from the National Institute on Aging, P30 AG062677, U01 AG006786 and consults for Roche Inc., Merck Inc., Genentech Inc. and Biogen. C.A.R. reports consulting or Scientific Advisory Boards for Huntington Study Group (HSG), Annexon, Mitoconix, NeuBase, NeuExcell, Roche/Genentech, SAGE, Spark, TEVA, uniQure and Wave, all unrelated to this paper. J.J.A. reports grants from the National Institutes of Health–National Institute on Aging and the National Cancer Institute, the Alzheimer’s Association, the Aging Research in Criminal Health Network and the Global Brain Health Institute. S.F. reports being employed by and holding stock in Biogen. J.S. reports research support from Amylyx, Biogen, Biotie Therapies (now Acorda Therapeutics), Cytokinetics Incorporated, Mitsubishi Tanabe Pharma America, Alexion, Medicinova, Ionis, Alector and Orphazyme, compensation received as a consultant for Apic Biosciences, Neurosense, Cytokinetics, Denali, GSK, Mitsubishi Tanabe Pharma America, Orphazyme, Pinteon, RRD, Swanbio, Helixsmith and royalties for serving as Neuromuscular Section Editor for UpToDate. S.A. reports grants from MND Scotland, the Motor Neuron Disease Association and the ALS Association. S.C. reports consulting fees from Sage Therapeutics and the Association for Frontotemporal Degeneration. P.M. A. reports grants from the Knut and Alice Wallenberg Foundation, the Swedish Research Council, Ulla-Carin Lindquist patient organization, the Umeå University Research Foundation and the Swedish Neuro Patient Organization; as well as consultancies for Biogen and Orphazyme A/S. R.S.F. reports grants to his institution to support his participation in clinical trials sponsored by AveXis/Novartis Gene Therapies, Biogen, Catabasis, Cytokinetics, Ionis, Muscular Dystrophy Association, National Institutes of Health, Lilly, ReveraGen, Roche, Sarepta, Scholar Rock and Summit. He has received honoraria for participating in symposia and on advisory boards from these same pharmaceutical companies. He serves without compensation as an advisor to the n-Lorem and EveryLife Foundations. His institution received funding from Biogen for the coordination of a SMA registry. He receives licensing fees from the Children’s Hospital of Philadelphia and publishing royalty fees from Elsevier. J.D.R. reports grants from the Bluefield Project, Medical Research Council, JPND and Brain Research UK. He has sat on medical advisory boards for Wave Life Sciences, Alector, Prevail Therapeutics and Arkuda Therapeutics. C.T.M. reports research funding outside the submitted work from NIH and Biogen, and provides consulting services for personal fees from Invicro and Axon Advisors on behalf of Translational Bioinformatics, LLC. He also receives an honorarium as Associate Editor of NeuroImage: Clinical. M.G. reports support from NIH, Biogen, Samuel Newhouse Foundation, Robinson Family Foundation, Wyncote Foundation; support in kind from LMI and Avid; consultant for Passage Bio, Bracco, Daneli; and participation in trials sponsored by Biogen, Alector, Passage Bio, Prevail. A.A.-C. reports grants from the MRC through the Joint Programme on Neurodegeneration (JPND) to support work contributing to the First International Pre-Symptomatic ALS Workshop; as well as consultancies for Biogen Idec, Wave Pharmaceuticals Ltd, Cytokinetics, OrionPharma, Amylyx, GSK, Novartis, Lilly, Brainstorm Therapeutics, Apellis Inc and Quralis outside the submitted work. M.R.T. reports grants from the Motor Neurone Disease Association and My Name’5 Doddie Foundation. He sits on the Scientific Advisory Board for Orphazyme. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- bvFTD
behavioural variant frontotemporal dementia
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- MBI
mild behavioural impairment
- MCI
mild cognitive impairment
- MMI
mild motor impairment
- NfL
neurofilament light chain
- PPA
primary progressive aphasia
- SMA
spinal muscular atrophy
Contributor Information
First International Pre-Symptomatic ALS Workshop:
