Abstract
WQ-3034 is a newly synthesized acidic fluoroquinolone. We assessed its in vitro activity against Mycobacterium tuberculosis and M. avium complex using levofloxacin (LVFX), ciprofloxacin (CPFX), sparfloxacin (SPFX), and KRM-1648 (KRM) as reference drugs. The MICs of these agents were determined by the agar dilution method with 7H11 medium. The MICs at which 50 and 90% of the test strains were inhibited (MIC50s, and MIC90s, respectively) for the test quinolones for rifampin (RMP)-susceptible M. tuberculosis strains were in the order SPFX < LVFX ≦ WQ-3034 ≦ CPFX, while those for RMP-resistant M. tuberculosis strains were in the order SPFX ≦ WQ-3034 ≦ LVFX < CPFX. The MICs of KRM for RMP-susceptible M. tuberculosis were much lower than those of the test quinolones, while the MIC90 of KRM for RMP-resistant M. tuberculosis strains was higher than those of the quinolones. The MIC50s and MIC90s of the test drugs for M. avium were in the order KRM < SPFX < CPFX ≦ WQ-3034 ≦ LVFX, while those for M. intracellulare were in the order KRM < SPFX < WQ-3034 ≒ LVFX ≦ CPFX. Next, we compared the antimicrobial activities of the test drugs against M. tuberculosis organisms residing in cells of the Mono Mac 6 macrophage (Mφ)-like cell line (MM6-Mφs) and of the A-549 type II alveolar cell line (A-549 cells). When drugs were added at the concentration that achieves the maximum concentration in blood, progressive killing or inhibition of the M. tuberculosis organisms residing in MM6-Mφs and A-549 cells was observed in the order KRM > SPFX ≧ LVFX > WQ-3034 > CPFX. The efficacies of all quinolones against intracellular M. tuberculosis organisms were significantly lower in A-549 cells than in MM6-Mφs. WQ-3034 at the MIC caused more marked growth inhibition of intramacrophage M. tuberculosis than did LVFX. These findings indicate that the in vitro anti-M. tuberculosis activity of WQ-3034 is greater than that of CPFX and is comparable to that of LVFX.
The recent epidemic of AIDS has caused a worldwide increase in intractable mycobacterial infections including multidrug-resistant tuberculosis (MDR-TB) and disseminated Mycobacterium avium complex (MAC) infections (8, 23). New quinolones are recommended for use for clinical control of MDR-TB (6), since they exhibit good bactericidal activity against Mycobacterium tuberculosis, achieve effective levels in serum, in tissue, and inside cells following oral administration, and produce few adverse effects (2, 7, 10). Quinolones including ofloxacin (OFLX), levofloxacin (LVFX), ciprofloxacin (CPFX), and sparfloxacin (SPFX) exhibited good therapeutic efficacy against experimental M. tuberculosis infection in mice (11, 13) and also exerted clinical efficacy against tuberculosis including MDR-TB when they were given in combination with other drugs including rifampin (RMP), isoniazid, pyrazinamide, ethambutol, and some aminoglycosides (2, 7).
WQ-3034, a new acidic fluoroquinolone (Fig. 1), has been reported to possess a broad spectrum of action against common bacteria. This drug has much stronger activity than other fluoroquinolones, including CPFX, LVFX, SPFX, grepafloxacin, gatifloxacin, and moxifloxacin, against Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, and Enterococcus faecalis (Y. Ohshita, N. Hayashi, H. Amano, Y. Hirao, Y. Nino, A. Yazaki, and Y. Kobayashi, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-84, 1998; Y. Ohshita and A. Yazaki, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-164, 1997). Moreover, WQ-3034 exhibits potent therapeutic efficacy against S. pneumoniae infection induced in mice (Ohshita et al., 38th ICAAC). In the present study, the in vitro antimicrobial activities of WQ-3034 against M. tuberculosis and MAC were compared with those of LVFX, CPFX, and SPFX, which have been shown to possess potent antimycobacterial activities (9, 11, 13, 16, 17). In Japan, OFLX and LVFX (which is the l isomer of OFLX and which is twice as active as OFLX) (16, 17) have been used by preference for the clinical control of intractable tuberculosis, since they have good pharmacokinetic properties and potent antimycobacterial activities (2, 5, 10). We therefore mainly compared the activity of WQ-3034 with that of LVFX. We also used a new benzoxazinorifamycin, KRM-1648 (KRM), which has strong activity against M. tuberculosis (21), as a comparison drug.
