Abstract
Traumatic brain injury is an important risk factor for development of Alzheimer’s disease and dementia. Unfortunately, no effective therapies are currently available for prevention and treatment of the traumatic brain injury-induced Alzheimer’s disease-like neurodegenerative disease. This is largely due to our limited understanding of the mechanisms underlying traumatic brain injury-induced neuropathology. Previous studies showed that pharmacological inhibition of monoacylglycerol lipase, a key enzyme degrading the endocannabinoid 2-arachidonoylglycerol, attenuates traumatic brain injury-induced neuropathology. However, the mechanism responsible for the neuroprotective effects produced by inhibition of monoacylglycerol lipase in traumatic brain injury remains unclear.
Here we first show that genetic deletion of monoacylglycerol lipase reduces neuropathology and averts synaptic and cognitive declines in mice exposed to repeated mild closed head injury. Surprisingly, these neuroprotective effects result primarily from inhibition of 2-arachidonoylglycerol metabolism in astrocytes, rather than in neurons. Single-cell RNA-sequencing data reveal that astrocytic monoacylglycerol lipase knockout mice display greater resilience to traumatic brain injury-induced changes in expression of genes associated with inflammation or maintenance of brain homeostasis in astrocytes and microglia. The monoacylglycerol lipase inactivation-produced neuroprotection is abrogated by deletion of the cannabinoid receptor-1 or by adeno-associated virus vector-mediated silencing of astrocytic peroxisome proliferator-activated receptor-γ. This is further supported by the fact that overexpression of peroxisome proliferator-activated receptor-γ in astrocytes prevents traumatic brain injury-induced neuropathology and impairments in spatial learning and memory.
Our results reveal a previously undefined cell type-specific role of 2-arachidonoylglycerol metabolism and signalling pathways in traumatic brain injury-induced neuropathology, suggesting that enhanced 2-arachidonoylglycerol signalling in astrocytes is responsible for the monoacylglycerol lipase inactivation-produced alleviation of neuropathology and deficits in synaptic and cognitive functions in traumatic brain injury.
Keywords: 2-arachidonoylglycerol, monoacylglycerol lipase, peroxisome proliferator-activated receptor-γ, neurodegeneration, Alzheimer’s disease
Hu et al. show that augmentation of 2-AG signalling in astrocytes alleviates TBI-induced neuropathology in mice, along with observed declines in synaptic and cognitive function. Inactivation of the enzyme that degrades 2-AG in astrocytes could have therapeutic potential for TBI-induced neuropathology.
See Loane (doi:10.1093/brain/awac014) for a scientific commentary on this article.
Introduction
Traumatic brain injury (TBI) resulting from external physical forces that cause damage to the brain is a serious worldwide public health problem. The severity of a TBI may range from mild to moderate to severe. Mild TBI, which includes concussion/subconcussion, accounts for ∼80% of all TBIs seen in the clinic.1,2 Repeated mild brain injury may lead to neurodegenerative disease.2,3 The features of the clinical symptoms and neuropathological changes in TBI are similar to those seen in Alzheimer’s disease,4-7 suggesting a close link between TBI and Alzheimer’s disease.8,9 Unfortunately, no effective therapies are currently available for prevention and treatment of TBI-induced Alzheimer’s disease-like neurodegenerative disease.4,10 This is largely due to our limited understanding of the mechanisms underlying TBI-induced neuropathology.
The endocannabinoid system, which consists of endocannabinoids, endocannabinoid receptors (CB1R and CB2R) that are targeted by Δ9-tetrahydrocannabinol (the primary psychoactive ingredient in marijuana), the enzymes that synthesize and degrade endocannabinoids, and transporters, is involved in a variety of physiological, pharmacological, and pathological processes. 2-Arachidonoylglycerol (2-AG), which is primarily synthesized from diacylglycerol by diacylglycerol lipases (DAGLα and β), is the most abundant endogenous cannabinoid displaying anti-inflammatory and neuroprotective properties in response to proinflammatory, excitotoxic and mechanical insults.11–15 Earlier studies showed that 2-AG is neuroprotective against closed head injury (CHI) and attenuates TBI-induced neuropathology,11 indicating that 2-AG is an important endogenous signalling mediator made ‘on demand’ that maintains brain homeostasis against harmful insults. However, 2-AG is a bioactive lipid mediator and is rapidly degraded upon its formation by several enzymes, including monoacylglycerol lipase (MAGL), α/β hydrolase domain-containing protein 6 and 12 (ABHD6/12), and cyclooxygenase-2 (COX-2). Although these enzymes are capable of degrading 2-AG, it has been estimated that 85% of 2-AG in the brain is hydrolysed by MAGL.16,17 The immediate metabolite of 2-AG is arachidonic acid, a precursor of both prostaglandins and leukotrienes catalysed through the enzymes COX-1/2 and arachidonate 5-lipoxygenase (LOX), respectively. Prostaglandins (i.e. PGE2) and leukotrienes appear to be proinflammatory mediators in neurodegenerative diseases.18,19 This suggests that inactivation of MAGL, by enhancing anti-inflammatory and neuroprotective 2-AG signalling while reducing proinflammatory and neurotoxic eicosanoid levels, could be a promising strategy as a therapy for inflammatory and neurodegenerative diseases.19,20 Indeed, previous studies show that pharmacological inhibition of MAGL resolves neuroinflammation and attenuates neuropathology as well as improves synaptic and cognitive functions in several animal models for brain disorders, including Alzheimer’s disease, amyotrophic lateral sclerosis, epilepsy, multiple sclerosis and Parkinson’s disease.12,20–24 The results from several recent studies provide further evidence that pharmacological inhibition of MAGL decreases neuropathological changes and improves synaptic plasticity, spatial learning and memory following mild TBI.25–28 However, the mechanisms by which pharmacological inactivation of MAGL exhibits neuroprotective effects in TBI are largely unclear.
Here we show that global deletion of MAGL significantly reduces neuroinflammation, neurodegeneration, TAR DNA-binding protein 43 (TDP-43) overproduction and phosphorylated tau (p-tau), and prevents synaptic and cognitive declines in animals exposed to repeated mild CHI (mCHI). These neuroprotective effects by global deletion of MAGL apparently result from inhibition of 2-AG metabolism in astrocytes, as the protective effects occur primarily in mice lacking MAGL in astrocytes, rather than in neurons. Single-cell RNA-sequencing (scRNA-seq) data reveal that astrocytic MAGL knockout mice exhibit resilience to TBI-induced changes in expression of genes associated with inflammation in astrocytes and microglia. Because the pharmacological MAGL inhibition-produced protective effects are diminished in mice deficient in CB1R, it is likely that the MAGL inactivation-produced beneficial effects are a result of enhanced 2-AG signalling. In addition, we provide evidence that neuroprotection by inactivation of astrocytic MAGL in TBI is primarily mediated via astrocytic 2-AG and downstream peroxisome proliferator-activated receptor-γ (PPARγ) signalling that inhibits nuclear factor-κB (NF-κB). Our results reveal a previously unidentified cell type-specific role of 2-AG metabolism and signalling pathways in TBI-induced neuropathology and synaptic and cognitive declines, suggesting that augmentation of 2-AG signalling in astrocytes promotes functional recovery from TBI.
Materials and methods
Animals
Mgll flox/flox mice were generated by the Texas A&M Institute for Genomic Medicine (Supplementary Figs 1 and 2A). A detailed description of generation of cell type-specific MAGL knockout mice is provided in the Supplementary material. The protocol for injection of 4-nitrophenyl-4-[bis(1,3-benzodioxol-5-yl)(hydroxy)methyl]piperidine-1-carboxylate (JZL184) was the same as described previously12,22,25,29 (Supplementary Fig. 2C).
Cell culture
Primary hippocampal neurons (astroglial cells < 2%) from embryos [embryonic Day 18 (E18)] and astrocytes (astrocytes > 95%) from postnatal Day 4–5 (P4–5) pups of wild-type, total MAGL knockout (tKO), neuronal MAGL knockout (nKO) and astrocytic MAGL knockout (aKO) were cultured as described previously13,22,30–32 (Supplementary material).
Repeated mild closed head injury
Induction of repeated mCHI with three impacts in mice was the same as described previously25 (Supplementary material and Supplementary Fig. 2).
Hippocampal slice preparation
Hippocampal slices were prepared from mice as described previously12,22,30,32 (Supplementary material).
Electrophysiological recordings
Long-term potentiation at CA3–CA1 synapses was recorded as described previously12,22,25,30,32 (Supplementary material).
Western blots
Expression of proteins in brain tissues by western blot assay was detected as described previously12,13,22,25,30 (Supplementary material).
Reverse transcription and real-time polymerase chain reaction
RNA expression of Magl, I1b, Il6, tumor necrosis factor α (Tnfa) and vimentin (Vim) in brain tissues was detected as described previously22,25 (Supplementary material).
Immunohistochemistry
Immunofluorescence analysis was performed to assess amyloid precursor protein (APP), MAGL, TDP-43, p-tau, Iba1 and GFAP in coronal brain sections as described previously12,22,25,30 (Supplementary material).
Histochemistry
Degenerating neurons were detected using Fluoro-Jade® C as described previously12,25 (Supplementary material).
Luciferase activity assay
PPARγ activity was assessed in NG108-15 cells transfected with pCMX-Gal-LBD-mPPARγ and TK-MH100x4-luc vectors, as described previously22 (Supplementary material).
Single-cell RNA sequencing
10x Genomics’ scRNA-seq technology was used to detect expression of genes in Mglllox/lox-non cre (wild-type), nKO, and aKO mice that received three impacts. Detailed single-cell suspension preparation, scRNA-seq library preparation, sequencing and data analysis are provided in the Supplementary material.
Morris water maze
The classic Morris water maze test was used to assess spatial learning and memory, as described previously12,22,24,25,30 (Supplementary material).
