Abstract
Recent research in tissue engineering and regenerative medicine has elucidated the importance of the matrisome. The matrisome, effectively the skeleton of an organ, provides physical and biochemical cues that drive important processes such as differentiation, proliferation, migration, and cellular morphology. Leveraging the matrisome to control these and other tissue-specific processes will be key to developing transplantable bioprosthetics. In the ovary, the physical and biological properties of the matrisome have been implicated in controlling the important processes of follicle quiescence and folliculogenesis. This expanding body of knowledge is being applied in conjunction with new manufacturing processes to enable increasingly complex matrisome engineering, moving closer to emulating tissue structure, composition, and subsequent functions which can be applied to a variety of tissue engineering applications.
Intersection of Biology and Engineering
Tissue engineering is a rapidly advancing field that leverages principles from multiple disciplines including cellular biology, developmental biology, stem cell biology, materials science, and engineering to develop new therapies that act by replacing damaged or diseased tissues with engineered ones. The extracellular matrix (ECM, see Glossary) and related factors (matrisome) regulate biochemical cues in the form of cellular adhesion molecules within the matrisome or by controlling the availability and orientation of paracrine and endocrine factors. This has been shown to influence the cellular morphology and subsequent function of several cell types, including islets and stem cells [1,2]. A correlation between cellular morphology and the proliferation of ovarian cells has also been made [3]. Further, physical cues are also important, and research shows that applying exogenous physical pressure maintains primordial follicle quiescence, reversing the effect that disrupting the ECM has on ovarian follicle activation [4]. At a time when traditional technologies are being expanded or refined to answer questions about the biochemical and physical properties of the microenvironment of a healthy organ, new ways to consider and incorporate both biochemical and physical cues into tissue engineering technologies are being developed [5-7]. Recent technological advances, including matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) and high-throughput screening of the ECM, substrates, and small molecules, have enabled an advanced understanding of the microenvironment and how it influences cellular behavior [8]. In addition, more sophisticated 3D and microfluidics culture systems have enabled in vitro analysis of crucial cell units and system-wide interactions that recapitulate in vivo morphology and function [9]. Furthermore, increasingly advanced biomaterial designs that integrate biologically relevant microstructures and features have informed how form can influence the function of cells [10-14]. These advances enable a clearer understanding of the organ microenvironment and how it influences the functional units, and will inform how a healthy organ can be built.
In this article we review and address fundamental concepts of tissue-specific microenvironments, the recent technologies or processes used to define these features, and emerging engineering approaches that support tissue function. Specific attention and focus will be given to the ovary, its biology, matrisome structure and composition, and the application of various top-down, bottom-up, and hybrid manufacturing approaches that have or could potentially be utilized to engineer an ideal tissue engineered (or bioprosthetic) ovary.
Identifying the Need for a Bioprosthetic Ovary and Current Transplant Options
Ovarian follicles are the spherical cell aggregates that contain the immature oocyte/gamete and supportive hormone-producing cells. When the ovary is exposed to chemotherapy or radiation, the number of ovarian follicles, or source of eggs and ovarian hormones, is depleted and accelerates the onset of menopause. Premature ovarian insufficiency (POI), or early menopause, can occur as a result of numerous genetic, autoimmune, iatrogenic, or idiopathic causes [15]. Fertility preservation is an option for those with a known treatment or progressive disorder that may disrupt normal ovarian function, and is a key quality-of-life concern for many cancer patients and survivors [16]. In addition, 55% of surveyed patients with differences in sexual development (DSD), or those with dysgenetic gonads, who can acquire POI, reported their desire to have biological children and that they wished they had explored fertility options [17]. One option for fertility preservation in adults, and the only option for prepubertal children, is ovarian tissue cryopreservation (OTC). In addition to considerations for fertility loss, the loss of ovarian hormones has detrimental systemic effects, and multiple cohorts have identified a life expectancy 2 years shorter in women with POI from various comorbidities that effect their cardiovascular system, metabolism, bone turnover, and ability to heal wounds [18,19].
Current options to restore fertility and hormone function are limited to transplantation of the intact tissue into the patient. To date, over 130 livebirths have been reported from transplantation of OTC tissue following eradication and recovery from cancer [20-22]. However, only 20–40% of transplants result in livebirth and produce an average of 2–5 years of hormones, leaving many without biological children and decades of post-cancer survival without essential hormone production [20-22]. In addition, this method is contraindicated in those with a moderate-to-high risk of metastatic or systemic disease within the ovaries [23]. Addressing these concerns and improving upon current transplantation outcomes will require a structure that supports isolated ovarian follicles, free of cancer cells. Current follicle isolation methods leverage both enzymatic and mechanical isolation. Processing steps that include a series of washing have been shown to remove cancer cells from primordial follicle preparations in murine ovaries that were seeded with breast cancer cells and in human ovaries from leukemia patients [24,25]. Because oocytes have not yet been made from human stem cells, the gametes will need to be isolated from the patient to produce biological offspring. This transplant would be integrated into a patient care plan to enable full restoration of hormones and fertility in patients. This transplant would feature an architectural design that supports isolated follicles through growth and ovulation, which was established for a murine bioprosthetic ovary transplant [26]. Improving upon this first iteration through investigations of how compartmentalization and biochemical and physical cues influence hormone production and egg quality will inform bioprosthetic ovary design and utilization for restorative therapies.
The Compartmentalization of the Ovary
The ovary is compartmentalized with a greater fibrous matrisome density in the cortical region [27,28] (Figure 1). This region houses quiescent primordial follicles which make up the bank of follicles that produce cyclical hormones and eggs. Both are controlled via the regulated activation of primordial follicles that grow and mature through stages of folliculogenesis. The cortex is the portion of the ovary that is cryopreserved for future use because it contains this bank of potential eggs and is more resistant to cryopreservation damage. In a normal ovary, primordial follicles are activated to undergo folliculogenesis until the onset of menopause, when folliculogenesis and the hormones and eggs that are released ceases. Folliculogenesis requires significant tissue remodeling, and the medullary region (where growing follicles reside) is less dense. Once activated, the primordial follicles grow to >600-fold their original size, recruiting additional somatic cell differentiation and vessels that support this growth and provide an outlet for systemic distribution of additional hormones [18]. Ovulation occurs through a process of ECM degradation and pressurized expulsion of the egg, and the remaining follicular cells form a temporary progesterone-producing gland that dissolves into a fibrous scar tissue. Each of these crucial follicular events is supported by, or remodels, the surrounding microenvironment.
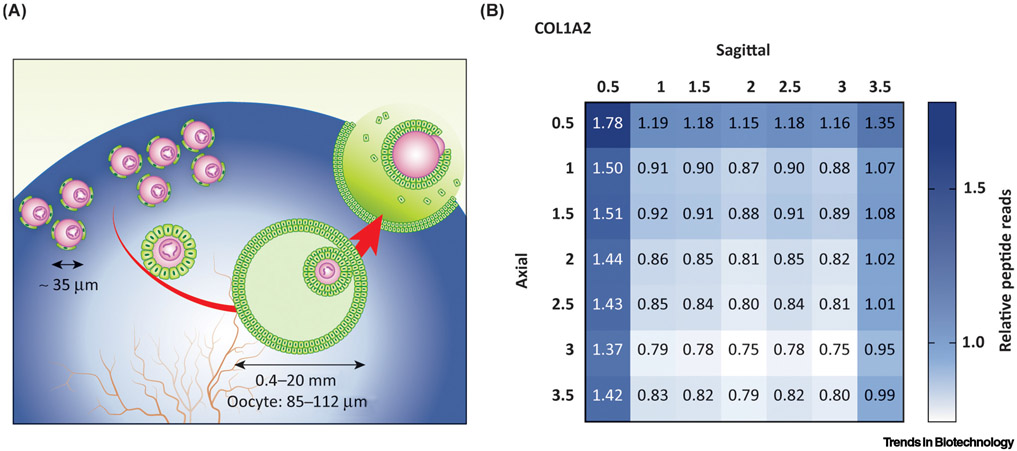
Figure 1. Schematic of Ovarian Compartmentalization.
