Abstract
Historically, identifying carcinogens has relied primarily on tumor studies in rodents, which require enormous resources in both money and time. In silico models have been developed for predicting rodent carcinogens but have not yet found general regulatory acceptance, in part due to the lack of a generally accepted protocol for performing such an assessment as well as limitations in predictive performance and scope. There remains a need for additional, improved in silico carcinogenicity models, especially ones that are more human-relevant, for use in research and regulatory decision-making. As part of an international effort to develop in silico toxicological protocols, a consortium of toxicologists, computational scientists, and regulatory scientists across several industries and governmental agencies evaluated the extent to which in silico models exist for each of the recently defined 10 key characteristics (KCs) of carcinogens. This position paper summarizes the current status of in silico tools for the assessment of each KC and identifies the data gaps that need to be addressed before a comprehensive in silico carcinogenicity protocol can be developed for regulatory use.
Keywords: In Silico, Computational Toxicology, Cancer, Carcinogenesis, Key Characteristics, Hazard Identification, Risk Assessment, (Q)SAR, Expert Alerts, Read-across
1. Introduction
Globally, cancer has generally been the second leading cause of death responsible for an estimated 9.6 million deaths in 2018 [1]. Carcinogenicity is a complex process that conceptually is often divided into three stages – initiation, promotion, and progression, the latter includes malignant conversion and metastasis (e.g., 2). Several public or private in silico models (e.g., CASE Ultra1, Derek2, Lazar3, the OECD QSAR Toolbox4, the Leadscope SAR Carcinogenicity Database5, the U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research rodent carcinogenicity (Q)SAR models6, VEGA Hub7) have been developed to predict the rodent carcinogenicity potential of chemicals. However, despite recent improvement in performance, the overall concordance of these models is not sufficient to serve as a primary basis for drug regulatory safety assessments [7] and therefore, their use is limited to supporting drug safety assessments and to its application in a tiered testing framework for prioritization of environmental chemicals by chemical regulators. Thus, there remains a need for improved in silico carcinogenicity models to be developed, especially ones that are more directly applicable to predicting the risk for cancer in humans rather than in rodents, for use in research and regulatory decision-making.
A major obstacle in the development of in silico methods for some endpoints is the lack of sufficient reliable and robust training datasets while the main obstacle for their regulatory adoption is the lack of widely accepted procedures for performing and documenting such assessments. In response, an international inter-disciplinary consortium representing a number of stakeholders was organized to develop publicly available in silico protocols to support the hazard assessment of major toxicological endpoints [8]. To date, this effort has resulted in the publication of in silico protocols for genetic toxicology [9] and skin sensitization [10] while the development of similar protocols for acute lethality, endocrine activity, and skin/eye irritation and corrosion are in progress. Here, we report on a working group within the consortium that focused on developing an in silico protocol for the hazard assessment of carcinogens, to be based on the Smith et al. [11] publication that recognized 10 key characteristics (KCs) of carcinogens. The use of these KCs to identify carcinogens is challenging given the lack of relevant standardized assays for most KCs and thus a concomitant lack of KC relevant computational methods. However, a KC framework might facilitate the combination of different pieces of evidence to create a systematic approach for the evaluation and integration of mechanistic evidence with other types of evidence while allowing for hazard classifications that are scientifically defensible and appropriate for regulatory decision-making [12], as demonstrated recently by California under Proposition 65 [13] and by Jaechke et al. [14] in their respective analyses of the potential carcinogenicity of acetaminophen. Thus, we decided to summarize the current status of in silico tools for the hazard assessment of carcinogens and to identify data gaps that need to be addressed before a KC-based in silico carcinogenicity protocol could be developed.
2. Key Characteristics of Carcinogens
2.1. KC1: Is Electrophilic or Can Be Metabolically Activated
Electrophiles play a key role in all stages of carcinogenicity by forming covalent adducts with electron-rich nucleophiles, such as DNA, RNA, proteins, and lipids [15–18]. While seen most prominently causing DNA damage, electrophiles are also involved in post-translational protein modification, which can alter protein enzymatic activity, the transduction of signals within and between cells, and gene expression [19]. Nonreactive substances may be transformed to reactive electrophilic species via metabolic processes in vivo, with biotransformation facilitated by mammalian and bacterial enzymes depending on the species, substrate, and route of exposure [15,20,21]. The type of interaction and ultimate effect depends on the inherent characteristics of the chemical and its biological target [16,22,23]. Measurement of a chemical’s electrophilicity or its propensity to be metabolically activated to an electrophile is generally performed indirectly, most frequently via examination of protein or DNA adducts, measured in vitro or in vivo [24].
Ashby and Tennant [25] identified a set of generally reactive chemical features potentially capable of covalently binding to DNA; this list has been refined and updated to predict additional electrophilic actions as well as structural alerts for genotoxicity [16,18,26,27]. In chemico knowledge of how individual functionalities of a structure interact and affect its overall potential for electrophilicity is needed to understand reactions with important cellular targets [22,28–33]. However, relying on the identification of a structural electrophilic mechanisms alone for predicting activity is over-predictive, leading to false positives [34]. The computational arena is replete with models for predicting bacterial mutagenicity, which is an indirect marker for a structure being an electrophile or able to be transformed into one [15,35]. Such computational models have been shown to adequately predict a structure’s potential for mutagenicity (with or without metabolic activation) and their use has been accepted as a replacement for the bacterial reverse mutation assay for pharmaceutical impurities [36–38]. Stand-alone models for prediction of metabolism exist that consider human metabolism (e.g., 39–42), though the potential electrophilicity of the products must be analyzed in separate quantitative structure-activity relationship (QSAR) models [43–46]. Multiple tools exist to determine a substance’s electrophilicity, although they generally measure only indirect endpoints. The ability to link these different tools into an integrated hazard assessment strategy that better reflects the physiological situation remains a work in progress that needs to be addressed to improve on the in silico prediction of electrophilicity.
2.2. KC2: Is Genotoxic
The term genotoxicity relates to the ability of a chemical to damage DNA and/or chromosome structure or segregation; such changes might lead to permanent changes to the genome which can be transmitted to successive generations of cells, potentially increasing the risk of cancer [47,48]. Genotoxicity resulting in cancer is the best understood and most experimentally measured of any of the KCs. It is also the most common characteristic identified when analyzing known human carcinogens [49].
A comprehensive in silico protocol for the hazard assessment of genotoxic chemicals was published recently [9]. This protocol contains information on the available test methods to assess genotoxicity, the in silico methods that are being used to predict genotoxicity, and approaches for their integration with experimental data. Table 1 summarizes information on the in vitro and in vivo tests for genotoxicity generally used for making regulatory decisions, many of which are harmonized in Organisation for Economic Co-operation and Development (OECD) Test Guidelines.
Table 1.
Genotoxicity Test Methods, OECD Test Guidelines, and In Silico Methods*
| Test Method | OECD Test Guideline | Endpoint | In Silico Methods |
|---|---|---|---|
| In vitro Bacterial Reverse Mutation Assay | OECD 471 [50] | Detects DNA point mutations. | An extensive number of compounds tested. Primary endpoint for genetic toxicity QSAR models (rule-based and statistical-based) [37,51]. |
| In vitro mammalian mutation tests | OECD 490 [52] | Detects gene mutations and chromosomal damage in endogenous thymidine kinase gene in the L5178Y TK+/− cell line (MLA) and the TK6 human lymphoblastoid cell line. | In silico MLA models are available, although limited expert rules exist. |
| OECD 476 [53] | Detects mutations at HPRT and at the XPRT transgene. | There is not enough data on HPRT or XPRT mutations to support useful in silico model development. Currently used for read-across/weight of evidence. | |
| In vitro micronucleus | OECD 487 [54] | Detects chromosome damage or other effects that lead to the formation of micronuclei in the cytoplasm of interphase cells. | Limited data published currently could be used for in silico derivation of structural alerts. |
| In vivo micronucleus | OECD 474 [55] | Detects chromosomal damage which results in the formation of micronuclei in erythrocytes in the bone marrow or peripheral blood cells of rats or mice | In silico in vivo micronucleus models available [56], although limited expert rules exist. |
| In vivo transgenic gene mutation assays | OECD 488 [57] | Measure mutagenic responses dependent upon in vivo metabolism, pharmacokinetics, DNA repair processes, and translesion DNA synthesis. | Limited data published, could be used for read-across/weight of evidence and derivation of expert alerts. |
| In vivo Pig-a assay | Validation documents only available [58,59] | Detects phenotypic mutation in an endogenous mammalian gene [60]. | |
| In vivo Comet assay | OECD 489 [61] | Measures DNA strand breaks and alkali-labile sites. |
Modified from Hasselgren et al. [9].
HPRT = hypoxanthine-guanine phosphoribosyl transferase gene; MLA = mouse lymphoma assay; OECD = Organisation for Economic Co-operation and Development; XPRT = xanthine guanine phosphoribosyl transferase
New assays for detecting genotoxic chemicals, primarily in vitro, have been developed that greatly increase throughput and/or sensitivity. These include quantitative high throughput screens (HTS) that evaluate the up-regulation of specific DNA damage response elements using reporter genes, (e.g., GADD45A [62], γ-H2AX [63], ATAD5 [64], TP53 [65]); the detection of multiple DNA damage response elements via targeted transcriptomic platforms [66–70] or multiple reporter cell lines [71]; using next-generation sequencing technologies to assess somatic mutations [72] and high throughput flow cytometric systems that integrate multiple endpoints relevant to genotoxicity and toxicity [73,74]. Data generated by HTS have been used for developing computational methods while others have not yet generated a sufficient chemical training dataset.
2.3. KC3: Alters DNA Repair or Causes Genomic Instability
Genomic instability, a common feature of cancer, refers to the increased tendency of a cell to acquire heritable alterations. This is typically associated with deficiencies in DNA replication and maintenance [75–78]. This instability is considered an enabling characteristic that drives tumor development and facilitates other cancer hallmarks [79]. To preserve genomic integrity, cells rely on a DNA damage response system that maintains DNA replication fidelity and activates cell cycle checkpoints and/or the DNA repair machinery in response to damaged DNA [76,80–82].
In a cell, a decrease in DNA repair competency and genomic stability is characterized by increased levels of mutations and chromosomal instability. Assays used to evaluate the ability of a chemical to induce DNA damage [9] (Table 1) can also be used to assess a chemical’s ability to reduce DNA repair activity [83]. Typically, cells are treated with a known DNA damaging agent (e.g., ionizing radiation, ultraviolet light, a direct acting genotoxic chemical) in the absence and presence of a chemical being tested for its ability to reduce a specific DNA repair pathway and one or more genotoxic endpoints measured. Other than for developing potential drug candidates for cancer therapy (e.g., 84), in silico methods to predict the ability of chemicals to inhibit DNA repair systems have not yet been developed.
2.4. KC4: Induces Epigenetic Alterations
Epigenetics refers to stable changes in gene expression and chromatin organization that occur without intrinsic changes in the primary DNA sequence. These changes can be mitotically inherited over cell divisions [85]. The epigenetic code is characterized by plasticity, with large variability across different cell types, in the same cells at different developmental stages, and under the influence of environmental stimuli [86]. Deregulation of the epigenome has been shown to occur during carcinogenesis and some carcinogens may disrupt various epigenetic mechanisms, including changes in DNA methylation, microRNA expression, histone modifications, and chromatin structure (e.g., 49,85–91). These can lead to altered gene expression and, among other effects, to tumor promotion [86,92]. Additional information about the chemical-induced epigenetic changes that can occur are provided by Chappell et al. [93], Herceg et al. [86], and Wang and Yang [94].
Changes in epigenetic patterns can be detected using standard molecular biology techniques and whole-genome approaches [24,95–98]. Alternatively, alterations in the protein levels and activity of epigenetic machinery can be measured. Appropriate in vivo models to study the impact of xenobiotics on the epigenome are lacking because of the complex nature of the epigenome among species, developmental stage, and across different tissues [86]. The OECD considers epigenetic mechanisms as crucial contributing information for an integrated approach to the testing and assessment of non-genotoxic carcinogens [97,99,100]. However, in silico methods for assessing this KC are limited. Epigenetic data are explicitly accounted for in the development of structural alerts for carcinogenicity [101,102], while computational approaches have been developed for identifying potential drugs and drug targets linked to mis-regulation of epigenetic mechanisms leading to disease [103–105]. A roadmap for investigating epigenome dysregulation and environmental origins of cancer includes strengthening causal relationships, finding consensus on an optimal approach for the analysis of population-based epigenome-wide studies, deciphering the complex exposure-to-phenotype pattern affected by early-life exposures, and understanding aging-associated epigenetic changes [89].
2.5. KC5: Induces Oxidative Stress
Oxidative stress is an imbalance between the generation and detoxification of reactive oxygen and/or nitrogen species (ROS/RNS). Sources of increased ROS formation include tissue inflammation, xenobiotic metabolism, interruption of mitochondrial oxidative phosphorylation, reduced turnover of oxidized cellular components, and an overwhelmed capacity of detoxifying enzymes [106]. Oxidative stress commonly occurs in neoplastic tissue and can be part of the tumor environment [107]. Major biomarkers and endpoints related to the formation of ROS and their effects include (1) cellular levels of oxidative stress/ROS, (2) oxidative changes to macromolecules (DNA, proteins, lipids), and (3) activation of the antioxidant response element (ARE) pathway and expression of downstream proteins and antioxidants [24]. Oxidative damage can alter the accuracy of DNA replication, integrity, and epigenetic features, inducing DNA replication stress and subsequent genome and epigenome instability [108]. The complexity of the carcinogenic process is demonstrated by the current understanding of the role that Nrf2 plays in oxidative damage and carcinogenesis (reviewed by He et al. [109] and Rojo de la Vega et al. [110]). This transcription factor is regarded as key to the cellular antioxidant response. However, Nrf2 activation in cancer cells promotes cancer progression and metastasis while conferring resistance to chemo- and radiotherapy. Thus, while activation in normal cells helps to prevent tumor initiation, prolonged or constitutive activation participates in tumor promotion and progression.
Several QSAR methods exist for the evaluation of the antioxidant properties of chemicals; however, no Model Reporting Format for one directly predicting oxidative stress, macromolecule oxidation, redox cycling, or ARE activation is listed in the Joint Research Centre QSAR database [35]. Published models are available that predict different aspects of oxidative stress, such as lipid peroxidation, redox-cycling, and ARE pathway activation [111–117]. Endpoints relevant to this KC, relevant assays, and the availability of corresponding in silico methods are detailed in Table 2. Some in silico models predict health outcomes based on ROS assays or oxidative stress as input parameters (e.g., insulin regulation [118], oxidative stress levels in workers [119], or phototoxicity [120]). Muller et al. [121] have developed a model for drug-induced liver injury, which, among other endpoints, uses oxidative stress as a descriptor. Most expert alert/rule-based systems recognize reactive structures like organic peroxides, hydroperoxides, or quinones, which are ROS or are known to release ROS. These alerts are then matched to endpoint damage like mutagenicity, micronucleus formation, or hepatotoxicity. While oxidative stress is not a direct predictor of cancer, it is considered a contributing factor in the prediction of carcinogenicity by in silico expert rule-based systems (e.g., Derek Nexus8).
Table 2:
List of endpoints and assays for the “Causes Oxidative Stress” (adapted from Smith et al.[24] with corresponding in silico models.
| Endpoint | Assay | In Silico Methods |
|---|---|---|
| ROS formation | Expert rule-based systems that recognize for example, peroxides and quinones. Redox-cycling capability is dependent on the one-electron reduction potential of a compound. Quantum chemical models are available to predict the one-electron reduction potential from chemical structure [112,114]. | |
| Oxidative DNA damage | ||
| Oxidation of other macromolecules |
|
|
| ARE/Nrf-2 activation | ||
| Glutathione oxidation | Measurement of GSH level, GSH/GSSG ratio [140] | None located. |
ARE = antioxidant responsive element; CAT = catalase; DSB = double-strand break; EPR = electron paramagnetic resonance; GFP = green fluorescent protein; GPx = glutathione peroxidase; GSH = glutathione; GSSG = oxidized glutathione; MDA = malondialdehyde; Nrf-2 = nuclear factor erythroid 2–related factor 2; qHTS = quantitative high throughput screening; ROS = reactive oxygen species; SOD = superoxide dismutase; TBARS = thiobarbituric acid reactive substances; YFP = yellow fluorescent protein
2.6. KC6: Induces Chronic Inflammation
Inflammation is an adaptive response of the body to a loss of tissue homeostasis triggered by internal or external stimuli, including non-infectious and infectious agents [141,142]. Chronic inflammation results in an imbalance of tissue homeostasis due to prolonged release of inflammation mediators and persistent recruitment and activation of inflammatory cells [87,143]. This can predispose the host to various disease conditions including cancer [144–148]. The perpetuated inflammatory conditions can affect all stages of carcinogenesis [145,146,149] by increasing the risk of cancer, promoting tumor progression, and supporting metastatic spread [79,150]. Poor sensitivity and complex aspects related to exposures and interindividual variability make it difficult to identify diagnostic biomarkers of chronic inflammation that lead to cancer [151,152]. Furthermore, inflammation that elicits cancer and cancer-induced inflammation are not easily distinguished [153].