Adeno-associated viruses injection
Wild-type or aKO mice at 2 months of age were stereotaxically injected with adeno-associated viruses (AAV) AAV5-GFAP-eGFP-m-PPARγ-shRNAmir(2), AAV5-GFAP-h-PPARγ-FLAG-WPRE or AAV5-GFAP-eGFP control vectors, as described previously.22,32 Mice received repeated mCHI 30 days following AAV injections and all assessments were made 30 days after TBI (Supplementary material and Supplementary Fig. 2D).
Liquid chromatography/mass spectrometry
The content of 2-AG in brain tissues from wild-type, tKO, nKO and aKO mice were detected using liquid-chromatography–tandem mass spectrometry, as described previously33 (Supplementary material).
Experimental design
The experimental study design and procedures and protocols are provided in Supplementary Fig. 2.
Data analysis
Results are presented as mean ± standard error of the mean (SEM). Unless stated otherwise, one or two-way ANOVA followed by post hoc tests were used for statistical comparison when appropriate. Differences were considered significant when P < 0.05.
Data availability
The data supporting the findings of this manuscript are available from the corresponding authors upon request. The scRNA-seq data have been deposited in the NCBI Gene Expression Omnibus under accession number GSE178226.
Results
Generation and characterization of MAGL conditional knockout mice
Previous studies shown that pharmacological inhibition of MAGL with JZL184 alleviates TBI-induced neuropathology.25–27 To determine whether genetic deletion of MAGL reduces TBI-induced neuropathology similar to pharmacological inhibition,25–27 and whether the MAGL-inactivation-produced neuroprotective effects in TBI are cell type-specific, in collaboration with the Texas A&M Institute for Genomic Medicine, we created Mgllflox/flox mice using the strategy shown in Supplementary Figs 1 and 2A. We used these mice to generate cell type-specific MAGL knockout mice, including tKO, nKO and aKO MAGL knockout mouse lines (Supplementary material We did not generate microglial MAGL knockout mice for the present study because MAGL in microglial cells does not play a significant role in degrading 2-AG. As demonstrated previously, the amount of 2-AG generated in microglial cells contributes an insignificant proportion of 2-AG to the overall 2-AG pool in the brain, and deletion of MAGL in microglial cells does not alter the brain 2-AG content,34 suggesting that MAGL in microglial cells may not play an important role in degrading 2-AG. This assumption was confirmed by a recent study where it was shown that 2-AG in microglial cells is primarily hydrolysed by ABHD12.35
Selective deletion of MAGL in tKO, nKO and aKO mice was first verified by immunoblot in vitro. As shown in Supplementary Fig. 3A, expression of MAGL was not detectable in cultured hippocampal neurons from tKO and nKO mice, but was seen in cultured hippocampal neurons from wild-type and aKO mice. Similarly, expression of MAGL was not detectable in cultured astrocytes from tKO and aKO mice, but was detected in cultured astrocytes from wild-type and nKO mice. These results indicate that MAGL was conditionally deleted. The cell type-specific deletion of MAGL was further confirmed in vivo by immunoblot (Supplementary Fig. 3B), quantitative polymerase chain reaction (qPCR; Supplementary Fig. 3C) and immunostaining (Supplementary Fig. 3D–F). In addition, no developmental or neurobehavioural changes were reported previously in cell type-specific MAGL knockout mice.34 No obvious developmental abnormalities or locomotor deficits were observed in the various MAGL knockout mice at ages (2–3 months) used in the present study (Supplementary Fig. 3G).
Inactivation of MAGL in astrocytes reduces neuroinflammation following repeated mild closed head injury
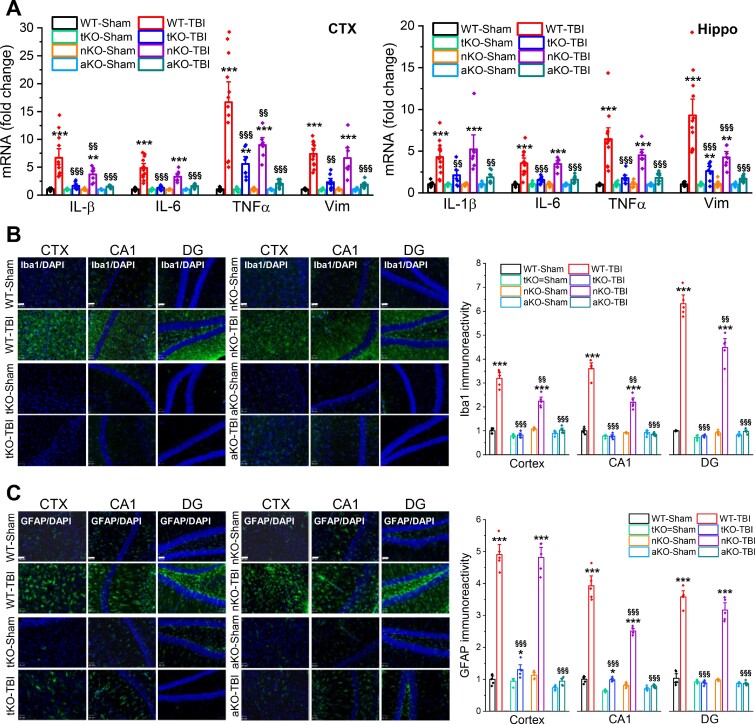
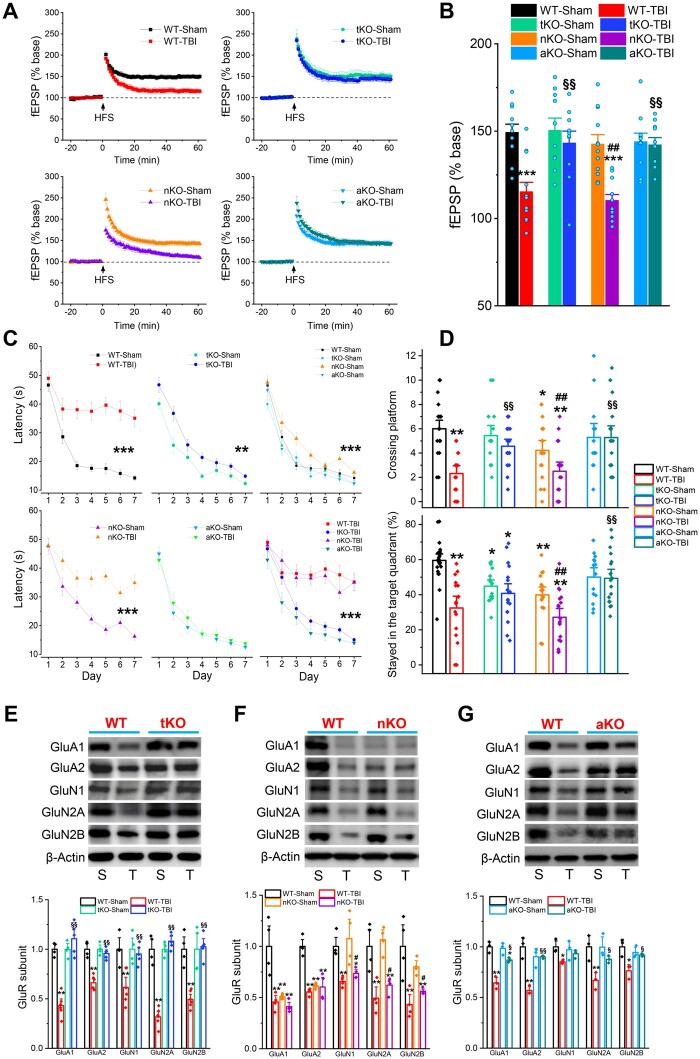
To determine whether genetic deletion of MAGL reduces TBI-induced neuropathology, we used a mouse model of repeated mCHI with three impacts at 24-h intervals (Supplementary Fig. 2B), as described previously.25 Neuroinflammation is one of the important neuropathological hallmarks in TBI,10,36 and contributes to secondary brain damage following the primary mechanical injury. To determine whether genetic inactivation of MAGL prevents TBI-induced neuroinflammation, we first assessed mRNA expression of proinflammatory factors, including Vim and cytokines (Il1b, Il6 and Tnfa), in the brain ipsilateral to the impact using qPCR analysis 24 h after the last impact. In addition, we assessed gliosis in the brain (reactive astrocytes and microglial cells) 30 days after the first impact. We found that expression of Il1b, Il6, Tnfa and Vim was robustly increased in both the cortex and hippocampus of wild-type animals that received three impacts as compared with wild-type-sham controls (Fig. 1A). The TBI-induced expression of these proinflammatory factors was significantly decreased in tKO and aKO mice, but less so in nKO mice, indicating that augmentation of 2-AG signalling in astrocytes is critically important in resolving TBI-triggered neuroinflammation. This finding was further supported by the results from immunostaining data which showed that TBI-increased immunoreactivity of Ib1a, a microglial marker, and GFAP, an astrocytic marker, was significantly reduced in tKO and aKO mice 30 days after TBI, but was only slightly or not reduced in nKO mice (Fig. 1B and C). These results suggest that anti-inflammatory effects produced by pharmacological or global genetic inactivation of MAGL in TBI result largely from limiting 2-AG metabolism in astrocytes, rather than in neurons.
Figure 1.