(A) Primordial follicles (left, 35 μm) remain quiescent within the ovarian cortex until they are recruited to grow. Follicles grow, accumulate antral fluid, and recruit vessels within the ovarian medulla until they ovulate through the surface. The ovary contains a gradient of denser to less-dense matrisome as represented by the blue gradient. (B) Matrisome proteins were mapped across porcine ovarian compartments using proteomics. Type I collagen (COL1) is more abundant in the cortical compartment (0.5 mm in sagittal and axial directions) than in the medullary compartment (1.0–3.5 mm). The values are relative to universal means of peptide reads. Image adapted, with permission, from [7].
Like human ovaries, bovine and porcine ovaries have distinct cortical and medullary regions, as visualized by scanning electron microscopy (SEM) [7,27]. Organized collagen bundles make up the tightly packed, uniform pore walls of the cortex. The medulla has larger pores made by the larger ovarian follicles and vessels, as well as more-permeable walls that include fibronectin fibers among less-uniform collagen bundles [27]. The density of collagen fibers is also greater around primordial follicles than around growing follicles in murine ovaries [28]. This ultrastructure called the matrisome is composed of >1000 proteins and includes core matrisome (e.g., collagen subunits, proteoglycans, and glycoproteins), ECM-modifying enzymes, ECM-binding proteins, ECM-associated proteins, and secreted factors (e.g., growth factors that are known to bind to the ECM, as well as receptors and modifying enzymes), as defined by The Matrisome Project [29]. These proteins are secreted by resident cells in a tissue-specific fashion, and although they were previously believed to largely provide structural support to the matrisome, they are now strongly implicated in modulating multiple cellular processes [30,31]. The matrisome is in a state of dynamic reciprocity with cells by providing biochemical and physical cues that modify cell signaling and behavior as cells transform the matrisome by assembly and disassembly. Recent research has shown that replicating the microenvironment including its spatial distribution, via 3D scaffolds, results in better outcomes by providing tissue-specific signaling cues for cell attachment, differentiation, vascularization, and function [32].
Defining the Microenvironment for Ideal Ovarian Function
Folliculogenesis is modulated by several endocrine, paracrine, and autocrine factors. In addition, the interstitial cells, including stromal and endothelial cells, are important for the influential microenvironment, especially during the later stages of follicular growth and ovulation. Small quiescent and growing follicles are activated and supported by gonadotropin-independent mechanisms [33]. Once a quiescent primordial follicle becomes activated, it either differentiates and matures into an ovulated egg (at a normal rate of one per month) or becomes atretic. This crucial point of activation coincides with the initiation of proliferation and differentiation in the primordial granulosa cells that surround and support the oocyte. Granulosa cell shape and packing density affect this process, and both biochemical and physical cues have been shown to influence cellular morphology [3,34]. Recently, cellular morphology has been used to predict transcriptional profiles and functional outputs in high-throughput cell screens [8,35]. Although the importance of interstitial cells is briefly discussed in the following text, this section focuses on two significant microenvironment factors, biochemical and physical cues, that can influence the longevity of a transplant (rate of primordial follicle activation).
Biochemical and Physical Cues Are Important for Regulating Folliculogenesis
Biochemical cues include binding sites along fibrous proteins, or matrisome-associated proteins, that bind to transmembrane complexes and translate the extracellular environment into responses within the cell [36-38], including proliferation, differentiation, and survival [39,40]. One example of a biochemical cue from the matrisome is Agrin (AGRN), a proteoglycan that works through the transmembrane dystrophin–glycoprotein complex to induce proliferation [41]. It was detected in the porcine ovarian matrisome and localized to activated follicles, making it a potential candidate for regulating granulosa cell proliferation and primordial follicle activation [7]. In addition, research has shown that the ECM can affect hormone availability and responsiveness by sequestering, trafficking, or presenting factors including growth factors, androgens, and estrogens via sequestration of sex hormone-binding globulin [42,43]. Incorporating the tissue- and compartment-specific matrisome with the appropriate biochemical cues within an engineered microenvironment can also induce metalloproteinases and tissue inhibitors of metalloproteinases, which regulate the activation and follicle growth and ovulation [44,45].
Proteomic analyses have been used to identify other matrisome proteins that may be similar or different across ovarian compartments. The ovary was processed into regular 0.5 mm slices in two anatomical directions and matrisome proteins were enriched using decellularization before liquid chromatography and tandem mass spectrometry [7]. Proteins from five matrisome categories were significantly differentially expressed between the cortex and medulla, and also within the medullary compartment. These included 11 proteins that were not previously identified within the ovary [7]. Table 1 summarizes matrisome proteins identified in other organs, their known cellular functions, and their abundance in the ovarian matrisome. Recent intense scrutiny of the matrisome as a tissue engineering toolkit has expanded experimental knowledge of the contribution of biochemical cues to biological processes of interest. These studies performed in other organs/tissues can inform future research in the ovary based on proteomic discovery and spatial mapping of matrisome proteins across ovarian compartments to discern contributors to follicle activation, quiescence, and folliculogenesis. Defining which matrisome proteins are most abundant within a compartment, and consequently in surrounding follicles at different stages of their development, may elucidate additional biochemical cues that facilitate folliculogenesis. This will be particularly important in controlling the rate of primordial follicle activation, a rate-limiting step that is rapidly depleted in ovarian tissue transplants [46].
Table 1.
