Abstract
Phosphorylated TDP-43 (pTDP-43) aggregates in the cytoplasm of motor neurons and neuroglia in the brain are one of the pathological hallmarks of amyotrophic lateral sclerosis. Although the axons exceed the total volume of motor neuron soma by several orders of magnitude, systematic studies investigating the presence and distribution of pTDP-43 aggregates within motor nerves are still lacking. The aim of this study is to define the TDP-43/pTDP-43 pathology in diagnostic motor nerve biopsies performed on a large cohort of patients presenting with a lower motor neuron syndrome and to assess whether this might be a discriminating tissue biomarker for amyotrophic lateral sclerosis and non-amyotrophic lateral sclerosis cases.
We retrospectively evaluated 102 lower motor neuron syndrome patients referred to our centre for a diagnostic motor nerve biopsy. Histopathological criteria of motor neuron disease and motor neuropathy were applied by two independent evaluators, who were blind to clinical data. TDP-43 and pTDP-43 were evaluated by immunohistochemistry, and results compared to final clinical diagnosis.
We detected significant differences between amyotrophic lateral sclerosis and non-amyotrophic lateral sclerosis cases in pTDP-43 expression in myelinated fibres: axonal accumulation was detected in 98.2% of patients with amyotrophic lateral sclerosis versus 30.4% of non-amyotrophic lateral sclerosis samples (P < 0.0001), while concomitant positive staining in Schwan cell cytoplasm was found in 70.2% of patients with amyotrophic lateral sclerosis versus 17.4% of patients who did not have amyotrophic lateral sclerosis (P < 0.001). Importantly, we were also able to detect pTDP-43 aggregates in amyotrophic lateral sclerosis cases displaying normal features at standard histopathological analysis.
Our findings demonstrated that a specific pTDP-43 signature is present in the peripheral nervous system of patients with amyotrophic lateral sclerosis, and could be exploited as a specific, accessible tissue biomarker. The detection of pTDP-43 aggregates within motor nerves of living patients with amyotrophic lateral sclerosis, occurring before axonal degeneration, suggests that this is an early event that may contribute to amyotrophic lateral sclerosis pathogenesis.
Keywords: peripheral nervous system, motor neuron disease, motor neuropathy, neuropathology, aggregates
Riva et al. identify a specific pTDP-43 signature in the peripheral nervous system of ALS patients. The detection of pTDP-43 aggregates within motor nerves of living ALS patients, prior to axonal degeneration, suggests that aggregation is an early event that may contribute to disease pathogenesis.
Introduction
Amyotrophic lateral sclerosis (ALS) is the most common and severe form of motor neuron disease (MND), a group of neurodegenerative disorders characterized by loss of motor neurons in the central brain and spinal cord.1 The absence of specific diagnostic biomarkers is a significant constraint in clinical diagnosis, which still relies on the demonstration of the presence and progression of signs of upper and lower motor neuron dysfunction, as defined by the revised El Escorial Criteria (R-EEC).2 Phenotypic heterogeneity represents a major challenge for the diagnosis of MNDs, in particular for patients presenting with isolated signs of lower motor neuron dysfunction, defined as lower motor neuron syndrome.1,3,4 Indeed, previous studies showed a percentage of misdiagnosis of about 10% in ALS population studies,5,6 rising to 19% when considering patients presenting with a lower motor neuron phenotype.7
We previously showed that in selected cases, motor nerve biopsy may be useful for an early differential diagnosis of patients presenting with atypical lower motor neuron syndrome,3 and for the recognition of certain rare forms of motor neuropathy8,9; however, motor nerve biopsy impact in clinical practice has not been fully assessed. Pathological hallmarks of ALS are the detection of selective motor neuron death, together with evidence of phosphorylated transactive response DNA-binding Protein 43 (pTDP-43)-positive aggregates in the cytoplasm of motor neurons and glia of the CNS.10-12 Additionally, the pivotal role of TDP-43 in the pathogenesis of ALS suggests promise for the development of novel TDP-43-based biomarkers for ALS; however, attempts in this direction have been non-conclusive so far.13,14 Systematic studies investigating the presence and distribution of TDP-43 aggregates within motor nerves are still lacking, despite signs of pTDP-43 pathology having been identified on small cutaneous nerves fibres and skeletal muscles of ALS patients.15–17
Herein, we aim to define the TDP-43/pTDP-43 pathology in diagnostic motor nerve biopsies performed on a large cohort of patients with lower motor neuron syndrome and to assess whether this might be a discriminating tissue biomarker for differentiating between ALS and non-ALS cases.
Materials and methods
Study design and participants
For the purpose of this study, we retrospectively reviewed the disease course of 113 patients who underwent motor nerve biopsy between December 1994 and March 2019. More common indications for motor nerve biopsy referral were a lower motor neuron syndrome, in most cases limited to one body region, often associated with atypical features such as sensory symptoms, anti-ganglioside antibodies or CSF abnormalities (Table 1 and Supplementary Tables 2 and 3). Motor nerve biopsies had been obtained for diagnostic purposes, after informed consent, and were stored in the Institute of Experimental Neurology (INSPE) tissue bank. All experimental protocols were approved by San Raffaele Scientific Institute Ethical Committee (Milan, Italy).
Table 1.