Ammar Al-Chalabi, Peter M Andersen, Jalayne Arias, Michael Benatar, Bradley Boeve, Stephanie Cosentino, Kuldip Dave, Toby Ferguson, Mary-Kay Floeter, Jonathan Rohrer, Stephanie Fradette, Tania Gendron, Volkan Granit, Anne-Laure Grignon, Murray Grossman, Amelie Gubitz, Petra Kaufman, Isabelle Le Ber, Suzee Lee, Andrea Malaspina, Michael P McDermott, Caroline McHutchison, Corey McMillan, Katie Nicholson, Ronald Petersen, Ronald Postuma, Richard Robinson, Howard Rosen, Christopher Ross, Jeremy Shefner, Christine Stanislaw, Nadine Tatton, Neil Thakur, Martin Turner, Jochen Weishaupt, and Joanne Wuu
References
- 1. Duara R, Loewenstein DA, Greig MT, et al. Pre-MCI and MCI: Neuropsychological, clinical, and imaging features and progression rates. Am J Geriatr Psychiatry. 2011;19(11):951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jessen F, Amariglio RE, van Boxtel M, et al. ; Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabin LA, Smart CM, Amariglio RE.. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13:369–396. [DOI] [PubMed] [Google Scholar]
- 5. van Harten AC, Mielke MM, Swenson-Dravis DM, et al. Subjective cognitive decline and risk of MCI: The Mayo Clinic Study of Aging. Neurology. 2018;91(4):e300–e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cacace R, Sleegers K, Van Broeckhoven C.. Molecular genetics of early-onset Alzheimer's disease revisited. Alzheimers Dement. 2016;12(6):733–748. [DOI] [PubMed] [Google Scholar]
- 7. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM.. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 8. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 9. Gillis C, Mirzaei F, Potashman M, Ikram MA, Maserejian N.. The incidence of mild cognitive impairment: A systematic review and data synthesis. Alzheimers Dement (Amst). 2019;11:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Maurik IS, van der Kall LM, de Wilde A, et al. ; Alzheimer's Disease Neuroimaging Initiative. Added value of amyloid PET in individualized risk predictions for MCI patients. Alzheimers Dement (Amst). 2019;11(1):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jack CR Jr, Bennett DA, Blennow K, et al. ; Contributors. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dementia. 2018;14(4):535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marks J, Syrjanen J, Graff-Radford J, et al. Comparison of neurofialment light and total tau as blood-based biomarkers of neurodegeneration: Associations with cognition and neuroimaging outcomes. In: 2021 Annual Meeting. American Academy of Neurology; 2021.
- 15. Preische O, Schultz SA, Apel A, et al. ; Dominantly Inherited Alzheimer Network. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25(2):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen RC. How early can we diagnose Alzheimer disease (and is it sufficient)? The 2017 Wartenberg lecture. Neurology. 2018;91(9):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. New Engl J Med. 2021;384(18):1691–1704. [DOI] [PubMed] [Google Scholar]
- 18. Rabinovici GD. Controversy and progress in Alzheimer's disease—FDA approval of aducanumab. New Engl J Med. 2021;385(9):771–774. [DOI] [PubMed] [Google Scholar]
- 19. Dunn B, Stein P, Cavazzoni P.. Approval of aducanumab for Alzheimer disease—the FDA's perspective. JAMA Intern Med. 2021;181(10):1276. [DOI] [PubMed] [Google Scholar]
- 20. Petersen RC. Aducanumab: What about the patient? Ann Neurol. 2021;90(3):334–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bateman RJ, Benzinger TL, Berry S, et al. ; DIAN-TU Pharma Consortium for the Dominantly Inherited Alzheimer Network. The DIAN-TU Next Generation Alzheimer's prevention trial: Adaptive design and disease progression model. Alzheimers Dement. 2017;13(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: Stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):228fs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tariot PN, Lopera F, Langbaum JB, et al. ; Alzheimer's Prevention Initiative. The Alzheimer's Prevention Initiative Autosomal-Dominant Alzheimer's Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer's disease, including a placebo-treated noncarrier cohort. Alzheimers Dement (N Y). 2018;4:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pringsheim T, Jette N, Frolkis A, Steeves TD.. The prevalence of Parkinson's disease: A systematic review and meta-analysis. Mov Disord. 2014;29(13):1583–1590. [DOI] [PubMed] [Google Scholar]