Biochemical and Physical Properties of Tissues, the Influence of Cellular Behavior, and Potential Locations of Matrisome Proteins in the Ovary
| Tissue | Physical properties |
Proteins | Function | Ovarian mapping | Refs |
|---|---|---|---|---|---|
| Intestine | 2.6–2.9 kPa | Collagens I, III, IV, V, VI, VII; laminin | Tensile strength to tissues | Collagen I was increased in cortex; collagen 4 was uniform throughout | [5,85,86] |
| Heparin, heparan sulfate, chondroitin sulfate, hyaluronic acid | Modulation of enzyme activity; ECM organization and assembly | Hyaluronic acid is increased in cortex compared to medulla | |||
| Bone | 25–40 kPa (osteoid matrix) | Collagen I | Primary structural ECM, overall bone strength, durability, and regulation of fibrillogenesis | Collagen I (the primary structural collagen of the ovary) was increased in cortex | [87-92] |
| 1.3–7.8 GPa (cancellous) | Decorin | Promotes collagen I fibrillogenesis; promotes bone formation | Detected by proteomic analysis; higher in cortex than in medulla | ||
| 12–20 GPa (cortical) | Biglycan | Promotes collagen I fibrillogenesis; promotes bone formation | Detected by proteomic analysis; no significant patterning between compartments was seen | ||
| Brain | 0.1–1 kPa | Laminins | Basal lamina, expansion; proliferation and differentiation of neural stem cells (NSCs) | Multiple laminins mapped; (major components of basal lamina) were higher in cortex than in medulla, | [88,93] |
| 130 Pa (white matter) | Agrin | Laminin network stabilization | Increased in cortex; involved in the Hippo kinase cascade | ||
| 70 Pa (grey matter) | Collagen IV | Major component of basal lamina at the neuromuscular junction | Collagen IV (major component of basement membranes) was expressed evenly throughout cortex and medulla | ||
| Human microvascular endothelial cells (HMECs) | N/Aa | Fibronectin | Cell adhesion | Fibronectin was increased in medulla | [94] |
| Liver | 100–200 kPa (tensile); 1–3.5 MPa (compression) | Collagen, biglycan, laminin, vitronectin | Sequestration of cytokines; stress-induced remodeling | Collagen I was high in cortex; laminin was increased in cortex; vitronectin (VTN) was increased in medulla | [95,96] |
| Pancreas | 0.14 MPa | Fibroblast growth factor 1, heparin | Cell proliferation and maintenance; insulin production | Fibroblast growth factor 1; heparin was detected by ovarian proteomics but there was no discernible patterning | [95,97] |
| Ovary | N/A | Collagen, elastin, fibrillin 1, emilin 1, glycosaminoglycans | ECM is age-dependent: collagen levels increase with age; fibrillin 1 decreases after menopause but is constant before | Collagen I and emilin 1 were both higher in the cortex | [34,98,99] |
| TGF-β, serine/threonine kinase receptor-associated protein | ECM formation and remodeling; follicle development | TGF-β was increased in cortex | |||
| Heart | 10–12 MPa | Cellular communication network factor 2 | Infarction reduction; attenuates ECM remodeling | Not detected by proteomic analysis; important downstream effector of Hippo and promotes primordial follicle activation | [100-103] |
| Tenascin C | Reduces inflammation | Detected; there was no specific patterning between compartments | |||
| Collagens | Structural component; proinflammatory in heart during infarction | Collagen I was significantly higher in cortex; multiple other collagens were detected, including IV, VI, III | |||
| Lung | 1.9 kPa | Collagens I, III, V; laminins; ECM1 | Quantification of matrisome in the lung | Collagen I, multiple laminins were mapped, and ECM1 were increased in cortex | [104,105] |
| Kidney | 4.1 MPa | EMILIN1, fibrillin 1 | ECM anchoring; human umbilical vein endothelial cell (HUVEC) proliferation and migration | EMILIN1 and fibrillin 1 were increased in cortex versus medulla | [105-107] |
| Collagens, agrin, perlecan | ECM structural proteins increase during fibrosis, | AGRN, perlecan, and collagen I were increased in cortex; other collagens vary (IV was the same in both compartments) | |||
| Muscle | 8–17 kPa | Laminin, collagen, fibronectin | ECM and integrin clustering | Laminin, some collagens, and fibronectin were increased in the cortex versus medulla | [108-110] |
| 30–50 kPa (skeletal) | Collagen VI | Functional integrity of muscles | Collagen VI was detected, but there was no significant patterning across compartments |
N/A, not available.
Physical cues are both intracellularly generated and externally applied forces, and, like biochemical cues, they have a broad impact on cell behavior (e.g., growth, differentiation, and function) [41,47,48]. These signals require mechanosensing units, which include cytoplasmic complexes such as integrins, extracellular components such as ECM cell adhesion molecules, and intracellular components including the cytoskeleton [49]. Significant in vitro work has examined the effects of rigidity on isolated follicles, including encapsulation of primordial follicles from rhesus macaque in 2% alginate, thus replicating a high-rigidity environment, which maintained optimal primordial follicle survival and morphology, whereas a more pliable 0.25–1.25% alginate environment supported increased hormone production in activated or growing follicles [50-54]. Recent investigations using shear-wave velocities across bovine ovaries indicated differences in the viscoelastic properties of the ovary across compartments [55]. These compartmental differences may be functional. Research using a light enzymatic treatment (CTK: collagenase type IV, trypsin, and knockout serum replacement) on murine ovaries resulted in ECM disruption and increased primordial follicle activation [56]. This activation in CTK-treated ovaries was counteracted by the application of hydrostatic pressure [56]. In an attempt to replicate a rigidity gradient that a growing follicle may feel within the ovary, secondary follicles were seeded into interpenetrating fibrin–alginate gels where degradation of fibrin reduced rigidity, created a more pliable environment, and follicles cultured in this way showed an 80% survival rate [53]. A study where murine ovaries were fragmented revealed changes in the polymerization of g-actin and f-actin, increases in the activity of the Hippo mechanotransduction pathway, leading to triggering of primordial follicle activation [57,58]. Dysregulated physical forces within the ovary are present in disease states, such as polycystic ovarian syndrome (PCOS) and Turner syndrome, where increased rigidity may contribute to anovulation in PCOS and loss of structural support may contribute to POI in Turner syndrome [50,59]. Together, these studies show that rigidity can influence follicle quiescence and folliculogenesis. We predict that advanced engineering solutions that can induce the production of, or recapitulate, the tissue-specific structural and biochemical support of the matrisome will enable tissue-specific function. By engineering these features into a scaffold, researchers could potentially reduce the rapid primordial follicle activation and subsequent depletion that occurs in current clinical solutions [4].
Fecundity Is a Bell Curve
Cyclical recruitment of ovarian follicles normally occurs under gonadotropin (follicle-stimulating hormone, FSH; and luteinizing hormone, LH)-dependent mechanisms that are established during puberty in humans. Although reproductive aging (a decline in eggs without chromosomal abnormalities) is a well-known phenomenon, the ability to make viable eggs that will result in a healthy offspring is also a challenge in younger individuals. In fact, ideal reproductive potential displays a bell-shaped age curve with a peak between the late 20s and early 30s [60,61]. The rates of in vitro maturation (IVM) to produce eggs in prepubertal populations are significantly lower that in postpubertal patients or animals [62]. In addition, there is broad evidence that altered egg quality during the pubertal transition may have significant effects on reproductive function and fertility [63]. Although this may not be a societal concern, it is particularly important for pediatric and adolescent cancer patients who preserve their ovarian tissue, or are stimulated to bank eggs, within the time-frame of this upward slope, but not the ideal peak, of reproductive potential. Prepubertal ovaries contain a deeper and more prominent cortex-like region, and there is less delineation between the cortex and medulla, relative to postpubertal ovaries. It has also been shown that stromal cells from postpubertal ovaries stimulate murine follicle growth and survival better than stromal cells from prepubertal ovaries [64]. The stromal cell population in human ovaries includes 19 different cell populations and has only been described in adult ovaries [65,66]. The ideal bioprosthetic ovary will not merely ovulate eggs but must be engineered with the microenvironment of the ideal reproductive age to produce good-quality eggs that result in successful pregnancies.
Assembling the Organ: Challenges and Advances in Manufacturing
It is increasingly clear that the biochemical and physical characteristics of the ovarian microenvironment are important to drive function within the ovary. It should be possible to apply this growing understanding to the engineering of organ matrisome products that can be applied to a variety of medical needs, including tissue modeling and tissue restoration and regeneration. However, even with the knowledge at hand, manufacturing these complex microenvironments remains a significant technical challenge.