Clinical features of ALS and non-ALS patients at baseline
| Total n = 102 |
ALS n = 71 |
Non-ALS mimics n = 31 |
|
|---|---|---|---|
| Demographic features | |||
| Sex, male | 73 (71.6%) | 49 (69%) | 24 (77.4%) |
| Age of onset, years | 52 (42–63) | 53 (43–65) | 51 (37–63) |
| Age at biopsy, years | 59 (44–67) | 59 (46–66) | 57 (39–69) |
| Disease duration at biopsy, months | 28 (13–51) | 25 (12–45) | 35 (17–88) |
| Clinical findings | |||
| Upper motor neuron score | 0 (0–3) | 1 (0–3) | 0 (0–0) |
| Sensory symptoms/signs | 36 (35.3%) | 18 (25.4%) | 18 (58.1%) |
| Diagnostic work-up | |||
| Abnormal CSF analysis | 15/61 (24.6%) | 10/39 (25.6%) | 5/22 (22.7%) |
| Anti-GM1 IgM antibody | 4/79 (5.3%) | 2/51 (3.9%) | 2/24 (8.3%) |
| Revised El Escorial Criteria | |||
| Possible ALS | 3 (2.9%) | 3 (4.2%) | 0 |
| Probable laboratory-supported ALS | 5 (4.9%) | 5 (7%) | 0 |
Data are presented as n (% of the total column unless specified) or median (IQR). Abnormal CFS albumino-cytological dissociation (protein level >60 mg/ml, cell count <5) in 11/15 cases; raised protein level together with cell count in 4/15.
Patients received a thorough prebiopsy clinical evaluation in five tertiary referral centres for neuromuscular disorders in Italy. Data were retrieved from hospital charts and fulfilment of standard revised El Escorial Criteria. An upper motor neuron score was calculated for each patient, as previously described (range 0–16).18 Disease duration was defined as the interval in months between the first symptom and motor nerve biopsy. Follow-up consisted of retrieval of outpatient/inpatient post-biopsy records and phone contacts; the time from biopsy to the date of last contact or death/tracheostomy was then calculated. Only patients with clinical data at follow-up sufficient to determine final diagnosis were included in the study. The good clinical practice of a complex clinical diagnosis, which included assessment of signs of upper and lower motor neuron dysfunction, ascertainment of a disease progression consistent with ALS and the exclusion of mimicking syndromes, was the reference standard for ALS diagnosis.1 A diagnosis other than ALS was established according to standard clinical criteria for motor neuropathies and other neuromuscular conditions.19–22
Procedures
The anterior motor branch of the obturator nerve was sampled and processed in all patients according to our standardized protocols, as described.3,9 Two independent evaluators, blinded to clinical data, reassessed the nerve biopsy of each patient with a series of at least three complete transverse sections of the nerve sample being analysed for each nerve. Histopathological criteria were applied to all biopsies and assigned to three main diagnostic categories: pathological MND, pathological motor neuropathy and non-diagnostic, as described.3 Doubtful cases were discussed by the study authors (A.Q., F.C., N.R.) in joint sample analysis with a Leica multiviews microscope until a consensus was reached. Inter-reader agreement was evaluated with Cohen’s kappa index. The level of axonal degeneration was morphometrically measured as the number of degenerating fibres/mm2; four groups were then constructed, according to quartiles (low, mild, moderate, severe). Fibre loss, regeneration, myelin pathology and inflammation were evaluated in a semiquantitative scale (none, mild, moderate, severe). A standardized protocol for image acquisition and morphometric measurement was applied, as described.3,23
All motor nerve samples were prescreened for paraffin block availability and for tissue quality and those which passed this first step were included in the study. Immunohistochemistry was performed on a complete transverse section of the nerve sample using commercially available anti-TDP-43 rabbit polyclonal (1:700, 10782–2-AP) and anti-phospho (pS409/410)-TDP-43 rabbit polyclonal (1:500, 22309–1-AP) antibodies. The immunoreactivity was amplified through the avidin-biotin complex (Supplementary material). In a subset of ALS and non-ALS samples (13 versus 13) pTDP-43 accumulation in motor nerves was further confirmed by immunohistochemistry in serial sections stained with anti-ubiquitin mouse monoclonal antibody (1:3000) (Supplementary Fig. 2). The pathological evaluation of TDP-43/pTDP-43 immunohistochemistry focused on the following five nerve areas: perineurial cells, vessels, endoneurial cells, axons and Schwann cells. The signal intensity was assessed according to a semiquantitative rating scale based on the proportion of positive cells over the total for each specific area examined (0 = all negative, 1 = number of positive < number of negative, 2 = number of positive ≈ number of negative, 3 = number of positive > number of negative, 4 ≈ all positive).
Statistical analysis
Data are expressed as n (%) or median (interquartile range, IQR). Descriptive parameters were compared by the chi-squared or Kruskal–Wallis tests, when appropriate. The association of TDP-43 and pTDP-43 positive versus negative staining within each nerve area with ALS was evaluated with the Fisher's exact test with a Bonferroni correction to account for multiple comparisons. The Kaplan–Meier method was used to construct curves of survival from disease onset (overall survival) and time of biopsy (post-biopsy survival) according to the level of axonal degeneration. A Cox multivariate analysis was subsequently applied; age at onset, disease duration at biopsy and revised El Escorial Criteria category, known predictors of survival were considered as covariates.24 Significant P-values, set at <0.05, and their 95% confidence intervals (CI) were reported. SPSS (SPSS Inc., USA) v.22 was used for all analyses.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
Dataset clinical characteristics
Of the 113 patients recorded in our database, 102 had both an informative nerve biopsy and sufficient follow-up, and were included in the study (Fig. 1). Clinical and demographic characteristics of study-patients are presented in Table 1. A final clinical diagnosis of ALS was determined in 71 patients (69.6%). A wide aetiological heterogeneity was observed within the group of non-ALS patients (n = 31, 30.4%) (Table 1 and Supplementary Tables 1 and 2): the most common were inflammatory neuropathies (n = 11, 35.5%), including three cases fulfilling the criteria for multifocal motor neuropathy (MMN, n = 3, 9.7%), one for chronic inflammatory demyelinating polyneuropathy (CIDP, n = 1, 3.2%), one Lewis–Sumner syndrome and one associated with systemic lupus erythematosus, followed by idiopathic neuropathy (n = 11, 35·5%), of which seven sensory-motor neuropathy and four motor neuropathy (n = 4) and myopathy (n = 3, 9.7%), of which two fulfilling the criteria for inclusion body myositis. Rare disease entities included amyloid polyneuropathy (n = 2, 6.5%) and paraneoplastic anti-Hu sensory-motor neuronopathy (n = 1, 3.2%); other clinical conditions were spondylotic myelopathy (n = 2, 6.5%) and femoral nerve entrapment (n = 1, 3.2%).