- 25. Kuhlenbaumer G, Berg D.. Parkinson disease genetics: Too early to predict progression? Nat Rev Neurol. 2019;15(11):625–626. [DOI] [PubMed] [Google Scholar]
- 26. Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. [DOI] [PubMed] [Google Scholar]
- 27. Heinzel S, Berg D, Gasser T, et al. ; MDS Task Force on the Definition of Parkinson's Disease. Update of the MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2019;34(10):1464–1470. [DOI] [PubMed] [Google Scholar]
- 28. Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB.. Evolution of prodromal Parkinson's disease and dementia with Lewy bodies: A prospective study. Brain. 2019;142(7):2051–2067. [DOI] [PubMed] [Google Scholar]
- 29. Postuma RB, Berg D.. Prodromal Parkinson's disease: The decade past, the decade to come. Mov Disord. 2019;34(5):665–675. [DOI] [PubMed] [Google Scholar]
- 30. Savica R, Carlin JM, Grossardt BR, et al. Medical records documentation of constipation preceding Parkinson disease: A case-control study. Neurology. 2009;73(21):1752–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaufmann H, Norcliffe-Kaufmann L, Palma JA, et al. ; Autonomic Disorders Consortium. Natural history of pure autonomic failure: A United States prospective cohort. Ann Neurol. 2017;81(2):287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berg D, Godau J, Seppi K, et al. ; PRIPS study group. The PRIPS study: Screening battery for subjects at risk for Parkinson's disease. Eur J Neurol. 2013;20(1):102–108. [DOI] [PubMed] [Google Scholar]
- 33. Jennings D, Siderowf A, Stern M, et al. ; PARS Investigators. Conversion to Parkinson disease in the PARS hyposmic and dopamine transporter-deficit prodromal cohort. JAMA Neurol. 2017;74(8):933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singer W, Schmeichel AM, Shahnawaz M, et al. Alpha-synuclein oligomers and neurofilament light chain in spinal fluid differentiate multiple system atrophy from Lewy body synucleinopathies. Ann Neurol. 2020;88(3):503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain. 2019;142(3):744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2015;30(12):1600–1611. [DOI] [PubMed] [Google Scholar]
- 37. Berg D, Postuma RB, Bloem B, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson's disease. Mov Disord. 2014;29(4):454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72(6):971–983. [DOI] [PubMed] [Google Scholar]
- 39. Ross CA, Aylward EH, Wild EJ, et al. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10(4):204–216. [DOI] [PubMed] [Google Scholar]
- 40. Huntington Study Group PHAROS Investigators. At risk for Huntington disease: The PHAROS (Prospective Huntington At Risk Observational Study) cohort enrolled. Arch Neurol. 2006;63(7):991–996. [DOI] [PubMed] [Google Scholar]
- 41. Biglan KM, Shoulson I, Kieburtz K, et al. ; The Huntington Study Group PHAROS Investigators. Clinical-Genetic Associations in the Prospective Huntington at Risk Observational Study (PHAROS): Implications for clinical trials. JAMA Neurol. 2016;73(1):102–110. [DOI] [PubMed] [Google Scholar]
- 42. Biglan KM, Ross CA, Langbehn DR, et al. Motor abnormalities in premanifest persons with Huntington's disease: The PREDICT-HD study. Mov Disord. 2009;24(12):1763–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paulsen JS, Hayden M, Stout JC, et al. ; Predict-HD Investigators of the Huntington Study Group. Preparing for preventive clinical trials: The Predict-HD study. Arch Neurol. 2006;63(6):883–890. [DOI] [PubMed] [Google Scholar]
- 44. Paulsen JS, Langbehn DR, Stout JC, et al. ; Predict-HD Investigators and Coordinators of the Huntington Study Group. Detection of Huntington's disease decades before diagnosis: The Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79(8):874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wild EJ, Tabrizi SJ.. Predict-HD and the future of therapeutic trials. Lancet Neurol. 2006;5(9):724–725. [DOI] [PubMed] [Google Scholar]