The ideal engineered microenvironment should exhibit similar compositional and microstructural characteristics to the natural target tissue, and provide relevant physical and biochemical cues (such as those listed in Table 1) to the ovary. Beyond being present, however, these components must also be arranged to emulate the characteristics of the distinct compartments of the target matrisomes. For the ovary, this requires that emulation of both the cortical and medullary regions, which are defined by microlayered ECM 'sheets' and nanoscale ECM 'webs', respectively. In addition, because the natural matrisome is non-static, the engineered matrisome should exhibit dynamic reciprocity through being able to change and remodel in response to the surrounding biological microenvironment. Finally, the engineered matrisome must be manufacturable – capable of being produced safely, consistently, and at rates and costs that do not inhibit their adoption.
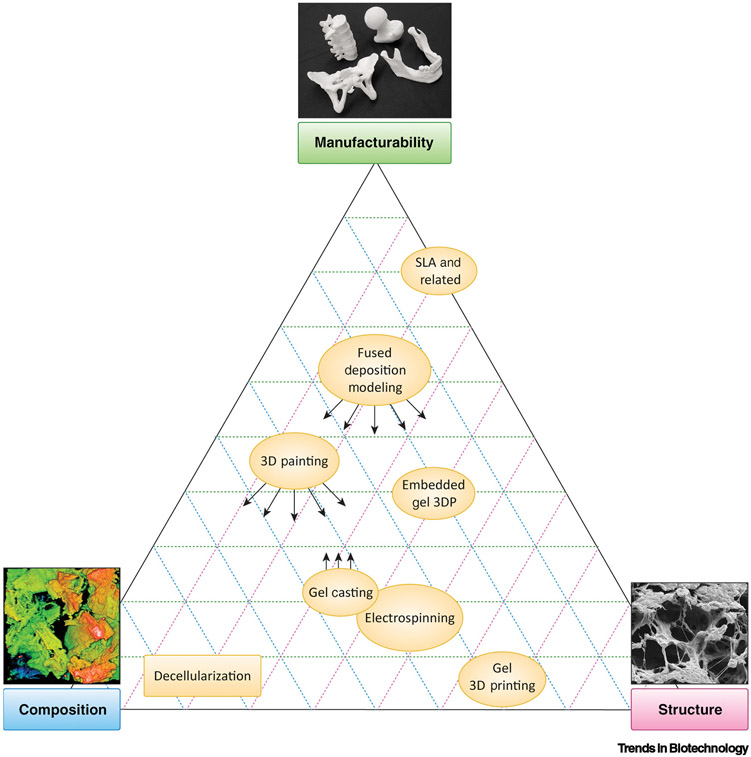
In efforts to capture these compositional, structural, and manufacturability characteristics, a variety of manufacturing approaches and fabrication techniques are currently being pursued (as highlighted in Figure 2) and positioned according to their relative strengths and weaknesses with respect to being manufacturable and their ability to capture matrisome composition and structure. These fabrication techniques comprise broader manufacturing approaches that can be divided into three major categories: top-down, bottom-up, and hybrid.
Figure 2. Relative Strengths and Weaknesses of Various Matrisome Manufacturing Approaches with Respect to Emulating Composition and Structure, and Their Corresponding Manufacturability.
Arrows indicate that strengths can be expanded towards the indicated qualities through integration with a hybrid approach. For example, the composition and structural characteristics of 3D painting can be strengthened by integration with gel casting (gel infusion into 3D-printed structures). Conversely, the manufacturability of gel casting processes can be improved by integrating them with 3D-printed structures. The composition image was adapted, with permission, from [12]. Abbreviations: 3DP, 3D printing; SLA, stereolithography.
Top-down approaches are characterized by minimal processing of natural materials and structures to yield a desired product. This approach is best exemplified by tissue decellularization. Bottom-up manufacturing encompasses methods that shape raw materials and reagents into the desired object. Hybrid approaches integrate characteristics of both top-down and bottom-up in an effort to capture matrisome composition and structure, while promoting overall manufacturability.
Top-down matrisome manufacturing methods are arguably the best established, and make use of decellularized organs and tissues to yield structures that retain compositional and structural components of their tissue of origin. Although decellularized organs and tissues have been extensively utilized in research as well as for numerous clinical products, their use in ovarian matrisome engineering has been limited, but very promising. Earlier work [27] demonstrated that decellularized bovine ovarian tissue could promote follicle maturation, restore hormonal function, and enable sexual maturation in previously ovariectomized mice after implantation. No additional processing beyond cutting the decellularized ovary to a transplantable size was required. These results highlight the effectiveness of specific tissue-derived decellularized ECM as a raw biological structure that retains much of its original composition. Although there are clear advantages to using top-down approaches such as tissue decellularization, there are also significant disadvantages. Beyond sourcing healthy, high-quality, and consistent tissue and organs, it is not practical to use top-down approaches to address the original problem – namely recellularization, in other words placing cells and large cell-aggregate structures such as ovarian follicles back into the appropriate locations within the dense, retained matrisome ECM structure without damage or significant modification. In fact, the aforementioned study that utilized decellularized ovary sheets was aware of this issue. Although a few follicles were present as aggregates, the majority of granulosa cells lacked an oocyte, resulting in a construct that behaved more like an estradiol transplant than one that would restore fertility [27]. Thus, although full tissue/organ decellularization approaches have demonstrated the value of the acellular tissue structure and its native composition, and have even been successfully applied in some applications, particularly in low-volume, surface-dominant tissues (epithelial tissues) such as bladder and dermis [67], these types of top-down approaches, on their own, may not be appropriate for engineering voluminous, multicompartment matrisomes such as those of the ovary.
Bottom-up approaches encompass a variety of techniques and processes, including freezecasting [68], foaming [69], electrospinning [70], and many types of additive manufacturing, also known as 3D (bio)printing [71]. These processes offer additional manufacturing versatility and allow the creation of designer structures from larger batches of processed raw materials – ultimately granting more manufacturing control than top-down processes. However, unlike decellularization, which begins with a compositionally and structurally suitable microenvironment, the compositional and structural components of bottom-up manufactured matrisomes must be engineered from scratch. To address this daunting challenge, both volumetric and layer-bylayer manufacturing approaches have been implemented. Volumetric bottom-up approaches, such as foaming, casting, and freeze-drying/lyophilization, can yield interconnected microporous structures, that are reminiscent of the natural ECM structure, from relevant materials including collagens and even solubilized, tissue-specific ECM [72]. However, these volumetric methods can suffer from many of the same deficiencies as top-down decellularized tissue approaches – namely the difficulty of effectively integrating cells throughout the structure volume.