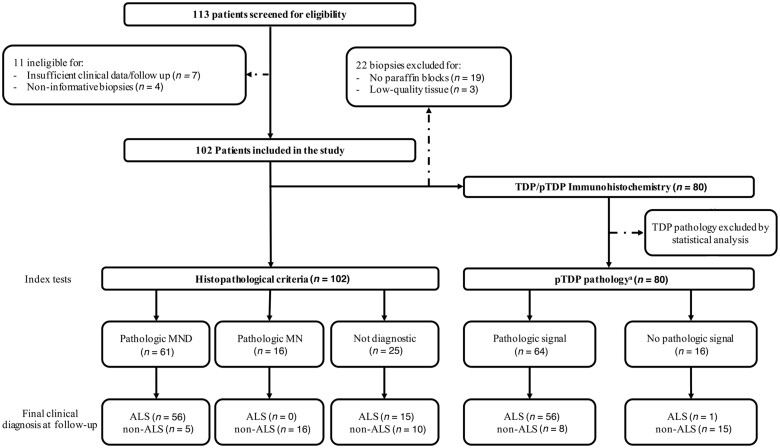
Figure 1.
Study flow chart. The reference standard is final clinical diagnosis at follow-up. *Defined, according to the results of our study, as pTDP-43 accumulation within myelinated fibres (either in axons or Schwann cell cytoplasm).
Histopathology
After evaluation of nerve morphology, 61 of the 102 eligible cases (59.8%) received a histopathological diagnosis of pathological MND (Fig. 1 and Table 2). Of these, 56 (91.8%) cases belonged to the ALS group (Fig. 2A–C). In the remaining five cases (8.2%), we observed signs of chronic axonal damage in two idiopathic motor neuropathies and one spondylotic myelopathy, while myelin ovoids, indicating acute axonal degeneration, were prominent in one amyloid neuropathy, which subsequently tested positive for Congo red stain, and one paraneoplastic anti-Hu sensory-motor neuronopathy, reflecting the subacute clinical course of the disease.8 Sixteen cases (15.7%) received a histopathological diagnosis of pathological motor neuropathy, which was confirmed by clinical follow-up in all cases. In most biopsies we detected signs of myelin pathology, such as small onion bulb formations (n = 8) or inflammation (n = 6), and high density of clusters of regenerating fibres (Fig. 2D–F). One patient had nerve oedema, demyelination and onion bulb formations associated with nerve inflammatory infiltrates, fulfilling the pathological diagnostic criteria for CIDP (Fig. 2G–J).19 Finally, 25 motor biopsies (24.5%) were considered not diagnostic for the reason that they either displayed normal morphology (n = 18, of which 11 with a final diagnosis of ALS), or did not fit any of the criteria for pathological MND or pathological motor neuropathy (n = 7, of which four were ALS). Within the latter subgroup, although one ALS biopsy fulfilled criteria for pathological MND it also showed an inflammatory infiltrate surrounding the wall of a small epineurial vessel, resembling microvasculitis (Fig. 2K–M); however, no fibrinoid necrosis of the vessel wall could be detected and we finally favoured a descriptive diagnosis, classifying this sample as uncertain.25,26 This case and the amyloid neuropathy case required discussion to reach consensus for a final histopathological diagnosis, resulting in an inter-reader agreement of 0.96 (Cohen’s κ; 95% CI: 0.92–1). A Kaplan–Meier analysis showed that in ALS the density of axonal degeneration is significantly associated with reduced overall survival [log-rank (Mantel–Cox) χ2 = 18.74, P> 0.001] and post-biopsy survival [log-rank (Mantel–Cox) χ2 = 9.66, P = 0.022], while multivariate analysis confirmed axonal degeneration as an independent negative factor (P = 0.001 and P = 0.009, respectively) (Supplementary Table 3).
Table 2.