Inactivation of MAGL in astrocytes suppresses TBI-induced neuroinflammation. (A) TBI-increased expression of proinflammatory factors vimentin and cytokines is alleviated by inactivation of MAGL in astrocytes. The experimental protocols for TBI induction and assessments are provided in Supplementary Fig. 2B. Expression of proinflammatory vimentin (Vim) and cytokines Il1b, Il6 and Tnfa (IL-1β, IL-6, TNFα) in the ipsilateral cortex (CTX) and hippocampus (Hippo) was analysed in wild-type (WT), tKO, nKO and aKO mice 24 h after three impacts. Data are means ± SEM. **P < 0.01, ***P < 0.001 compared with WT-sham, §§P < 0.01, §§§P < 0.001 compared with WT-TBI (ANOVA with Fisher's PLSD post hoc test, n = 7–12 animals/group). (B) Immunoreactivity of Iba1 (microglial marker) in the ipsilateral cortex, hippocampal CA1 and dentate gyrus (DG) was imaged in wild-type, tKO, nKO and aKO mice 30 days after the first TBI. Scale bars = 40 μm. Data are means ± SEM. ***P < 0.001 compared with WT-sham; §§P < 0.01, §§§P < 0.001 compared with WT-TBI (ANOVA with Bonferroni post hoc test, n = 5 animals/group). (C) Immunostaining analysis of reactivity of GFAP (astrocytic marker) in the ipsilateral cortex, CA1 and dentate gyrus of wild-type, tKO, nKO and aKO mice. Scale bars = 40 μm. Data are means ± SEM. **P < 0.01, ***P < 0.001 compared with WT-sham; §§§P < 0.01 compared with WT-TBI (ANOVA with Bonferroni post hoc test, n = 5 animals/group).
Inhibition of 2-AG metabolism in astrocytes strengthens resilience to TBI-induced changes in expression of genes involved in inflammation
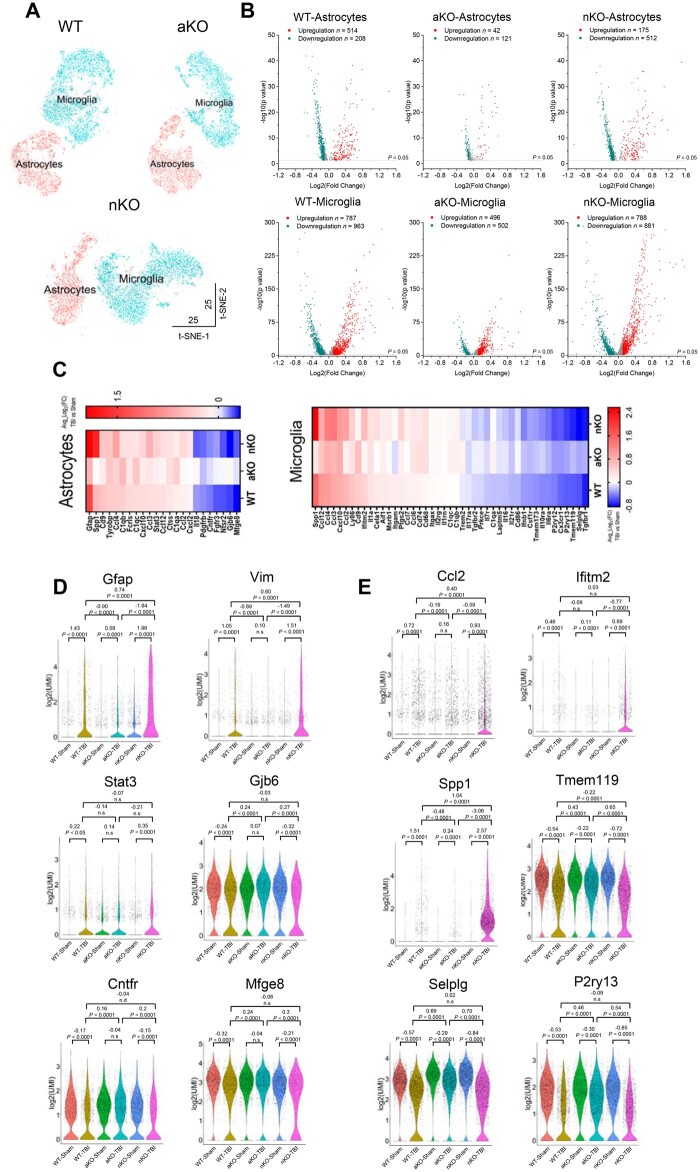
Because neuroinflammation is a key role in secondary brain injury following primary mechanical injury, and astrocytes and microglia are the primary players in neuroinflammation, we used the 10x Genomics Chromium scRNA-seq technology to assess expression of genes in astrocytes and microglia from wild-type, nKO and aKO mice that received three mCHI. Aqp4, Gja1, Slc1a2 and Gpr37l1 were used as specific cell markers for identification of astrocytes, while Aif1, Itgam, Csf1r and Tmem119 were used as specific cell markers for microglia. The numbers of astrocytes and microglia were assessed as shown in Supplementary Table 3. Figure 2A shows t-distributed stochastic neighbour embedding (t-SNE) plots of astrocytic and microglial cell clusters from wild-type, aKO and nKO mice. We found that TBI resulted in up- or downregulation of a significant number of genes in astrocytes and microglia from wild-type and nKO mice, but fewer from aKO mice (Fig. 2B). Importantly, TBI resulted in upregulation of genes involved in inflammation and downregulation of genes associated with anti-inflammatory responses, or associated with maintenance of brain homeostasis in wild-type and nKO mice, whereas effects in aKO mice are minor (Fig. 2C–E and Supplementary Fig. 4). In addition, gene ontology analysis of differentially expressed genes showed that more genes involved in immune and inflammatory signalling pathways were upregulated in astrocytes and microglia in wild-type and nKO mice than in aKO mice (Supplementary Figs 5 and 6). The scRNA-seq data suggest that inhibition of 2-AG metabolism in astrocytes renders the brain more resilient to TBI-induced changes in expression of genes involved in neuroinflammation.
Figure 2.
TBI-induced changes in expression of genes involved in inflammatory responses in astrocytes and microglia. (A) Visualization of the scRNA-seq data using t-SNE embedding. (B) Volcano plots for TBI-induced up- and downregulated genes in astrocytes and microglia from wild-type (WT), aKO and nKO mice (TBI versus sham). (C) Heat maps of differentially expressed genes in astrocytes and microglia in wild-type, aKO and nKO mice (TBI versus sham). (D) Violin plots of a few representative genes that are significantly up- or downregulated in astrocytes resulting from TBI in wild-type, nKO and aKO mice. The Wilcoxon rank sum test was used for statistical significance. (E) Violin plots of a few representative genes that are significantly up- or downregulated in microglia resulting from TBI in wild-type, nKO and aKO mice.
TBI-induced neuropathology is prevented by selective inactivation of MAGL in astrocytes
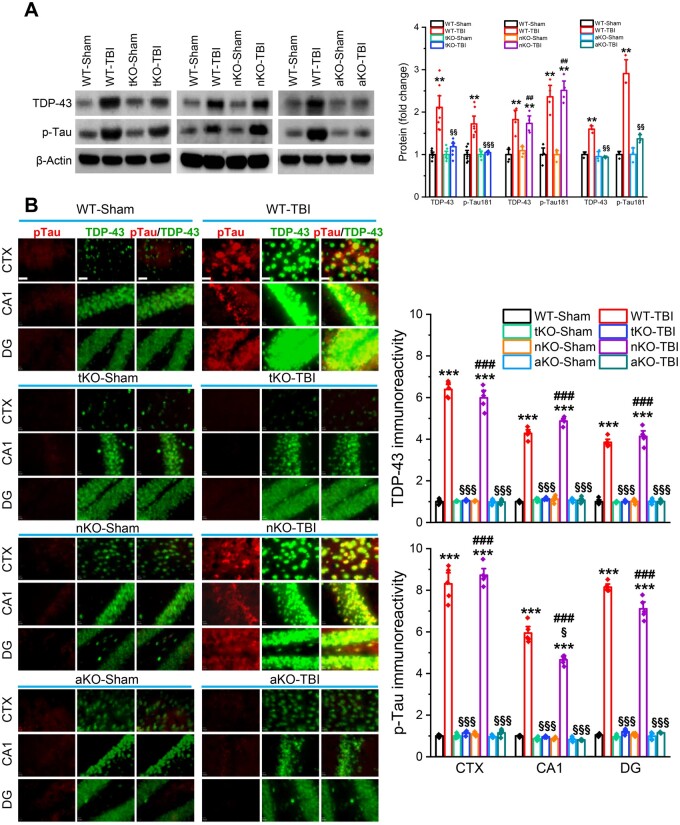
Aggregation of TDP-43 and hyperphosphorylated tau (p-tau) are hallmarks of TBI-induced neuropathology.5,7,10,37 We demonstrated previously that pharmacological inhibition of MAGL significantly reduces TDP-43 expression and p-tau in TBI animals.25 To determine whether genetic deletion of MAGL also prevents TBI-induced TDP-43 overproduction and p-tau, we assessed TDP-43 and p-tau-T181 (p-tau) in wild-type, tKO, nKO and aKO mice 30 days after the first impact. We found that repeated mCHI robustly elevated TDP-43 and p-tau in wild-type mice, but not in tKO and aKO mice (Fig. 3A and B). Surprisingly, production of TDP-43 and p-tau were significantly elevated in nKO mice following TBI, similar to wild-type mice. To determine whether TBI increases tau phosphorylation at additional phosphorylation sites, we assessed p-tau-Ser202, p-tau-Thr231, p-tau-Ser396 and p-tau-Ser404 in the hippocampus of wild-type, tKO, nKO and aKO mice that received impacts. We found that TBI resulted in significant increases in p-tau Ser202, Thr231, Ser396 and Ser404 in wild-type and nKO mice, but not in tKO and aKO (Supplementary Fig. 7). These results indicate that 2-AG degradation in astrocytes plays an important role in TBI-induced TDP-43 overproduction and tau phosphorylation at multiple sites, and that limiting 2-AG metabolism by inactivation of MAGL in astrocytes prevents TBI-induced neuropathological changes.
Figure 3.