To our knowledge, computational toxicology has not been applied to predict either tissue inflammation or inflammatory signaling as detected by biomarkers or in vitro phenotypic profiling. Phenotypic in vitro assays such as those used in the ToxCast and Tox21 programs detect the ability of chemicals to up- or down-regulate key pathways associated with chronic inflammation [154]. These assays provide reasonable training sets, although the short-term nature of these assays poses a limitation for the evaluation of the prolonged inflammatory responses [24]. Specifically, NF-κB transcription factors have been the subject of several computational studies focusing on their inhibition; however, further investigation is needed to assess their predictive accuracy for chronic inflammation [155]. The mechanisms underlying chronic inflammation are complex and there is a limited understanding of the factors that induce this state or promote its prolongation in a spatiotemporal framework [156]. A high throughput in vitro ROS assay coupled with structure-activity relationship analysis of the known carcinogens might be a good starting point for in silico predictive methods for supporting the development of an inflammation-mediated adverse outcome pathway (AOP). Datasets including inflammatory responses based on biomarkers or measurements of tissue inflammation in humans may also offer a possible means for developing in silico models for chronic inflammation. However, the use of biomarkers is hampered by the difficulty in establishing a causal relationship between inflammatory biomarkers and cancer development [24].
2.7. KC7: Is Immunosuppressive
Immunosuppression is a reduction in the capacity of the immune system to respond effectively to foreign antigens, including antigens on tumor cells, and is associated in humans with an increased risk of certain tumors. Mechanisms have been proposed to explain the inter-relationship between the immune system and cancer [157,158]: these include tumor immunoediting [159], oncogenic viruses (e.g., HIV [160]), chronic inflammation [161], and chronic B cell stimulation [162]. Neoplasm types associated with drug-induced immunosuppression include a 20-fold increase in lymphoma, non-melanoma skin cancer, and Kaposi sarcoma seen in renal transplant patients [163]. Immunosuppression may not directly transform normal cells into potential tumor cells but rather potentiate neoplasia by facilitating the survival and proliferation of pre-neoplastic cells [157,163]. Furthermore, immune suppression of the adaptive immune system can lead to activation of the innate immune system to cause inflammation [164], another KC of carcinogens.
Experimental methods for assessing immunosuppression that focus on the ability of chemicals to interfere with various immune pathways are described by the FDA [165]; this draft document states that the standard 2-year carcinogenicity studies are not specifically designed to detect carcinogenicity caused by drug-induced decreases in tumor surveillance particularly when the increased tumor risk is caused by recrudescence of latent viral oncogenes, infectious agents, or chronic inflammatory states. However, as reviewed by Alden et al. [166], the p53+/− and the AKR mouse strains harbor a lymphoma virus and viral oncogene, respectively, and both develop lymphomas upon chronic treatment with immunosuppressive drugs suggesting these strains might be useful for assessing the cancer risk from immunosuppressive drugs. The diversity and mechanisms of action (MOA) for immunosuppressive reagents make both measurement and in silico predictions of this endpoint challenging.
2.8. KC8: Modulates Receptor-Mediated Effects
Receptor-mediated effects can involve alterations in receptor expression and activation or altered levels of receptor ligands or cofactors [167]. Receptors are broadly divided into two categories: intracellular receptors (e.g., nuclear receptors) and cell-surface receptors (e.g., ligand-gated ion channels, G-protein coupled receptors (GPCRs), receptor tyrosine kinases (RTKs)) [168]. Many human carcinogens display receptor-mediated activity [49,167,169]. While many receptors can play a role in carcinogenesis (e.g., activation of PI3/AKT signaling through GPCRs and RTKs [170]), the focus here is primarily on nuclear receptors as these [171] and their co-regulators [172] are critically involved in normal physiological processes and therefore, serve as important targets in carcinogenesis. Hormone-dependent cancers such as estrogen-dependent breast [173] and endometrial [174] cancers and androgen-dependent prostate cancer [175] represent the most well-defined examples of nuclear receptor involvement in cancer.
Many chemicals that have endocrine disrupting potential (e.g., phthalates, bisphenol A, polychlorinated biphenyls) display carcinogenic potential as well [176–178]. The molecular initiating event associated with receptor-based effects is binding of ligand to receptor. However, receptor binding alone is insufficient to determine downstream effects as the nature of such interactions (i.e., agonism versus antagonism) determines subsequent gene and protein expression alterations. An example of an AOP leading from estrogen receptor (ER) activation to breast cancer is presented in Morgan et al. [179].
There are numerous nuclear receptor assay methods in the literature. Methods that directly or indirectly measure receptor modulation are reviewed by Smith et al. [24]. However, these assays do not directly indicate carcinogenic potential and virtually all of the in vitro assays lack metabolizing systems which significantly limits their sensitivity and relevance. Open source computational models for ER [180] and androgen receptor (AR) [181] modulation that integrate results from multiple high throughput in vitro assays are bridging the gap between in vitro and in silico methods. Computational models that predict ER activity include read-across, molecular docking, and virtual screening tools are available for in silico hazard identification [182–187]. However, generating the necessary carcinogenicity data can be challenging due to knowledge gaps regarding the tissue-specificity of many receptor-mediated effects as well as uncertainty about which receptor-mediated effects contribute to carcinogenesis. Empirical data are required to identify the receptor-mediated events and the corresponding tissues and cancer types for which receptor activity is the critical initiating event in carcinogenesis, rather than being a downstream indicator of disease progression. Research efforts should be directed toward identifying the inflection point between normal receptor function and inappropriate receptor activity that triggers disease. Of interest is the observation that the conclusion of a recent evaluation of non-genotoxic agrochemical rodent carcinogens was that the most common MOA involved nuclear receptor activation [188]. Another concern is that some receptors (e.g., aryl hydrocarbon receptor (AhR), peroxisome proliferator-activated receptor (PPARα)) have a critical role in rodent liver carcinogenesis through non-genotoxic mechanisms, but with questionable relevance to humans [189]. These and other species- and sex-specific tumors highlight the need to better understand the specificity of receptor involvement in human carcinogenesis.
2.9. KC9: Causes Immortalization
Escaping cellular senescence and becoming immortal is a prerequisite step in carcinogenesis [190]. However, while immortalization per se is not sufficient for tumorigenesis, it may allow for the accumulation of the additional genetic changes needed for malignancy [191,192]. Senescence is regulated by telomere shortening and some carcinogens have been shown to activate the enzyme telomerase, thus preventing their shortening and/or increasing their length [193]. Several other mechanisms involving altered signaling pathways (e.g., p53/p21 and p16/Rb pathways) can influence senescence and immortalization [191,192]. Genotoxicants are thought to make cells immortal through direct inactivation (via point mutations and deletions) of the effector pathways (e.g., direct inactivation of the tumor suppressors p53 and p16 [194], while epigenetic mechanisms have been linked to immortalization induced by some non-genotoxic carcinogens [191].
Table 3 provides a list of endpoints and assays, adapted from Smith et al. [24], as well as corresponding in silico methods. Data for the Syrian hamster embryo (SHE) in vitro cell transformation assays are collected in the OECD QSAR toolbox12 [185]. However, the molecular mechanism governing the processes in cell transformation are not fully understood and therefore are not used for making regulatory decisions [195–197]. No other in silico methods currently exist for predicting the ability of chemicals to induce immortalization.
Table 3.
List of endpoints and assays with corresponding in silico models for the “Causes immortalization” KC.
| Endpoint | Assay | Type of assay | In Silico |
|---|---|---|---|
| Changes of in vitro transformation activity | Cell transformation assays | Syrian hamster embryo (SHE) assay [198–201] | QSAR models for SHE cell transformation from public literature [5,6,202,203] and Instem13. |
| Alterations of cellular senescence markers | Changes in β-galactosidase, p16, p21, and p53 protein levels | Non-human and human in vitro assays and in vivo assays and biomarkers [204] | - |
| Alterations of telomere length and/or telomerase activity | Telomerase activity assay | Non-human and human in vitro assay and in vivo assays and biomarkers [205] | - |
| Telomere length by real-time PCR | In vivo assays and biomarkers [206–209] | - | |
| Alterations in stem cell genes | Expression of Myc, Oct3/4, Klf4, Sox-2 | Non-human and human in vitro assay [210,211] and in vivo assays and biomarkers [210,212] | - |
SHE = Syrian hamster embryo
2.10. KC10: Alters Cell Proliferation, Cell Death, or Nutrient Supply
Tumor size is determined by cell proliferation, cell loss via apoptosis or necrosis, and the vascular supply. Cancer cells often exhibit unusual cellular energetics, relying on glycolysis for energy even under aerobic conditions [213] and converting glucose to lactate. Alterations in cellular replication and/or cell-cycle control linked to carcinogenesis have been described as predisposing to unrepaired DNA damage leading to cancer-causing mutations in replicating cells, sustained replication, and the ability of a transformed cell to escape normal cell-cycle control. A component common to these is evasion of apoptosis or other terminal programming [214]. Factors involved in cell proliferation and cell death include inflammatory signals [215–217], apoptosis and autophagy [218] and angiogenesis [49]. Dysregulation of gap junction intercellular communication (GJIC) can cause loss of contact inhibition, leading to abnormal cell growth [87]. GJIC is dysregulated or absent in a majority of human cancer cells [219–221]. GJIC is also involved in metastasis as reduced connexin expression may promote physical detachment of the cells from their substrate [222,223]. Nongenotoxic mechanisms may be responsible for GJIC inhibition [224,225], including inhibition by tumor promoters [226–230].
Methods for assessing cell proliferation, cell death/apoptosis, cell viability, and angiogenesis in vivo or in vitro as well as methods for assessing cellular bioenergetics and metabolism are presented in Table 4. Other than for potential drug candidates, global in silico methods to assess the ability of chemicals to induce cell death, cell proliferation, and alteration of nutrient supply have not been developed. However, local QSARs (i.e., those dedicated to a specific group or class of chemicals) have been developed to: (1) facilitate the discovery of novel inhibitors of enzymes and signaling molecules associated with cellular processes, whose aberration might lead to cancer (e.g., 231–237); (2) predict the antiproliferative activity of specific classes of candidate anticancer agents [238,239]; and (3) predict the ability of structurally-related compounds such as perfluorinated fatty acids, dialkyl phthalates, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls, to alter GJIC function [102,240–242].
Table 4:
List of endpoints and assays for the KC “Alters Cell Proliferation, Cell Death, or Nutrient Supply”. For these endpoints, corresponding in silico models have not been identified.
| Endpoint | In Vitro and In Vivo |
|---|---|
| Cell proliferation |
|
| Cell viability |
|
| Apoptosis |
|
| Nutrient supply, as metabolism |
|
| Cell oxygen consumption & bioenergetics |
|
| Angiogenesis | |
| Gap junction intercellular communication (GJIC) |
|
Akt = “Ak” refers to the AKR mouse strain while the t stands for thymoma, ATP = adenosine triphosphate, AR = androgen receptor, ELISA = enzyme-linked immunosorbent assay, EPR = electron paramagnetic resonance, ER = estrogen receptor, IGF – insulin growth factor, mTOR = mechanistic target of rapamycin, NF-kB = nuclear factor kappa-light-chain-enhancer of activated B cells, Raf = Rapidly Accelerated Fibrosarcoma, TGF = transforming growth factor, TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling, VEGF = Vascular endothelial growth factor, Wnt = a blending of Wingless and Int-1.
3. Discussion and Conclusion
Anticipating the carcinogenic potential of small molecules remains a major challenge that poses a serious threat to the population when unintentionally hazardous drugs or impurities reach the market [258]. Furthermore, humans are exposed to thousands of chemicals that have inadequate data on which to predict their potential for toxicological effects, including cancer [259]. The KC construct provides a pragmatic platform for collecting data and organizing information regarding carcinogens and it has recently been used in combination with read-across principles to support a recommendation to include chemicals in the NTP Report on Carcinogens that did not have animal cancer data [260]. Such structured information may help to support the development of in silico models possibly using AOP concepts. Here, we indicate where experimental methods and in silico models currently exist for each KC to support the hazard assessment for carcinogenicity and, importantly, where gaps exist. The “is genotoxic” KC is the only characteristic where global in silico methods for predicting carcinogenicity hazard have been developed that are being used for making regulatory decisions [37,38]. In silico predictions of electrophilicity exist also but are not accepted for making regulatory decisions alone due to low specificity. Mechanistic challenges as well as the absence of relevant methods for measuring certain KCs and/or the lack of robust experimental data, along with their complex causal relationship to carcinogenesis, make the other nine KCs less tractable for the development and application of in silico prediction models. This is especially true for non-genotoxic carcinogens where achieving a dose that exceeds homeostasis and exposure duration are important elements. Many of the KCs lack specificity for carcinogenicity by being involved in non-carcinogenicity-related disease processes as well. A critically important requirement for their regulatory use is a specific effect related directly to tumor development. However, as proposed by Madia et al [261], integration of relevant KC data derived from other toxicological studies into carcinogen hazard assessments is useful as it will maximize exploitation of existing data while minimizing the need to develop additional resources to evaluate non-genotoxic mechanisms.
There is widespread industry and regulatory interest in developing a framework for the prediction of human carcinogenicity using a combination of available experimental in vitro and in vivo data and inputs from in silico models. Such frameworks, termed “In Silico Toxicology Protocols,” [8] have been successfully developed for genetic toxicology [9] and skin sensitization [10] following a pattern that is generally applicable to any toxicological endpoint. For carcinogenicity, data would be integrated across the 10 KCs to give an overall hazard assessment. Each KC would be evaluated using available experimental data and the results of statistical models, expert alerts, and read-across evaluation to make an assessment in terms of hazard and the associated reliability of hazard determination. Once all 10 KCs are evaluated to the extent possible, the data are combined into an overall assessment of carcinogenicity and an associated confidence level (low, medium, high) assigned.
Integration of the 10 KCs into an overall hazard assessment is complex and not simply additive. The majority of KCs are not truly independent but rather interact with each other in a complex network that differs depending on the species, sex, individual, tissue, and cancer type, as well as with other factors related to environment and personal life-styles. For example, although electrophilicity is a common attribute of genotoxicants, electrophilic targets also include cellular constituents other than DNA that might result in altered homeostasis, increased genomic instability, and/or altered gene expression.
The contributions of the different KCs to carcinogenesis are not equal. Rather, the 10 KCs are meant to comprehensively account for the different mechanisms involved in tumor initiation, promotion, and progression, regardless of prevalence. Krewski et al. [49] analyzed the key characteristics of 86 agents known to cause cancer in humans for which mechanistic information was available. The most prevalent key characteristic was “is genotoxic” with 85 of the 86 known human carcinogens demonstrating that activity, followed by 47 agents with “alters cell proliferation, cell death, or nutrient supply” and 40 agents with “induces oxidative stress”. The 86 human carcinogens were associated with one to nine of the 10 KCs, with an average of four KCs. However, it is likely that the agents associated with very few KCs and especially a single KC (e.g., thiotepa with KC2 only) reflect the lack of relevant studies rather than a unique profile. Illustratively, we used these data published by Krewski and colleagues [49] to generate Figure 1 This figure provides a model that places the 10 KCs in perspective with the three stages of carcinogenesis and with each other. The KC boxes are color-coded based on the percentage of the 86 human carcinogens with data supporting the involvement of that KC [49]; these percentages might not reflect reality but are currently the best available. The arrows indicate likely interactions at the molecular and cellular levels and effects. Given the lack of experimental data supporting some of these interactions, we consider the relationships to be biologically plausible but not necessarily demonstrated experimentally. However, this figure aids in demonstrating the complexity of the interactions between the various KCs and the 3 stages of carcinogenesis. We also hope that by indicating where likely intersections occur, it will prompt the development of useful AOPs perhaps based on those intersections. In this figure, interactions between the various KCs are not specified but rather from each KC to its primary molecular or cellular level events/effects and from there to one or more downstream events and/or to one or more of the three stages of carcinogenesis.