Study cohort final diagnosis and histopathological patterns
| Patients | Histopathology | |||
|---|---|---|---|---|
| Pathological MND | Pathological MN | Not diagnostic | ||
| ALS | 71 (69.6%) | 56 (78.9%) | 0 | 15 (21.1%) |
| Revised El Escorial Criteria at time of biopsy | ||||
| Negative | 63 (88.8%) | 50 (79.4%) | 0 | 13 (20.6%) |
| Possible ALS | 3 (4.2%) | 3 (100%) | 0 | 0 |
| Probable laboratory-supported ALS | 5 (7.0%) | 3 (60.0%) | 0 | 2 (40.0%) |
| Non-ALS | 31 (30.4%) | 5 (16.1%) | 16 (51.6%) | 10 (32.3%) |
| Final diagnosis: | ||||
| Inflammatory neuropathya | 11 (35.5%) | 0 | 10 (90.1%) | 1 (9.9%) |
| Idiopathic neuropathyb | 11 (35.5%) | 2 (18.2%) | 6 (54.5%) | 3 (27.3%) |
| Myopathyc | 3 (9.7%) | 0 | 0 | 3 (100%) |
| Amyloid neuropathy | 2 (6.5%) | 1 (50.0%) | 0 | 1 (50.0%) |
| Spondylotic myelopathy | 2 (6.5%) | 1 (50.0%) | 0 | 1 (50.0%) |
| HU-PSMN | 1 (3.2%) | 1 (100%) | 0 | 0 |
| Femoral nerve entrapment | 1 (3.2%) | 0 | 0 | 1 (100%) |
Histopathological data are expressed as n (% of the total row). HU-PSMN = anti-Hu paraneoplastic sensory-motor neuronopathy. Pathological features consistent with MND were detected in almost 80% of ALS; while none satisfied criteria for pathological motor neuropathy (MN). In the ALS group, not diagnostic samples were either normal (n= 11) or not conclusive (n= 4). Among non-ALS mimics, biopsy highlighted pathological features diagnostic for motor neuropathy in 16 of 26 motor neuropathies of different aetiologies, while in five cases the pathological features suggested an MND.
Three multifocal motor neuropathy (n= 3), CIPD (n= 1), chronic motor axonal neuropathy (n= 1), Lewis–Sumner syndrome (n= 1), systemic lupus erythematosus-associated inflammatory motor neuropathy (n= 1), inflammatory neuropathy, not otherwise specified (n= 4).
Idiopathic sensory-motor neuropathy (n= 7) and four idiopathic motor neuropathy (n= 4).
Including two inclusion body myositis;.
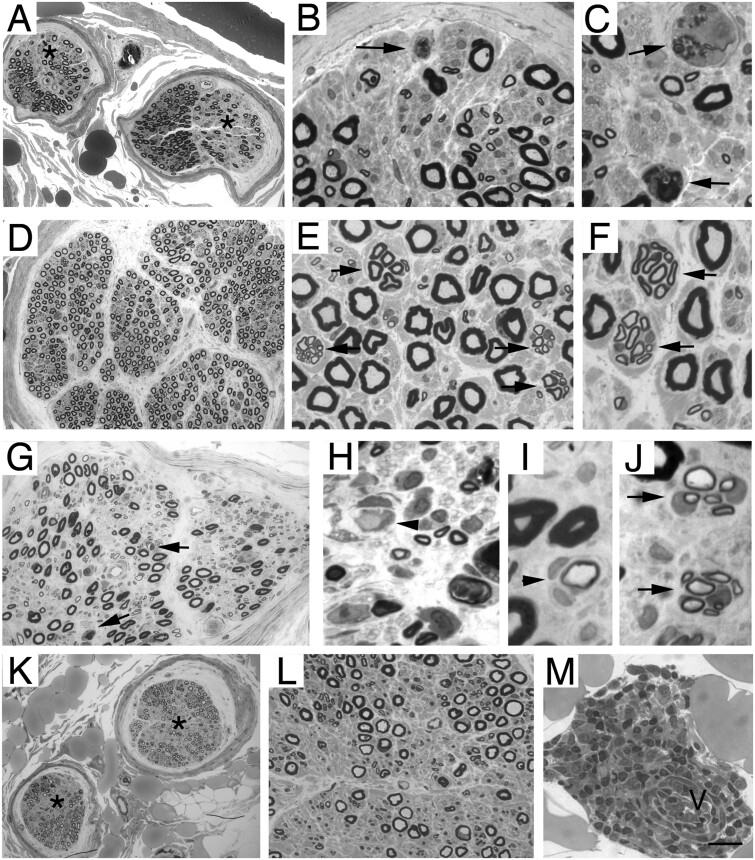
Figure 2.
Representative neuropathological cases. Transverse semi-thin sections of biopsy of motor nerve from an ALS case and motor neuropathy patients. In ALS patients (A–C), focal decreased density of myelinated nerve fibres (A: asterisks) is evident in two nerve fascicles. At higher magnification (B and C), axonal degeneration (arrows). (D–F) Diffuse mild reduction of myelinated nerve fibres is present in a representative section from patients with definite diagnosis of motor neuropathy; there are many clusters of small myelinated fibres (E and F, arrows), indicating axonal regeneration. (G–J) Transverse section of nerve from a patient with motor CIDP: reduction of myelinated nerve fibres (G) associated with axonal degeneration (G, arrows) demyelination (H, arrowhead), small onion bulbs (I, arrowhead), indicating remyelination and clusters of small myelinated fibres (J, arrows), indicating axonal regeneration. (K–M) Transverse sections of nerve from a patient with a final diagnosis of ALS: focal decreased density of myelinated nerve fibres (K: asterisks) is evident in two nerve fascicles; perivascular inflammatory infiltration (M) surrounds an epineurial blood vessel (M, v). Scale bar in M = 50 μm (A, D, G and K) ; 20 μm (B, E, H, L and M); and 10 μm (C, F, I and J).