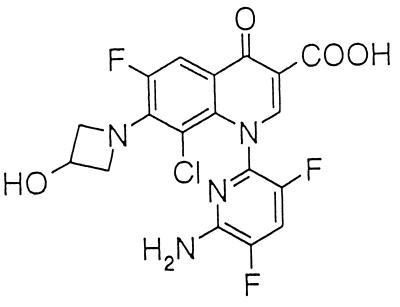
FIG. 1.
Chemical structure of WQ-3034.
MATERIALS AND METHODS
Organisms.
M. tuberculosis (45 strains), M. avium (20 strains), and Mycobacterium intracellulare (20 strains) isolated from sputum specimens of patients with tuberculosis or MAC infections in Japan were grown in 7H9 medium. The M. tuberculosis and MAC strains were isolated in several hospitals in different districts of Japan. The M. tuberculosis strains were divided into two groups on the basis of their susceptibilities to RMP, as follows. One is RMP-susceptible MTB (RMP MIC, ≦0.78 μg/ml; 22 strains) and the other is RMP-resistant MTB (RMP MIC, ≧1.56 μg/ml; 23 strains); the groups were divided according to the standards of the Centers for Disease Control and Prevention's method of proportions test (12). M. avium and M. intracellulare strains were identified by DNA probe testing (Gen-Probe or AccuProbe test).
Cell lines.
Cells of the Mono Mac 6 human macrophage (Mφ)-like cell line (MM6-Mφs; German Collection of Microorganisms and Cell Cultures, Mascheroder, Germany) and of the A-549 cell line, a human type II lung epithelial cell line (A-549 cells; American Type Culture Collection, Manassas, Va.), were used.
Drugs.
WQ-3034 (Wakunaga Pharmaceutical Co., Hiroshima, Japan), LVFX (Daiichi Pharmaceutical Co., Tokyo, Japan), CPFX (Bayer Pharmaceutical Co., Osaka, Japan), SPFX (Dai-Nippon Pharmaceutical Co., Osaka, Japan), RMP (Daiichi Pharmaceutical Co.), and KRM (Kaneka Coorporation, Hyogo, Japan) were used. The quinolones and rifamycin derivatives were initially dissolved in 0.1 N NaOH and dimethyl sulfoxide, respectively, at a concentration of 2 mg/ml.
MIC and MBC determinations.
The MICs of the test drugs were determined as reported previously (18) by either the agar dilution method with Middlebrook 7H11 medium or the broth dilution method with 7HSF medium, a broth medium with the same composition as 7H11 agar but without malachite green. The minimal bactericidal concentrations (MBCs) were determined as described previously (18). Briefly, after measurement of the MICs with 7HSF medium, the MBCs were determined by inoculating 10-μl samples from wells in which test agents allowed no visible growth of the organisms onto a 7H11 agar plate, followed by a 14-day cultivation. MBCs were read as the minimum concentrations of drugs that caused >99.9% killing of the inoculated organisms.
Intracellular growth of organisms.
Cultured MM-6 Mφs and A-549 cells (4 × 104 cells) suspended in 0.2 ml of RPMI 1640 medium and Ham's F-12K medium containing 5% fetal bovine serum, respectively, were seeded on round-bottom microculture wells. MM-6 Mφs and A-549 cells were then infected with M. tuberculosis Kurono or 93062 strain (both are RMP susceptible) at a multiplicity of infection of 30 for 3 h and at a multiplicity of infection of 10 for 2 h, respectively (these conditions yielded comparable loads of mycobacterial infection for MM6-Mφs and A-549 cells). After washing with 2% fetal bovine serum-Hanks' balanced salt solution to remove extracellular bacteria (MM6-Mφs were washed three times by centrifugation at 150 × g for 5 min), M. tuberculosis-infected cells were cultured in 0.2 ml of the corresponding medium in the presence or absence of test drugs for up to 7 days. At intervals, the cells were lysed with 0.07% sodium dodecyl sulfate followed by subsequent neutralization with 6% bovine serum albumin, and the recovered M. tuberculosis organisms were then washed with distilled water by centrifugation (2,000 × g for 30 min). The number of CFU of recovered M. tuberculosis was counted on 7H11 agar plates. In this experiment, the numbers of recovered host cells were increased by about two and three times during 7-day cultivations with MM6-Mφs and A-549 cells, respectively.