- 46. Tabrizi SJ, Langbehn DR, Leavitt BR, et al. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: Cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tabrizi SJ, Scahill RI, Durr A, et al. Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: The 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31–42. [DOI] [PubMed] [Google Scholar]
- 48. Unified Huntington's Disease Rating Scale: Reliability and consistency. Huntington Study Group. Mov Disord. 1996;11(2):136–142. [DOI] [PubMed] [Google Scholar]
- 49. Ross CA, Reilmann R, Cardoso F, et al. Movement Disorder Society Task Force Viewpoint: Huntington's disease diagnostic categories. Mov Disord Clin Pract. 2019;6(7):541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Penney JB Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH.. CAG repeat number governs the development rate of pathology in Huntington's disease. Ann Neurol. 1997;41(5):689–692. [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Long JD, Mills JA, et al. ; PREDICT-HD Investigators and Coordinators of the Huntington Study Group. Indexing disease progression at study entry with individuals at-risk for Huntington disease. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(7):751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Langbehn DR, Hayden MR, Paulsen JS.; and the PREDICT-HD Investigators of the Huntington Study Group. CAG-repeat length and the age of onset in Huntington disease (HD): A review and validation study of statistical approaches. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(2):397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium. Identification of genetic factors that modify clinical onset of Huntington's disease. Cell. 2015;162(3):516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aylward EH. Magnetic resonance imaging striatal volumes: A biomarker for clinical trials in Huntington's disease. Mov Disord. 2014;29(11):1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paulsen JS, Long JD, Ross CA, et al. ; PREDICT-HD Investigators and Coordinators of the Huntington Study Group. Prediction of manifest Huntington's disease with clinical and imaging measures: A prospective observational study. Lancet Neurol. 2014;13(12):1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tabrizi SJ, Scahill RI, Owen G, et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: Analysis of 36-month observational data. Lancet Neurol. 2013;12(7):637–649. [DOI] [PubMed] [Google Scholar]
- 57. Aylward EH, Liu D, Nopoulos PC, et al. ; PREDICT-HD Investigators and Coordinators of the Huntington Study Group. Striatal volume contributes to the prediction of onset of Huntington disease in incident cases. Biol Psychiatry. 2012;71(9):822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Southwell AL, Smith SE, Davis TR, et al. Ultrasensitive measurement of huntingtin protein in cerebrospinal fluid demonstrates increase with Huntington disease stage and decrease following brain huntingtin suppression. Sci Rep. 2015;5:12166- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wild EJ, Boggio R, Langbehn D, et al. Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington's disease patients. J Clin Invest. 2015;125(5):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Byrne LM, Rodrigues FB, Johnson EB, et al. Evaluation of mutant huntingtin and neurofilament proteins as potential markers in Huntington's disease. Sci Transl Med. 2018;10(458):eaat7108. [DOI] [PubMed] [Google Scholar]
- 61. Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, et al. ; Phase 1–2a IONIS-HTTRx Study Site Teams. Targeting Huntingtin expression in patients with Huntington's disease. New Engl J Med. 2019;380(24):2307–2316. [DOI] [PubMed] [Google Scholar]