TDP-43 and pTDP-43 pathology in motor nerves
Of the 102 cases included in the study, 22 paraffin blocks were either unavailable or showed low tissue quality (Fig. 1), therefore, 80 patients (57 ALS and 23 non-ALS) were included in the TDP-43 and pTDP-43 analysis. TDP-43 confirmed its ubiquitous physiological nuclear staining, present in all cases of both disease groups and particularly expressed in endoneurial, perineurial and vessel-wall nuclei (Fig. 3A–C). Focusing on myelinated fibres, we identified TDP-43 accumulation in a higher proportion of ALS compared with non-ALS patients, a difference that, however, did not reach statistical significance: in ALS patients we identified TDP-43 accumulation in Schwann cells cytoplasm in 38 cases (66.7%) and in the axon in 26 ALS samples (45.6%), while in non-ALS patients TDP-43 accumulation was detected in Schwann cells cytoplasm in eight cases (34.8%) and in the axon in five samples (21.7%) (Supplementary Tables 1, 2 and 4). The differences between ALS and non-ALS cases were significantly more pronounced when analysing pTDP-43 expression in myelinated fibres: axonal accumulation was detected in 56 ALS cases (98.2%) versus seven in non-ALS samples (30.4%) (P < 0.0001), while concomitant positive Schwann cell cytoplasmic staining was found in 40 ALS patients (70.2%) versus four non-ALS cases (17.4%) (P < 0.001) (Fig. 3 D–H and Supplementary Tables 1, 2 and 4). Therefore, the best predictors for a diagnosis of ALS were pTDP-43 accumulation in axons and Schwann cell cytoplasm, as detailed next. In a minority of the non-ALS cases, however, we observed abnormal pTDP-43 accumulation within myelinated fibres, in Schwann cell cytoplasm in four cases and in the axon in seven cases (eight cases in total; 34.8%), including idiopathic motor neuropathy (n = 4), myopathy (n = 3) and amyloid neuropathy (n = 1) (Supplementary Tables 1, 2 and 4).
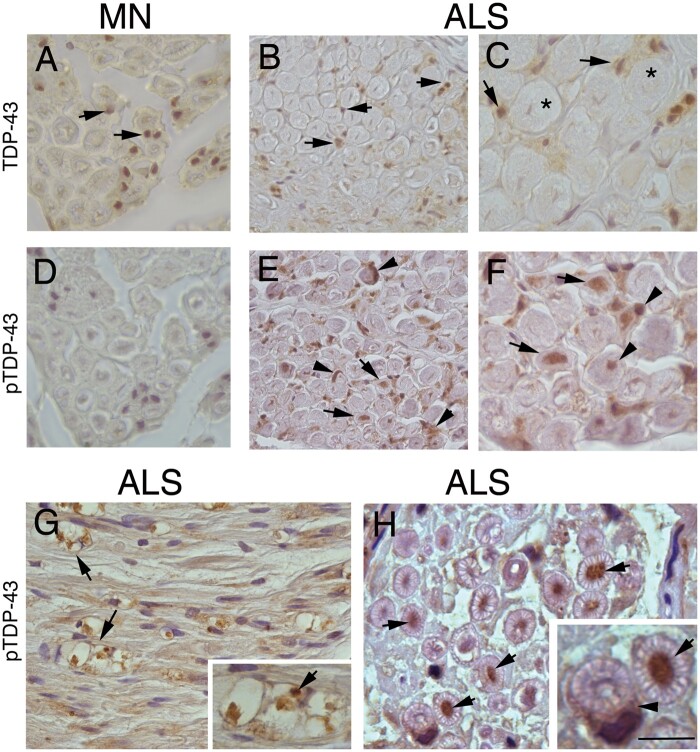
Figure 3.
Immunohistochemistry localization of TDP-43 and pTDP-43 in motor nerve biopsies. TDP-43 immunoreactivity is present in the endoneurium in nuclei of cells, including Schwann cells (A–C, arrows), in both motor neuropathy (A, arrows) and ALS nerves (B and C, arrows); in C, asterisks mark two large myelinated nerve fibres. (D) Staining for pTDP-43 is negative in motor neuropathy; in ALS patients with normal morphology at biopsy, pTDP-43 immunoreactivity is present in cytoplasm of Schwann cells (E and F, arrowheads) and axons (E and F, arrows). In biopsy-proven ALS with features of axonal degeneration, pTDP-43 is expressed in ovoids (G, arrows) indicating axonal degeneration and axons (H, arrows); two large myelinated nerve fibres showing strong pTDP-43 immunoreactivity in a different pattern: axon (H, high magnification, arrow) and Schwann cell (H, high magnification, arrowhead). Scale bar = 50 μm (A–D); 30 μm (E–H); and 10 μm (inset in G and H).
Notably, when focusing on the 11 ALS cases whose motor biopsies showed normal features at standard histopathology analysis, we were able to demonstrate a positive immunohistochemistry pTDP-43 signal in the axons of all samples (100%) and in Schwann cells cytoplasm in six ALS cases (54.5%), suggesting that these findings may represent an early pathological event, preceding axonal degeneration and fibres loss. Indeed, Kaplan–Meier analysis showed no association between of pTDP-43 pathology and ALS survival. In the non-ALS group, we demonstrated a positive pTDP-43 signal in axons and in Schwann cells cytoplasm in respectively four (50.0%) and two (25.0%) cases of the eight biopsies showing normal or specific features at standard histopathology analysis. Specifically, one patient with amyloid neuropathy showed pTDP-43 aggregates in both axons and Schwann cells, three patients with myopathy (of which two fulfilled the criteria for inclusion body myositis) showed pTDP-43 aggregates in axons, while in one idiopathic motor neuropathy patient pTDP-43 was detected in Schwann cells.