RESULTS
MICs of WQ-3034 for M. tuberculosis and MAC.
We determined the MICs of WQ-3034, CPFX, LVFX, SPFX, KRM, and RMP for M. tuberculosis and MAC strains by the agar dilution method using 7H11 medium. Table 1 summarizes the MICs at which 50 and 90% of the test strains were inhibited (MIC50 and MIC90, respectively). First, the MICs of the test quinolones for RMP-susceptible M. tuberculosis strains were in the order SPFX < LVFX ≦ WQ-3034 ≦ CPFX, whereas their MICs for RMP-resistant M. tuberculosis were in the order SPFX ≦ WQ-3034 ≦ LVFX < CPFX. Thus, it appeared that WQ-3034 had anti-M. tuberculosis activity comparable to that of LVFX and was more active than CPFX. WQ-3034 was somewhat more efficacious than LVFX against RMP-resistant M. tuberculosis isolates. Notably, the MICs of the four quinolones for RMP-resistant M. tuberculosis isolates were 4 to 16 times higher than their MICs for RMP-susceptible M. tuberculosis isolates. However, this does not necessarily indicate the cross-resistance of wild-type M. tuberculosis isolates to RMP and quinolones, since most RMP-resistant M. tuberculosis strains were isolated from patients who had been given multiple drug treatments, including treatments with quinolones such as OFLX and CPFX for long periods (personal communications, S. Kawahara, Minami-Okayama Hospital, and M. Sakatani, Kinki-Central Hospital). Although KRM had much lower MICs than those of quinolones for RMP-susceptible M. tuberculosis isolates, its MICs were much higher for RMP-resistant M. tuberculosis isolates.
TABLE 1.
MICs of WQ-3034, CPFX, LVFX, SPFX, KRM, and RMP against M. tuberculosis and MAC strainsa
| Organism | No. of strains | MIC50 (μg/ml)
|
MIC90 (μg/ml)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WQ-3034 | CPFX | LVFX | SPFX | KRM | RMP | WQ-3034 | CPFX | LVFX | SPFX | KRM | RMP | ||
| M. tuberculosis | |||||||||||||
| RMP susceptible | 22 | 0.39 | 0.78 | 0.39 | 0.2 | ≦0.05 | ≦0.05 | 0.78 | 0.78 | 0.39 | 0.2 | ≦0.05 | 0.39 |
| RMP resistant | 23 | 1.56 | 6.25 | 3.13 | 1.56 | 1.56 | 50 | 6.25 | 12.5 | 6.25 | 3.13 | 25 | 100 |
| M. avium | 20 | 3.13 | 3.13 | 6.25 | 1.56 | 0.1 | 50 | 25 | 12.5 | 25 | 6.25 | 0.39 | >100 |
| M. intracellulare | 20 | 25 | 25 | 25 | 12.5 | ≦0.05 | 3.13 | 25 | 50 | 25 | 12.5 | 0.1 | 6.25 |
MICs were determined by the agar dilution method with 7H11 medium.
Second, the MICs of the test quinolones for M. avium were in the order SPFX < CPFX ≦ WQ-3034 ≦ LVFX, while those for M. intracellulare were in the order SPFX < WQ-3034 ≒ LVFX ≦ CPFX (Table 1). As reported previously (22), the MIC50s of the four quinolones for M. avium were four to eight times lower than those for M. intracellulare. In contrast, the MIC50s of rifamycin derivatives (KRM and RMP) for M. avium were ≧2 to 16 times higher than those for M. intracellulare.
By the broth dilution method with 7HSF medium, the MICs and MBCs of WQ-3034 and LVFX for M. tuberculosis Kurono were as follows: for WQ-3034, the MIC and MBC were both 1.0 μg/ml (MBC/MIC = 1); for LVFX, the MIC and MBC were both 0.25 μg/ml (MBC/MIC = 1). This indicates that these quinolones preferably exert bactericidal activity against M. tuberculosis organisms.
Antimicrobial activity of WQ-3034 against intracellular M. tuberculosis.