- 62. Byrne LM, Rodrigues FB, Blennow K, et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington's disease: A retrospective cohort analysis. Lancet Neurol. 2017;16(8):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Constantinescu R, Holmberg B, Rosengren L, Corneliusson O, Johnels B, Zetterberg H.. Light subunit of neurofilament triplet protein in the cerebrospinal fluid after subthalamic nucleus stimulation for Parkinson's disease. Acta Neurol Scand. 2011;124(3):206–210. [DOI] [PubMed] [Google Scholar]
- 64. Niemela V, Landtblom AM, Blennow K, Sundblom J.. Tau or neurofilament light—which is the more suitable biomarker for Huntington's disease? PLoS ONE. 2017;12(2):e0172762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rodrigues FB, Byrne LM, Tortelli R, et al. Mutant huntingtin and neurofilament light have distinct longitudinal dynamics in Huntington's disease. Sci Transl Med. 2020;12(574):eabc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Niemela V, Landtblom AM, Nyholm D, et al. Proenkephalin decreases in cerebrospinal fluid with symptom progression of Huntington's disease. Mov Disord. 2021;36(2):481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Al Shweiki MR, Oeckl P, Pachollek A, et al. Cerebrospinal fluid levels of prodynorphin-derived peptides are decreased in Huntington's disease. Mov Disord. 2021;36(2):492–497. [DOI] [PubMed] [Google Scholar]
- 68. Darras B, Monani U, De Vivo D.. Genetic disorders affecting the motor neuron: Spinal muscular atrophy. In: Swaiman K, Ashwal S, Ferriero D, et al. eds. Swaiman’s pediatric neurology: Principles and practice. 6th edn.Elsevier; 2017:1057–1064. [Google Scholar]
- 69. Lefebvre S, Burlet P, Liu Q, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16(3):265–269. [DOI] [PubMed] [Google Scholar]
- 70. Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B.. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: Fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Human Genet. 2002;70(2):358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus Sham control in later-onset spinal muscular atrophy. New Engl J Med. 2018;378(7):625–635. [DOI] [PubMed] [Google Scholar]
- 73. Finkel RS. Electrophysiological and motor function scale association in a pre-symptomatic infant with spinal muscular atrophy type I. Neuromuscul Disord. 2013;23(2):112–115. [DOI] [PubMed] [Google Scholar]
- 74. Lowes L, Alfano L, Chen D, et al. Presymptomatic spinal muscular atrophy: Reality or myth? Neuromuscul Disord. 2019;29:S129. [Google Scholar]
- 75. Zerres K, Rudnik-Schoneborn S.. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52(5):518–523. [DOI] [PubMed] [Google Scholar]
- 76. De Vivo DC, Bertini E, Swoboda KJ, et al. ; NURTURE Study Group. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. US National Library of Medicine. A Study of Risdiplam in Infants with Genetically Diagnosed and Presymptomatic Spinal Muscular Atrophy (Rainbowfish). Accessed May 2021. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03779334?term=NCT03779334&draw=2&rank=1
- 78. US National Library of Medicine Pre-Symptomatic Study of Intravenous Onasemnogene Abeparvovec-xioi in Spinal Muscular Atrophy (SMA) for Patients with Multiple Copies of SMN2 (SPR1NT). Accessed May 2021. https://clinicaltrials.gov/ct2/show/NCT03505099?term=NCT03505099&draw=2&rank=1
- 79. Darras BT, Crawford TO, Finkel RS, et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann Clin Transl Neurol. 2019;6(5):932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dangouloff T, Servais L.. Clinical evidence supporting early treatment of patients with spinal muscular atrophy: Current perspectives. Ther Clin Risk Manag. 2019;15:1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus Sham Control in infantile-onset spinal muscular atrophy. New Engl J Med. 2017;377(18):1723–1732. [DOI] [PubMed] [Google Scholar]
- 82. Day JW, Finkel RS, Chiriboga CA, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284–293. [DOI] [PubMed] [Google Scholar]
- 83. Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. New Engl J Med. 2017;377(18):1713–1722. [DOI] [PubMed] [Google Scholar]