TBI-induced TDP-43 overproduction and tau phosphorylation are mitigated by inactivation of MAGL in astrocytes, but not in neurons. (A) Western blot analysis of TDP-43, and p-tau-T181 (p-tau) in the hippocampus of wild-type (WT), tKO, nKO and aKO mice 30 days after the first impact. Data are means ± SEM. **P < 0.01 compared with WT-sham; §§P < 0.01, §§§P < 0.001 compared with WT-TBI; ##P < 0.01 compared with nKO-sham (ANOVA with Fisher's PLSD post hoc test, n = 5–7 animals/group). (B) Immunostaining analysis of TDP-43 and p-tau in the brain [cortex (CTX), CA1 and dentate gyrus (DG)] of wild-type, tKO, nKO and aKO mice 30 days after the first injury. Immunoreactivity signals of TDP-43 or p-tau were normalized to WT-sham. Scale bars = 40 μm. ***P < 0.001, compared with WT-sham; §P < 0.05, §§§P < 0.001 compared with WT-TBI; ###P < 0.001 compared with nKO-sham (ANOVA with Bonferroni post hoc test, n = 5 animals/group).
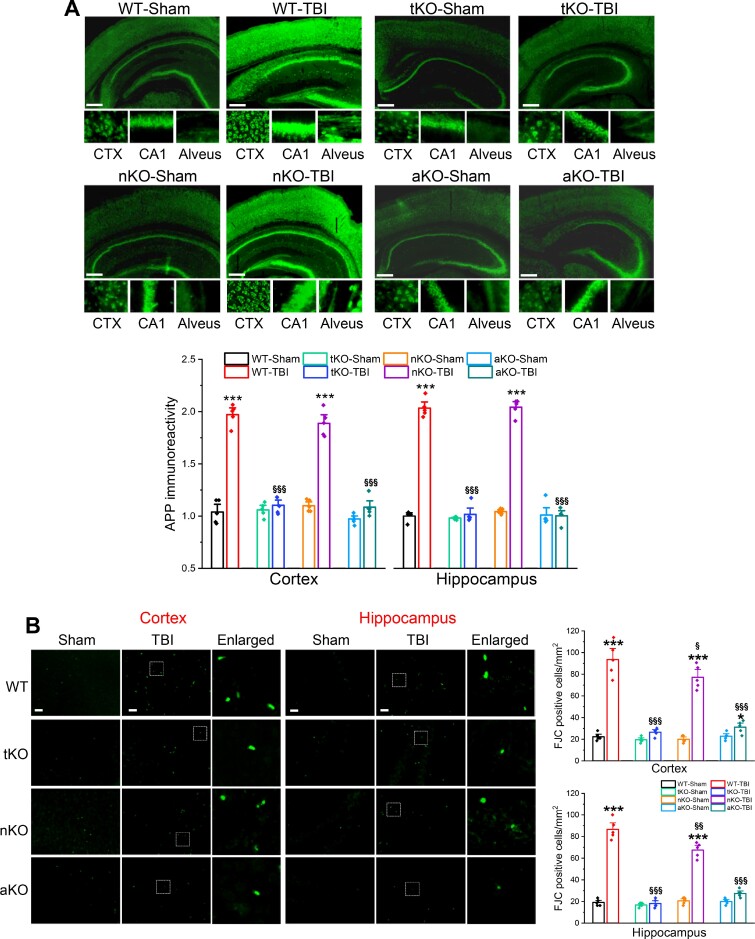
Diffuse axonal injury has been recognized as one of the most common and important pathological features of TBI.38 Expression of APP is increased following TBI.7,10,25,37 APP is not only a precursor of amyloid-β, but is also an important marker for diffuse axonal injury after TBI.39 To determine whether genetic deletion of MAGL ameliorates TBI-induced axonal injury, we assessed APP in the brain 30 days after three impacts. We found that production of APP in the cortex and hippocampus was significantly elevated in wild-type and nKO mice that received impacts, but not in tKO and aKO mice (Fig. 4A). The signal for APP immunoreactivity in wild-type and nKO mice, but not in tKO and aKO mice, was enhanced not only in the cell bodies, but also in the alveus, where the axons of CA1 pyramidal neurons are located. These results suggest that limiting astrocytic 2-AG degradation prevents TBI-induced axonal injury.
Figure 4.
Inactivation of MAGL in astrocytes prevents TBI-induced axonal injury and neurodegeneration. (A) TBI-induced axonal injury is diminished in aKO mice, but not in nKO mice. Immunostaining analysis of APP (axonal injury marker) in the cortex (CTX) and CA1 of wild-type, tKO, nKO and aKO mice 30 days after the first impact. Scale bars = 40 μm. Data are means ± SEM. ***P < 0.001, compared with WT-sham; §§§P < 0.001 compared with WT-TBI (ANOVA with Bonferroni post hoc test, n = 5 animals/group). (B) TBI-induced neurodegeneration is attenuated by genetic inactivation of MAGL. Fluoro-Jade® C (FJC, a specific marker for degenerating neurons)-positive cells in the ipsilateral cortex and hippocampus were detected 30 days after the first impact. Data are means ± SEM. *P < 0.05, ***P < 0.001 compared with WT-sham; §P < 0.05, §§P < 0.01, §§§P< 0.001 compared with WT-TBI (ANOVA with Bonferroni post hoc test, n = 5 animals/group). Scale bars = 40 μm.
Neurodegeneration is one of the important neuropathological features in TBI. We previously demonstrated that the number of degenerating neurons after TBI is significantly decreased in animals treated with JZL184, a potent MAGL inhibitor.25 To determine whether knockout of MAGL also reduces neurodegeneration after TBI, we assessed Fluoro-Jade® C (a neurodegenerating marker)-positive neurons in the brains of animals expose to repeated mCHI. We found that repeated mCHI markedly increased the number of Fluoro-Jade® C-positive neurons in the cortex and hippocampus in wild-type mice assessed 30 days after the impacts (Fig. 4B). As expected, the number of Fluoro-Jade® C-positive neurons was significantly reduced in MAGL knockout mice. Although the number of degenerating neurons was also decreased in nKO mice, the magnitude of the decrease in nKO mice was much less than in tKO or aKO mice. These data suggest that reduced neurodegeneration produced by pharmacological inhibition or global deletion of MAGL inactivation following TBI largely results from inhibition of 2-AG metabolism in astrocytes.
MAGL inactivation in astrocytes prevents TBI-induced deteriorations of synaptic and cognitive functions
The long-term consequences of repeated mCHI are synaptic and cognitive declines, which eventually lead to dementia. To determine whether inhibition of 2-AG degradation by genetic deletion of MAGL prevents TBI-induced impairments in long-term synaptic plasticity, learning and memory, we assessed long-term potentiation at hippocampal Schaffer collateral synapses using electrophysiological recordings. Assays were conducted 30 days after the first TBI impact. We found that repeated mCHI significantly impaired hippocampal long-term potentiation in wild-type mice (Fig. 5A and B). However, TBI-induced impairments in long-term synaptic plasticity were averted in tKO and aKO mice, but not in nKO mice. We used the Morris water maze test to assess spatial learning and memory. In comparison to wild-type mice, TBI-induced deficits in spatial learning and memory were prevented in tKO and aKO mice, but not in nKO mice (Fig. 5C and D and Supplementary Fig. 8). Unexpectedly, sham nKO mice displayed impaired spatial learning and memory (Fig. 5C and D), suggesting that proper 2-AG degradation in neurons is required for normal cognitive function. We also noted that the TBI-induced impairments in learning and memory were completely absent in aKO mice, but not in tkO mice (Fig. 5C and D). This may be because inactivation of neuronal MAGL in tKO mice worsens performance in the Morris water maze test. Our data provide evidence that confining 2-AG metabolism by inactivation of MAGL in astrocytes maintains homeostasis of brain function following brain injury.
Figure 5.
Selective inactivation of MAGL in astrocytes prevents TBI-induced synaptic and cognitive declines. (A) Long-term potentiation recordings at hippocampal Shaffer-collateral synapses 30 days after the first injury. (B) Mean values of the potentiation of field excitatory postsynaptic potentials (fEPSPs) averaged from 56 to 60 min following high-frequency stimulation (HFS). Data are means ± SEM. ***P < 0.001 compared with WT-Sham; §§P < 0.001 compared with WT-TBI; ##P < 0.001 compared with nKO-sham (ANOVA with Bonferroni post hoc test, n = 10–12 slices/4–5 animals). (C) Spatial learning and memory were assessed using the Morris water maze test 30 days after the first injury. Data are means ± SEM. **P < 0.01, ***P < 0.001 (ANOVA repeated measures with Bonferroni post hoc test, n = wild-type mice: 22 sham and 16 TBI; tKO mice: 16 sham and 18 TBI; nKO mice: 18 sham and 18 TBI; and aKO mice: 14 sham and 18 TBI). (D) The probe test was conducted 24 h following 7 days of learning acquisition training. Data are means ± SEM. *P < 0.05, **P < 0.01 compared with WT-sham; §§P < 0.01 compared with WT-TBI; ##P < 0.01 compared with nKO-sham (ANOVA with Bonferroni post hoc test). The number of animals in each groups the same as in C. (E–G) TBI-induced downregulation of glutamate receptor subunits is attenuated in aKO, but not in nKO mice. Immunoblot analysis of glutamate receptor subunits in the hippocampus of wild-type and tKO mice, wild-type and nKO and wild-type and aKO mice that received sham or three impacts. The analysis was performed 30 days after the first injury. Data are means ± SEM. *P < 0.05, **P < 0.01 compared with WT-sham, §P < 0.05, §§P < 0.01 compared with WT-TBI; #P < 0.05, ##P < 0.01 compared with nKO-sham (ANOVA with Fisher's PLSD test post hoc test, n = 3–5 animals/group).