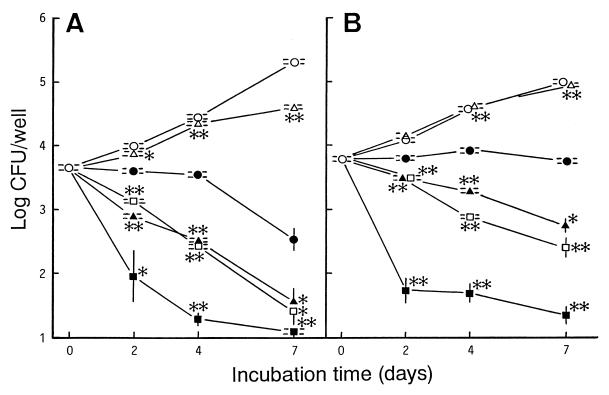
We compared the activity of WQ-3034 against intracellular M. tuberculosis Kurono organisms with those of CPFX, LVFX, SPFX, and KRM. When the drugs were added to the culture medium at concentrations equivalent to the maximum concentration achievable in blood (Cmax), their activities against the M. tuberculosis organisms residing in MM6-Mφs and A-549 cells were in the order KRM > SPFX ≧ LVFX > WQ-3034 > CPFX, irrespective of the host cell type (Fig. 2). Notably, the efficacies of quinolones in killing or inhibiting the growth of intracellular M. tuberculosis isolates were significantly lower for A-549 cells than for MM6-Mφs (P < 0.05; Student's t test). While SPFX, LVFX, and WQ-3034 progressively eliminated the organisms within MM6-Mφs, only the first two were capable of killing the organisms residing in A-549 cells. Notably, among the test drugs KRM had the most potent bactericidal activity against the M. tuberculosis organisms within MM6-Mφs and A-549 cells, irrespective of the host cell type. The same results were obtained when M. tuberculosis 93062 was used (data not shown).
FIG. 2.
Antimicrobial activities of WQ-3034 (●), CPFX (▵), LVFX (▴), SPFX (□), and KRM (■) against M. tuberculosis Kurono residing in MM6-Mφs (A) and A-549 cells (B). The drugs were added to the culture medium of M. tuberculosis-infected cells at the Cmax achievable in the blood after oral administration: 3.7, 0.56, 2.0, 0.39, and 0.05 μg/ml for WQ-3034, CPFX, LVFX, SPFX, and KRM, respectively. ○, control culture without drugs. Each symbol indicates the mean ± standard error of the mean (n = 3). ∗ and ∗∗, significantly different between bacterial CFU recovered from cells treated with each comparison drug and cells treated with WQ-3034 (∗, P < 0.05; ∗∗, P < 0.01; Student's t test).
When WQ-3034 and LVFX were added at their MICs (MICs by the broth dilution method, 1.0 and 0.25 μg/ml, respectively), WQ-3034 displayed bacteriostatic activity against the M. tuberculosis Kurono isolates multiplying in MM6-Mφs, while LVFX did not exhibit such activity (data not shown). Thus, the activities of these drugs against intramacrophage M. tuberculosis isolates were ranked WQ-3034 > LVFX. Weak bacteriostatic effects were observed for these drugs against M. tuberculosis isolates within A-549 cells (data not shown).
DISCUSSION
In this study, we compared the in vitro activity of a new fluoroquinolone, WQ-3034, with those of CPFX, LVFX, SPFX, and KRM as reference drugs. First, MIC determinations revealed that the MICs of WQ-3034 for M. tuberculosis were comparable to those of LVFX, and in most cases, the WQ-3034 MICs were two or four times lower than those of CPFX. Among the four quinolones, SPFX had the lowest MICs for M. tuberculosis; in most cases, the SPFX MICs were two or four times lower than those of WQ-3034 and LVFX. Therefore, the anti-M. tuberculosis activities of these quinolones were ranked SPFX > WQ-3034 ≒ LVFX > CPFX. Second, for MAC isolates, the WQ-3034 MICs were comparable to those of CPFX and LVFX, but the WQ-3034 MICs were two to four times those of SPFX. Thus, the anti-MAC activities of the test quinolones can be ranked SPFX > WQ-3034 ≒ CPFX ≒ LVFX.