- 84. Bang J, Spina S, Miller BL.. Frontotemporal dementia. Lancet. 2015;386(10004):1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rademakers R, Neumann M, Mackenzie IR.. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8(8):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rosen HJ, Boeve BF, Boxer AL.. Tracking disease progression in familial and sporadic frontotemporal lobar degeneration: Recent findings from ARTFL and LEFFTDS. Alzheimers Dement. 2020;16(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moore KM, Nicholas J, Grossman M, et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: An international retrospective cohort study. Lancet Neurol. 2020;19(2):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Petersen RC. Clinical practice. Mild cognitive impairment. New Engl J Med. 2011;364(23):2227–2234. [DOI] [PubMed] [Google Scholar]
- 91. Boeve B, Bove J, Brannelly P, et al. ; LEFFTDS Consortium. The longitudinal evaluation of familial frontotemporal dementia subjects protocol: Framework and methodology. Alzheimers Dement. 2020;16(1):22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Olney NT, Ong E, Goh SM, et al. ; ARTFL and LEFFTDS consortia. Clinical and volumetric changes with increasing functional impairment in familial frontotemporal lobar degeneration. Alzheimers Dement. 2020;16(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ismail Z, Smith EE, Geda Y, et al. ; ISTAART Neuropsychiatric Symptoms Professional Interest Area. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: A cross-sectional analysis. Lancet Neurol. 2015;14(3):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Thijssen EH, La Joie R, Wolf A, et al. ; Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. van der Ende EL, Meeter LH, Poos JM, et al. Serum neurofilament light chain in genetic frontotemporal dementia: A longitudinal, multicentre cohort study. Lancet Neurol. 2019;18(12):1103–1111. [DOI] [PubMed] [Google Scholar]
- 97. Chen Q, Boeve BF, Schwarz CG, et al. ; LEFFTDS Consortium. Tracking white matter degeneration in asymptomatic and symptomatic MAPT mutation carriers. Neurobiol Aging. 2019;83:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen Q, Boeve BF, Senjem M, et al. Trajectory of lobar atrophy in asymptomatic and symptomatic GRN mutation carriers: A longitudinal MRI study. Neurobiol Aging. 2020;88:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen Q, Boeve BF, Senjem M, et al. ; LEFFTDS Consortium. Rates of lobar atrophy in asymptomatic MAPT mutation carriers. Alzheimers Dement (N Y). 2019;5:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen Q, Boeve BF, Tosakulwong N, et al. Brain MR spectroscopy changes precede frontotemporal lobar degeneration phenoconversion in Mapt mutation carriers. J Neuroimaging. 2019;29(5):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Le Blanc G, Jette Pomerleau V, McCarthy J, et al. ; Genetic Frontotemporal Dementia Initiative (GENFI). Faster cortical thinning and surface area loss in presymptomatic and symptomatic C9orf72 repeat expansion adult carriers. Ann Neurol. 2020;88(1):113–122. [DOI] [PubMed] [Google Scholar]
- 102. Mutsaerts H, Mirza SS, Petr J, et al. ; GENetic Frontotemporal dementia Initiative (GENFI). Cerebral perfusion changes in presymptomatic genetic frontotemporal dementia: A GENFI study. Brain. 2019;142(4):1108–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Staffaroni AM, Ljubenkov PA, Kornak J, et al. Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain. 2019;142(2):443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tsai RM, Bejanin A, Lesman-Segev O, et al. F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimers Res Ther. 2019;11(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jones DT, Knopman DS, Graff-Radford J, et al. In vivo (18)F-AV-1451 tau PET signal in MAPT mutation carriers varies by expected tau isoforms. Neurology. 2018;90(11):e947–e954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. van der Ende EL, Jackson JL, White A, Seelaar H, van Blitterswijk M, Van Swieten JC.. Unravelling the clinical spectrum and the role of repeat length in C9ORF72 repeat expansions. J Neurol Neurosurg Psychiatry. 2021;92(5):502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Benatar M, Turner MR, Wuu J.. Defining pre-symptomatic amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(5-6):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shefner JM, Al-Chalabi A, Baker MR, et al. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol. 2020;131(8):1975–1978. [DOI] [PubMed] [Google Scholar]