Figure 1. Interactions among the 10 KCs and with the Three Stages of Carcinogenesis.
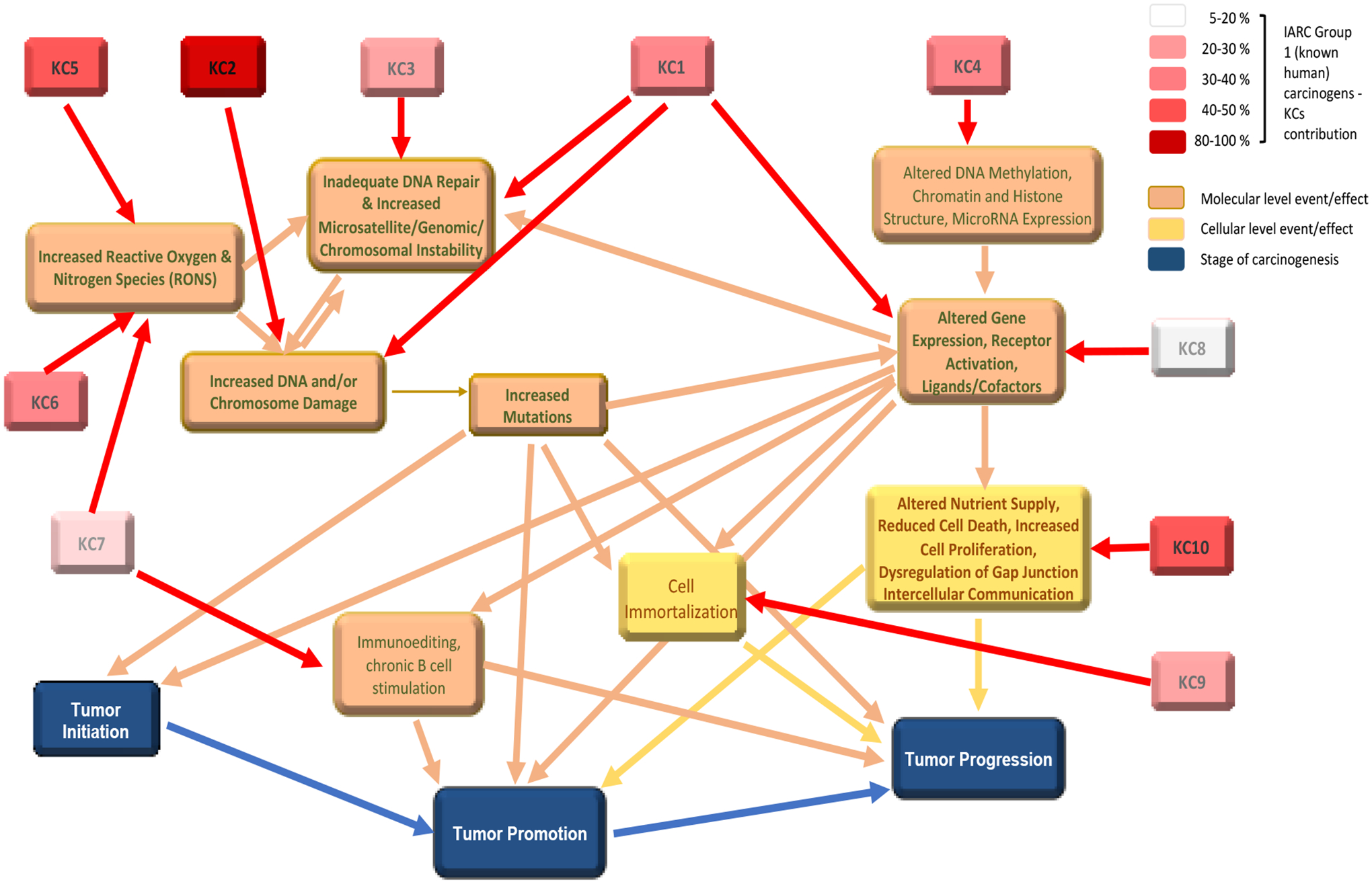
KC1 = is electrophilic, KC2 = is genotoxic, KC3 = alters DNA repair or causes genomic instability, KC4 = induces epigenetic alterations, KC5 = induces oxidative stress, KC6 = induces chronic inflammation, KC7 = is immunosuppressive, KC8 = modulates receptor-mediated effects, KC9 = causes immortalization, KC10 = alters cell proliferation, cell death, or nutrient supply. Supporting information for the different arrows can be found in the text or are considered by the working group to be biologically plausible. KCs are color coded according to the association between KCs and 86 chemicals carcinogenic to humans (International Agency for Research on Cancer Group 1 Agents) as determined by Krewski et al. [49].
Related to Figure 1 is Table 5, which summarizes the in silico methods currently available for each KC and the extent to which these methods are currently used for making regulatory cancer-related decisions. The table also summarizes the likely relationships among the different KCs in terms of the initial (i.e., first level) common molecular or cellular level event/effect each KC affects.
Table 5:
Relationships between the different KCs, their contribution to one or more stages of carcinogenesis, and availability of global in silico methods and whether used for making regulatory decisions related to cancer.
| Key Characteristic (KC) | First level, common molecular or cellular level event/effect among KCs | In Silico Methods | ||
|---|---|---|---|---|
| Availability | Used for making cancer-related regulatory decisions | |||
| KC1: Is Electrophilic or Can Be Metabolically Activated | KC2, KC3, KC8 | Well-established for adduct formation, but otherwise limited | Yes, but limited to DNA reactivity | |
| KC2: Is Genotoxic | KC1 | Well-established | Yes | |
| KC3: Alters DNA Repair Or Causes Genomic Instability | KC1 | None | No | |
| KC4: Induces Epigenetic Alterations | Limited | No | ||
| KC5: Induces Oxidative Stress | KC6, KC7 | Not for cancer but for assay specific endpoints | No | |
| KC6: Induces Chronic Inflammation | KC5, KC7 | Not for cancer but could be developed for assay specific endpoints | No | |
| KC7: Is Immunosuppressive | KC5, KC6 | None | No | |
| KC8: Modulates Receptor-Mediated Effects | KC1 | Yes, for multiple nuclear receptors1 | No | |
| KC9: Causes Immortalization | Only for in vitro Syrian hamster embryo cell transformation | No | ||
| KC10: Alters cell proliferation, cell death, or nutrient supply | None | No | ||
First level refers to arrows from two or more KCs that point to the same first molecular or cellular level event/effect in Figure 1. As noted in Figure 1, KCs 1–8 contribute to all three stages of carcinogenesis (initiation, promotion, progression), while KCs 9 and 10 contribute to promotion and progression.
The 99% prevalence of the “Is Genotoxic” KC among the known human carcinogens supports a regulatory decision tree approach for carcinogenicity hazard assessment that stops at in vitro or rodent genotoxicity if positive, with the other KCs evaluated only if the genotoxicity results are negative or inconclusive [18]. However, the more universal approach presented here would, once the data gaps have been filled, potentially provide for a better mechanistic understanding of carcinogenicity hazard as well as human relevance. Such data would also be useful for risk assessment, especially in situations where multiple chemicals or mixtures are being considered. A firmer grasp of the relationships between chemical structure, relative potency, and mechanisms of carcinogenicity would help to facilitate rational molecular design strategies during chemical development and strengthen risk-based decision-making when identifying lead compounds [262,263]. There is also tremendous value in having effective in silico carcinogenicity models which can provide rapid and inexpensive assessments to support the development of new drugs and treatments.
As noted earlier, global and regulatory accepted in silico models for the prediction of 9 of the 10 KCs do not currently exist. This is due in part because the development of relevant, sensitive, and specific assays depends on a better understanding of the biological processes involved in most of the KCs as well as because experimental data for these specific KCs are generally not required by regulatory agencies. Therefore, robust datasets on which to develop useful in silico models are generally not available. Chiu et al. [154] determined that the high throughput screening (HTS) assays conducted within the U.S. Environmental Protection Agency’s ToxCast program included assays that were relevant to all but three KCs (3, 7, and 9). However, the extent of coverage within the seven KCs with HTS assays varied widely. Subsequently, Iyer et al. [264] identified and mapped 137 ToxCast cancer pathway-related assays to 5 of the 10 KCs, concluding that the biological coverage of the ToxCast assays related to cancer pathways was currently limited. These two publications indicate where the development of additional HTS assays would be useful in terms of the KCs and also where chemical rich datasets already exist for the development of in silico models. Currently, a major limitation of these HTS assays is the general lack of metabolic competency, meaning that test results are limited to the parent compound and any known metabolites that also might have been tested.
The development of approaches integrating in silico predictions with in vitro and/or in vivo experimental data could serve as a more mechanistic, hypothesis-driven and tailored carcinogenicity assessment strategy in the future. Furthermore, the concept of a predictive computational model built upon pathways of toxicity (i.e., a quantitative AOP (qAOP)) is gaining interest due to its ability to address the question of how much perturbation in any of the upstream key events and under what conditions will adverse outcomes likely occur [265]. There is an increased interest in global genomics methods that evaluate changes in transcriptomics and/or proteomics in tissues and cells when exposed to carcinogens. The goal is to identify responding pathways and the point at which cellular homeostasis is exceeded (i.e., the tipping point), potentially leading to an increased risk for tumor development [266,267]. A network perspective on cancer encourages the application of computational modeling approaches [268], which aid the integration of multiple assays’ results to generate either qualitative or quantitative outcomes. Methodologies for computational analysis can vary widely depending on the question being posed and the available experimental data, ranging from highly abstracted models using correlative regression to networks and logic modeling techniques and highly specific models using differential equations [268]. It is important to ensure that the developed computational models are fit for their intended purpose, as determined by problem formulation of the question to be addressed or the regulatory use under consideration [269].
Currently, there are no integrated approaches to testing and assessment (IATAs) for the assessment of carcinogenicity. However, an OECD working group has addressed the possibility of an IATA for the detection of non-genotoxic carcinogens [97,99,270,271]. In this context, the ‘Hallmarks of Cancer’ [79] and key characteristics defined by Smith et al. [11] were further analyzed with the goal to elaborate grouping format/overarching KCs to address specifically non-genotoxic carcinogens. Such IATAs could also serve as a framework to integrate the various existing in silico methods for the evaluation of absorption, distribution, metabolism, excretion (ADME) and toxicokinetics [272–275] into carcinogenicity hazard assessment. The assays useful for evaluating the ability of chemicals to induce a specific KC can also be integrated (and weighted) with historical pathology data (e.g., pre-neoplastic lesions [276–278]) and/or human molecular epidemiological data. These may provide evidence that reinforces biological plausibility [279] and therefore establish more meaningful relationships across biology, exposures, susceptibility, and disease that significantly contribute to an understanding of the underlying pathways of carcinogenicity [85]. Although it remains a significant challenge to integrate all of the modelled and observational data into a single in silico carcinogenicity protocol, developing such a protocol would contribute to a more complete mechanistic understanding of cancer and its development, significantly advancing other areas of research and medicine.
This position paper summarizes the current availability of in silico methods for each of the 10 KCs of carcinogenicity as well as indicating where experimental methods need to be implemented and robust databases generated to support the development of relevant in silico methods for underrepresented KCs. Given our increased scientific understanding of processes related to carcinogenicity and other diseases that occur in response to some or many of the same key characteristics, as well as the continued development of better assays and computational methods, we can expect incremental advances in our ability to predict the carcinogenic hazard of compounds.
Highlights.
Summarizes the 10 key characteristics (KCs) of carcinogens
Assesses how current in silico methods address each of the KCs
Indicates where experimental methods need to be implemented and robust databases generated
Highlights interactions among the KCs with the different stages of carcinogenesis
Acknowledgements
The authors acknowledge with appreciation the contributions of the other members of the in silico carcinogenicity working group throughout this effort, and the comments and suggested edits provided by the different organizational internal reviewers.
Funding
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R44ES026909. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
☆ FDA Disclaimer: This article reflects the views of the authors and should not be construed to represent FDA’s views or policies. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
MHRA Disclaimer: Any opinions expressed in this document are the author’s and are not necessarily shared by other assessors at the Medicines and Healthcare products Regulatory Agency (MHRA). As such, they cannot be considered to be UK policy. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the UK’s MHRA.
Health Canada Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of Health Canada. The mention of commercial products, their sources, or their use should not be construed as either an actual or implied endorsement of such products by Health Canada.
European Commission Disclaimer: The views expressed are solely those of the authors and the content does not necessarily represent the views or position of the European Commission.
Declaration of interests
Dr. Myatt reports grants from NIH during the conduct of the study; and FDA’s Center for Drug Evaluation and Research (CDER) and Leadscope Inc. (an Instem company) are parties to a formal Research Collaboration Agreement (RCA). Dr. N. Kruhlak is the FDA CDER Principal Investigator for this agreement, and Dr. K. Cross is the Leadscope Principal Investigator for this agreement.
MultiCase Inc. - http://www.multicase.com/case-ultra
Organisation for Economic Co-operation and Development - https://www.oecd.org/chemicalsafety/risk-assessment/oecd-qsar-toolbox.htm
Leadscope Inc. - https://www.instem.com/solutions/insilico/computational-toxicology.php
Lhasa Ltd - https://www.lhasalimited.org/products/sarah-nexus.htm
MultiCase Inc. - http://www.multicase.com/case-ultra
Author Statement
Raymond R. Tice: Conceptualization, Writing - Original Draft, Writing - Review & Editing. Arianna Bassan: Conceptualization, Writing - Original Draft, Writing - Review & Editing. Alexander Amberg: Writing - Review & Editing. Lennart T. Anger: Writing - Review & Editing. Marc A. Beal: Writing - Review & Editing. Phillip Bellion: Writing - Review & Editing. Romualdo Benigni: Writing - Review & Editing. Jeffrey Birmingham: Writing - Review & Editing. Alessandro Brigo: Writing - Review & Editing. Frank Bringezu: Writing - Review & Editing. Lidia Ceriani: Writing - Review & Editing. Ian Crooks: Writing - Review & Editing. Kevin Cross: Writing - Review & Editing. Rosalie Elespuru: Writing - Review & Editing. David M. Faulkner: Writing - Review & Editing. Marie C. Fortin: Writing - Review & Editing. Paul Fowler: Writing - Review & Editing. Markus Frericks: Writing - Review & Editing. Helga H.J. Gerets: Writing - Review & Editing. Gloria D. Jahnke: Writing - Review & Editing. David R. Jones: Writing - Review & Editing. Naomi L. Kruhlak: Writing - Review & Editing. Elena Lo Piparo: Writing - Review & Editing. Juan Lopez-Belmonte: Writing - Review & Editing. Amarjit Luniwal: Writing - Review & Editing. Alice Luu: Writing - Review & Editing. Federica Madia: Writing - Review & Editing. Serena Manganelli: Writing - Review & Editing. Balasubramanian Manickam: Writing - Review & Editing. Jordi Mestres: Writing - Review & Editing. Amy L. Mihalchik-Burhans: Writing - Review & Editing. Louise Neilson: Writing - Review & Editing. Arun Pandiri: Writing - Review & Editing. Manuela Pavan: Writing - Review & Editing. Cynthia V. Rider: Writing - Review & Editing. John P. Rooney: Writing - Review & Editing. Alejandra Trejo-Martin: Writing - Review & Editing. Karen H. Watanabe-Sailor: Writing - Review & Editing. Angela T. White: Writing - Review & Editing. David Woolley: Writing - Review & Editing. Glenn J. Myatt: Review & Editing, Funding acquisition.
References
- [1].WHO, World Health Organization Cancer Fact Sheet, (2020). https://www.who.int/news-room/fact-sheets/detail/cancer (accessed October 20, 2020).