In pharmacological studies, WQ-3034 exhibited a long elimination half-life in cynomolgus monkeys (Ohshita et al., 38th ICAAC). In dogs to which WQ-3034 was administered orally at a dose of at 10 mg/kg of body weight, the following pharmacokinetic data were obtained: for WQ-3034 the Cmax was 1.82 μg/ml, the half-life was 5.0 h, and the area under the concentration-time curve from 0 to 8 h was 5.00 μg · h/ml; for CPFX, the Cmax was 1.56 μg/ml, the half-life was 3.9 h, and the area under the concentration-time curve from 0 to 8 h was 7.61 μg · h/ml (Y. Ohshita, personal communication). Moreover, WQ-3034 has less convulsion-inducing activity and phototoxicity than the other reference quinolones (Ohshita et al., 3th ICAAC). Moreover, its cytotoxicity against CHL cells is as low as that of CPFX (50% inhibitory concentrations, 47 and 65 μg/ml for WQ-3034 and CPFX, respectively). Thus, WQ-3034 has somewhat promising properties as a chemotherapeutic agent for the clinical management of tuberculosis.
Comparison of the activities of the four quinolones at the Cmax against intracellular M. tuberculosis indicated that their efficacies against the organisms residing in MM6-Mφs and A-549 cells were in the order SPFX ≧ LVFX > WQ-3034 > CPFX, irrespective of the test M. tuberculosis strains or host cells used. Notably, WQ-3034 at the MIC was more efficacious than LVFX at inhibiting the intramacrophage growth of M. tuberculosis. This suggests either that WQ-3034 is more easily delivered than LVFX to the phagosomes of the MM6-Mφs that engulf bacteria or that WQ-3034 is more stable than LVFX in the intramacrophage environment. Alternatively, this phenomenon may be related to the fact that the antimicrobial activity of WQ-3034 is potentiated under acidic conditions, such as below pH 6.0 (Ohshita and Yazaki, 37th ICAAC). Indeed, Mφ phagosomes that engulf mycobacterial pathogens are equilibrated to pH 5.5 to 5.7 (15).
M. tuberculosis and MAC invade and replicate within A-549 alveolar cells, and the degree of their invasiveness is related to the degree of virulence of the organisms (3, 14, 19). The production of reactive nitrogen intermediates, which are the major effector molecules of Mφ-mediated killing of mycobacterial pathogens (1, 4), is markedly increased in Mφs after MAC infection, but such an increase is not observed in A-549 cells (19). As shown in Fig. 2, the antimicrobial activities of test quinolones against M. tuberculosis organisms multiplying in A-549 cells were significantly lower than those against M. tuberculosis organisms residing in MM6-Mφs. The same situation was observed for the activity of KRM against intracellular MAC organisms (19). Thus, mobilization of reactive nitrogen intermediate-dependent antimicrobial mechanisms in host cells appears to be critical for quinolone-mediated elimination of intracellular M. tuberculosis.
Notably, WQ-3034 has much lower (8 to 32 time lower) MICs than those of LVFX and SPFX for gram-positive bacteria including S. aureus, S. pneumoniae, S. pyogenes, and E. faecalis (Ohshita et al., 38th ICAAC; Ohshita and Yazaki, 37th ICAAC). However, the present study revealed that the in vitro anti-M. tuberculosis activity of WQ-3034 is comparable to or somewhat weaker than that of LVFX and is significantly less than that of SPFX. This was also the case for HSR-903, a newly synthesized quinolone with superior activity against gram-positive cocci (20). It thus appears that an increase in the activity of a given quinolone against common gram-positive bacteria does not necessarily lead to the increased activity of that drug against M. tuberculosis organisms. This finding is important for drug design and for the screening of new quinolones with potent anti-M. tuberculosis activities.
ACKNOWLEDGMENTS
This study was partly supported by grants from the Ministry of Public Welfare of Japan and the Ministry of Education, Science, and Culture of Japan.