Layer-by-layer bottom-up approaches, including electrospinning and 3D printing, offer more control over porosity and microstructure than volumetric processes, but typically suffer from reduced manufacturing rates. Electrospinning processes can be used to produce structures with consistent and relevant feature sizes and tailored pore-size ranges by using a variety of materials, including synthetic polymers [73], collagens, and solubilized, tissue-specific ECM [74]; however, the nature of the electrospinning process inhibits the creation of thick or architecturally controlled pore structures – making them more suitable for planar, non-volumetric tissues matrisomes. 3D-printing processes offer additional manufacturing control over pore architecture. This control, combined with the increasing introduction of relevant biomaterials, has made 3D printing a significant focus of research and development over the past 15 years. The distinct categories of 3D printing and their advantages and disadvantages were previously discussed by Jakus et al. [71], and are only briefly mentioned here. Vat polymerization methods, including emerging volumetric 3D printing [74], are able to achieve excellent resolution but are restricted to photopolymeric materials, most of which are not compositionally similar to natural ECM. Conversely, extrusion-based 3D-printing methods, which may include hydrogels [75], with or without encapsulated cells, or advanced non-hydrogel biomaterial matrices, such as those characteristic of the emerging 3D-painting process [10,76], have not been able to achieve the same resolution as vat polymerization methods, or even the same complex geometries owing to material collapse, but are generally more compositionally relevant to the matrisome microenvironment. Thus, although bottom-up approaches offer more control and are rapidly improving with respect to resolution, material compatibility, and manufacturing rates, no single bottom-up technique is currently able to capture the compositional and structural complexity or spatial resolution of the natural matrisome.
Fortunately, it may not be necessary to recreate every single component of the natural matrisome to impart at least some of its intended function within an engineered structure. This was effectively demonstrated in prior work by Laronda et al. who created a bioprosthetic ovary from a 3D-printed gelatin hydrogel seeded with ovarian follicles [26]. These structures, 3D printed with a highly specific and selected pore-architecture, not only supported follicular attachment, growth, and hormone production but restored hormonal and reproductive function when implanted into previously ovariectomized mice. These results demonstrate that relatively simple but thoughtfully engineered structures, whose compositions and designs are informed by fundamental knowledge of the tissue target, can still be effective at emulating the natural matrisome.
Although top-down and bottom-up approaches continue to progress, it remains likely that no single manufacturing approach will be able to fully capture the structure and composition of the matrisome, including the dynamic complexity of the human ovary. Therefore, hybrid processes that combine the advantages of multiple techniques may be necessary to achieve these goals. For example, 3D-printed structures can be infused with decellularized ECM-based hydrogels, optionally containing cells to impart multiple scales of structure and composition [77]. This circumvents the resolution limitations of 3D-printing hydrogels, allows ordered, microporous architectures as well as nanoporous fibrous networks, and introduces compositionally relevant materials, while permitting the incorporation of cells. This hybrid process has frequently been used to add biologically active components to otherwise low-bioactivity but mechanically robust 3D-printed materials such as fused deposition modeled polyesters [78]. More recently, ECM hydrogels derived from decellularized tissues are being integrated with advanced 3D-printed materials that not only impart structural integrity but actively participate in the biological function and emulation of the matrisome. 3D-painted Fluffy-PLG™, an ultraporous synthetic material that emulates the fibrous nano- and microstructure of soft-tissue matrisome [10,79], is one such material that is being actively utilized in conjunction with ECM hydrogels to yield early prototypes of compositionally and structural complex engineered matrisomes. Continued refinement of top-down and bottom-up approaches, and their integration with each other, has potential to generate functional ovarian matrisomes composed of distinct compartments. Such engineered matrisomes would not only be able to support follicle viability but could also promote selective follicular maturation and function, resulting in a dynamic microenvironment that is able to maintain proper hormone function, restore fertility, and effectively act as a fully functional bioprosthetic organ.
Concluding Remarks
The emergence and use of new characterization and analytical techniques, including high-precision spatial proteomics and advanced micro-3D imaging technologies of whole organs, have moved the field from protein lists to localization of proteins within the tissue [80,81]. The expanding datasets of RNA-seq data from human tissues and recent advances in quantitative RNA in situ hybridization facilitate our understanding of specific cell types within healthy human organs and their transcriptional profiles [82]. However, questions remain concerning the properties of the microenvironment are necessary to support the cells within a manufactured structure and produce the desired function (see Outstanding Questions).
Outstanding Questions.
What is the contribution of matrisome components to the maintenance of follicle quiescence and folliculogenesis? Are these specific to particular matrisome compartments (e.g., cortex and medulla)?
Do the physical properties of the ovary vary significantly between the cortex and the medulla? Do these properties modulate follicle activation and folliculogenesis in vivo, and can they be leveraged in future bioprosthetic ovary scaffolds?
Can we utilize developments in spatial mapping of the matrisome and physical properties of organs such as the ovary to build more biologically relevant scaffolds? Are compartment-specific signatures discovered via these maps important to the function of organs such as the ovary?
How can we design, engineer, and manufacture, in a robust and repeatable fashion, biomaterials and corresponding scaffolds that recapitulate the structures and compositions of tissue-specific microenvironments? How can we reduce the batch-to-batch variability that is observed in decellularized ECM (dECM)-derived biomaterials?
The matrisome of the ovary was recently mapped, and this revealed significantly different compositions and quantities of matrisome proteins across and within compartments [7]. It will be essential to determine which biochemical properties of the matrisome are necessary to regulate the initial activation of quiescent follicles and then to promote the controlled development and cyclical release of a fertilizable egg. High-throughput screens of how granulosa cell morphology and subsequent function are influenced by an ECM substrate, or how proliferation rates are affected by other biochemical cues from the matrisome, may reveal how these components from the ovarian microenvironment influence primordial follicle activation. However, because the granulosa cell is part of a cell aggregate that contains a central oocyte connected with transzonal projections, a more accurate representation of follicular behavior would be to test the effects of matrisome factors in 3D. The next set of experiments will include the use of modified ECM bioinks within a 3D-printed architecture – that has been demonstrated to support isolated follicles – to determine which matrisome proteins, such as AGRN, influence primordial follicle activation in vitro. Options for incorporating these ECM proteins are detailed in the previous text.
A similar map needs to be created that quantifies the changes in physical properties across ovarian compartments and around follicles of different stages. As with biochemical cues, the effects of physical properties on follicle activation and folliculogenesis will need to be tested on follicles from large animal models that more closely resemble the human condition, and eventually on human follicles. Atomic force microscopy (AFM) has been used to map the physical properties of biological samples such as brain slices, and will be used on the ovary [83]. In addition, AFM experiments can be performed to distinguish the physical properties of ovarian cells versus the ECM to define the properties that should be engineered versus those that are acquired by the interstitial cells or follicles themselves. Recent advances of this technology could elucidate the viscoelastic properties of cell–cell and cell–matrix interactions that may influence functional outputs, such as granulosa cell proliferation and differentiation that steer oocyte differentiation and maturation [84]. The next set of experiments to define the ideal physical properties that enable primordial follicle quiescence versus activation could use biologically inert materials that separate biochemical cues from physical cues. However, in vivo these cues are not in isolation and are likely to act synergistically. Therefore, the use of hybrid manufacturing approaches that modulate both biochemical and physical cues across a gradient may be the most informative for the development of a bioprosthetic ovary construct.