- [2].Pitot HC, Dragan YP, Facts and theories concerning the mechanisms of carcinogenesis, FASEB J. 5 (1991) 2280–2286. 10.1096/fasebj.5.9.1860619. [DOI] [PubMed] [Google Scholar]
- [3].Contrera JF, Matthews EJ, Daniel Benz R, Predicting the carcinogenic potential of pharmaceuticals in rodents using molecular structural similarity and E-state indices, Regul. Toxicol. Pharmacol 38 (2003) 243–259. 10.1016/s0273-2300(03)00071-0. [DOI] [PubMed] [Google Scholar]
- [4].Guo D, Kruhlak NL, Stavitskaya L, Cross KP, Bower DA, Characterizing compound classes by rodent carcinogenicity tumor severity and type, in: Baltimore, MD, 2016. 10.1177/1091581816686042. [DOI] [Google Scholar]
- [5].Matthews EJ, Kruhlak NL, Cimino MC, Benz RD, Contrera JF, An analysis of genetic toxicity, reproductive and developmental toxicity, and carcinogenicity data: I. Identification of carcinogens using surrogate endpoints, Regul. Toxicol. Pharmacol 44 (2006) 83–96. 10.1016/j.yrtph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- [6].Matthews EJ, Kruhlak NL, Cimino MC, Benz RD, Contrera JF, An analysis of genetic toxicity, reproductive and developmental toxicity, and carcinogenicity data: II. Identification of genotoxicants, reprotoxicants, and carcinogens using in silico methods, Regul. Toxicol. Pharmacol 44 (2006) 97–110. 10.1016/j.yrtph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- [7].Kruhlak NL, Hsu C-W, Kim MT, Validating (Q)SAR models to predict rodent carcinogenicity, in: 2020. https://cdn.ymaws.com/toxforum.site-ym.com/resource/resmgr/meeting_presentations/carcinogenesis_workshop/validating__q_sar_models_for.pdf.
- [8].Myatt GJ, Ahlberg E, Akahori Y, Allen D, Amberg A, Anger LT, Aptula A, Auerbach S, Beilke L, Bellion P, Benigni R, Bercu J, Booth ED, Bower D, Brigo A, Burden N, Cammerer Z, Cronin MTD, Cross KP, Custer L, Dettwiler M, Dobo K, Ford KA, Fortin MC, Gad-McDonald SE, Gellatly N, Gervais V, Glover KP, Glowienke S, Van Gompel J, Gutsell S, Hardy B, Harvey JS, Hillegass J, Honma M, Hsieh J-H, Hsu C-W, Hughes K, Johnson C, Jolly R, Jones D, Kemper R, Kenyon MO, Kim MT, Kruhlak NL, Kulkarni SA, Kümmerer K, Leavitt P, Majer B, Masten S, Miller S, Moser J, Mumtaz M, Muster W, Neilson L, Oprea TI, Patlewicz G, Paulino A, Lo Piparo E, Powley M, Quigley DP, Reddy MV, Richarz A-N, Ruiz P, Schilter B, Serafimova R, Simpson W, Stavitskaya L, Stidl R, Suarez-Rodriguez D, Szabo DT, Teasdale A, Trejo-Martin A, Valentin J-P, Vuorinen A, Wall BA, Watts P, White AT, Wichard J, Witt KL, Woolley A, Woolley D, Zwickl C, Hasselgren C, In silico toxicology protocols, Regul. Toxicol. Pharmacol 96 (2018) 1–17. 10.1016/j.yrtph.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hasselgren C, Ahlberg E, Akahori Y, Amberg A, Anger LT, Atienzar F, Auerbach S, Beilke L, Bellion P, Benigni R, Bercu J, Booth ED, Bower D, Brigo A, Cammerer Z, Cronin MTD, Crooks I, Cross KP, Custer L, Dobo K, Doktorova T, Faulkner D, Ford KA, Fortin MC, Frericks M, Gad-McDonald SE, Gellatly N, Gerets H, Gervais V, Glowienke S, Van Gompel J, Harvey JS, Hillegass J, Honma M, Hsieh J-H, Hsu C-W, Barton-Maclaren TS, Johnson C, Jolly R, Jones D, Kemper R, Kenyon MO, Kruhlak NL, Kulkarni SA, Kümmerer K, Leavitt P, Masten S, Miller S, Moudgal C, Muster W, Paulino A, Lo Piparo E, Powley M, Quigley DP, Reddy MV, Richarz A-N, Schilter B, Snyder RD, Stavitskaya L, Stidl R, Szabo DT, Teasdale A, Tice RR, Trejo-Martin A, Vuorinen A, Wall BA, Watts P, White AT, Wichard J, Witt KL, Woolley A, Woolley D, Zwickl C, Myatt GJ, Genetic toxicology in silico protocol, Regul. Toxicol. Pharmacol 107 (2019) 104403. 10.1016/j.yrtph.2019.104403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johnson C, Ahlberg E, Anger LT, Beilke L, Benigni R, Bercu J, Bobst S, Bower D, Brigo A, Campbell S, Cronin MTD, Crooks I, Cross KP, Doktorova T, Exner T, Faulkner D, Fearon IM, Fehr M, Gad SC, Gervais V, Giddings A, Glowienke S, Hardy B, Hasselgren C, Hillegass J, Jolly R, Krupp E, Lomnitski L, Magby J, Mestres J, Milchak L, Miller S, Muster W, Neilson L, Parakhia R, Parenty A, Parris P, Paulino A, Paulino AT, Roberts DW, Schlecker H, Stidl R, Suarez-Rodrigez D, Szabo DT, Tice RR, Urbisch D, Vuorinen A, Wall B, Weiler T, White AT, Whritenour J, Wichard J, Woolley D, Zwickl C, Myatt GJ, Skin sensitization in silico protocol, Regul. Toxicol. Pharmacol 116 (2020) 104688. 10.1016/j.yrtph.2020.104688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, DeMarini DM, Caldwell JC, Kavlock RJ, Lambert PF, Hecht SS, Bucher JR, Stewart BW, Baan RA, Cogliano VJ, Straif K, Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis, Environ. Health Perspect 124 (2016) 713–721. 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goodman J, Lynch H, Improving the International Agency for Research on Cancer’s consideration of mechanistic evidence, Toxicol Appl Pharmacol 319 (2017) 39–46. 10.1016/j.taap.2017.01.020. [DOI] [PubMed] [Google Scholar]
- [13].OEHHA, Proposition 65 - Evidence on the carcinogenicity of acetaminophen. Reproductive and cancer hazard assessment branch office of environmental health hazard assessment California environmental protection agency, Sacramento, CA, 2019. https://oehha.ca.gov/proposition-65/crnr/acetaminophen-under-consideration-listing-carcinogen-identification-committee. [Google Scholar]
- [14].Jaeschke H, Murray FJ, Monnot AD, Jacobson-Kram D, Cohen SM, Hardisty JF, Atillasoy E, Hermanowski-Vosatka A, Kuffner E, Wikoff D, Chappell GA, Bandara SB, Deore M, Pitchaiyan SK, Eichenbaum G, Assessment of the biochemical pathways for acetaminophen toxicity: Implications for its carcinogenic hazard potential, Regul Toxicol Pharmacol 120 (2021) 104859. 10.1016/j.yrtph.2020.104859. [DOI] [PubMed] [Google Scholar]
- [15].Benigni R, Bossa C, Mechanisms of chemical carcinogenicity and mutagenicity: a review with implications for predictive toxicology, Chem. Rev 111 (2011) 2507–2536. 10.1021/cr100222q. [DOI] [PubMed] [Google Scholar]
- [16].Enoch SJ, Cronin MTD, A review of the electrophilic reaction chemistry involved in covalent DNA binding, Crit. Rev. Toxicol 40 (2010) 728–748. 10.3109/10408444.2010.494175. [DOI] [PubMed] [Google Scholar]
- [17].Miller EC, Miller JA, Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules, Cancer. 47 (1981) 2327–2345. . [DOI] [PubMed] [Google Scholar]
- [18].OECD, Fundamental and Guiding Principles for (Q)SAR Analysis of Chemical Carcinogens with Mechanistic Considerations, OECD Environment, Health and Safety Publications, Paris, 2015. 10.1787/9789264274792-en. [DOI] [Google Scholar]
- [19].Groeger AL, Freeman BA, Signaling actions of electrophiles: anti-inflammatory therapeutic candidates, Mol. Interventions. 10 (2010) 39–50. 10.1124/mi.10.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kemper R, Taub M, Bogdanffy M, Metabolism: a determinant of toxicity, in: Hayes A, Kruger C (Eds.), Hayes’ Principles and Methods of Toxicology, Sixth Edition, CRC Press, London, 2014: pp. 141–214. 10.1201/b17359. [DOI] [Google Scholar]
- [21].Klaunig JE, Chemical carcinogenesis, in: Klaassen CD, Amdur MO (Eds.), Casarett and Doull’s Toxicology: The Basic Science of Poisons, New York: McGraw-Hill, 2013: p. 189. [Google Scholar]
- [22].LoPachin RM, Geohagen BC, Nordstroem LU, Mechanisms of soft and hard electrophile toxicities, Toxicology. 418 (2019) 62–69. 10.1016/j.tox.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sakanyan V, Reactive chemicals and electrophilic stress in cancer: a minireview, High-Throughput. 7 (2018). 10.3390/ht7020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Smith MT, Guyton KZ, Kleinstreuer N, Borrel A, Cardenas A, Chiu WA, Felsher DW, Gibbons CF, Goodson WH, Houck KA, Kane AB, La Merrill MA, Lebrec H, Lowe L, McHale CM, Minocherhomji S, Rieswijk L, Sandy MS, Sone H, Wang A, Zhang L, Zeise L, Fielden M, The key characteristics of carcinogens: relationship to the hallmarks of cancer, relevant biomarkers, and assays to measure them, Cancer Epidemiol., Biomarkers Prev 29 (2020) 1887–1903. 10.1158/1055-9965.EPI-19-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ashby J, Tennant RW, Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP, Mutat. Res 204 (1988) 17–115. 10.1016/0165-1218(88)90114-0. [DOI] [PubMed] [Google Scholar]
- [26].Beheshti A, Norouzi P, Ganjali M, A simple and robust model for predicting the reduction potential of quinones family: Electrophilicity index effect, Int. J. Electrochem. Sci 7 (2012) 4811–4821. [Google Scholar]
- [27].Schwöbel JAH, Wondrousch D, Koleva YK, Madden JC, Cronin MTD, Schüürmann G, Prediction of Michael-type acceptor reactivity toward glutathione, Chem. Res. Toxicol 23 (2010) 1576–1585. 10.1021/tx100172x. [DOI] [PubMed] [Google Scholar]
- [28].Ahlberg E, Amberg A, Beilke LD, Bower D, Cross KP, Custer L, Ford KA, Van Gompel J, Harvey J, Honma M, Jolly R, Joossens E, Kemper RA, Kenyon M, Kruhlak N, Kuhnke L, Leavitt P, Naven R, Neilan C, Quigley DP, Shuey D, Spirkl H-P, Stavitskaya L, Teasdale A, White A, Wichard J, Zwickl C, Myatt GJ, Extending (Q)SARs to incorporate proprietary knowledge for regulatory purposes: A case study using aromatic amine mutagenicity, Regul. Toxicol. Pharmacol 77 (2016) 1–12. 10.1016/j.yrtph.2016.02.003. [DOI] [PubMed] [Google Scholar]
- [29].Chamorro E, Melin J, On the intrinsic reactivity index for electrophilicity/nucleophilicity responses, J. Mol. Model 21 (2015) 53. 10.1007/s00894-015-2608-2. [DOI] [PubMed] [Google Scholar]
- [30].Parr RG, Szentpály L. v., Liu S, Electrophilicity index, J. Am. Chem. Soc 121 (1999) 1922–1924. 10.1021/ja983494x. [DOI] [Google Scholar]
- [31].Putz MV, Ionaşcu C, Putz A-M, Ostafe V, Alert-QSAR. Implications for electrophilic theory of chemical carcinogenesis, Int. J. Mol. Sci 12 (2011) 5098–5134. 10.3390/ijms12085098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Roy DR, Sarkar U, Chattaraj PK, Mitra A, Padmanabhan J, Parthasarathi R, Subramanian V, Van Damme S, Bultinck P, Analyzing toxicity through electrophilicity, Mol. Diversity. 10 (2006) 119–131. 10.1007/s11030-005-9009-x. [DOI] [PubMed] [Google Scholar]
- [33].Shalini A, Tandon H, Chakraborty T, Molecular electrophilicity index - A promising descriptor for predicting toxicological property, J. Bioequivalence Bioavailability. 09 (2017). 10.4172/jbb.1000356. [DOI] [Google Scholar]
- [34].Schwöbel JAH, Koleva YK, Enoch SJ, Bajot F, Hewitt M, Madden JC, Roberts DW, Schultz TW, Cronin MTD, Measurement and estimation of electrophilic reactivity for predictive toxicology, Chem. Rev 111 (2011) 2562–2596. 10.1021/cr100098n. [DOI] [PubMed] [Google Scholar]
- [35].EC JRC, JRC QSAR Model Database, European Commission, Joint Research Centre (JRC), JRC QSAR Model Database. (2020). https://ec.europa.eu/jrc/en/scientific-tool/jrcqsar-model-database (accessed September 17, 2020).