Discussion
Here we provide first evidence that pathological pTDP-43 accumulates within the motor nerves of living ALS patients from the diagnostic stages and may be considered to be a tissue biomarker for the diagnosis of ALS. Notably, all ALS motor nerves with a normal morphology at standard histopathological examination displayed signs of pTDP-43 aggregation, suggesting that this is an early event in the disease course that may have potential pathogenetic implications.
The present study of a large retrospective cohort of patients with lower motor neuron syndrome confirms that motor nerve biopsy may be a useful add-on tool for the aetiological diagnosis of difficult cases presenting with a lower motor neuron syndrome.3 More recently, there have been significant advances in neurophysiology, imaging, ALS genetics and the development of fluid biomarkers.27–29 Additionally, the presence of cognitive and behavioural changes, now known to occur in up to 50% cases of ALS, and the association of ALS with the frontotemporal lobar degeneration (FTLD) spectrum have now been clearly recognized.30 Therefore, there is increasing awareness of the limitations and weaknesses of the El Escorial Criteria,31 their 2000 revision2 as well as the Awaji amendment,32 with particular reference to their lack of sensitivity. The new Gold Coast criteria for ALS have, therefore, been recently proposed, to overcome these limitations.33 These criteria at this stage do not include the presence or absence of specific mutations or cognitive and behavioural changes; however, they would be able to capture patients who have pure lower motor neuron forms of ALS with the involvement of at least two body regions.33 Within this changing context, even if these criteria still require validation, motor nerve biopsy may be considered in a limited number of difficult cases presenting with a lower motor neuron syndrome. It is also important to acknowledge that while motor nerve biopsy can support a pathological diagnosis of MND (pathological MND), the diagnosis of ALS remains clinical, and can usually be achieved through a combination of careful history taking, clinical examination, electrophysiological testing, laboratory studies, imaging and close follow-up, without the necessity of performing a nerve or muscle biopsy.3,33 Identification of specific pathological features also allows the recognition of potentially treatable and rare disorders, such as motor CIDP or amyloid neuropathy.8,9 Interestingly, in one ALS patient the motor nerve biopsy showed signs of inflammation, an intriguing feature that was previously observed in sural nerve biopsies of ALS patients with sensory deficits.34 The pathogenic implication of these findings, however, are not clear,34–36 and were not replicated in our case-series. Our study additionally showed that myelin ovoids, indicating ongoing axonal degeneration, proved to be associated with poor survival in MND, independently from other previously reported prognostic factors.24 Importantly, we demonstrated a pathological pTDP-43 signature occurring before axonal degeneration in ALS motor nerves obtained for diagnostic purposes. This observation is unprecedented, but consistent with previous post-mortem studies in the CNS that established cytoplasmic aggregation and phosphorylation of TDP-43 in the brain and spinal cord as a pathological hallmark of ALS.10 We therefore further investigated the diagnostic sensibility and specificity of pTDP-43 aggregates in motor nerve biopsies and found that testing for pTDP-43 pathology in myelinated fibres structures revealed high sensitivity and specificity and was able to reliably distinguish between ALS and non-ALS mimic disorders, including inflammatory motor neuropathy. We, however, found some positive cases in some specific non-ALS neuromuscular disorders, such as inclusion body myositis and idiopathic motor neuropathy. Specifically, pTDP-43 pathology is detected in over 70% of inclusion body myositis muscle biopsies,21 and patients with mutated valosin containing protein (VCP), an ALS-linked gene, may develop either ALS or inclusion body myositis with frontotemporal dementia, highlighting a link between these disorders. We additionally observed pathological pTDP-43 signature in four patients with a final diagnosis of idiopathic motor neuropathy. While this finding limits the diagnostic potential of the technique it, more importantly, highlights potential shared pathogenic mechanisms of motor neuron/axonal degeneration.26 Unfortunately, the clinico-pathological diagnosis was not complemented by an etiological or molecular characterization; this is unsurprising, since currently known disease-related genes cover only up to one-third of hereditary motor neuropathy cases leaving as a consequence the majority as idiopathic.37 Intriguingly, an altered TDP-43-dependent splicing function was previously observed in the muscle tissue of a patient with distal hereditary motor neuropathy and myofibrillar myopathy mutated for heat shock protein family B8 (HSPB8).38 Additionally, increased levels of HSPB8 protein, a molecular chaperone, protected against TDP-43-mediated toxicity and extended survival in the hSOD1G93A ALS mice.39 We also observed a peripheral pTDP-43 pathology in amyloid motor neuropathy8; however, further studies are needed to confirm the potential links between pTDP-43 pathology and amyloidosis.
The demonstration of pTDP-43 aggregates within motor nerves of ALS patients at the diagnostic stages of disease as well as in cases displaying normal features at standard histopathological analysis, before the occurrence of axonal degeneration, leads to the speculation that they might also exert a pathogenetic effect.26 Motor neurons are extremely polarized cells and the length of axons by far exceeds the dimension of the motor neuron cell body. Hence, a better comprehension of the molecular events occurring in the peripheral nervous system could be crucial for a better understanding of ALS pathogenic mechanisms.26 Axonal transport of RNA granules is disrupted by ALS-linked TDP-43 mutations,40 and may deprive distal compartments, including neuromuscular junctions, of mRNAs essential for local function, making them susceptible to neurodegeneration. A strict control of TDP-43 concentration and localization is essential for axonal homeostasis. Increased levels of axonal TDP-43 were observed following axotomy,41 suggesting a role in nerve regeneration while overexpression of wild-type or mutated p.Gly348Cys TDP-43 in mice led to prolonged cytoplasmic translocation, negatively affecting axonal regeneration and impairing axonal sprouting.42 Interestingly, we also showed that pTDP-43 accumulates in the cytoplasm of Schwann cells, suggesting a role is played by peripheral glia in the disease pathogenesis, in line with recent findings showing a non-cell autonomous pathogenic nature of the disease.43 Schwann cells are essential for axonal homeostasis and repair after injury, and Schwann cells impairment has been reported in ALS,44 although their contribution to disease still needs to be clarified. In the CNS, pathological pTDP-43 aggregates have been found in oligodendrocytes in both grey and white matter of the spinal cord, even preceding inclusions in motor neurons and potentially exerting a negative effect on cellular functioning and survival.43,45 Similarly, pTDP-43 aggregates in Schwann cells may hamper their critical role for axonal homeostasis.