- 109. Makki AA, Benatar M.. Diagnostic accuracy of thoracic paraspinal electromyography in amyotrophic lateral sclerosis. J Clin Neurophysiol. 2007;24(3):298–300. [DOI] [PubMed] [Google Scholar]
- 110. Makki AA, Benatar M.. The electromyographic diagnosis of amyotrophic lateral sclerosis: Does the evidence support the El Escorial criteria? Muscle Nerve. 2007;35(5):614–619. [DOI] [PubMed] [Google Scholar]
- 111. Strong MJ, Grace GM, Freedman M, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10(3):131–146. [DOI] [PubMed] [Google Scholar]
- 112. Ellis D. Delis-Kaplan Executive Function System–D-KEFS. The Psychological Corporation; 2001. [Google Scholar]
- 113. Heaton RK. Wisconsin Card Sorting Test Manual. Psychological assessment resources; 1981. [Google Scholar]
- 114. Kongs SK, Thompson LL, Iverson GL, Heaton RK.. Wisconsin Card Sorting Test-64 Card Version: WCST-64. PAR; 2000. [Google Scholar]
- 115. Kaplan EF, Goodglass H, Weintraub S.. The Boston Naming Test. 2nd ed. Lea and Febiger; 1983. [Google Scholar]
- 116. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I.. The "Reading the Mind in the Eyes" Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- 117. Benton AL, deS K, Sivan AB.. Multilingual Aphasia Examination Third Edition. PAR Inc; 1994. [Google Scholar]
- 118. Benton AL, Varney NR, Hamsher KD.. Visuospatial judgment. A clinical test. Archives of Neurology. 1978;35(6):364–367. [DOI] [PubMed] [Google Scholar]
- 119. Warrington EK, James M.. The Visual Object and Space Perception Battery: VOSP. Pearson; 1991. [Google Scholar]
- 120. Wechsler D. Wechsler Memory Scale (WMS-IV) technical and interpretive manual. Pearson; 2009. [Google Scholar]
- 121. Delis D, Kramer J, Kaplan E, Ober B.. California Verbal Learning Test: Adult Version Manual. The Psychological Corporation; 1987. [Google Scholar]
- 122. Raaphorst J, De Visser M, Linssen WHJP, De Haan RJ, Schmand B.. The cognitive profile of amyotrophic lateral sclerosis: A meta-analysis. Amyotroph Lateral Scler. 2010;11(1-2):27–37. [DOI] [PubMed] [Google Scholar]
- 123. Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grisé D, Goldstein LH.. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia. 2000;38(6):734–747. [DOI] [PubMed] [Google Scholar]
- 124. Gollan TH, Weissberger GH, Runnqvist E, Montoya RI, Cera CM.. Self-ratings of Spoken Language Dominance: A Multi-Lingual Naming Test (MINT) and Preliminary Norms for Young and Aging Spanish-English Bilinguals. Biling (Camb Engl). 2012;15(3):594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B.. Are amyotrophic lateral sclerosis patients cognitively normal?. Neurology. 2003;60(7):1094–1097. [DOI] [PubMed] [Google Scholar]
- 126. Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH.. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer's disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pocock SJ. The combination of randomized and historical controls in clinical trials. J Chronic Dis. 1976;29(3):175–188. [DOI] [PubMed] [Google Scholar]
- 128. Benatar M, Stanislaw C, Reyes E, et al. Presymptomatic ALS genetic counseling and testing: Experience and recommendations. Neurology. 2016;86(24):2295–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fanos JH, Gronka S, Wuu J, Stanislaw C, Andersen PM, Benatar M.. Impact of presymptomatic genetic testing for familial amyotrophic lateral sclerosis. Genet Med. 2011;13(4):342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. United States Department of Labor. The Genetic Information Nondiscrimination Act of 2008 (GINA). In: US Department of Labor EBSA, ed. Public Law No. 110-233. Vol. H.R. 493. Washington, DC; 2008.