- [36].Benigni R, Battistelli CL, Bossa C, Giuliani A, Fioravanzo E, Bassan A, Fuart Gatnik M, Rathman J, Yang C, Tcheremenskaia O, Evaluation of the applicability of existing (Q)SAR models for predicting the genotoxicity of pesticides and similarity analysis related with genotoxicity of pesticides for facilitating of grouping and read across, EFSA Supporting Publications. 16 (2019). 10.2903/sp.efsa.2019.EN-1598. [DOI] [PubMed] [Google Scholar]
- [37].ICH, M7 (R1) Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk, European Medicines Agency, 2017. https://database.ich.org/sites/default/files/M7_R1_Guideline.pdf. [Google Scholar]
- [38].Landry C, Kim MT, Kruhlak NL, Cross KP, Saiakhov R, Chakravarti S, Stavitskaya L, Transitioning to composite bacterial mutagenicity models in ICH M7 (Q)SAR analyses, Regul. Toxicol. Pharmacol 109 (2019) 104488. 10.1016/j.yrtph.2019.104488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cruciani G, Carosati E, De Boeck B, Ethirajulu K, Mackie C, Howe T, Vianello R, MetaSite: understanding metabolism in human cytochromes from the perspective of the chemist, J. Med. Chem 48 (2005) 6970–6979. 10.1021/jm050529c. [DOI] [PubMed] [Google Scholar]
- [40].Djoumbou-Feunang Y, Fiamoncini J, Gil-de-la-Fuente A, Greiner R, Manach C, Wishart DS, BioTransformer: a comprehensive computational tool for small molecule metabolism prediction and metabolite identification, J. Cheminf 11 (2019) 2. 10.1186/s13321-018-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Peach ML, Zakharov AV, Liu R, Pugliese A, Tawa G, Wallqvist A, Nicklaus MC, Computational tools and resources for metabolism-related property predictions. 1. Overview of publicly available (free and commercial) databases and software, Future Med. Chem 4 (2012) 1907–1932. 10.4155/fmc.12.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tyzack JD, Kirchmair J, Computational methods and tools to predict cytochrome P450 metabolism for drug discovery, Chem. Biol. Drug Des 93 (2019) 377–386. 10.1111/cbdd.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Long A, Fielding K, McSweeney N, Payne M, Smoraczewska E, Chapter 11. Expert systems: the use of expert systems in drug design-toxicity and metabolism, in: Livingstone DJ, Davis AM (Eds.), RSC Drug Discovery, Royal Society of Chemistry, Cambridge, 2011: pp. 279–311. 10.1039/9781849733410-00279. [DOI] [Google Scholar]
- [44].Mekenyan O, Dimitrov S, Pavlov T, Veith G, A systematic approach to simulating metabolism in computational toxicology. I. the TIMES heuristic modelling framework, Curr. Pharm. Des 10 (2004) 1273–1293. 10.2174/1381612043452596. [DOI] [PubMed] [Google Scholar]
- [45].Rydberg P, Gloriam DE, Zaretzki J, Breneman C, Olsen L, SMARTCyp: A 2D method for prediction of cytochrome P450-mediated drug metabolism, ACS Med. Chem. Lett 1 (2010) 96–100. 10.1021/ml100016x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rydberg P, Gloriam DE, Olsen L, The SMARTCyp cytochrome P450 metabolism prediction server, Bioinformatics. 26 (2010) 2988–2989. 10.1093/bioinformatics/btq584. [DOI] [PubMed] [Google Scholar]
- [47].Levine AJ, Puzio-Kuter AM, The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes, Science. 330 (2010) 1340–1344. 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- [48].Stehr H, Jang S-HJ, Duarte JM, Wierling C, Lehrach H, Lappe M, Lange BMH, The structural impact of cancer-associated missense mutations in oncogenes and tumor suppressors, Mol. Cancer. 10 (2011) 54. 10.1186/1476-4598-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Krewski D, Bird M, Al-Zoughool M, Birkett N, Billard M, Milton B, Rice JM, Grosse Y, Cogliano VJ, Hill MA, Baan RA, Little J, Zielinski JM, Key characteristics of 86 agents known to cause cancer in humans, J. Toxicol. Environ. Health, Part B. 22 (2019) 244–263. 10.1080/10937404.2019.1643536. [DOI] [PubMed] [Google Scholar]
- [50].OECD, Test No. 471: Bacterial Reverse Mutation Test, OECD Publishing, Paris, 1997. 10.1787/9789264071247-en. [DOI] [Google Scholar]
- [51].EFSA PPR, Guidance on the establishment of the residue definition for dietary risk assessment, EFSA J. 14 (2016) 4549. 10.2903/j.efsa.2016.4549. [DOI] [Google Scholar]
- [52].OECD, Test No. 490: In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, OECD Publishing, Paris, 2016. 10.1787/9789264264908-en. [DOI] [Google Scholar]
- [53].OECD, Test No. 476: In Vitro Mammalian Cell Gene Mutation Tests using the Hprt and xprt genes, OECD Publishing, 2016. 10.1787/9789264264809-en. [DOI] [Google Scholar]
- [54].OECD, Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Publishing, Paris, 2016. 10.1787/9789264264861-en. [DOI] [Google Scholar]
- [55].OECD, Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Publishing, Paris, 2016. 10.1787/9789264264762-en. [DOI] [Google Scholar]
- [56].Yoo JW, Kruhlak NL, Landry C, Cross KP, Sedykh A, Stavitskaya L, Development of improved QSAR models for predicting the outcome of the in vivo micronucleus genetic toxicity assay, Regul. Toxicol. Pharmacol 113 (2020) 104620. 10.1016/j.yrtph.2020.104620. [DOI] [PubMed] [Google Scholar]
- [57].OECD, Test No. 488: Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays, OECD Publishing, Paris, 2020. 10.1787/9789264203907-en. [DOI] [Google Scholar]
- [58].OECD, The in vivo erythrocyte Pig-a gene mutation assay - Part 1 - Detailed review paper and performance assessment, OECD Environment, Health and Safety Publications, Paris, 2020. http://www.oecd.org/chemicalsafety/testing/series-testing-assessment-publications-number.htm. [Google Scholar]
- [59].OECD, The in vivo erythrocyte Pig-a gene mutation assay - Part 2 - Validation report, OECD Environment, Health and Safety Publications, Paris, 2020. http://www.oecd.org/chemicalsafety/testing/series-testing-assessment-publications-number.htm. [Google Scholar]
- [60].Gollapudi BB, Lynch AM, Heflich RH, Dertinger SD, Dobrovolsky VN, Froetschl R, Horibata K, Kenyon MO, Kimoto T, Lovell DP, Stankowski LF, White PA, Witt KL, Tanir JY, The in vivo Pig-a assay: A report of the International Workshop On Genotoxicity Testing (IWGT) Workgroup, Mutat. Res., Genet. Toxicol. Environ. Mutagen 783 (2015) 23–35. 10.1016/j.mrgentox.2014.09.007. [DOI] [PubMed] [Google Scholar]
- [61].OECD, Test No. 489: In Vivo Mammalian Alkaline Comet Assay, OECD Publishing, Paris, 2016. 10.1787/9789264264885-en. [DOI] [Google Scholar]
- [62].Hughes C, Rabinowitz A, Tate M, Birrell L, Allsup J, Billinton N, Walmsley RM, Development of a high-throughput Gaussia Luciferase reporter assay for the activation of the GADD45a gene by mutagens, promutagens, clastogens, and aneugens, J. Biomol. Screening 17 (2012) 1302–1315. 10.1177/1087057112453312. [DOI] [PubMed] [Google Scholar]
- [63].Mishima M, Chromosomal aberrations clastogens vs aneugens, Front. Biosci 9 (2017) 1–16. 10.2741/s468. [DOI] [PubMed] [Google Scholar]
- [64].Fox JT, Sakamuru S, Huang R, Teneva N, Simmons SO, Xia M, Tice RR, Austin CP, Myung K, High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death, Proc. Natl. Acad. Sci. U.S.A 109 (2012) 5423–5428. 10.1073/pnas.1114278109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Witt KL, Hsieh J-H, Smith-Roe SL, Xia M, Huang R, Zhao J, Auerbach SS, Hur J, Tice RR, Assessment of the DNA damaging potential of environmental chemicals using a quantitative high-throughput screening approach to measure p53 activation, Environ. Mol. Mutagen 58 (2017) 494–507. 10.1002/em.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Aubrecht J, Caba E, Gene expression profile analysis: An emerging approach to investigate mechanisms of genotoxicity, Pharmacogenomics. 6 (2005) 419–428. 10.1517/14622416.6.4.419. [DOI] [PubMed] [Google Scholar]
- [67].Corvi R, Vilardell M, Aubrecht J, Piersma A, Validation of transcriptomics-based in vitro methods, in: Eskes C, Whelan M (Eds.), Validation of Alternative Methods for Toxicity Testing, Springer International Publishing, Cham, 2016: pp. 243–257. 10.1007/978-3-319-33826-2_10. [DOI] [PubMed] [Google Scholar]
- [68].Li H-H, Chen R, Hyduke DR, Williams A, Frötschl R, Ellinger-Ziegelbauer H, O’Lone R, Yauk CL, Aubrecht J, Fornace AJ, Development and validation of a high-throughput transcriptomic biomarker to address 21st century genetic toxicology needs, Proc. Natl. Acad. Sci. U.S.A 114 (2017) E10881–E10889. 10.1073/pnas.1714109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Li H-H, Hyduke DR, Chen R, Heard P, Yauk CL, Aubrecht J, Fornace AJ, Development of a toxicogenomics signature for genotoxicity using a dose-optimization and informatics strategy in human cells, Environ. Mol. Mutagen 56 (2015) 505–519. 10.1002/em.21941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sakai R, Kondo C, Oka H, Miyajima H, Kubo K, Uehara T, Utilization of CDKN1A/p21 gene for class discrimination of DNA damage-induced clastogenicity, Toxicology. 315 (2014) 8–16. 10.1016/j.tox.2013.10.009. [DOI] [PubMed] [Google Scholar]
- [71].Hendriks G, Derr RS, Misovic B, Morolli B, Calléja FMGR, Vrieling H, The extended ToxTracker assay discriminates between induction of DNA damage, oxidative Stress, and protein misfolding, Toxicol. Sci 150 (2016) 190–203. 10.1093/toxsci/kfv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Salk JJ, Kennedy SR, Next-generation genotoxicology: using modern sequencing technologies to assess somatic mutagenesis and cancer risk, Environ. Mol. Mutagen 61 (2020) 135–151. 10.1002/em.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bryce SM, Bernacki DT, Smith-Roe SL, Witt KL, Bemis JC, Dertinger SD, Investigating the generalizability of the MultiFlow® DNA damage assay and several companion machine learning models with a set of 103 diverse test chemicals, Toxicol. Sci 162 (2018) 146–166. 10.1093/toxsci/kfx235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wilde S, Dambowsky M, Hempt C, Sutter A, Queisser N, Classification of in vitro genotoxicants using a novel multiplexed biomarker assay compared to the flow cytometric micronucleus test: classification of in vitro genotoxicants, Environ. Mol. Mutagen 58 (2017) 662–677. 10.1002/em.22130. [DOI] [PubMed] [Google Scholar]
- [75].Aguilera A, García-Muse T, Causes of genome instability, Annu. Rev. Genet 47 (2013) 1–32. 10.1146/annurev-genet-111212-133232. [DOI] [PubMed] [Google Scholar]
- [76].Ahmad SS, Ahmed K, Venkitaraman AR, Science in focus: genomic instability and its implications for clinical cancer care, Clin. Oncol 30 (2018) 751–755. 10.1016/j.clon.2018.09.001. [DOI] [PubMed] [Google Scholar]
- [77].Lee J-K, Choi Y-L, Kwon M, Park PJ, Mechanisms and consequences of cancer genome instability: lessons from genome sequencing studies, Annu. Rev. Pathol.: Mech. Dis 11 (2016) 283–312. 10.1146/annurev-pathol-012615-044446. [DOI] [PubMed] [Google Scholar]
- [78].Yao Y, Dai W, Genomic instability and cancer, J. Carcinog. Mutagen 5 (2014) 1000165. 10.4172/2157-2518.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell. 144 (2011) 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [80].Chatterjee N, Walker GC, Mechanisms of DNA damage, repair, and mutagenesis, Environ. Mol. Mutagen 58 (2017) 235–263. 10.1002/em.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Friedberg EC, A brief history of the DNA repair field, Cell Res 18 (2008) 3–7. 10.1038/cr.2007.113. [DOI] [PubMed] [Google Scholar]
- [82].Valdiglesias V, Pásaro E, Méndez J, Laffon B, Assays to determine DNA repair ability, J. Toxicol. Environ. Health Part A. 74 (2011) 1094–1109. 10.1080/15287394.2011.582320. [DOI] [PubMed] [Google Scholar]
- [83].Figueroa-González G, Pérez-Plasencia C, Strategies for the evaluation of DNA damage and repair mechanisms in cancer, Oncol. Lett 13 (2017) 3982–3988. 10.3892/ol.2017.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhu Y, Hu J, Hu Y, Liu W, Targeting DNA repair pathways: a novel approach to reduce cancer therapeutic resistance, Cancer Treat. Rev 35 (2009) 590–596. 10.1016/j.ctrv.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [85].IARC, Tumour site concordance and mechanisms of carcinogenesis, WHO Press, Switzerland, 2019. https://publications.iarc.fr/_publications/media/download/5592/a0d74b9a500b62a4fb6d2ee6c2926b54b82cb9dc.pdf. [PubMed] [Google Scholar]
- [86].Herceg Z, Lambert M-P, van Veldhoven K, Demetriou C, Vineis P, Smith MT, Straif K, Wild CP, Towards incorporating epigenetic mechanisms into carcinogen identification and evaluation, Carcinogenesis. 34 (2013) 1955–1967. 10.1093/carcin/bgt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Al-Zoughool M, Bird M, Rice J, Baan RA, Billard M, Birkett N, Krewski D, Zielinski JM, Development of a database on key characteristics of human carcinogens, J. Toxicol. Environ. Health, Part B. 22 (2019) 264–287. 10.1080/10937404.2019.1642593. [DOI] [PubMed] [Google Scholar]
- [88].Beetch M, Harandi-Zadeh S, Yang T, Boycott C, Chen Y, Stefanska B, Mohammed S, DNA methylation landscape of triple-negative ductal carcinoma in situ (DCIS) progressing to the invasive stage in canine breast cancer, Sci. Rep 10 (2020) 2415. 10.1038/s41598-020-59260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Herceg Z, Ghantous A, Wild CP, Sklias A, Casati L, Duthie SJ, Fry R, Issa J-P, Kellermayer R, Koturbash I, Kondo Y, Lepeule J, Lima SCS, Marsit CJ, Rakyan V, Saffery R, Taylor JA, Teschendorff AE, Ushijima T, Vineis P, Walker CL, Waterland RA, Wiemels J, Ambatipudi S, Esposti DD, Hernandez-Vargas H, Roadmap for investigating epigenome deregulation and environmental origins of cancer, Int. J. Cancer. 142 (2018) 874–882. 10.1002/ijc.31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Morgensztern D, Devarakonda S, Mitsudomi T, Maher C, Govindan R, 11 - Mutational Events in Lung Cancer: Present and Developing Technologies, in: Pass HI, Ball D, Scagliotti GV (Eds.), IASLC Thoracic Oncology (Second Edition), Second Edition, Elsevier, Philadelphia, 2018: pp. 95–103.e2. 10.1016/B978-0-323-52357-8.00011-1. [DOI] [Google Scholar]
- [91].Tryndyak VP, Role of epigenetics in tumor induction by non-genotoxic carcinogens, Current Opinion in Toxicology. 6 (2017) 42–49. 10.1016/j.cotox.2017.08.004. [DOI] [Google Scholar]
- [92].Chappell GA, Rager JE, Epigenetics in chemical-induced genotoxic carcinogenesis, Current Opinion in Toxicology. 6 (2017) 10–17. 10.1016/j.cotox.2017.06.007. [DOI] [Google Scholar]
- [93].Chappell G, Pogribny IP, Guyton KZ, Rusyn I, Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review, Mutat. Res., Rev. Mutat. Res 768 (2016) 27–45. 10.1016/j.mrrev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang Z, Yang C, Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: A novel mechanism of metal carcinogenesis, Semin. Cancer Biol 57 (2019) 95–104. 10.1016/j.semcancer.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dusinska M, Tulinska J, El Yamani N, Kuricova M, Liskova A, Rollerova E, Rundén-Pran E, Smolkova B, Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing?, Food Chem. Toxicol 109 (2017) 797–811. 10.1016/j.fct.2017.08.030. [DOI] [PubMed] [Google Scholar]
- [96].Greally JM, Jacobs MN, In vitro and in vivo testing methods of epigenomic endpoints for evaluating endocrine disruptors, ALTEX. 30 (2013) 445–471. 10.14573/altex.2013.4.445. [DOI] [PubMed] [Google Scholar]
- [97].Jacobs MN, Colacci A, Louekari K, Luijten M, Hakkert BC, Paparella M, Vasseur P, International regulatory needs for development of an IATA for non-genotoxic carcinogenic chemical substances, ALTEX. 33 (2016) 359–392. 10.14573/altex.1601201. [DOI] [PubMed] [Google Scholar]
- [98].Ren N, Atyah M, Chen W-Y, Zhou C-H, The various aspects of genetic and epigenetic toxicology: testing methods and clinical applications, J. Transl. Med 15 (2017) 110. 10.1186/s12967-017-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jacobs MN, Colacci A, Corvi R, Vaccari M, Aguila MC, Corvaro M, Delrue N, Desaulniers D, Ertych N, Jacobs A, Luijten M, Madia F, Nishikawa A, Ogawa K, Ohmori K, Paparella M, Sharma AK, Vasseur P, Chemical carcinogen safety testing: OECD expert group international consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens, Arch. Toxicol 94 (2020) 2899–2923. 10.1007/s00204-020-02784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Parfett CL, Desaulniers D, A Tox21 approach to altered epigenetic landscapes: assessing epigenetic toxicity pathways leading to altered gene expression and oncogenic transformation in vitro, Int. J. Mol. Sci 18 (2017) 1179. 10.3390/ijms18061179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Benigni R, Bossa C, Tcheremenskaia O, Nongenotoxic carcinogenicity of chemicals: mechanisms of action and early recognition through a new set of structural alerts, Chem. Rev 113 (2013) 2940–2957. 10.1021/cr300206t. [DOI] [PubMed] [Google Scholar]
- [102].Woo Y-T, Lai DY, Mechanisms of action of chemical carcinogens and their role in structure–activity relationships (SAR) analysis and risk assessment, in: Benigni R (Ed.), Quantitative Structure-Activity Relationship (QSAR) Models of Mutagens and Carcinogens, CRC Press, Taylor & Francis Group, 2003. [Google Scholar]
- [103].Aguayo-Ortiz R, Fernández-de Gortari E, Chapter 2 - Overview of computer-aided drug design for epigenetic targets, in: Medina-Franco JL (Ed.), Epi-Informatics, Academic Press, Boston, 2016: pp. 21–52. 10.1016/B978-0-12-802808-7.00002-2. [DOI] [Google Scholar]
- [104].Lu W, Zhang R, Jiang H, Zhang H, Luo C, Computer-aided drug design in epigenetics, Front. Chem 6 (2018) 57. 10.3389/fchem.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Somogyvari EE, Akl SG, Winn LM, Computational approaches to epigenetic drug discovery, in: Adamatzky A (Ed.), Emergent Computation : A Festschrift for Selim G. Akl, Springer International Publishing, Cham, 2017: pp. 453–465. 10.1007/978-3-319-46376-6_21. [DOI] [Google Scholar]