This study has some limitations. The retrospective design and recruitment from tertiary referral centres may have indeed biased our study population. Furthermore, our analysis does not include healthy controls, due to its clinically based retrospective study design. A prospective study including a larger population of both ALS and potentially treatable ALS mimicking conditions would be indeed ideally needed to confirm our results. Moreover, motor nerve biopsy, being a technique requiring specific surgical skills and experience to guarantee low complication rates and overall high-quality standards, is a diagnostic procedure with limited clinical indications. The development of a widely accessible specific TDP-43-based tissue or wet biomarker is urgently needed for ALS. Notwithstanding these limitations, we were able to study the specific motor nerve ALS endophenotype, including axons, which represent >95% of the total cellular volume of motor neurons. It is important to point out that further studies are needed to unravel the potential pathogenetic implications of pTDP-43 aggregates within the peripheral nervous system in ALS pathogenesis.
In conclusion, we demonstrated a specific pTDP-43 signature in the peripheral nervous system of ALS patients, that may be exploited as a specific, accessible tissue biomarker. The combined evaluation of pTDP-43 and histopathology was effective as an add-on test in differentiating ALS from non-ALS mimics.
With the hoped-for advent of more effective treatments, an early diagnosis will be of increasing and utmost importance, since the efficacy of any treatment would be maximized if started at the earlies stages of disease and prior to the occurrence of axonal degeneration. Finally, our findings open the way to the hypothesis that pTDP-43 aggregates within the peripheral nervous system may represent a novel therapeutic target for innovative strategies aimed at preventing ALS-linked axonal degeneration.
Funding
This work was supported by ARiSLA, Italian Research Foundation for ALS (ExAlta), the Italian Ministry of Health (RF-2019–12369320) and Giovanni Marazzina Foundation.
Competing interests
M.F. is Editor-in-Chief of the Journal of Neurology and Associate Editor of Neurological Sciences, received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla and ARiSLA (Fondazione Italiana di Ricerca per la SLA). All other authors report no competing interests related to this paper.
Supplementary Material
Abbreviations
- ALS
amyotrophic lateral sclerosis
- CIDP
chronic inflammatory demyelinating polyneuropathy
- MND
motor neuron disease
- TDP-43
transactive response DNA-binding protein 43
References
- 1. van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet. 2017;390(10107):2084–2098. [DOI] [PubMed] [Google Scholar]
- 2. Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. [DOI] [PubMed] [Google Scholar]
- 3. Riva N, Iannaccone S, Corbo M, et al. Motor nerve biopsy: Clinical usefulness and histopathological criteria. Ann Neurol. 2011;69(1):197–201. [DOI] [PubMed] [Google Scholar]
- 4. Garg N, Park SB, Vucic S, et al. Differentiating lower motor neuron syndromes. J Neurol Neurosurg Psychiatry. 2017;88(6):474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davenport RJ, Swingler RJ, Chancellor AM, Warlow CP.. Avoiding false positive diagnoses of motor neuron disease: Lessons from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry. 1996;60(2):147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O.. Amyotrophic lateral sclerosis mimic syndromes: A population-based study. Arch Neurol. 2000;57(1):109–113. [DOI] [PubMed] [Google Scholar]
- 7. Visser J, van den Berg-Vos RM, Franssen H, et al. Mimic syndromes in sporadic cases of progressive spinal muscular atrophy. Neurology. 2002;58(11):1593–1596. [DOI] [PubMed] [Google Scholar]
- 8. Quattrini A, Nemni R, Sferrazza B, et al. Amyloid neuropathy simulating lower motor neuron disease. Neurology. Aug 1998;51(2):600–602. [DOI] [PubMed] [Google Scholar]
- 9. Riva N, Gallia F, Iannaccone S, et al. Chronic motor axonal neuropathy. J Peripher Nerv Syst. 2011;16(4):341–346. [DOI] [PubMed] [Google Scholar]
- 10. Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74(1):20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mackenzie IR, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61(5):427–434. [DOI] [PubMed] [Google Scholar]
- 12. Hasegawa M, Arai T, Nonaka T, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64(1):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feneberg E, Gray E, Ansorge O, Talbot K, Turner MR.. Towards a TDP-43-based biomarker for ALS and FTLD. Mol Neurobiol. 2018;55(10):7789–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Majumder V, Gregory JM, Barria MA, Green A, Pal S.. TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: A systematic review and meta-analysis. BMC Neurol. 2018;18(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cykowski MD, Powell SZ, Appel JW, Arumanayagam AS, Rivera AL, Appel SH.. Phosphorylated TDP-43 (pTDP-43) aggregates in the axial skeletal muscle of patients with sporadic and familial amyotrophic lateral sclerosis. Acta Neuropathol Commun. 2018;6(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ren Y, Liu W, Li Y, et al. Cutaneous somatic and autonomic nerve TDP-43 deposition in amyotrophic lateral sclerosis. J Neurol. 2018;265(8):1753–1763. [DOI] [PubMed] [Google Scholar]