- 131. Prince AE, Berkman BE.. When does an illness begin: Genetic discrimination and disease manifestation. J Law Med Ethics. 2012;40(3):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Harkins K, Sankar P, Sperling R, et al. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther. 2015;7(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Benatar M, Wuu J.. Presymptomatic studies in ALS: Rationale, challenges, and approach. Neurology. 2012;79(16):1732–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Benatar M, Wuu J, Andersen P, et al. Design of a Phase 3, randomized, placebo-controlled trial of Tofersen in clinically pre-symptomatic SOD1 mutation carriers: The ATLAS study. In: American Academy of Neurology Annual Meeting 2021. American Academy of Neurology; 2021. [Google Scholar]
- 135. Miller T, Cudkowicz M, Shaw PJ, et al. Phase 1-2 trial of antisense oligonucleotide Tofersen for SOD1 ALS. New Engl J Med. 2020;383(2):109–119. [DOI] [PubMed] [Google Scholar]
- 136. Abrahams S, Newton J, Niven E, Foley J, Bak TH.. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(1-2):9–14. [DOI] [PubMed] [Google Scholar]
- 137. Crockford C, Kleynhans M, Wilton E, et al. ECAS A-B-C: Alternate forms of the Edinburgh cognitive and behavioural ALS screen. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(1-2):57–64. [DOI] [PubMed] [Google Scholar]
- 138. Niven E, Newton J, Foley J, et al. Validation of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS): A cognitive tool for motor disorders. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(3-4):172–179. [DOI] [PubMed] [Google Scholar]
- 139. Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers’ neuropsychological test battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord. 2018;32(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Woolley SC, York MK, Moore DH, et al. Detecting frontotemporal dysfunction in ALS: Utility of the ALS Cognitive Behavioral Screen (ALS-CBS™). Amyotroph Lateral Scler. 2010;11(3):303–311. [DOI] [PubMed] [Google Scholar]
- 141. Gavett BE, Ashendorf L, Gurnani AS.. Reliable change on neuropsychological tests in the Uniform Data Set. J Int Neuropsychol Soc. 2015;21(7):558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jacobson NS, Truax P.. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. [DOI] [PubMed] [Google Scholar]
- 143. McSweeny AJ, Naugle RI, Chelune GJ, Lüders H.. ‘T scores for change’: An illustration of a regression approach to depicting change in clinical neuropsychology. Clin Neuropsychol. 1993;7(3):300–312. [Google Scholar]
- 144. Duff K. Evidence-based indicators of neuropsychological change in the individual patient: Relevant concepts and methods. Arch Clin Neuropsychol. 2012;27(3):248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Blair JR, Spreen O.. Predicting premorbid IQ: A revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3(2):129–136. [Google Scholar]
- 146. Pearson N. Advanced clinical solutions for WAIS-IV and WMS-IV: Administration and scoring manual. The Psychological Corporation; 2009. [Google Scholar]
- 147. Wechsler D. Test of premorbid functioning. UK version (TOPF UK). Pearson Corporation; 2011. [Google Scholar]
- 148. Barona A, Reynolds CR, Chastain R.. A demographically based index of premorbid intelligence for the WAIS—R. J Consult Clin Psychol. 1984;52(5):885–887. [Google Scholar]
- 149. Taylor MJ, Heaton RK.. Sensitivity and specificity of WAIS–III/WMS–III demographically corrected factor scores in neuropsychological assessment. J Int Neuropsychol Soc. 2001;7(7):867–874. [PubMed] [Google Scholar]
- 150. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3-4):153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kertesz A, Davidson W, Fox H.. Frontal Behavioral Inventory: Diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24(1):29–36. [DOI] [PubMed] [Google Scholar]
- 152. Wear HJ, Wedderburn CJ, Mioshi E, et al. The Cambridge Behavioural Inventory Revised. Dement Neuropsychol. 2008;2(2):102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Elamin M, Pinto-Grau M, Burke T, et al. Identifying behavioural changes in ALS: Validation of the Beaumont Behavioural Inventory (BBI). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(1-2):68–73. [DOI] [PubMed] [Google Scholar]
- 154. Grace J, Malloy PF.. Frontal systems behavior scale: FrSBe. Psychological Assessment Resources Lutz; 2001. [Google Scholar]
- 155. Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A.. Neurofilament light: A candidate biomarker of pre-symptomatic ALS and phenoconversion. Ann Neurol. 2018;84(1):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Benatar M, Wuu J, Lombardi V, et al. Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(7-8):538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ghadessi M, Tang R, Zhou J, et al. A roadmap to using historical controls in clinical trials - by Drug Information Association Adaptive Design Scientific Working Group (DIA-ADSWG). Orphanet J Rare Dis. 2020;15(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lim J, Walley R, Yuan J, et al. Minimizing patient burden through the use of historical subject-level data in innovative confirmatory clinical trials: Review of methods and opportunities. Ther Innov Regul Sci. 2018;52(5):546–559. [DOI] [PubMed] [Google Scholar]
- 159. Viele K, Berry S, Neuenschwander B, et al. Use of historical control data for assessing treatment effects in clinical trials. Pharm Stat. 2014;13(1):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.