- [106].Sies H, Berndt C, Jones DP, Oxidative stress, Annu. Rev. Biochem 86 (2017) 715–748. 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- [107].Hayes JD, Dinkova-Kostova AT, Tew KD, Oxidative stress in cancer, Cancer Cell. 38 (2020) 167–197. 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tsegay PS, Lai Y, Liu Y, Replication stress and consequential instability of the genome and epigenome, Molecules. 24 (2019) 3870. 10.3390/molecules24213870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].He F, Ru X, Wen T, NRF2, a transcription factor for stress response and beyond, Int. J. Mol. Sci 21 (2020) 4777. 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rojo de la Vega M, Chapman E, Zhang DD, NRF2 and the hallmarks of cancer, Cancer Cell. 34 (2018) 21–43. 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bai F, Hong D, Lu Y, Liu H, Xu C, Yao X, Prediction of the antioxidant response elements’ response of compound by deep learning, Front. Chem 7 (2019) 385. 10.3389/fchem.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Bradbury SP, Mekenyan O, Veith GD, Zaharieva N, SAR models for futile metabolism: one-electron reduction of quinones, phenols and nitrobenzenes, SAR QSAR Environ. Res 4 (1995) 109–124. 10.1080/10629369508029908. [DOI] [Google Scholar]
- [113].Mayr A, Klambauer G, Unterthiner T, Hochreiter S, DeepTox: toxicity prediction using deep learning, Front. Environ. Sci 3 (2016). 10.3389/fenvs.2015.00080. [DOI] [Google Scholar]
- [114].Mekenyan OG, Bradbury SP, Kamenska VB, Estimating one-electron reduction potentials of quinones, SAR QSAR Environ. Res 5 (1996) 255–268. 10.1080/10629369608031715. [DOI] [Google Scholar]
- [115].Sierra M, Bragg-Gonzalo L, Grasa J, Muñoz MJ, González D, Miana-Mena FJ, Oxidative stress prediction: A preliminary approach using a response surface based technique, Toxicol. In Vitro. 46 (2018) 273–283. 10.1016/j.tiv.2017.10.016. [DOI] [PubMed] [Google Scholar]
- [116].Williamson TP, Amirahmadi S, Joshi G, Kaludov NK, Martinov MN, Johnson DA, Johnson JA, Discovery of potent, novel Nrf2 inducers via quantum modeling, virtual screening, and in vitro experimental validation, Chem. Biol. Drug Des 80 (2012) 810–820. 10.1111/cbdd.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zgheib E, Limonciel A, Jiang X, Wilmes A, Wink S, van de Water B, Kopp-Schneider A, Bois FY, Jennings P, Investigation of Nrf2, AhR and ATF4 activation in toxicogenomic databases, Front. Genet 9 (2018) 429. 10.3389/fgene.2018.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Smith GR, Shanley DP, Computational modelling of the regulation of Insulin signalling by oxidative stress, BMC Syst. Biol 7 (2013) 41. 10.1186/1752-0509-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Zendehdel R, Shetab-Boushehri SV, Azari MR, Hosseini V, Mohammadi H, Chemometrics models for assessment of oxidative stress risk in chrome-electroplating workers, Drug Chem. Toxicol 38 (2015) 174–179. 10.3109/01480545.2014.922096. [DOI] [PubMed] [Google Scholar]
- [120].Onoue S, Ohtake H, Suzuki G, Seto Y, Nishida H, Hirota M, Ashikaga T, Kouzuki H, Comparative study on prediction performance of photosafety testing tools on photoallergens, Toxicol. In Vitro. 33 (2016) 147–152. 10.1016/j.tiv.2016.03.003. [DOI] [PubMed] [Google Scholar]
- [121].Muller C, Pekthong D, Alexandre E, Marcou G, Horvath D, Richert L, Varnek A, Prediction of drug induced liver injury using molecular and biological descriptors, Comb. Chem. High Throughput Screening. 18 (2015) 315–322. 10.2174/1386207318666150305144650. [DOI] [PubMed] [Google Scholar]
- [122].Bou R, Codony R, Tres A, Decker EA, Guardiola F, Determination of hydroperoxides in foods and biological samples by the ferrous oxidation–xylenol orange method: A review of the factors that influence the method’s performance, Anal. Biochem 377 (2008) 1–15. 10.1016/j.ab.2008.02.029. [DOI] [PubMed] [Google Scholar]
- [123].Marrocco I, Altieri F, Peluso I, Measurement and clinical significance of biomarkers of oxidative stress in humans, Oxid. Med. Cell. Longevity. 2017 (2017) 1–32. 10.1155/2017/6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Woolley JF, Stanicka J, Cotter TG, Recent advances in reactive oxygen species measurement in biological systems, Trends Biochem. Sci 38 (2013) 556–565. 10.1016/j.tibs.2013.08.009. [DOI] [PubMed] [Google Scholar]
- [125].Hancock J, Conway M, eds., Redox-mediated signal transduction, 2nd edition, Humana Press, New York, 2019. 10.1007/978-1-4939-9463-2. [DOI] [Google Scholar]
- [126].Bime C, Zhou T, Wang T, Slepian MJ, Garcia JGN, Hecker L, Reactive Oxygen Species–associated molecular signature predicts survival in patients with sepsis, Pulm. Circ 6 (2016) 196–201. 10.1086/685547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Møller P, The comet assay: ready for 30 more years, Mutagenesis. 33 (2018) 1–7. 10.1093/mutage/gex046. [DOI] [PubMed] [Google Scholar]
- [128].Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF, Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing, Environ. Mol. Mutagen 35 (2000) 206–221. . [DOI] [PubMed] [Google Scholar]
- [129].Patrineli A, Clifford MN, Ioannides C, Contribution of phenols, quinones and reactive oxygen species to the mutagenicity of white grape juice in the Ames test, Food Chem. Toxicol 34 (1996) 869–872. 10.1016/s0278-6915(96)00048-8. [DOI] [PubMed] [Google Scholar]
- [130].Wilcox P, Naidoo A, Wedd DJ, Gatehouse DG, Comparison of Salmonella Typhimurium TA102 with Escherichia coli WP2 tester strains, Mutagenesis. 5 (1990) 285–291. 10.1093/mutage/5.3.285. [DOI] [PubMed] [Google Scholar]
- [131].Mourad R, Ginalski K, Legube G, Cuvier O, Predicting double-strand DNA breaks using epigenome marks or DNA at kilobase resolution, Genome Biol 19 (2018) 34. 10.1186/s13059-018-1411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Cipak Gasparovic A, Zarkovic N, Zarkovic K, Semen K, Kaminskyy D, Yelisyeyeva O, Bottari SP, Biomarkers of oxidative and nitro-oxidative stress: conventional and novel approaches, Br. J. Pharmacol 174 (2017) 1771–1783. 10.1111/bph.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Tsikas D, Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges, Anal. Biochem 524 (2017) 13–30. 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- [134].Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska T, Gasparovic AC, Cuadrado A, Weber D, Poulsen HE, Grune T, Schmidt HHHW, Ghezzi P, Clinical relevance of biomarkers of oxidative stress, Antioxid. Redox Signaling. 23 (2015) 1144–1170. 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kim MT, Huang R, Sedykh A, Wang W, Xia M, Zhu H, Mechanism profiling of hepatotoxicity caused by oxidative stress using antioxidant response element reporter gene assay models and big data, Environ. Health Perspect 124 (2016) 634–641. 10.1289/ehp.1509763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Shukla SJ, Huang R, Simmons SO, Tice RR, Witt KL, Vanleer D, Ramabhadran R, Austin CP, Xia M, Profiling environmental chemicals for activity in the antioxidant response element signaling pathway using a high throughput screening approach, Environ. Health Perspect 120 (2012) 1150–1156. 10.1289/ehp.1104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zhao J, Shukla SJ, Xia M, Cell-based assay for identifying the modulators of antioxidant response element signaling pathway, Methods Mol. Biol 1473 (2016) 55–62. 10.1007/978-1-4939-6346-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ramírez-Expósito MJ, Mayas MD, Carrera-González MP, Martínez-Martos JM, Gender differences in the antioxidant response to oxidative stress in experimental brain tumors, Curr. Cancer Drug Targets. 19 (2019) 641–654. 10.2174/1568009618666181018162549. [DOI] [PubMed] [Google Scholar]
- [139].Weydert CJ, Cullen JJ, Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue, Nat. Protoc 5 (2010) 51–66. 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Giustarini D, Colombo G, Garavaglia ML, Astori E, Portinaro NM, Reggiani F, Badalamenti S, Aloisi AM, Santucci A, Rossi R, Milzani A, Dalle-Donne I, Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells, Free Radical Biol. Med 112 (2017) 360–375. 10.1016/j.freeradbiomed.2017.08.008. [DOI] [PubMed] [Google Scholar]
- [141].Medzhitov R, Origin and physiological roles of inflammation, Nature. 454 (2008) 428–435. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- [142].Vendramini-Costa DB, Carvalho JE, Molecular link mechanisms between inflammation and cancer, Curr. Pharm. Des 18 (2012) 3831–3852. 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- [143].Multhoff G, Radons J, Radiation, inflammation, and immune responses in cancer, Front. Oncol 2 (2012) 58. 10.3389/fonc.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Coussens LM, Werb Z, Inflammation and cancer, Nature. 420 (2002) 860–867. 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Greten FR, Grivennikov SI, Inflammation and cancer: triggers, mechanisms, and consequences, Immunity. 51 (2019) 27–41. 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kane A, Inflammation - Chapter 17, in: Baan RA, Stewart BW, Straif K (Eds.), Tumour Site Concordance and Mechanisms of Carcinogenesis, WHO Press, World Health Organization, Switzerland, 2019. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Tumour-Site-Concordance-And-Mechanisms-Of-Carcinogenesis-2019. [PubMed] [Google Scholar]
- [147].Liu CH, Abrams ND, Carrick DM, Chander P, Dwyer J, Hamlet MRJ, Macchiarini F, PrabhuDas M, Shen GL, Tandon P, Vedamony MM, Biomarkers of chronic inflammation in disease development and prevention: challenges and opportunities, Nat. Immunol 18 (2017) 1175–1180. 10.1038/ni.3828. [DOI] [PubMed] [Google Scholar]
- [148].Wu Y, Antony S, Meitzler JL, Doroshow JH, Molecular mechanisms underlying chronic inflammation-associated cancers, Cancer Lett 345 (2014) 164–173. 10.1016/j.canlet.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Piotrowski I, Kulcenty K, Suchorska W, Interplay between inflammation and cancer, Rep. Pract. Oncol. Radiother 25 (2020) 422–427. 10.1016/j.rpor.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Multhoff G, Molls M, Radons J, Chronic inflammation in cancer development, Front. Immunol 2 (2012). 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Brenner DR, Scherer D, Muir K, Schildkraut J, Boffetta P, Spitz MR, Le Marchand L, Chan AT, Goode EL, Ulrich CM, Hung RJ, A review of the application of inflammatory biomarkers in epidemiologic cancer research, Cancer Epidemiol., Biomarkers Prev 23 (2014) 1729–1751. 10.1158/1055-9965.EPI-14-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM, Chronic inflammation in the etiology of disease across the life span, Nat. Med 25 (2019) 1822–1832. 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Allin KH, Bojesen SE, Nordestgaard BG, Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population, Int. J. Cancer. 139 (2016) 1493–1500. 10.1002/ijc.30194. [DOI] [PubMed] [Google Scholar]
- [154].Chiu WA, Guyton KZ, Martin MT, Reif DM, Rusyn I, Use of high-throughput in vitro toxicity screening data in cancer hazard evaluations by IARC Monograph Working Groups, ALTEX. 35 (2018) 51–64. 10.14573/altex.1703231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Jackson PJM, Jamshidi S, Farag DB, Computational approaches in the development of small-molecule transcription factor inhibitors, in: Rahman KM, Thurston DE (Eds.), Small-Molecule Transcription Factor Inhibitors in Oncology:, Royal Society of Chemistry, Cambridge, 2018. 10.1039/9781782624011. [DOI] [Google Scholar]
- [156].Miyasaka M, Takatsu K, eds., Chronic inflammation, Springer Japan, Tokyo, 2016. 10.1007/978-4-431-56068-5. [DOI] [Google Scholar]
- [157].Lebrec H, Brennan FR, Haggerty H, Herzyk D, Kamperschroer C, Maier CC, Ponce R, Preston BD, Weinstock D, Mellon RD, HESI/FDA workshop on immunomodulators and cancer risk assessment: Building blocks for a weight-of-evidence approach, Regul. Toxicol. Pharmacol 75 (2016) 72–80. 10.1016/j.yrtph.2015.12.018. [DOI] [PubMed] [Google Scholar]
- [158].Ponce R, Immunomodulation and cancer: Using mechanistic paradigms to inform risk assessment, Current Opinion in Toxicology. 10 (2018) 98–110. 10.1016/j.cotox.2018.06.002. [DOI] [Google Scholar]
- [159].Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD, Cancer immunoediting: from immunosurveillance to tumor escape, Nat. Immunol 3 (2002) 991–998. 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- [160].Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, Goedert JJ, Cancer risk in people infected with human immunodeficiency virus in the United States, Int. J. Cancer. 123 (2008) 187–194. 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- [161].Mantovani A, Allavena P, Sica A, Balkwill F, Cancer-related inflammation, Nature. 454 (2008) 436–444. 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- [162].Küppers R, Mechanisms of B-cell lymphoma pathogenesis, Nat. Rev. Cancer. 5 (2005) 251–262. 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- [163].Bugelski PJ, Volk A, Walker MR, Krayer JH, Martin P, Descotes J, Critical review of preclinical approaches to evaluate the potential of immunosuppressive drugs to influence human neoplasia, Int. J. Toxicol 29 (2010) 435–466. 10.1177/1091581810374654. [DOI] [PubMed] [Google Scholar]
- [164].Guo H, Ahn S, Zhang L, Benzene-associated immunosuppression and chronic inflammation in humans: a systematic review, Occup. Environ. Med (2020) oemed-2020–106517. 10.1136/oemed-2020-106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].FDA, FDA draft nonclinical safety evaluation of the immunotoxic potential of drugs and biologics - Guidance for industry, in: Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, 2020. https://www.fda.gov/media/135312/download. [Google Scholar]
- [166].Alden CL, Lynn A, Bourdeau A, Morton D, Sistare FD, Kadambi VJ, Silverman L, A critical review of the effectiveness of rodent pharmaceutical carcinogenesis testing in predicting for human risk, Vet. Pathol 48 (2011) 772–784. 10.1177/0300985811400445. [DOI] [PubMed] [Google Scholar]
- [167].Bosland MC, Receptor-mediated mechanisms - Chapter 14, in: Baan RA, Stewart BW, Straif K (Eds.), Tumour Site Concordance and Mechanisms of Carcinogenesis, WHO Press, World Health Organization, Switzerland, 2019. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Tumour-Site-Concordance-And-Mechanisms-Of-Carcinogenesis-2019. [Google Scholar]
- [168].Roberts MF, Kruchten AE, Introduction, in: Receptor Biology, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016. https://www.academia.edu/30128984/Receptor_Biology (accessed September 30, 2020). [Google Scholar]
- [169].Guyton KZ, Rusyn I, Chiu WA, Corpet DE, van den Berg M, Ross MK, Christiani DC, Beland FA, Smith MT, Application of the key characteristics of carcinogens in cancer hazard identification, Carcinogenesis. 39 (2018) 614–622. 10.1093/carcin/bgy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E, PI3K/AKT signaling pathway and cancer: an updated review, Ann. Med 46 (2014) 372–383. 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- [171].Dhiman VK, Bolt MJ, White KP, Nuclear receptors in cancer - uncovering new and evolving roles through genomic analysis, Nat. Rev. Genet 19 (2018) 160–174. 10.1038/nrg.2017.102. [DOI] [PubMed] [Google Scholar]
- [172].Lonard DM, O’Malley BW, Nuclear receptor coregulators: modulators of pathology and therapeutic targets, Nat. Rev. Endocrinol 8 (2012) 598–604. 10.1038/nrendo.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Gil EMC, Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer, Cancer Treat. Rev 40 (2014) 862–871. 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- [174].Llauradó M, Ruiz A, Majem B, Ertekin T, Colás E, Pedrola N, Devis L, Rigau M, Sequeiros T, Montes M, Garcia M, Cabrera S, Gil-Moreno A, Xercavins J, Castellví J, Garcia A, Ramón y Cajal S, Moreno G, Alameda F, Vázquez-Levin M, Palacios J, Prat J, Doll A, Matías-Guiu X, Abal M, Reventós J, Molecular bases of endometrial cancer: new roles for new actors in the diagnosis and the therapy of the disease, Mol. Cell. Endocrinol 358 (2012) 244–255. 10.1016/j.mce.2011.10.003. [DOI] [PubMed] [Google Scholar]
- [175].Auchus RJ, Sharifi N, Sex hormones and prostate cancer, Annu. Rev. Med 71 (2020) 33–45. 10.1146/annurev-med-051418-060357. [DOI] [PubMed] [Google Scholar]
- [176].Fénichel P, Chevalier N, Is testicular germ cell cancer estrogen dependent? The role of endocrine disrupting chemicals, Endocrinology. 160 (2019) 2981–2989. 10.1210/en.2019-00486. [DOI] [PubMed] [Google Scholar]
- [177].Gibson DA, Saunders PTK, Endocrine disruption of oestrogen action and female reproductive tract cancers, Endocr.-Relat. Cancer. 21 (2014) T13–31. 10.1530/ERC-13-0342. [DOI] [PubMed] [Google Scholar]
- [178].Rutkowska AZ, Szybiak A, Serkies K, Rachoń D, Endocrine disrupting chemicals as potential risk factor for estrogen-dependent cancers, Pol. Arch. Med. Wewn 126 (2016) 562–570. 10.20452/pamw.3481. [DOI] [PubMed] [Google Scholar]
- [179].Morgan MM, Johnson BP, Livingston MK, Schuler LA, Alarid ET, Sung KE, Beebe DJ, Personalized in vitro cancer models to predict therapeutic response: Challenges and a framework for improvement, Pharmacol. Ther 165 (2016) 79–92. 10.1016/j.pharmthera.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Judson RS, Magpantay FM, Chickarmane V, Haskell C, Tania N, Taylor J, Xia M, Huang R, Rotroff DM, Filer DL, Houck KA, Martin MT, Sipes N, Richard AM, Mansouri K, Setzer RW, Knudsen TB, Crofton KM, Thomas RS, Integrated Model of Chemical Perturbations of a Biological Pathway Using 18 In Vitro High-Throughput Screening Assays for the Estrogen Receptor, Toxicol. Sci 148 (2015) 137–154. 10.1093/toxsci/kfv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Kleinstreuer NC, Ceger P, Watt ED, Martin M, Houck K, Browne P, Thomas RS, Casey WM, Dix DJ, Allen D, Sakamuru S, Xia M, Huang R, Judson R, Development and validation of a computational model for androgen receptor activity, Chem. Res. Toxicol 30 (2017) 946–964. 10.1021/acs.chemrestox.6b00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Cotterill JV, Palazzolo L, Ridgway C, Price N, Rorije E, Moretto A, Peijnenburg A, Eberini I, Predicting estrogen receptor binding of chemicals using a suite of in silico methods - Complementary approaches of (Q)SAR, molecular docking and molecular dynamics, Toxicol. Appl. Pharmacol 378 (2019) 114630. 10.1016/j.taap.2019.114630. [DOI] [PubMed] [Google Scholar]
- [183].Garcia-Serna R, Vidal D, Remez N, Mestres J, Large-scale predictive drug safety: from structural alerts to biological mechanisms, Chem. Res. Toxicol 28 (2015) 1875–1887. 10.1021/acs.chemrestox.5b00260. [DOI] [PubMed] [Google Scholar]
- [184].IRCSS, VEGA HUB, Istituto di Ricerche Farmacologiche Mario Negri, https://www.vegahub.eu/, 2020.