Disruption of 2-AG metabolism in astrocytes prevents TBI-reduced expression of glutamate receptor subunits
We previously demonstrated that repeated mCHI leads to downregulation of expression of glutamate receptor subunits, which are key components for excitatory synaptic transmission and plasticity, and that pharmacological inhibition of MAGL prevents TBI-induced downregulation of these subunits.25 We predicted that knockout of MAGL should prevent TBI-induced deterioration in expression of the glutamate receptor subunits. We found that TBI resulted in robust downregulation of AMPA receptor subunits, including GluA1 and GluA2 and NMDA receptor subunits, including GluN1, GluN2A and GluN2B, in the hippocampus of wild-type mice 30 days after impacts (Fig. 5E–G). However, TBI-induced downregulation of these subunits was significantly attenuated in tKO and aKO mice, but not in nKO mice (Fig. 5E–G). We also observed that expression of GluA1 and GluA2 was significantly reduced in sham nKO mice compared with sham wild-type mice (Fig. 5F). This may underlie the impaired spatial learning and memory in nKO mice as shown in Fig. 5C and D. These results suggest that inhibition of 2-AG metabolism in astrocytes prevents TBI-induced synaptic and cognitive declines by maintaining the integrity of excitatory glutamatergic synapses.
Expression of MAGL is elevated in astrocytes
The data presented above indicate that 2-AG metabolism in astrocytes promotes TBI-induced neuropathology and synaptic and cognitive declines. We hypothesized that TBI may accelerate degradation of 2-AG by elevating expression of MAGL in astrocytes, which in turn promotes neuroinflammatory responses and neuropathological changes. To test this hypothesis, we assessed expression of MAGL following TBI in wild-type mice. We found that expression of MAGL in the brain was elevated following TBI (Supplementary Fig. 9A). As the samples extracted from the homogenized brain tissue for immunoblot analysis contain all types of cells, we asked whether the increased expression of MAGL resulted primarily from an increase in astrocytes following TBI. To this end, we used double immunostaining to detect expression of MAGL in astrocytes, and observed that expression of MAGL in GFAP-positive astrocytes was significantly elevated (Supplementary Fig. 9B). In light of these findings, we assessed brain 2-AG by mass spectrometry analysis in cell type-specific MAGL knockout mice. We found that the 2-AG content was increased more than 7-fold in tKO mice, 5-fold in nKO mice and about 2-fold in aKO mice under the control condition, suggesting that a large proportion of 2-AG in the brain was metabolized by MAGL in neurons (Supplementary Fig. 9C). These data are consistent with previous reports.34 However, no significant changes in the levels of brain 2-AG between sham and TBI in each genotype were detected 24 h after TBI (Supplementary Fig. 9C). This might be due to the relatively small amount of 2-AG produced in astrocytes that contributes to the overall 2-AG pool in the brain.34
MAGL inactivation-alleviated neuropathology and cognitive decline are mediated via CB1R
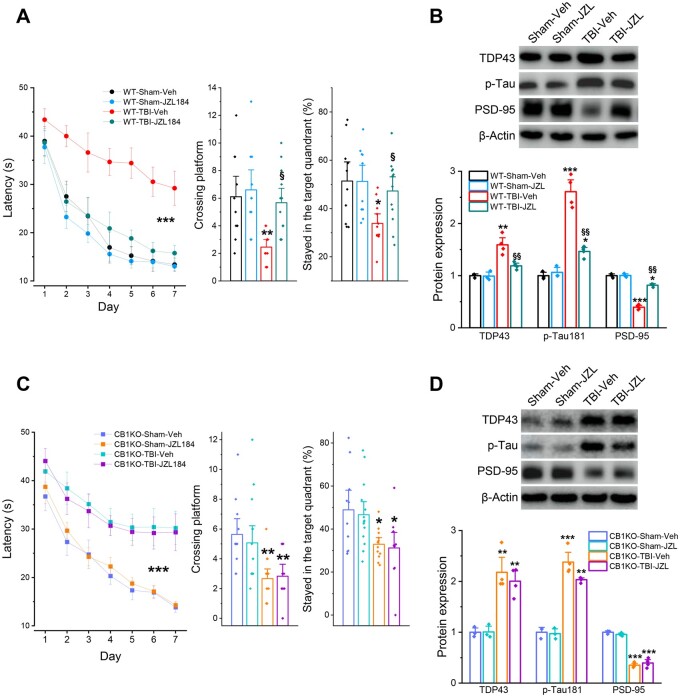
The data shown above indicate that genetic deletion of MAGL in astrocytes mitigates TBI-induced neuropathology and prevents synaptic and cognitive declines, similar to effects of JZL184, as reported previously.25 However, it remains unclear whether the beneficial effects produced by inactivation of astrocytic MAGL in TBI are mediated via enhanced 2-AG signalling and its downstream mediators CB1R or CB2R. To determine whether CB1R mediates MAGL inactivation-produced neuroprotective effects, CB1R knockout mice were intraperitoneally injected with JZL184 (10 mg/kg) or vehicle 30 min after each impact and then once a day for four consecutive days (total seven injections), as described previously25 (Supplementary Fig. 2C). While JZL184 prevented TBI-induced neuropathology and cognitive impairments in wild-type mice (Fig. 6A and B), similar to previous observations,25 we found that, in CB1R knockout mice exposed to TBI, JZL184 failed to avert TBI-induced impairments of spatial learning and memory and neuropathological changes, including TDP-43, p-tau and synaptic marker PSD-95 (Fig. 6C and D). These results indicate that MAGL inactivation-produced neuroprotective effects against TBI resulted primarily from augmentation of 2-AG signalling, which activates downstream CB1R-mediated signalling pathways.
Figure 6.
CB1 receptors mediate MAGL inactivation-produced alleviation of neuropathology and cognitive decline in TBI. (A) Assessment of spatial learning and memory in wild-type mice that received three impacts. The dosing regimen of JZL184 injection and the experimental protocols are provided in Supplementary Fig. 2C. Data are means ± SEM. ***P < 0.001 (ANOVA repeated measures with Bonferroni post hoc test, n = 10–12 animals/group). The probe test was conducted 24 h following 7 days of invisible platform training. Data are means ± SEM. *P < 0.05, **P < 0.01 compared with WT-sham-Veh, §P < 0.05 compared with WT-TBI-Veh (ANOVA with Bonferroni post hoc test). (B) Immunoblot analysis of TDP-43, p-tau and PSD-95 expression in the hippocampus of wild-type mice 30 days after TBI. Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared with WT-sham-Veh, §§P < 0.01 compared with WT-TBI-Veh (ANOVA with Fisher's PLSD test post hoc test, n = 3–4 animals/group). (C) Assessment of spatial learning and memory in CB1R knockout mice that received impacts. Data are means ± SEM. ***P < 0.001 (ANOVA repeated measures with Bonferroni post hoc test, n = 11–14 animals/group). The probe test was conducted 24 h following 7 days of invisible platform training. Data are means ± SEM. *P < 0.05, **P < 0.01 with CB1KO-sham-Veh (ANOVA with Bonferroni post hoc test). (D) Immunoblot analysis of TDP-43, p-tau and PSD-95 expression in the hippocampus of CB1KO mice 30 days after TBI. Data are means ± SEM. **P < 0.01, ***P < 0.001 compared with CB1KO-sham-Veh (ANOVA with Fisher's PLSD test post hoc test, n = 3–4 animals/group).
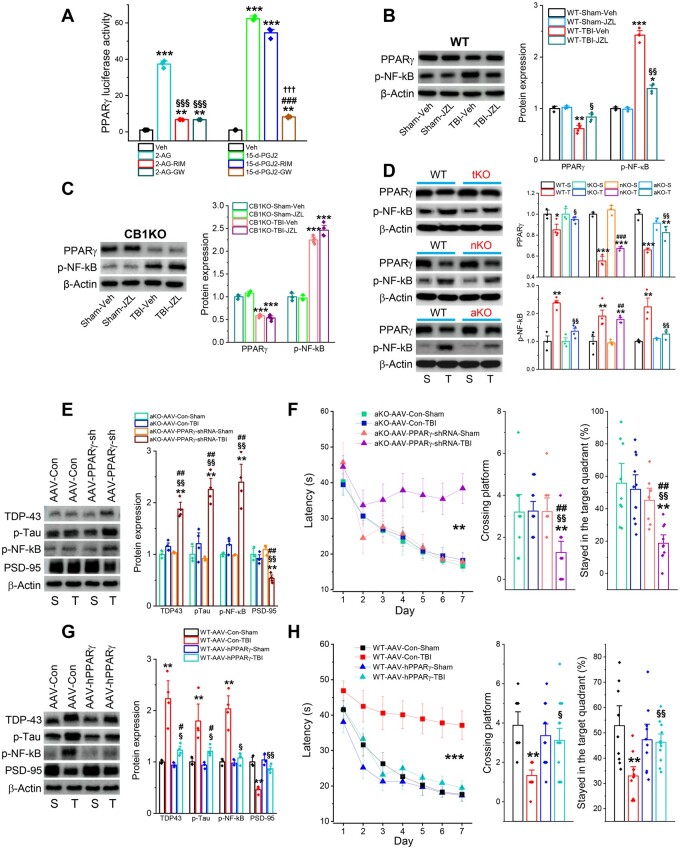
PPARγ in astrocytes mediates 2-AG-produced neuroprotective effects in TBI
Previous studies showed that neuroprotective effects of 2-AG in TBI are mediated via CB1R.11,40 Our present study also showed that the protective effects produced by pharmacological inhibition of MAGL in TBI are mediated via CB1R, shown by the fact that the effects were abolished in CB1R knockout mice (Fig. 6). However, no studies have been conducted to decipher downstream signalling pathways mediating beneficial effects in TBI resulting from MAGL inactivation in astrocytes. Our previous studies demonstrated that anti-inflammatory effects induced by 2-AG signalling are mediated through stimulating activity and expression of PPARγ, which inhibits activity of NF-κB, an important transcription factor regulating expression of genes involved in inflammation.15,19,22 Because an interplay between PPARγ and NF-κB plays an important role in controlling of neuroinflammation in the CNS,41–43 we postulated that PPARγ acts downstream of CB1R in mediating neuroprotective effects in TBI resulting from MAGL inactivation in astrocytes. To test this idea, we first assessed whether 2-AG increases in PPARγ activity, and whether the activity could be blocked by CB1R and PPARγ antagonists. We also used 15d-PGJ2, an endogenous PPARγ agonist, as a positive control. We found that 2-AG robustly elevated PPARγ luciferase activity, and that the elevation was blocked by rimonabant, a potent and selective CB1R antagonist, or by GW9662, a PPARγ antagonist (Fig. 7A). However, induction of PPARγ luciferase activity by 15d-PGJ2 was not blocked by rimonabant, but was blocked by GW9662. These results indicate that the increase in PPARγ activity stimulated by 2-AG was mediated via CB1R. Next, we determined whether TBI causes downregulation of PPARγ and increases phosphorylation of NF-κB (p-NF-κB), and whether these effects are revoked by augmentation of 2-AG signalling through pharmacological inhibition of MAGL. We found that TBI resulted in a decrease in hippocampal expression of PPARγ and an increase in p-NF-κB in wild-type mice (Fig. 7B). However, these changes were diminished or attenuated by JZL184, suggesting that enhancement of 2-AG signalling mitigates TBI-induced downregulation of PPARγ, resulting in upregulation of p-NF-κB. As predicted, these effects of JZL184 on PPARγ and p-NF-κB were not seen in CB1KO mice (Fig. 7C).