As promising as any emerging technology or new product might be, it is only effective if it can be repeatedly produced at the scale that is needed and reaches the population for which it is intended. This statement highlights additional challenges that must be overcome if engineered matrisome products are to reach the patients who are most likely to benefit from them: these challenges include consistent-quality manufacturing, regulatory clearance, clinical adoption, and cost. Each of these four challenges was briefly addressed here, but each also warrants its own extended, focused discussion because the outcomes greatly impact on which new technologies can make it to the market and benefit patients. The main features that should be addressed to resolve these challenges include techniques for isolating ovarian follicles that maintain their integrity, and manufacturing the microenvironment as an off-the-shelf product. The isolation of primordial follicles from their matrisome-dense microenvironment while maintaining oocyte–granulosa cell connections, and not inducing follicle activation, is challenging, and studies are ongoing to improve the efficiency and effectiveness of this process by using a combination of enzymatic digestions and engineered platforms. In addition, an engineered matrisome solution should fit within existing clinical practice and norms if it is to be utilized for its intended purpose. Successful implementation of these types of matrisome products then lays the foundation for more complex, cellularized, and even personalized solutions that can be implemented in the clinic. This pipeline approach, in which at least a portion of the bioprosthetic is off-the-shelf, is particularly relevant to the highly personalized nature of the ovary and its functional component, the gamete-containing follicles.
Current and future patients who utilize OTC for fertility preservation have limited options for restoring their fertility and hormones. Although the ultimate goal is to create a bioprosthetic ovary that supports long-term fertility and hormones, smaller incremental advances along this path that maximize options for fertility and hormone restoration would increase options and satisfy some patient needs. Therefore, although the combination of different biochemical and physical cues within ovarian compartments is challenging to construct, separate constructs that are manufactured to support growth in vitro could be achieved in the short term. In addition, ovarian hormone-replacement therapies that provide the full milieu and respond to paracrine and endocrine signals within the patient to regulate hormone secretion may enable long-term ovarian hormone restoration separately from the restoration of fertility. Identification of a microenvironment that supports the proliferation, differentiation, and functional output of granulosa and theca cells (the hormone-producing cells of the ovary) would contribute to the development of a cell-based hormone-replacement therapy.
Engineering the ideal organ requires an understanding of the biochemical and physical cues that drive function. In all likelihood, a single top-down or bottom-up approach is unlikely to achieve this, but instead a combination of hybrid approaches will be necessary, each with complementary strengths that can overcome each other's inherent limitations. Recreating the full functionality of an organ and how it will be implemented into the care plan of a patient is complex. Biologists, engineers, and clinicians need to work closely together so that the target product can be designed, fabricated, and iteratively improved upon. In so doing, a bioprosthetic ovary that supports long-term hormonal restoration and produces good-quality eggs for women with POI can be manufactured and used to improve the lives of patients.
Highlights.
The matrisome – the extracellular matrix and associated proteins – provides both biochemical and physical cues to cells. Recent research has shown that the matrisome composition is tissue-specific, and that matrisome derived from tissue can be used to support important biological processes more robustly.
There is different intra- and intercompartmental variation in the composition and abundance of ovarian matrisome proteins.
Biochemical and physical cues in the ovary have been shown to modulate folliculogenesis and follicle activation.
Recent advances in manufacturing methods, including bottom-up approaches such as 3D printing in combination with emerging advanced materials, are allowing the design and engineering of matrisomal objects and products that increasingly emulate the natural structure and composition of matrisome, including that of the ovary.
Acknowledgments
This work was supported in part by the Stanley Manne Children’s Research Institute and the Ann and Robert H. Lurie Children’s Hospital of Chicago (to N.F.C.H.), the Warren and Eloise Batts endowment (M.M.L.), and a Burroughs Wellcome Fund Career Award at the Scientific Interface (1014568, M.M.L.).
Glossary
- Bioink
an acellular or cell-laden biomaterial that is compatible with an additive manufacturing or 3D-printing modality and provides a biologically relevant composition and structure that emulate the target tissue microenvironment and/or support cell viability, proliferation, differentiation, and/or function
- Biomaterial design
the process by which a new biomaterial or biomaterial derivative, such as a bioink, is defined so as to emulate and/or recapitulate the intended targeted biological function and/or be compatible with a specific means of manufacturing (i.e., 3D printing)
- 3D (bio)printing
the process of additively manufacturing biomaterial structures, that are intended for biological or medical applications, and that may or may not contain living cells, growth factors, ECM components, polymers, bioceramics, or other bioactive components
- Dynamic reciprocity
the two-way relationship between cells and their environments. The environment modifies cellular behavior and gene expression patterns, and in turn gene expression patterns and secreted proteins affect the extracellular environment (matrisome, etc.)
- Extracellular matrix (ECM)
the non-cellular component that makes up the skeleton of an organ or other soft tissue. The ECM provides both physical and biochemical support to tissues. ECM is composed of proteins and polysaccharides in a tissue-specific fashion
- Folliculogenesis
the maturation process undergone by ovarian follicles in which the supported primordial oocyte develops into an ovulated egg
- Manufacturability
the ability to reliably produce quality-controlled products at the necessary scale and rate to meet the demand for that product
- Matrisome
the dataset made up of the ECM and associated proteins
- Microstructure
the physical tissue environment, irrespective of its composition, that can be defined by characteristics such as porosity and nano/microfiber size and/or arrangement, for example parallel, orthogonal, random, and/or web-like configurations
- Ovarian follicle
the functional unit of the ovary that is composed of the oocyte and supporting steroidogenic cells (granulosa and theca cells)
Footnotes
Declaration of Interests
A.J. is a co-founder and CTO of the company, Dimension Inx., and has ownership and other financial interests in this company. Dimension Inx. did not influence the contents of this article. The other authors declare no conflicts of interest.
References
- 1.Tran R et al. (2020) Developmentally-inspired biomimetic Culture models to produce functional islet-like cells from pluripotent precursors. Front. Bioeng. Biotechnol 8, 583970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xing F et al. (2019) Regulation and directing stem cell fate by tissue engineering functional microenvironments: scaffold physical and chemical cues. Stem Cells Int. 2019, 2180925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva-Buttkus PD et al. (2008) Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J. Cell Sci 121, 3890–3900 [DOI] [PubMed] [Google Scholar]
- 4.Hayashi K et al. (2020) Environmental factors for establishment of the dormant state in oocytes. Develop. Growth Differ 62, 150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee A et al. (2019) 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487 [DOI] [PubMed] [Google Scholar]
- 6.Arteel GE and Naba A (2020) The liver matrisome – looking beyond collagens. JHEP Rep. 2, 100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henning NF et al. (2019) Proteomic analyses of decellularized porcine ovaries identified new matrisome proteins and spatial differences across and within ovarian compartments. Sci. Rep 9, 20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marklein RA et al. (2018) Functionally-relevant morphological profiling: a tool to assess cellular heterogeneity. Trends Biotechnol. 36, 105–118 [DOI] [PubMed] [Google Scholar]
- 9.Xiao S et al. (2017) A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun 8, 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakus AE et al. (2018) 3D-printing porosity: a new approach to creating elevated porosity materials and structures. Acta Biomater. 72, 94–109 [DOI] [PubMed] [Google Scholar]