- [185].OECD, The OECD QSAR Toolbox, Toolbox 4.4, https://www.oecd.org/chemicalsafety/risk-assessment/oecd-qsar-toolbox.htm, 2020. https://www.oecd.org/chemicalsafety/risk-assessment/oecd-qsar-toolbox.htm.
- [186].Porta N, Roncaglioni A, Marzo M, Benfenati E, QSAR methods to screen endocrine disruptors, Nucl. Recept. Res 3 (2016). 10.11131/2016/101203. [DOI] [Google Scholar]
- [187].Vedani A, Dobler M, Smieško M, VirtualToxLab - a platform for estimating the toxic potential of drugs, chemicals and natural products, Toxicol. Appl. Pharmacol 261 (2012) 142–153. 10.1016/j.taap.2012.03.018. [DOI] [PubMed] [Google Scholar]
- [188].Heusinkveld H, Braakhuis H, Gommans R, Botham P, Corvaro M, van der Laan JW, Lewis D, Madia F, Manou I, Schorsch F, Wolterink G, Woutersen R, Corvi R, Mehta J, Luijten M, Towards a mechanism-based approach for the prediction of nongenotoxic carcinogenic potential of agrochemicals, Crit. Rev. Toxicol (2020) 1–15. 10.1080/10408444.2020.1841732. [DOI] [PubMed] [Google Scholar]
- [189].Felter SP, Foreman JE, Boobis A, Corton JC, Doi AM, Flowers L, Goodman J, Haber LT, Jacobs A, Klaunig JE, Lynch AM, Moggs J, Pandiri A, Human relevance of rodent liver tumors: Key insights from a Toxicology Forum workshop on nongenotoxic modes of action, Regul. Toxicol. Pharmacol 92 (2018) 1–7. 10.1016/j.yrtph.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Fridman AL, Tainsky MA, Critical pathways in cellular senescence and immortalization revealed by gene expression profiling, Oncogene. 27 (2008) 5975–5987. 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Carnero A, Blanco-Aparicio C, Kondoh H, Lleonart ME, Martinez-Leal JF, Mondello C, Scovassi AI, Bisson WH, Amedei A, Roy R, Woodrick J, Colacci A, Vaccari M, Raju J, Al-Mulla F, Al-Temaimi R, Salem HK, Memeo L, Forte S, Singh N, Hamid RA, Ryan EP, Brown DG, Wise JP, Wise SS, Yasaei H, Disruptive chemicals, senescence and immortality, Carcinogenesis. 36 Suppl 1 (2015) S19–37. 10.1093/carcin/bgv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Reddel RR, The role of senescence and immortalization in carcinogenesis, Carcinogenesis. 21 (2000) 477–484. 10.1093/carcin/21.3.477. [DOI] [PubMed] [Google Scholar]
- [193].Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A, Kronenberg F, Kiechl S, Telomere length and risk of incident cancer and cancer mortality, JAMA. 304 (2010) 69–75. 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- [194].Yasaei H, Gilham E, Pickles JC, Roberts TP, O’Donovan M, Newbold RF, Carcinogen-specific mutational and epigenetic alterations in INK4A, INK4B and p53 tumour-suppressor genes drive induced senescence bypass in normal diploid mammalian cells, Oncogene. 32 (2013) 171–179. 10.1038/onc.2012.45. [DOI] [PubMed] [Google Scholar]
- [195].Corvi R, Spielmann H, Hartung T, Chapter 3.6 - Alternative approaches for carcinogenicity and reproductive toxicity, in: Balls M, Combes R, Worth A (Eds.), The History of Alternative Test Methods in Toxicology, Academic Press, 2019: pp. 209–217. 10.1016/B978-0-12-813697-3.00024-X. [DOI] [Google Scholar]
- [196].OECD, Guidance Document on the In Vitro BHAS 42 Cell Transformation Assay, OECD Environment, Health and Safety Publications, Paris, 2016. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2016)1&docLanguage=En. [Google Scholar]
- [197].OECD, Guidance Document on the In Vitro Syrian Hamster Embryo (SHE) Cell Transformation Assay, OECD Environment, Health and Safety Publications, Paris, 2015. http://www.oecd.org/env/ehs/testing/Guidance-Document-on-the-in-vitro-Syrian-Hamster-Embryo-Cell-Transformation-Assay.pdf. [Google Scholar]
- [198].Benigni R, Bossa C, Tcheremenskaia O, Battistelli CL, Giuliani A, The Syrian hamster embryo cells transformation assay identifies efficiently nongenotoxic carcinogens, and can contribute to alternative, integrated testing strategies, Mutat. Res., Genet. Toxicol. Environ. Mutagen 779 (2015) 35–38. 10.1016/j.mrgentox.2015.02.001. [DOI] [PubMed] [Google Scholar]
- [199].Creton S, Aardema MJ, Carmichael PL, Harvey JS, Martin FL, Newbold RF, O’Donovan MR, Pant K, Poth A, Sakai A, Sasaki K, Scott AD, Schechtman LM, Shen RR, Tanaka N, Yasaei H, Cell transformation assays for prediction of carcinogenic potential: state of the science and future research needs, Mutagenesis. 27 (2012) 93–101. 10.1093/mutage/ger053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Isfort RJ, Kerckaert GA, LeBoeuf RA, Comparison of the standard and reduced pH Syrian hamster embryo (SHE) cell in vitro transformation assays in predicting the carcinogenic potential of chemicals, Mutat. Res 356 (1996) 11–63. 10.1016/0027-5107(95)00197-2. [DOI] [PubMed] [Google Scholar]
- [201].OECD, Detailed Review Paper On Cell Transformation Assays For Detection Of Chemical Carcinogens, OECD Environment, Health and Safety Publications, Paris, 2007. http://www.oecd.org/chemicalsafety/testing/series-testing-assessment-publications-number.htm. [Google Scholar]
- [202].Guan D, Fan K, Spence I, Matthews S, QSAR ligand dataset for modelling mutagenicity, genotoxicity, and rodent carcinogenicity, Data in Brief. 17 (2018) 876–884. 10.1016/j.dib.2018.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Guan D, Fan K, Spence I, Matthews S, Combining machine learning models of in vitro and in vivo bioassays improves rat carcinogenicity prediction, Regul. Toxicol. Pharmacol 94 (2018) 8–15. 10.1016/j.yrtph.2018.01.008. [DOI] [PubMed] [Google Scholar]
- [204].Hooten NN, Evans MK, Techniques to induce and quantify cellular senescence, J. Visualized Exp (2017). 10.3791/55533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Skvortsov DA, Zvereva ME, Shpanchenko OV, Dontsova OA, Assays for detection of telomerase activity, Acta Naturae. 3 (2011) 48–68. [PMC free article] [PubMed] [Google Scholar]
- [206].Carugno M, Maggioni C, Crespi E, Bonzini M, Cuocina S, Dioni L, Tarantini L, Consonni D, Ferrari L, Pesatori AC, Night shift work DNA methylation and telomere length: an investigation on hospital female nurses, Int. J. Environ. Res. Public Health. 16 (2019). 10.3390/ijerph16132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Cawthon RM, Telomere length measurement by a novel monochrome multiplex quantitative PCR method, Nucleic Acids Res 37 (2009) e21. 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Liang G, Schernhammer E, Qi L, Gao X, De Vivo I, Han J, Associations between rotating night shifts, sleep duration, and telomere length in women, PLoS ONE. 6 (2011) e23462. 10.1371/journal.pone.0023462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, Tyrka AR, Early life adversity and telomere length: a meta-analysis, Mol. Psychiatry. 23 (2018) 858–871. 10.1038/mp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Wakao S, Kitada M, Kuroda Y, Ogura F, Murakami T, Niwa A, Dezawa M, Morphologic and gene expression criteria for identifying human induced pluripotent stem cells, PLoS ONE. 7 (2012) e48677. 10.1371/journal.pone.0048677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Zhu L-F, Xiao M, Chen Y-Q, Wang L-Y, Luo X-F, Yuan X-H, Ren J-H, Chen Z-Z, Hu J-D, Yang T, In vitro effects of reprogramming factors on the expressions of pluripotent genes and CD34 gene in human acute promyelocytic leukemia HL-60 cells, Genomics. 109 (2017) 331–335. 10.1016/j.ygeno.2017.05.006. [DOI] [PubMed] [Google Scholar]
- [212].Noguchi K, Eguchi H, Konno M, Kawamoto K, Nishida N, Koseki J, Wada H, Marubashi S, Nagano H, Doki Y, Mori M, Ishii H, Susceptibility of pancreatic cancer stem cells to reprogramming, Cancer Sci 106 (2015) 1182–1187. 10.1111/cas.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Rajendran JG, Mankoff DA, O’Sullivan F, Peterson LM, Schwartz DL, Conrad EU, Spence AM, Muzi M, Farwell DG, Krohn KA, Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging, Clin. Cancer Res 10 (2004) 2245–2252. 10.1158/1078-0432.ccr-0688-3. [DOI] [PubMed] [Google Scholar]
- [214].Ryter SW, Mizumura K, Choi AMK, The impact of autophagy on cell death modalities, International Journal of Cell Biology. 2014 (2014) 502676. 10.1155/2014/502676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Coussens LM, Zitvogel L, Palucka AK, Neutralizing tumor-promoting chronic inflammation: a magic bullet?, Science. 339 (2013) 286–291. 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Coussens LM, Pollard JW, Leukocytes in mammary development and cancer, Cold Spring Harbor Perspect. Biol 3 (2011). 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Pollard JW, Macrophages define the invasive microenvironment in breast cancer, J. Leukoc. Biol 84 (2008) 623–630. 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, Kimmelman A, Kumar S, Levine B, Maiuri MC, Martin SJ, Penninger J, Piacentini M, Rubinsztein DC, Simon H-U, Simonsen A, Thorburn AM, Velasco G, Ryan KM, Kroemer G, Autophagy in malignant transformation and cancer progression, EMBO J 34 (2015) 856–880. 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Trosko JE, Chang CC, Madhukar BV, Klaunig JE, Chemical, oncogene and growth factor inhibition gap junctional intercellular communication: an integrative hypothesis of carcinogenesis, Pathobiology. 58 (1990) 265–278. 10.1159/000163596. [DOI] [PubMed] [Google Scholar]
- [220].Trosko JE, Ruch RJ, Cell-cell communication in carcinogenesis, Front. Biosci 3 (1998) d208–236. 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- [221].Yamasaki H, Gap junctional intercellular communication and carcinogenesis, Carcinogenesis. 11 (1990) 1051–1058. 10.1093/carcin/11.7.1051. [DOI] [PubMed] [Google Scholar]
- [222].Mao X-Y, Li Q-Q, Gao Y-F, Zhou H-H, Liu Z-Q, Jin W-L, Gap junction as an intercellular glue: Emerging roles in cancer EMT and metastasis, Cancer Lett 381 (2016) 133–137. 10.1016/j.canlet.2016.07.037. [DOI] [PubMed] [Google Scholar]
- [223].Sinyuk M, Mulkearns-Hubert EE, Reizes O, Lathia J, Cancer connectors: connexins, gap junctions, and communication, Front. Oncol 8 (2018) 646. 10.3389/fonc.2018.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Krutovskikh V, Yamasaki H, Gap junctional intercellular communication as a method to detect and predict carcinogenicity, in: Kitchin KT (Ed.), Carcinogenicity: Testing, Predicting, and Interpreting Chemical Effects, M. Dekker, New York, 1999: pp. 267–286. [Google Scholar]
- [225].Rosenkranz HS, Pollack N, Cunningham AR, Exploring the relationship between the inhibition of gap junctional intercellular communication and other biological phenomena, Carcinogenesis. 21 (2000) 1007–1011. 10.1093/carcin/21.5.1007. [DOI] [PubMed] [Google Scholar]
- [226].Chipman JK, Mally A, Edwards GO, Disruption of gap junctions in toxicity and carcinogenicity, Toxicol. Sci 71 (2003) 146–153. 10.1093/toxsci/71.2.146. [DOI] [PubMed] [Google Scholar]
- [227].Kalimi GH, Sirsat SM, Phorbol ester tumor promoter affects the mouse epidermal gap junctions, Cancer Lett 22 (1984) 343–350. 10.1016/0304-3835(84)90173-3. [DOI] [PubMed] [Google Scholar]
- [228].Rivedal E, Yamasaki H, Sanner T, Inhibition of gap junctional intercellular communication in Syrian hamster embryo cells by TPA, retinoic acid and DDT, Carcinogenesis. 15 (1994) 689–694. 10.1093/carcin/15.4.689. [DOI] [PubMed] [Google Scholar]
- [229].Yotti LP, Chang CC, Trosko JE, Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter, Science. 206 (1979) 1089–1091. 10.1126/science.493994. [DOI] [PubMed] [Google Scholar]
- [230].Zefferino R, Piccoli C, Di Gioia S, Capitanio N, Conese M, Gap junction intercellular communication in the carcinogenesis hallmarks: is this a phenomenon or epiphenomenon?, Cells. 8 (2019) 896. 10.3390/cells8080896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Fei J, Zhou L, Liu T, Tang X-Y, Pharmacophore modeling, virtual screening, and molecular docking studies for discovery of novel Akt2 inhibitors, Int. J. Med. Sci 10 (2013) 265–275. 10.7150/ijms.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Gao J, Che D, Zheng VW, Zhu R, Liu Q, Integrated QSAR study for inhibitors of Hedgehog Signal Pathway against multiple cell lines: a collaborative filtering method, BMC Bioinformatics. 13 (2012) 186. 10.1186/1471-2105-13-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Goudarzi N, Arab Chamjangali M, Kalhor P, Linear and nonlinear QSAR study of N2 and O6 substituted guanine derivatives as cyclin-dependent kinase 2 inhibitors, ISRN Anal. Chem 2013 (2013) e151464. 10.1155/2013/151464. [DOI] [Google Scholar]