Figure 7.
PPARγ in astrocytes mediates the MAGL inactivation-produced neuroprotective effects in TBI. (A) PPARγ luciferase reporter activity in NG108-15 cells transfected with pCMX-Gal-LBD-mPPARγ and TK-MH100x4-luc vectors in the presence of 2-AG (10 µM), 15d-PGJ2 (10 µM) with and without rimonabant (RIM, 1 µM) or GW9662 (GW, 1 µM). Luciferase activity values were normalized to the level of β-galactosidase activity. Data are means ± SEM. **P < 0.01, ***P < 0.001, compared with the vehicle control; §§§P < 0.001 compared with 2-AG; ###P < 0.001 compared with 15d-PGJ2; †††P < 0.001 compared with 15d-PGJ2-RIM (ANOVA with Fisher's PLSD test post hoc test, n = 3–4/group). (B) TBI-induced downregulation of PPARγ and upregulation of p-NF-κB in the hippocampus are attenuated by pharmacological inhibition of MAGL with JZL184 (10 mg/kg) in wild-type (WT) mice. The data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the WT-sham-Veh; §P < 0.05, §§P < 0.01 compared with WT-TBI-Veh. (C) Immunoblot analysis of hippocampal expression of PPARγ and p-NF-κB in CB1R knockout mice that received JZL184 (10 mg/kg, i.p.). The analysis was performed 30 days after the first impact. ***P < 0.001 compared with CB1KO-sham-Veh (ANOVA with Fisher's PLSD test post hoc test, n = 3–4 animals/group). (D) Immunoblot analysis of hippocampal expression of PPARγ and p-NF-κB in wild-type, tKO, nKO and aKO mice. Data are means ± SEM. **P < 0.01, ***P < 0.001 compared with WT-sham, §P < 0.05, §§P < 0.01 compared with WT-TBI, ##P < 0.01, ###P < 0.001 compared with nKO-sham (ANOVA with Fisher's PLSD test post hoc test, n = 3–4 animals/group). (E) Effects of astrocytic PPARγ silencing on TBI-induced neuropathology in aKO mice. AAV5-GFAP-eGFP-m-PPARγ-shRNAmir vectors or AAV5-GFAP-eGFP control vectors were injected into the left side of hippocampus in aKO mice 30 days prior to TBI (Supplementary Fig. 2D). All the assessments were made 30 days after the impacts. Immunoblot analysis of TDP-43, p-tau, p-NF-κB and PSD-95 in the hippocampus of aKO mice 30 days after TBI. The data are means ± SEM. **P < 0.01 compared with AAV-Con-sham; §§P < 0.01 compared with AAV-Con-TBI; ##P < 0.01 compared with AAV-shRNA-sham (ANOVA with Fisher's PLSD test post hoc test, n = 3–4 animals/group). (F) Silencing of astrocytic PPARγ in TBI-induced changes of spatial learning and memory in aKO mice injected with AAV5-GFAP-eGFP-PPARγ-shRNA or AAV5 control vectors. The Morris water maze test was conducted 30 days after the first injury. Data are means ± SEM. **P < 0.01 (ANOVA repeated measures with Bonferroni post hoc test, n = 8–11 animals/group). The probe test was conducted 24 h following 7 days of invisible platform training. Data are means ± SEM. **P < 0.01, compared with AAV-Con-sham; §§P < 0.01 compared with AAV-Con-TBI; ##P < 0.01 compared with AAV-shRNA-sham (ANOVA with Bonferroni post hoc test). (G) Effects of astrocytic PPARγ overexpression on TBI-induced neuropathology in wild-type mice. AAV5-GFAP-h-PPARγ-FALG-WPRE vectors or AAV5-GFAP control vectors were injected into the left side of hippocampus in wild-type mice 30 prior to TBI (Supplementary Fig. 2D). Immunoblot analysis of TDP-43, p-tau, p-NF-κB, and PSD-95 in the hippocampus of wild-type mice 30 days after TBI. Data are means ± SEM. *P < 0.05, **P < 0.01 compared with AAV-Con-sham; §P < 0.05, §§P < 0.01 compared with AAV-Con-TBI; #P < 0.05 compared with AAV-PPARγ-sham (ANOVA with Fisher's PLSD test post hoc test, n = 3–4 animals/group). (H) Overexpression of astrocytic PPARγ in TBI-induced changes in spatial learning and memory in wild-type mice injected with AAV5-GFAP-h-PPARγ-FALG-WPRE or AAV5-GFAP control vectors. Data are means ± SEM. ***P < 0.001 (ANOVA repeated measures with Bonferroni post hoc test, n = 10–14 animals/group). The probe test was conducted 24 h following 7 days of invisible platform training. Data are means ± SEM. **P < 0.01 compared with AAV-Con-sham; §P < 0.05, §§P < 0.01 compared with AAV-Con-TBI (ANOVA with Bonferroni post hoc test, n = 10–14 animals/group).
To confirm that MAGL inactivation resulted in the observed changes in PPARγ and p-NF-κB in TBI, we assessed PPARγ and p-NF-κB in MAGL knockout mice. We observed that repeated mCHI significantly reduced hippocampal expression of PPARγ and increased p-NF-κB in wild-type mice. However, TBI-induced decrease in PPARγ and increase in NF-κB were attenuated in tKO and aKO mice, but not in nKO mice (Fig. 7D). These results indicate that 2-AG degradation in astrocytes promotes TBI-induced neuropathological changes by downregulation of PPARγ and upregulation of p-NF-κB, suggesting that augmentation of 2-AG signalling in astrocytes, rather than in neurons, prevents these TBI-induced changes.
If PPARγ in astrocytes is an important downstream signalling molecule in neuroprotective effects mediated by 2-AG-CB1R in TBI, then knockdown of PPARγ in aKO mice should eliminate or attenuate the protective effects against brain trauma. To test this hypothesis, we used the AAV vector-mediated gene silencing technique to specifically knock down astrocytic PPARγ in aKO mice. AAV5-GFAP-eGFP-m-PPARγ-shRNA or AAV5-GFAP-eGFP-control vectors were stereotaxically injected into the hippocampus of aKO mice 30 days prior to TBI impacts and assessments were conducted 30 days after TBI (Supplementary Figs 2D, 10A and B). While there were no significant changes in TDP-43, p-tau or p-NF-κB between sham and TBI in aKO mice that received AAV control vectors, there were significant increases in TDP-43, p-tau and p-NF-κB after TBI in aKO mice that received AAV-PPARγ-shRNA vectors (Fig. 7E). Similarly, TBI did not significantly reduce PSD-95 in aKO mice injected with AA control vector (Fig. 7E). However, expression of PSD-95 was significantly reduced in aKO mice injected with AAV-PPARγ-shRNA vectors. Importantly, alleviation of TBI-induced deficits in spatial learning and memory in aKO mice was abolished by silencing of PPARγ in astrocytes (Fig. 7F). These results indicate that neuroprotective effects in TBI produced by inactivation of MAGL in astrocytes are likely mediated through 2-AG-CB1R-PPARγ signalling.
To confirm PPARγ as a signalling molecule that mediates beneficial effects in TBI produced by astrocytic 2-AG signalling, we used AAV5-GFAP-h-PPARγ-FLAG-WPRE vectors to overexpress human PPARγ in astrocytes of wild-type mice that received repeated mCHI (Supplementary Figs 2D and 10C). We found that while TBI significantly increased TDP-43, p-tau and p-NF-κB and decreased PSD-95, in wild-type mice treated with AAV control vectors, neuropathological changes induced by TBI were mitigated in wild-type mice overexpressing PPARγ in astrocytes (Fig. 7G). Of significance, overexpression of PPARγ in astrocytes prevented TBI-induced cognitive decline in wild-type mice (Fig. 7H). Our data provide evidence that PPARγ functions as an important downstream signalling molecule of 2-AG and CB1R in mediating the beneficial effects of astrocytic MAGL inactivation in TBI (Supplementary Fig. 11).