- 17. Mori F, Tada M, Kon T, et al. Phosphorylated TDP-43 aggregates in skeletal and cardiac muscle are a marker of myogenic degeneration in amyotrophic lateral sclerosis and various conditions. Acta Neuropathol Commun. 2019;7(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turner MR, Cagnin A, Turkheimer FE, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: An [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15(3):601–609. [DOI] [PubMed] [Google Scholar]
- 19. Van den Bergh PYK, Hadden RDM, Bouche P, et al. ; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. Eur J Neurol. 2010;17(3):356–363. [DOI] [PubMed] [Google Scholar]
- 20. Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of multifocal motor neuropathy. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripheral Nervous Syst. 2010;15(4):295–301. [DOI] [PubMed] [Google Scholar]
- 21. Weihl CC, Temiz P, Miller SE, et al. TDP-43 accumulation in inclusion body myopathy muscle suggests a common pathogenic mechanism with frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2008;79(10):1186–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dimachkie MM, Barohn RJ.. Inclusion body myositis. Semin Neurol. 2012;32(3):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riva N, Domi T, Lopez ID, et al. The brachial plexus branches to the pectoral muscles in adult rats: Morphological aspects and morphometric normative data. Front Neuroanat. 2012;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calvo A, Moglia C, Lunetta C, et al. Factors predicting survival in ALS: A multicenter Italian study. J Neurol. 2017;264(1):54–63. [DOI] [PubMed] [Google Scholar]
- 25. Collins MP, Dyck PJB, Hadden RDM.. Update on classification, epidemiology, clinical phenotype and imaging of the nonsystemic vasculitic neuropathies. Curr Opin Neurol. 2019;32(5):684–695. [DOI] [PubMed] [Google Scholar]
- 26. Gentile F, Scarlino S, Falzone YM, et al. The peripheral nervous system in amyotrophic lateral sclerosis: Opportunities for translational research. Front Neurosci. 2019;13:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falzone YM, Russo T, Domi T, et al. Current application of neurofilaments in amyotrophic lateral sclerosis and future perspectives. Review . Neural Regen Res. 2021;16(10):1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riva N, Agosta F, Lunetta C, Filippi M, Quattrini A.. Recent advances in amyotrophic lateral sclerosis. J Neurol. 2016;263(6):1241–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turner MR. Progress and new frontiers in biomarkers for amyotrophic lateral sclerosis. Biomark Med. 2018;12(7):693–696. [DOI] [PubMed] [Google Scholar]
- 30. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3-4):153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial ‘Clinical limits of amyotrophic lateral sclerosis’ workshop contributors. J Neurol Sci. 1994;124:96–107. [DOI] [PubMed] [Google Scholar]
- 32. de Carvalho M, Dengler R, Eisen A, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119(3):497–503. [DOI] [PubMed] [Google Scholar]
- 33. Shefner JM, Al-Chalabi A, Baker MR, et al. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol. 2020;131(8):1975–1978. [DOI] [PubMed] [Google Scholar]
- 34. Devigili G, Uceyler N, Beck M, et al. Vasculitis-like neuropathy in amyotrophic lateral sclerosis unresponsive to treatment. Acta Neuropathol. 2011;122(3):343–352. [DOI] [PubMed] [Google Scholar]
- 35. Nardo G, Trolese MC, Verderio M, et al. Counteracting roles of MHCI and CD8(+) T cells in the peripheral and central nervous system of ALS SOD1(G93A) mice. Mol Neurodegener. 2018;13(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kano O, Beers DR, Henkel JS, Appel SH., WNL. Peripheral nerve inflammation in ALS mice: Cause or consequence. Neurology. 2012;78(11):833–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bansagi B, Griffin H, Whittaker RG, et al. Genetic heterogeneity of motor neuropathies. Neurology. 2017;88(13):1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cortese A, Laura M, Casali C, et al. Altered TDP-43-dependent splicing in HSPB8-related distal hereditary motor neuropathy and myofibrillar myopathy. Eur J Neurol. 2018;25(1):154–163. [DOI] [PubMed] [Google Scholar]
- 39. Crippa V, Sau D, Rusmini P, et al. The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet. 2010;19(17):3440–3456. [DOI] [PubMed] [Google Scholar]
- 40. Alami NH, Smith RB, Carrasco MA, et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81(3):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moisse K, Volkening K, Leystra-Lantz C, Welch I, Hill T, Strong MJ.. Divergent patterns of cytosolic TDP-43 and neuronal progranulin expression following axotomy: Implications for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009;1249:202–211. [DOI] [PubMed] [Google Scholar]
- 42. Swarup V, Audet JN, Phaneuf D, Kriz J, Julien JP.. Abnormal regenerative responses and impaired axonal outgrowth after nerve crush in TDP-43 transgenic mouse models of amyotrophic lateral sclerosis. J Neurosci. 2012;32(50):18186–18195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee J, Hyeon SJ, Im H, Ryu H, Kim Y, Ryu H.. Astrocytes and microglia as non-cell autonomous players in the pathogenesis of ALS. Exp Neurobiol. 2016;25(5):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arbour D, Tremblay E, Martineau E, Julien JP, Robitaille R.. Early and persistent abnormal decoding by glial cells at the neuromuscular junction in an ALS model. J Neurosci. 2015;35(2):688–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang SH, Li Y, Fukaya M, et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16(5):571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.