- [234].Halder AK, Giri AK, Cordeiro MNDS, Multi-target chemometric modelling, fragment analysis and virtual screening with ERK inhibitors as potential anticancer agents, Molecules. 24 (2019). 10.3390/molecules24213909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Halder AK, Cordeiro MNDS, Development of multi-target chemometric models for the inhibition of class I PI3K enzyme isoforms: a case study using QSAR-Co tool, Int. J. Mol. Sci 20 (2019). 10.3390/ijms20174191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Lakhlili W, Yasri A, Ibrahimi A, Structure-activity relationships study of mTOR kinase inhibition using QSAR and structure-based drug design approaches, OncoTargets Ther 9 (2016) 7345–7353. 10.2147/OTT.S108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Zanni R, Galvez-Llompart M, Morell C, Rodríguez-Henche N, Díaz-Laviada I, Recio-Iglesias MC, Garcia-Domenech R, Galvez J, Novel cancer chemotherapy hits by molecular topology: dual Akt and Beta-catenin inhibitors, PLoS ONE. 10 (2015) e0124244. 10.1371/journal.pone.0124244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Matias M, Campos G, Santos AO, Falcão A, Silvestre S, Alves G, Synthesis, in vitro evaluation and QSAR modelling of potential antitumoral 3,4-dihydropyrimidin-2-(1H)-thiones, Arabian J. Chem 12 (2019) 5086–5102. 10.1016/j.arabjc.2016.12.007. [DOI] [Google Scholar]
- [239].Mullen LMA, Duchowicz PR, Castro EA, QSAR treatment on a new class of triphenylmethyl-containing compounds as potent anticancer agents, Chemom. Intell. Lab. Syst 107 (2011) 269–275. 10.1016/j.chemolab.2011.04.011. [DOI] [Google Scholar]
- [240].Bruce ED, Autenrieth RL, Burghardt RC, Donnelly KC, McDonald TJ, Using quantitative structure-activity relationships (QSAR) to predict toxic endpoints for polycyclic aromatic hydrocarbons (PAH), J. Toxicol. Environ. Health Part A. 71 (2008) 1073–1084. 10.1080/15287390802114337. [DOI] [PubMed] [Google Scholar]
- [241].Ruiz P, Faroon O, Moudgal CJ, Hansen H, De Rosa CT, Mumtaz M, Prediction of the health effects of polychlorinated biphenyls (PCBs) and their metabolites using quantitative structure–activity relationship (QSAR), Toxicol. Lett 181 (2008) 53–65. 10.1016/j.toxlet.2008.06.870. [DOI] [PubMed] [Google Scholar]
- [242].Stenberg M, Hamers T, Machala M, Fonnum F, Stenius U, Lauy A-A, van Duursen MBM, Westerink RHS, Fernandes ECA, Andersson PL, Multivariate toxicity profiles and QSAR modeling of non-dioxin-like PCBs--an investigation of in vitro screening data from ultra-pure congeners, Chemosphere. 85 (2011) 1423–1429. 10.1016/j.chemosphere.2011.08.019. [DOI] [PubMed] [Google Scholar]
- [243].Wood CE, Hukkanen RR, Sura R, Jacobson-Kram D, Nolte T, Odin M, Cohen SM, Scientific and Regulatory Policy Committee (SRPC) review: interpretation and use of cell proliferation data in cancer risk assessment, Toxicol. Pathol 43 (2015) 760–775. 10.1177/0192623315576005. [DOI] [PubMed] [Google Scholar]
- [244].Lecoeur H, Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases, Exp. Cell Res 277 (2002) 1–14. 10.1006/excr.2002.5537. [DOI] [PubMed] [Google Scholar]
- [245].Cheah R, Srivastava R, Stafford ND, Beavis AW, Green V, Greenman J, Measuring the response of human head and neck squamous cell carcinoma to irradiation in a microfluidic model allowing customized therapy, Int. J. Oncol 51 (2017) 1227–1238. 10.3892/ijo.2017.4118. [DOI] [PubMed] [Google Scholar]
- [246].Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, Minor L, Cell viability assays, in: Markossian S, Sittampalam GS, Grossman A, Brimacombe K, Arkin M, Auld D, Austin CP, Baell J, Caaveiro JMM, Chung TDY, Coussens NP, Dahlin JL, Devanaryan V, Foley TL, Glicksman M, Hall MD, Haas JV, Hoare SRJ, Inglese J, Iversen PW, Kahl SD, Kales SC, Kirshner S, Lal-Nag M, Li Z, McGee J, McManus O, Riss T, Saradjian P, Trask OJ, Weidner JR, Wildey MJ, Xia M, Xu X (Eds.), Assay Guidance Manual, Eli Lilly & Company and the National Center for Advancing Translational Sciences, Bethesda (MD), 2013. http://www.ncbi.nlm.nih.gov/books/NBK144065/. [Google Scholar]
- [247].Diepart C, Verrax J, Calderon PB, Feron O, Jordan BF, Gallez B, Comparison of methods for measuring oxygen consumption in tumor cells in vitro, Anal. Biochem 396 (2010) 250–256. 10.1016/j.ab.2009.09.029. [DOI] [PubMed] [Google Scholar]
- [248].Plitzko B, Loesgen S, Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in culture cells for assessment of the energy metabolism, Bio-Protoc 8 (2018) e2850. 10.21769/BioProtoc.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Kalyanaraman B, Cheng G, Hardy M, Ouari O, Lopez M, Joseph J, Zielonka J, Dwinell MB, A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds, Redox Biol 14 (2018) 316–327. 10.1016/j.redox.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Salminen A, Kaarniranta K, Kauppinen A, AMPK and HIF signaling pathways regulate both longevity and cancer growth: the good news and the bad news about survival mechanisms, Biogerontology. 17 (2016) 655–680. 10.1007/s10522-016-9655-7. [DOI] [PubMed] [Google Scholar]
- [251].Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA, Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells, Am. J. Physiol.: Cell Physiol 292 (2007) C125–136. 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- [252].LaRocca J, Costa E, Sriram S, Hannas BR, Johnson KJ, Short-term toxicogenomics as an alternative approach to chronic in vivo studies for derivation of points of departure: A case study in the rat with a triazole fungicide, Regul. Toxicol. Pharmacol 113 (2020) 104655. 10.1016/j.yrtph.2020.104655. [DOI] [PubMed] [Google Scholar]
- [253].Tran TB, Baek C, Min J, Electric cell-substrate impedance sensing (ECIS) with microelectrode arrays for investigation of cancer cell-fibroblasts interaction, PLoS ONE. 11 (2016) e0153813. 10.1371/journal.pone.0153813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Vinken M, Adverse Outcome Pathways as Tools to Assess Drug-Induced Toxicity, in: Benfenati E (Ed.), In Silico Methods for Predicting Drug Toxicity, Humana Press, New York, NY, 2016: pp. 325–337. 10.1007/978-1-4939-3609-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Krutovskikh VA, Oyamada M, Yamasaki H, Sequential changes of gap-junctional intercellular communications during multistage rat liver carcinogenesis: direct measurement of communication in vivo, Carcinogenesis. 12 (1991) 1701–1706. 10.1093/carcin/12.9.1701. [DOI] [PubMed] [Google Scholar]
- [256].Sai K, Kanno J, Hasegawa R, Trosko JE, Inoue T, Prevention of the down-regulation of gap junctional intercellular communication by green tea in the liver of mice fed pentachlorophenol, Carcinogenesis. 21 (2000) 1671–1676. 10.1093/carcin/21.9.1671. [DOI] [PubMed] [Google Scholar]
- [257].Upham BL, Deocampo ND, Wurl B, Trosko JE, Inhibition of gap junctional intercellular communication by perfluorinated fatty acids is dependent on the chain length of the fluorinated tail, Int. J. Cancer. 78 (1998) 491–495. . [DOI] [PubMed] [Google Scholar]
- [258].FDA, FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market, FDA. (2020). https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market (accessed October 19, 2020).
- [259].Judson R, Richard A, Dix DJ, Houck K, Martin M, Kavlock R, Dellarco V, Henry T, Holderman T, Sayre P, Tan S, Carpenter T, Smith E, The toxicity data landscape for environmental chemicals, Environ. Health Perspect 117 (2009) 685–695. 10.1289/ehp.0800168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Atwood ST, Lunn RM, Garner SC, Jahnke GD, New perspectives for cancer hazard evaluation by the report on carcinogens: a case study using read-across methods in the evaluation of haloacetic acids found as water disinfection by-products, Environ. Health Perspect 127 (2019) 125003. 10.1289/EHP5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Madia F, Pillo G, Worth A, Corvi R, Prieto P, Integration of data across toxicity endpoints for improved safety assessment of chemicals: the example of carcinogenicity assessment, Arch Toxicol 95 (2021) 1971–1993. 10.1007/s00204-021-03035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Coish P, Brooks BW, Gallagher EP, Kavanagh TJ, Voutchkova-Kostal A, Zimmerman JB, Anastas PT, Current status and future challenges in molecular design for reduced hazard, ACS Sustainable Chem. Eng 4 (2016) 5900–5906. 10.1021/acssuschemeng.6b02089. [DOI] [Google Scholar]
- [263].Zimmerman JB, Anastas PT, Erythropel HC, Leitner W, Designing for a green chemistry future, Science. 367 (2020) 397–400. 10.1126/science.aay3060. [DOI] [PubMed] [Google Scholar]
- [264].Iyer S, Pham N, Marty M, Sandy M, Solomon G, Zeise L, An Integrated Approach Using Publicly Available Resources for Identifying and Characterizing Chemicals of Potential Toxicity Concern: Proof-of-Concept With Chemicals That Affect Cancer Pathways, Toxicol Sci 169 (2019) 14–24. 10.1093/toxsci/kfz017. [DOI] [PubMed] [Google Scholar]
- [265].Spinu N, Cronin MTD, Enoch SJ, Madden JC, Worth AP, Quantitative adverse outcome pathway (qAOP) models for toxicity prediction, Arch. Toxicol 94 (2020) 1497–1510. 10.1007/s00204-020-02774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Liu L, Shao Z, Lv J, Xu F, Ren S, Jin Q, Yang J, Ma W, Xie H, Zhang D, Chen X, Identification of early warning signals at the critical transition point of colorectal cancer based on dynamic network analysis, Front. Bioeng. Biotechnol 8 (2020) 530. 10.3389/fbioe.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Yang B, Li M, Tang W, Liu W, Zhang S, Chen L, Xia J, Dynamic network biomarker indicates pulmonary metastasis at the tipping point of hepatocellular carcinoma, Nat. Commun 9 (2018) 678. 10.1038/s41467-018-03024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Kreeger PK, Lauffenburger DA, Cancer systems biology: a network modeling perspective, Carcinogenesis. 31 (2010) 2–8. 10.1093/carcin/bgp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Parish ST, Aschner M, Casey W, Corvaro M, Embry MR, Fitzpatrick S, Kidd D, Kleinstreuer NC, Lima BS, Settivari RS, Wolf DC, Yamazaki D, Boobis A, An evaluation framework for new approach methodologies (NAMs) for human health safety assessment, Regul. Toxicol. Pharmacol 112 (2020) 104592. 10.1016/j.yrtph.2020.104592. [DOI] [PubMed] [Google Scholar]
- [270].Luijten M, Corvi R, Mehta J, Corvaro M, Delrue N, Felter S, Haas B, Hewitt NJ, Hilton G, Holmes T, Jacobs MN, Jacobs A, Lamplmair F, Lewis D, Madia F, Manou I, Melching-Kollmuss S, Schorsch F, Schütte K, Sewell F, Strupp C, van der Laan JW, Wolf DC, Wolterink G, Woutersen R, Zvonar Z, Heusinkveld H, Braakhuis H, A comprehensive view on mechanistic approaches for cancer risk assessment of nongenotoxic agrochemicals, Regul. Toxicol. Pharmacol 118 (2020) 104789. 10.1016/j.yrtph.2020.104789. [DOI] [PubMed] [Google Scholar]
- [271].Paparella M, Colacci A, Jacobs MN, Uncertainties of testing methods: What do we (want to) know about carcinogenicity?, ALTEX. 34 (2017) 235–252. 10.14573/altex.1608281. [DOI] [PubMed] [Google Scholar]
- [272].Ghosh J, Lawless MS, Waldman M, Gombar V, Fraczkiewicz R, Modeling ADMET, in: Benfenati E (Ed.), In Silico Methods for Predicting Drug Toxicity, Humana Press, New York, NY, 2016: pp. 63–83. 10.1007/978-1-4939-3609-0_4. [DOI] [PubMed] [Google Scholar]
- [273].Madden JC, In Silico Approaches for Predicting ADME Properties, in: Puzyn T, Leszczynski J, Cronin MT (Eds.), Recent Advances in QSAR Studies, Springer, Dordrecht, 2010: pp. 283–304. 10.1007/978-1-4020-9783-6_10. [DOI] [Google Scholar]
- [274].Mostrag-Szlichtyng A, Worth A, Review of QSAR Models and Software Tools for predicting Biokinetic Properties, Luxembourg, 2010. 10.2788/94537. [DOI] [Google Scholar]
- [275].Shin HK, Kang Y-M, No KT, Predicting ADME Properties of Chemicals, in: Leszczynski J (Ed.), Handbook of Computational Chemistry, Springer, Dordrecht, Netherlands, 2016: pp. 1–37. 10.1007/978-94-007-6169-8_59-1. [DOI] [Google Scholar]
- [276].Coleman WB, Neoplasia, in: Coleman WB, Tsongalis GJ (Eds.), Molecular Pathology, 2nd Edition, Elsevier, 2018: pp. 71–97. 10.1016/B978-0-12-802761-5.00004-3. [DOI] [Google Scholar]
- [277].Feo F, Preneoplastic lesions, in: Schwab M (Ed.), Encyclopedia of Cancer, Springer Berlin Heidelberg, Berlin, Heidelberg, 2011: pp. 2977–2984. 10.1007/978-3-642-16483-5_4724. [DOI] [Google Scholar]
- [278].Malarkey DE, Hoenerhoff MJ, Maronpot RR, Carcinogenesis: manifestation and mechanism, in: Fundamentals of Toxicologic Pathology, Elsevier, 2018: pp. 83–104. 10.1016/B978-0-12-809841-7.00006-X. [DOI] [Google Scholar]
- [279].IARC, Some organophosphate insecticides and herbicides, WHO Press, World Health Organization, Switzerland, 2017. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Organophosphate-Insecticides-And-Herbicides-2017. [Google Scholar]