- 11.Licht C et al. (2019) Synthetic 3D PEG-anisogel tailored with fibronectin fragments induce aligned nerve extension. Biomacromolecules 20, 4075–4087 [DOI] [PubMed] [Google Scholar]
- 12.Jakus AE et al. (2017) 'Tissue papers' from organ-specific decellularized extracellular matrices. Adv. Funct. Mater 27, 1700992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omidinia-Anarkoli A et al. (2020) Hierarchical fibrous guiding cues at different scales influence linear neurite extension. Acta Biomater. 113, 350–359 [DOI] [PubMed] [Google Scholar]
- 14.Cutiongco MFA et al. (2018) Functional differences between healthy and diabetic endothelial cells on topographical cues. Biomaterials 153, 70–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meacham LR et al. (2020) Standardizing risk assessment for treatment-related gonadal insufficiency and infertility in childhood adolescent and young adult cancer: the pediatric initiative network risk stratification system. J. Adolesc. Young Adult 9, 662–666 [DOI] [PubMed] [Google Scholar]
- 16.Armuand GM et al. (2014) Desire for children, difficulties achieving a pregnancy, and infertility distress 3 to 7 years after cancer diagnosis. Support Care Cancer 22, 2805–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Słowikowska-Hilczer J et al. (2017) Fertility outcome and information on fertility issues in individuals with different forms of disorders of sex development: findings from the dsd-LIFE study. Fertil. Steril 108, 822–831 [DOI] [PubMed] [Google Scholar]
- 18.Muka T et al. (2016) Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 1, 767–776 [DOI] [PubMed] [Google Scholar]
- 19.Chemaitilly W et al. (2017) Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude Lifetime Cohort. J. Clin. Endocrinol. Metab 102, 2242–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacheco F and Oktay K (2017) Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod. Sci 24, 1111–1120 [DOI] [PubMed] [Google Scholar]
- 21.Donnez J and Dolmans M-M (2017) Fertility preservation in women. New Engl. J. Med 377, 1657–1665 [DOI] [PubMed] [Google Scholar]
- 22.Shapira M et al. (2020) Evaluation of ovarian tissue transplantation: results from three clinical centers. Fertil. Steril 114, 388–397 [DOI] [PubMed] [Google Scholar]
- 23.Rosendahl M et al. (2010) Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil. Steril 94, 2186–2190 [DOI] [PubMed] [Google Scholar]
- 24.Kniazeva E et al. (2015) Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci. Rep 5, 17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soares M et al. (2017) Eliminating malignant cells from cryopreserved ovarian tissue is possible in leukaemia patients. Brit. J. Haematol 28, 255–259 [DOI] [PubMed] [Google Scholar]
- 26.Laronda MM et al. (2017) A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun 8, 15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laronda MM et al. (2015) Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 50, 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bochner F et al. (2015) A novel intravital imaging window for longitudinal microscopy of the mouse ovary. Sci. Rep 5, 12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao X et al. (2019) MatrisomeDB: the ECM-protein knowledge database. Nucleic Acids Res. 48, D1136–D1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah JS et al. (2018) Biomechanics and mechanical signaling in the ovary: a systematic review. J. Assist. Reprod. Genet 321, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keane TJ et al. (2015) Tissue-specific effects of esophageal extracellular matrix. Tissue Eng. A 21, 2293–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T et al. (2020) Mechanophysical cues in extracellular matrix regulation of cell behavior. Chembiochem 21, 1254–1264 [DOI] [PubMed] [Google Scholar]
- 33.Hsueh AJW et al. (2014) Intraovarian control of early folliculogenesis. Endocr. Rev 36, er20141020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy K et al. (2018) Nuclear exclusion of SMAD2/3 in granulosa cells is associated with primordial follicle activation in the mouse ovary. J. Cell Sci 131, jcs.218123. [DOI] [PubMed] [Google Scholar]
- 35.Nassiri I and McCall MN (2018) Systematic exploration of cell morphological phenotypes associated with a transcriptomic query. Nucleic Acids Res. 46, e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouni E et al. (2020) Spatiotemporal changes in mechanical matrisome components of the human ovary from prepuberty to menopause. Hum. Reprod 35, 1391–1410 [DOI] [PubMed] [Google Scholar]
- 37.Kurylo MP et al. (2016) Effect of proteoglycans at interfaces as related to location, architecture, and mechanical cues. Arch. Oral Biol 63, 82–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ernst EH et al. (2017) Dormancy and activation of human oocytes from primordial and primary follicles: molecular clues to oocyte regulation. Hum. Reprod 32, 1684–1700 [DOI] [PubMed] [Google Scholar]
- 39.Muncie JM and Weaver VM (2018) The physical and biochemical properties of the extracellular matrix regulate cell fate. Curr. Top. Dev. Biol 130, 1–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shridhar A et al. (2019) Investigating the effects of tissue-Specific extracellular matrix on the adipogenic and osteogenic differentiation of human adipose-derived stromal cells within composite hydrogel scaffolds. Front. Bioeng. Biotechnol 7, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bassat E et al. (2017) The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng K-M et al. (2006) Evidence that fibulin family members contribute to the steroid-dependent extravascular sequestration of sex hormone-binding globulin. J. Biol. Chem 281, 15853–15861 [DOI] [PubMed] [Google Scholar]
- 43.Teixeira SPB et al. (2020) Biomaterials for sequestration of growth factors and modulation of cell behavior. Adv. Funct. Mater 30, 1909011 [Google Scholar]
- 44.Cabral-Pacheco GA et al. (2020) The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci 21, 9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouni E et al. (2019) The human ovary and future of fertility assessment in the post-genome era. Int. J. Mol. Sci 20, 4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silber S et al. (2018) Cryopreservation and transplantation of ovarian tissue: results from one center in the USA. J. Assist. Reprod. Genet 35, 2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latorre E et al. (2018) Active superelasticity in three-dimensional epithelia of controlled shape. Nature 563, 203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thorne JT et al. (2015) Dynamic reciprocity between cells and their microenvironment in reproduction. Biol. Reprod 92, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finch-Edmondson M and Sudol M (2016) Framework to function: mechanosensitive regulators of gene transcription. Cell. Mol. Biol. Lett 21, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood CD et al. (2015) Multi-modal magnetic resonance elastography for noninvasive assessment of ovarian tissue rigidity in vivo. Acta Biomater. 13, 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hornick JE et al. (2012) Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum. Reprod 27, 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hornick JE et al. (2013) Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction 145, 19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shikanov A et al. (2009) Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 30, 5476–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J et al. (2013) Fibrin promotes development and function of macaque primary follicles during encapsulated threedimensional culture. Hum. Reprod 28, 2187–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gargus ES et al. (2020) Ultrasound shear wave velocity varies across anatomical region in ex vivo bovine ovaries. Tissue Eng. A 26, 720–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagamatsu G et al. (2019) Mechanical stress accompanied with nuclear rotation is involved in the dormant state of mouse oocytes. Sci. Adv 5, eaav9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawamura K et al. (2013) Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc National Acad Sci 110, 17474–17479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawashima I and Kawamura K (2017) Regulation of follicle growth through hormonal factors and mechanical cues mediated by Hippo signaling pathway. Syst. Biol. Reprod. Med 64, 3–11 [DOI] [PubMed] [Google Scholar]
- 59.Reindollar RH (2011) Turner syndrome: contemporary thoughts and reproductive issues. Semin. Reprod. Med 29, 342–352 [DOI] [PubMed] [Google Scholar]