Discussion
Based on a 2015 Centers for Disease Control and Prevention report, the number of new cases of TBI in the USA is ∼2.8 million annually. It is estimated that there are 5.3 million individuals living with a TBI-related disability. Clinically, the majority of mild TBI cases result from a closed head injury.1,2 Repeated mild TBI from multiple concussions or subconcussions may lead to Alzheimer’s disease-like neuropathological changes, suggesting that TBI is a significant risk factor for development of AD and dementia.2,10,37,44 Therefore, it is imperative to understand the mechanisms that contribute to TBI-induced Alzheimer’s disease-like neurodegenerative disease.
In the present study, we show that genetic deletion of MAGL mitigated TBI-induced neuroinflammation, TDP-43 overproduction, phosphorylated tau and neurodegeneration, and prevents downregulation of glutamate receptor subunits and deficits in long-term synaptic plasticity, learning and memory in mice exposed to repeated mCHI, similar to effects produced by pharmacological inhibition of MAGL.25–27 The neuroprotective effects of global deletion of MAGL apparently result from inhibition of 2-AG metabolism in astrocytes, as the protective effects occurred primarily in mice lacking MAGL in astrocytes, rather than in neurons. Analysis by scRNA-seq also showed that following TBI, significant numbers of genes were up- or downregulated in astrocytes and microglia from wild-type and nKO mice, but this effect was not apparent in astrocytes and microglia from aKO mice. In particular, TBI-induced upregulation of genes involved in inflammation and downregulation of genes involved in anti-inflammatory responses were greater in wild-type and nKO mice than in aKO mice, suggesting that limiting 2-AG metabolism in astrocytes strengthens resilience of the brain to TBI. Our results clearly show that the beneficial effects produced by inactivation of MAGL were the result of enhanced 2-AG signalling in astrocytes because these effects are revoked in CB1R knockout mice. We also show that PPARγ is a downstream signalling molecule of 2-AG and CB1R. This is supported by evidence showing that silencing of PPARγ in astrocytes eliminates the protective effects against TBI in aKO mice. This is further confirmed by the fact that overexpression of human PPARγ in astrocytes averts TBI-induced neuropathological changes and cognitive decline in wild-type mice. In addition, we observed that selective inactivation of MAGL in neurons caused downregulation of glutamate AMPA receptor subunits and resulted in impairments in spatial learning and memory in sham mice, suggesting that proper 2-AG metabolism in neurons is important for normal cognitive function. Our study reveals a previously undefined cell type-specific role of 2-AG metabolism in TBI-induced neuropathology, synaptic and cognitive deficits and signalling pathways that mediate beneficial effects resulting from inactivation of MAGL in astrocytes (Supplementary Fig. 11).
Endocannabinoids are produced naturally in the body. 2-AG is the most abundant endocannabinoid displaying anti-inflammatory and neuroprotective properties.11,13–15,45 Previous studies showed that inhibition of 2-AG metabolism by pharmacological or genetic inactivation of MAGL, which augments 2-AG signalling and reduces 2-AG metabolites, reduces neuroinflammation and neuropathology and improves synaptic and cognitive functions in several animal models of disease.12,21–23,25–28,46 Panikashvili et al.11,45 first reported 2-AG as a neuroprotective mediator in TBI. We and others also demonstrated that pharmacological inhibition of MAGL decreases neuroinflammation and neuropathology and improves neurobehavioural function and blood–brain barrier integrity as well as synaptic and cognitive functions following mild TBI.25–27 However, it remains unclear whether genetic deletion of MAGL prevents TBI-induced neuropathological changes. In particular, no information is available as to the role of 2-AG metabolism in specific cell types plays in promoting TBI-induced neuropathological changes. In the present study, we first show that genetic deletion of MAGL and pharmacological inhibition of MAGL produce similar neuroprotective effects. Surprisingly, alleviation of TBI-induced neuropathology and synaptic and cognitive declines occur primarily in aKO mice, but not in nKO mice, even though the amount of 2-AG in the brain of nKO mice is higher than in aKO mice (Supplementary Fig. 9C), suggesting that 2-AG metabolism in promoting TBI-induced neuropathology and synaptic and cognitive declines is cell type-specific.
Neuroinflammation is a critical factor in neuropathogenesis following TBI,3,10 suggesting that resolving or attenuating neuroinflammation may prevent long-term sequelae of TBI. Activated astrocytes are an important player in neuroinflammation as they release cytokines and chemokines in response to harmful insults, which in turn promote inflammatory responses in microglial cells.47–52 Our qPCR and immunohistochemical data show that TBI induced expression of proinflammatory cytokines and reactivity of astrocytes and microglia in wild-type mice. The TBI-induced neuroinflammatory responses were mitigated in tKO and aKO mice, but not in nKO mice, indicating an important role of astrocytic 2-AG metabolism in promoting TBI-induced neuropathology. Data from scRNA-seq provide further evidence that upregulation of genes involved in neuroinflammation and downregulation of genes associated with anti-inflammation or maintenance of CNS homeostasis were greater in wild-type and nKO mice than in aKO mice following TBI. For example, expression of Gfap, Vim, Stat3, Ccl2, Spp1 and Cxcl10 in astrocytes and Ccl2, Ccl3, Ccl4, Ccl7, Ifitm2, Spp1, Ccl12 and Ptgs2 in microglia were robustly upregulated in wild-type and nKO mice following TBI, but expression of these genes was not significantly changed or was less upregulated in aKO mice. In contrast, expression of Gjb6, Cntfr, Mfge8, Fgfr3, Ntsr2 and Pdgfrb in astrocytes and Cx3cr1, Tmem119, Selplg, P2ry12, P2ry13 and Cd86 in microglia was significantly downregulated in wild-type and nKO mice after TBI, but was not changed or was less downregulated in aKO mice. Our observations indicate that restraining 2-AG metabolism in astrocytes enhances resilience to brain trauma by suppressing TBI-induced upregulation of genes associated with inflammation, and preventing TBI-induced downregulation of genes with anti-inflammatory properties or maintenance of brain homeostasis. In particular, changes in expression of genes in both astrocytes and microglia, and reduction of reactivity of astrocytes and microglia, suggest that resolving inflammatory responses in astrocytes by inactivation of astrocytic MAGL is capable of curbing inflammatory responses in microglia.
MAGL hydrolyses 2-AG to arachidonic acid, a precursor of prostaglandins and leukotrienes. Prostaglandins and leukotrienes are proinflammatory and neurotoxic mediators in neurodegenerative diseases.18 It has been demonstrated previously that inflammatory prostaglandins either in vitro or in vivo are largely derived from 2-AG in astrocytes, rather than in neurons.34 This may be one of the mechanisms underlying anti-inflammatory and neuroprotective effects produced by inactivation of astrocytic MAGL in TBI. However, in the present study, we provide evidence that neuroprotective effects produced by pharmacological inhibition of MAGL are mediated via CB1R, indicating that the protective effects from inactivation of MAGL in TBI largely result from 2-AG-mediated signalling. As demonstrated previously, 2-AG is capable of activating PPARγ, and induces CB1R-dependent and independent anti-inflammatory and neuroprotective effects in response to proinflammatory insults in experimental models and in Alzheimer’s disease neuropathology.15,22 In the present study, we provide further evidence that PPARγ luciferase activity induced by 2-AG is blocked by CB1R or PPARγ antagonists, suggesting that PPARγ is a downstream signalling mediator of CB1R.15,22,53 Importantly, we observed that TBI suppresses expression of PPARγ in wild-type mice, and that the suppression is reversed by inhibition of 2-AG metabolism in astrocytes, but not in neurons. Silencing of PPARγ in astrocytes diminishes both neuroprotective effects and improvement of cognitive function in aKO mice exposed to repeated mCHI. In contrast, overexpression of human PPARγ in astrocytes prevents TBI-induced neuropathology and cognitive decline in wild-type mice. These results provide evidence of the important role of astrocytic PPARγ in mediating 2-AG-produced neuroprotective effects in TBI. The anti-inflammatory and neuroprotective effects of PPARγ are likely mediated through interactions with the transcription factor, NF-κB, resulting in inhibition of NF-κB activity and transcription of genes involved in inflammation.15,22,54 This conclusion warrants further investigation.
As reported previously, administration of 2-AG attenuates TBI-induced neuropathological changes.11 The anti-inflammatory and neuroprotective effects of 2-AG signalling suggest that augmentation of 2-AG signalling might provide a therapeutic approach ameliorating neuropathological changes in neurodegenerative diseases. Indeed, it has been proposed that MAGL is a promising therapeutic target for neuroinflammatory and neurodegenerative diseases.12,20,55,56 However, we observed in the present study that deletion of MAGL in neurons did not prevent TBI-induced neuropathological changes or deterioration in learning and memory; instead, sham nKO mice displayed reduced expression of AMPA glutamate receptor subunits and impaired spatial learning and memory. These results suggest that proper 2-AG degradation in neurons is important for maintaining the integrity of cognitive function and that global inactivation of MAGL might result in some undesirable adverse effects as a consequence of disruption of 2-AG metabolism in neurons. In contrast, inactivation of MAGL in astrocytes to prevent 2-AG degradation would likely achieve better anti-inflammatory and neuroprotective effects in TBI, while avoiding potential unwanted effects induced by inactivation of MAGL in neurons. As observed in the present study, expression of MAGL in astrocytes is elevated following TBI. Although no significant changes in the 2-AG content were detected in the brains of TBI animals, elevated expression of MAGL suggests that 2-AG degradation in astrocytes is likely accelerated following TBI.
Accumulated information indicates that TBI is an important risk factor for development of Alzheimer’s disease-like neurodegenerative disease and dementia. Unfortunately, no effective therapies are currently available to treat or prevent TBI-induced neuropathology and cognitive decline. The data presented in this report reveal a previously undefined cell type-specific role of 2-AG metabolism and CB1R-PPARγ-mediated signalling pathways in TBI-induced neuropathology, and synaptic and cognitive deficits, suggesting that augmentation of 2-AG or PPARγ signalling in astrocytes may provide a promising therapeutic approach for preventing or treating TBI-induced Alzheimer’s disease-like neurodegenerative disease.