REFERENCES
- 1.Akaki T, Sato K, Shimizu T, Sano C, Kajitani H, Dekio S, Tomioka H. Effector molecules in expression of the antimicrobial activity of macrophages against Mycobacterium avium complex: roles of reactive nitrogen intermediates, reactive oxygen intermediates, and free fatty acids. J Leukoc Biol. 1997;62:795–804. doi: 10.1002/jlb.62.6.795. [DOI] [PubMed] [Google Scholar]
- 2.Alangaden G J, Lerner S A. The clinical use of fluoroquinolones for the treatment of mycobacterial diseases. Clin Infect Dis. 1997;25:1213–1221. doi: 10.1086/516116. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez L E, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alverolar cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien S-C, Rogge M C, Gisclon L G, Curtin C, Wong F, Natarajan J, Williams R R, Fowler C L, Cheung W K, Chow A T. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother. 1997;41:2256–2260. doi: 10.1128/aac.41.10.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohn D L, Iseman M D. Treatment and prevention of multidrug-resistant tuberculosis. Res Microbiol. 1993;144:150–153. doi: 10.1016/0923-2508(93)90030-6. [DOI] [PubMed] [Google Scholar]
- 7.Grassi C. New drugs for tuberculosis. Exp Opin Invest Drugs. 1997;6:1211–1226. doi: 10.1517/13543784.6.9.1211. [DOI] [PubMed] [Google Scholar]
- 8.Hart C A, Beeching N J, Duerden B I. Tuberculosis into the next century. J Med Microbiol. 1996;44:1–34. [Google Scholar]
- 9.Hoffner S E, Genzelius L, Olsson-Liljequist B. In-vitro activity of fluorinated quinolones against drug-resistant Mycobacterium tuberculosis. J Antimicrob Chemother. 1997;40:885–888. doi: 10.1093/jac/40.6.885. [DOI] [PubMed] [Google Scholar]
- 10.Hooper D C, Wolfson J S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985;28:716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji B, Lounis N, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of levofloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:1341–1344. doi: 10.1128/aac.39.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kent P T, Kubica G P. Public health mycobacteriology: a guide for the level III laboratory. Atlanta, Ga: Centers for Disease Control; 1985. [Google Scholar]
- 13.Klemens S P, Sharpe C A, Rogge M C, Cynamon M H. Activity of levofloxacin in a murine model of tuberculosis. Antimicrob Agents Chemother. 1994;38:1470–1479. doi: 10.1128/aac.38.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonough K A, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–4811. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh Y-K, Straubinger R M. Intracellular fate of Mycobacterium avium: use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization of phagosomal pH and phagosome-lysosome interaction. Infect Immun. 1996;64:319–325. doi: 10.1128/iai.64.1.319-325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rastogi N, Goh K E, Bryskier A, Devallois A. In vitro activities of levofloxacin used alone and in combination with first- and second-line antituberculous drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:1610–1616. doi: 10.1128/aac.40.7.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito H, Sato K, Tomioka H, Dekio S. In vitro antimycobacterial activity of a new quinolone, levofloxacin (DR-3355) Tuberc Lung Dis. 1995;76:377–380. doi: 10.1016/0962-8479(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 18.Sato K, Akaki T, Tomioka H. Antimicrobial activities of benzoxazinorufamycin KRM-1648, clarithromycin, and levofloxacin against intracellular Mycobacterium avium complex phagocytosed by murine preitoneal macrophages. J Antimicrob Chemother. 1998;41:77–83. doi: 10.1093/jac/41.1.77. [DOI] [PubMed] [Google Scholar]
- 19.Sato K, Tomioka H. Antimicrobial activities of benzoxazinorifamycin (KRM-1648) and clarithromycin against Mycobacterium avium-intracellulare complex residing in murine peritoneal macrophages, human macrophage-like cells and human alveolar epithelial cells. J Antimicrob Chemother. 1999;43:351–357. doi: 10.1093/jac/43.3.351. [DOI] [PubMed] [Google Scholar]
- 20.Tomioka H, Sato K, Akaki T, Kajitani H, Kawahara S, Sakatani M. Comparative in vitro antimicrobial activities of the newly synthesized quinolone HSR-903, sitafloxacin (DU-6859a), gatifloxacin (AM-1155), and levofloxacin against Mycobacterium tuberculosis and Mycobacterium avium complex. Antimicrob Agents Chemother. 1999;43:3001–3004. doi: 10.1128/aac.43.12.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomioka H, Sato K, Hidaka T, Saito H. In vitro and in vivo antimycobacterial activities of KRM-1648, a new benzoxazinorifamycin. In: Pandalai S G, editor. Recent research developments in antimicrobial agents and chemotherapy. Trivandrum, India: Research Signpost; 1997. pp. 37–68. [Google Scholar]
- 22.Tomioka H, Sato K, Saito H, Yamada Y. Susceptibility of Mycobacterium avium and Mycobacterium intracellulare to various antibacterial drugs. Microbiol Immunol. 1989;33:509–514. doi: 10.1111/j.1348-0421.1989.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 23.Young L S. Mycobacterial infections in immunocompromised patients. Curr Opin Infect Dis. 1996;9:240–245. [Google Scholar]