- 60.Gruhn JR et al. (2019) Chromosome errors in human eggs shape natural fertility over reproductive life span. Science 365, 1466–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duncan FE (2017) Egg quality during the pubertal transition – is youth all it’s cracked up to be? Front. Endocrinol 8, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson RA et al. (2013) The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum. Reprod 29, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Granados A et al. (2015) Relationship between timing of peak height velocity and pubertal staging in boys and girls. J. Clin. Res. Pediatr. Endocrinol 7, 235–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tingen CM et al. (2011) A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction 141, 809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan X et al. (2019) Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun 10, 3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao H et al. (2019) Single-cell transcriptomics of human oocytes: environment-driven metabolic competition and compensatory mechanisms during oocyte maturation. Antioxid. Redox Signal 30, 542–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Milan PB et al. (2020) Decellularization and preservation of human skin: a platform for tissue engineering and reconstructive surgery. Methods 171, 62–67 [DOI] [PubMed] [Google Scholar]
- 68.Dewey MJ et al. (2020) Anisotropic mineralized collagen scaffolds accelerate osteogenic response in a glycosaminoglycan-dependent fashion. RSC Adv. 10, 15629–15641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuang T et al. (2017) Facile preparation of open-cellular porous poly (l-lactic acid) scaffold by supercritical carbon dioxide foaming for potential tissue engineering applications. Chem. Eng. J 307, 1017–1025 [Google Scholar]
- 70.Grant R et al. (2019) Blended electrospinning with human liver extracellular matrix for engineering new hepatic microenvironments. Sci. Rep 9, 6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jakus AE (2019) An introduction to 3D printing – past, present, and future promise. In 3D Printing in Orthopaedic Surgery (Dipaola MD and Wodajo FM, eds), pp. 1–15, Elsevier [Google Scholar]
- 72.Wang B et al. (2016) Functional maturation of induced pluripotent stem cell hepatocytes in extracellular matrix – a comparative analysis of bioartificial liver microenvironments. Stem Cell Transl. Med 5, 1257–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wunner FM et al. (2018) Melt electrospinning writing of highly ordered large volume scaffold architectures. Adv. Mater 30, 1706570. [DOI] [PubMed] [Google Scholar]
- 74.Schoen B et al. (2017) Electrospun extracellular matrix: paving the way to tailor-made natural scaffolds for cardiac tissue regeneration. Adv. Funct. Mater 27, 1700427 [Google Scholar]
- 75.Rutz AL et al. (2017) Toward next-generation bioinks: tuning material properties pre- and post-printing to optimize cell viability. MRS Bull. 42, 563–570 [Google Scholar]
- 76.Jakus AE et al. (2016) Hyperelastic 'bone': a highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci. Transl. Med 8, 358ra127. [DOI] [PubMed] [Google Scholar]
- 77.Jakus AE et al. (2015) Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 9, 4636–4648 [DOI] [PubMed] [Google Scholar]
- 78.Kim JH et al. (2018) 3D bioprinted human skeletal muscle constructs for muscle function restoration. Sci. Rep 8, 12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Y-H et al. (2019) Three-dimensionally printed hyperelastic bone scaffolds accelerate bone regeneration in critical-size calvarial bone defects. Plast. Reconstr. Surg 143, 1397–1407 [DOI] [PubMed] [Google Scholar]
- 80.Sun N et al. (2018) High-resolution tissue mass spectrometry imaging reveals a refined functional anatomy of the human adult adrenal gland. Endocrinology 159, 1511–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gessel M et al. (2015) Decellularization of intact tissue enables MALDI imaging mass spectrometry analysis of the extracellular matrix. J. Mass Spectrom 50, 1288–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erben L and Buonanno A (2019) Detection and quantification of multiple RNA sequences using emerging ultrasensitive fluorescent in situ hybridization techniques. Curr. Protoc. Neurosci 87, e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Babu PKV and Radmacher M (2019) Mechanics of brain tissues studied by atomic force microscopy: a perspective. Front. Neurosci 13, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Efremov YM et al. (2019) Measuring viscoelasticity of soft biological samples using atomic force microscopy. Soft Matter 16, 64–81 [DOI] [PubMed] [Google Scholar]
- 85.Brown B et al. (2006) The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 12, 519–526 [DOI] [PubMed] [Google Scholar]
- 86.Hodde JP et al. (1996) Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 2, 209–217 [DOI] [PubMed] [Google Scholar]
- 87.Lu T et al. (2015) Enhanced osteointegration on tantalum-implanted polyetheretherketone surface with bone-like elastic modulus. Biomaterials 51, 173–183 [DOI] [PubMed] [Google Scholar]
- 88.Koser DE et al. (2015) CNS cell distribution and axon orientation determine local spinal cord mechanical properties. Biophys. J 108, 2137–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saito M and Marumo K (2015) Effects of collagen crosslinking on bone material properties in health and disease. Calcif. Tissue Int 97, 242–261 [DOI] [PubMed] [Google Scholar]
- 90.Coulson-Thomas YM et al. (2015) The identification of proteoglycans and glycosaminoglycans in archaeological human bones and teeth. PLoS One 10, e0131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierret C et al. (2010) Developmental cues and persistent neurogenic potential within an in vitro neural niche. BMC Dev. Biol 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Massoulié J and Millard CB (2009) Cholinesterases and the basal lamina at vertebrate neuromuscular junctions. Curr. Opin. Pharmacol 9, 316–325 [DOI] [PubMed] [Google Scholar]
- 93.Sanes JR (2003) The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem 278, 12601–12604 [DOI] [PubMed] [Google Scholar]
- 94.Hodde J et al. (2002) Fibronectin peptides mediate HMEC adhesion to porcine-derived extracellular matrix. Biomaterials 23, 1841–1848 [DOI] [PubMed] [Google Scholar]
- 95.Peloso A et al. (2016) The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Ann. Surg 264, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Massey VL et al. (2017) The hepatic 'matrisome' responds dynamically to injury: characterization of transitional changes to the extracellular matrix in mice. Hepatology 65, 969–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Opara EC et al. (2010) Design of a bioartificial pancreas. J. Investig. Med 58, 831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fang X et al. (2016) SMAD3 activation: a converging point of dysregulated TGF-beta superfamily signaling and genetic aberrations in granulosa cell tumor development? Biol. Reprod 95, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharum IB et al. (2017) Serine threonine kinase receptor associated protein regulates early follicle development in the mouse ovary. Reproduction 153, 221–231 [DOI] [PubMed] [Google Scholar]
- 100.Gershlak JR et al. (2013) Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochem. Biophys. Res. Commun 439, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gravning J et al. (2012) Myocardial connective tissue growth factor (CCN2/CTGF) attenuates left ventricular remodeling after myocardial infarction. PLoS One 7, e52120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mercer SE et al. (2013) A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev. Biol 382, 457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jugdutt BI (2003) Ventricular remodeling after infarction and the extracellular collagen matrix. Circulation 108, 1395–1403 [DOI] [PubMed] [Google Scholar]
- 104.Booth AJ et al. (2012) Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care 186, 866–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Dijk CGM et al. (2020) Extracellular matrix analysis of human renal arteries in both quiescent and active vascular state. Int. J. Mol. Sci 21, 3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Snedeker JG et al. (2005) Strain-rate dependent material properties of the porcine and human kidney capsule. J. Biomech 38, 1011–1021 [DOI] [PubMed] [Google Scholar]
- 107.Bülow RD and Boor P (2019) Extracellular matrix in kidney fibrosis: more than just a scaffold. J. Histochem. Cytochem 67, 643–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gillies AR and Lieber RL (2011) Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44, 318–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boppart MD and Mahmassani ZS (2019) Integrin signaling: linking mechanical stimulation to skeletal muscle hypertrophy. Am. J. Physiol. Cell Physiol 317, C629–C641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cescon M et al. (2016) Lack of collagen VI promotes neurodegeneration by impairing autophagy and inducing apoptosis during aging. Aging Albany 8, 1083–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]