Supplementary Material
Acknowledgements
The authors thank Dr Ronald Evans of Salk Institute for providing PPARγ luciferase vectors, NIH Mental Health Institute transgenic core for providing CB1R knockout mice and NIH Mental Health Institute Chemical Synthesis and Drug Supply Program for providing JZL184 and rimonabant.
Funding
This work was supported by National Institutes of Health grants R01NS076815, R01MH113535, and R01AG058621 (to C.C.) and by start-up funds from UT Health San Antonio, Joe R. & Teresa Lozano Long School of Medicine (to C.C.).
Competing interests
The authors report no competing interests.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AAV
adeno-associated virus
- a/n/tKO
astrocytic/neuronal/total MAGL knockout
- CB1R
cannabinoid receptor 1
- JZL184
4-nitrophenyl-4-[bis(1,3-benzodioxol-5-yl)(hydroxy)methyl]piperidine-1-carboxylate
- MAGL
monoacylglycerol lipase
- mCHI
mild closed head injury
- p-NF-κB
phosphorylated nuclear factor-κB
- PPARγ
peroxisome proliferator-activated receptor-γ
- TBI
traumatic brain injury
- scRNA-seq
single-cell RNA sequencing
- TDP-43
TAR DNA-binding protein 43
References
- 1. Levin HS, Robertson CS.. Mild traumatic brain injury in translation. J Neurotrauma. 2013;30(8):610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McKee AC, Robinson ME.. Military-related traumatic brain injury and neurodegeneration. Alzheimer's Dement. 2014;10(Suppl 3):S242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC.. Long-term consequences of repetitive brain trauma: Chronic traumatic encephalopathy. PM R. 2011;3(10 Suppl 2):S460–467. [DOI] [PubMed] [Google Scholar]
- 4. DeKosky ST, Blennow K, Ikonomovic MD, Gandy S.. Acute and chronic traumatic encephalopathies: Pathogenesis and biomarkers. Nat Rev Neurol. 2013;9(4):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(157):157lr5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lakis N, Corona RJ, Toshkezi G, Chin LS.. Chronic traumatic encephalopathy–neuropathology in athletes and war veterans. Neurol Res. 2013;35(3):290–299. [DOI] [PubMed] [Google Scholar]
- 7. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson VE, Stewart W, Smith DH.. Traumatic brain injury and amyloid-beta pathology: A link to Alzheimer's disease? Nat Rev Neurosci. 2010;11(5):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graves AB, White E, Koepsell TD, et al. The association between head trauma and Alzheimer's disease. Am J Epidemiol. 1990;131(3):491–501. [DOI] [PubMed] [Google Scholar]
- 10. Blennow K, Hardy J, Zetterberg H.. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76(5):886–899. [DOI] [PubMed] [Google Scholar]
- 11. Panikashvili D, Simeonidou C, Ben-Shabat S, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413(6855):527–531. [DOI] [PubMed] [Google Scholar]
- 12. Chen R, Zhang J, Wu Y, et al. Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease. Cell Reports. 2012;2(5):1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, Chen C.. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J Biol Chem. 2008;283(33):22601–22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X, Zhang J, Chen C.. Endocannabinoid 2-arachidonoylglycerol protects neurons against beta-amyloid insults. Neuroscience. 2011;178:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Du H, Chen X, Zhang J, Chen C.. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-gamma. Br J Pharmacol. 2011;163(7):1533–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blankman JL, Simon GM, Cravatt BF.. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chemistry & Biology. 2007;14(12):1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Long JZ, Nomura DK, Cravatt BF.. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16(7):744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salmon JA, Higgs GA.. Prostaglandins and leukotrienes as inflammatory mediators. Br Med Bull. 1987;43(2):285–296. [DOI] [PubMed] [Google Scholar]
- 19. Xu JY, Chen C.. Endocannabinoids in synaptic plasticity and neuroprotection. Neuroscientist. 2015;21(2):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gil-Ordonez A, Martin-Fontecha M, Ortega-Gutierrez S, Lopez-Rodriguez ML.. Monoacylglycerol lipase (MAGL) as a promising therapeutic target. Biochem Pharmacol. 2018;157:18–32. [DOI] [PubMed] [Google Scholar]
- 21. Nomura DK, Morrison BE, Blankman JL, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334(6057):809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J, Hu M, Teng Z, Tang YP, Chen C.. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer's disease. J Neurosci. 2014;34(45):14919–14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piro JR, Benjamin DI, Duerr JM, et al. A dysregulated endocannabinoid–eicosanoid network supports pathogenesis in a mouse model of Alzheimer's disease. Cell Rep. 2012;1(6):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J, Chen C.. Alleviation of neuropathology by inhibition of monoacylglycerol lipase in APP transgenic mice lacking CB2 receptors. Mol Neurobiol. 2018;55(6):4802–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Teng Z, Song Y, Hu M, Chen C.. Inhibition of monoacylglycerol lipase prevents chronic traumatic encephalopathy-like neuropathology in a mouse model of repetitive mild closed head injury. J Cerebral Blood Flow Metabol. 2015;35(3):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayeux J, Katz P, Edwards S, Middleton JW, Molina PE.. Inhibition of endocannabinoid degradation improves outcomes from mild traumatic brain injury: A mechanistic role for synaptic hyperexcitability. J Neurotrauma. 2017;34(2):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katz PS, Sulzer JK, Impastato RA, Teng SX, Rogers EK, Molina PE.. Endocannabinoid degradation inhibition improves neurobehavioral function, blood–brain barrier integrity, and neuroinflammation following mild traumatic brain injury. J Neurotrauma. 2015;32(5):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schurman LD, Lichtman AH.. Endocannabinoids: A promising impact for traumatic brain injury. Front Pharmacol. 2017;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hashem J, Hu M, Zhang J, Gao F, Chen C.. Inhibition of 2-arachidonoylglycerol metabolism alleviates neuropathology and improves cognitive function in a tau mouse model of Alzheimer's disease. Mol Neurobiol. 2021;58(8):4122–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen R, Zhang J, Fan N, et al. Delta9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell. 2013;155(5):1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C.. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25(43):9858–9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song Y, Hu M, Zhang J, Teng ZQ, Chen C.. A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer's disease. EBioMedicine. 2019;39:409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morgan AJ, Kingsley PJ, Mitchener MM, et al. Detection of cyclooxygenase-2-derived oxygenation products of the endogenous cannabinoid 2-arachidonoylglycerol in mouse brain. ACS Chem Neurosci. 2018;9(7):1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Viader A, Blankman JL, Zhong P, et al. Metabolic interplay between astrocytes and neurons regulates endocannabinoid action. Cell Rep. 2015;12(5):798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viader A, Ogasawara D, Joslyn CM, et al. A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation. Elife. 2016;5:e12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Djordjevic J, Sabbir MG, Albensi BC.. Traumatic brain injury as a risk factor for Alzheimer's disease: Is inflammatory signaling a key player? Curr Alzheimer Res. 2016;13(7):730–738. [DOI] [PubMed] [Google Scholar]
- 37. Smith DH, Johnson VE, Stewart W.. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat Rev Neurol. 2013;9(4):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson VE, Stewart W, Smith DH.. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW.. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993;160(2):139–144. [DOI] [PubMed] [Google Scholar]
- 40. Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, Shohami E.. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J Cereb Blood Flow Metabol. 2005;25(4):477–484. [DOI] [PubMed] [Google Scholar]
- 41. Bright JJ, Kanakasabai S, Chearwae W, Chakraborty S.. PPAR regulation of inflammatory signaling in CNS diseases. PPAR Res. 2008;2008:658520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Villapol S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell Mol Neurobiol. 2018;38(1):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bernardo A, Minghetti L.. Regulation of glial cell functions by PPAR-gamma natural and synthetic agonists. PPAR Res. 2008;2008:864140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stein TD, Alvarez VE, McKee AC.. Chronic traumatic encephalopathy: A spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimer's Res Ther. 2014;6(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Panikashvili D, Shein NA, Mechoulam R, et al. The endocannabinoid 2-AG protects the blood–brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22(2):257–264. [DOI] [PubMed] [Google Scholar]
- 46. Pihlaja R, Takkinen J, Eskola O, et al. Monoacylglycerol lipase inhibitor JZL184 reduces neuroinflammatory response in APdE9 mice and in adult mouse glial cells. J Neuroinflamm. 2015;12(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aschner M. Astrocytes as mediators of immune and inflammatory responses in the CNS. Neurotoxicology. 1998;19(2):269–281. [PubMed] [Google Scholar]
- 48. Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20(2):160–172. [DOI] [PubMed] [Google Scholar]
- 49. Kaur D, Sharma V, Deshmukh R.. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer's disease. Inflammopharmacology. 2019;27(4):663–677. [DOI] [PubMed] [Google Scholar]
- 50. Kempuraj D, Thangavel R, Natteru PA, et al. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine. 2016;1(1). [PMC free article] [PubMed] [Google Scholar]
- 51. Linnerbauer M, Wheeler MA, Quintana FJ.. Astrocyte crosstalk in CNS inflammation. Neuron. 2020;108(4):608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu J, Dong H, Qian Q, et al. Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behav Brain Res. 2017;332:145–153. [DOI] [PubMed] [Google Scholar]
- 53. O'Sullivan SE. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152(5):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bensinger SJ, Tontonoz P.. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. [DOI] [PubMed] [Google Scholar]
- 55. Fowler CJ. Monoacylglycerol lipase—A target for drug development? Br J Pharmacol. 2012;166(5):1568–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grabner GF, Zimmermann R, Schicho R, Taschler U.. Monoglyceride lipase as a drug target: At the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther. 2017;175:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this manuscript are available from the corresponding authors upon request. The scRNA-seq data have been deposited in the NCBI Gene Expression Omnibus under accession number GSE178226.