Abstract
Spatially, division site selection is one of the most precisely controlled processes in bacterial physiology. Despite its obvious importance to the production of properly sized, viable daughter cells, the mechanisms underlying division site selection have remained largely mysterious. In this issue of Molecular Microbiology, Hajduk et al. provide new insight into this essential process. Overturning previous models, including one of their own, they discover that two factors involved in chromosome remodeling—the ParB-like protein Spo0J, and the nucleoid associated protein Noc—work together to coordinate early steps in DNA replication with establishment of a medial division site in the Gram-positive bacterium, Bacillus subtilis.
Finding That Sweet-Spot for FtsZ Assembly
In most bacteria and archaea, cell division is initiated by assembly of the tubulin-like GTPase FtsZ at the future division site. FtsZ forms a ring-like array of treadmilling polymers that serve as a platform for the cell division machinery (Haeusser and Margolin, 2016). Proper placement of the FtsZ ring in time and space is required to ensure newborn cells reach adequate size and contain a full genetic complement. To achieve this, placement of the FtsZ ring at mid-cell and subsequent division are highly precise, with less than a 1% margin of error, suggesting a highly regulated process (Trueba, 1982; Yu and Margolin, 1999). Unsurprisingly, the factors responsible for the spatial localization of FtsZ assembly are of great interest.
In Escherichia coli and B. subtilis, the prevailing model has been that two sets of factors—the Min proteins and nucleoid occlusion (NO)— coordinate FtsZ assembly with chromosome replication and segregation to ensure the ring forms at mid-cell (Monahan et al., 2014; Rowlett and Margolin, 2015). This two-factor model proposes that the Min system inhibits FtsZ assembly at or near cell poles, while NO inhibits FtsZ assembly over the unsegregated chromosome (the “nucleoid”) through the activity of a DNA-binding protein (SlmA or Noc, in E. coli or B. subtilis respectively) (Figure 1A). Separation of the replicated chromosomes reveals a NO-free zone of reduced DNA concentration where FtsZ could assemble unperturbed. In other words, Min and Noc “corral” FtsZ, allowing it to only reach critical concentration for assembly at midcell.
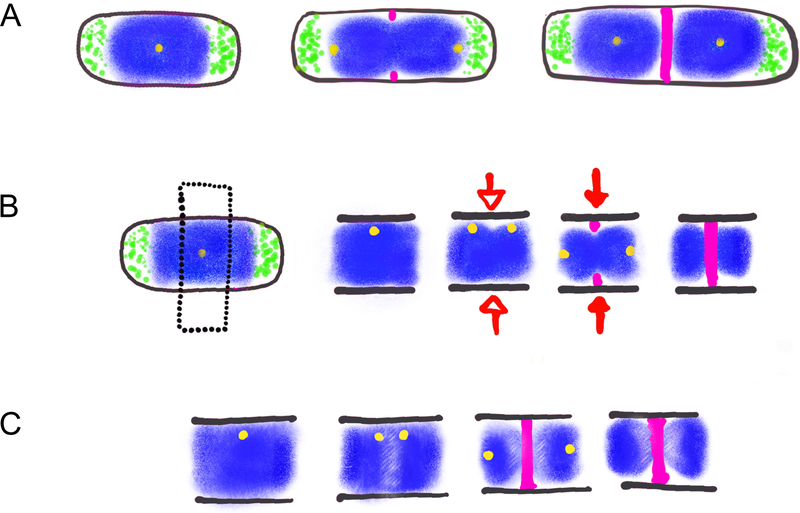
Figure 1: Three models for division site selection.
A. Nucleoid Occlusion. The nucleoid (blue) and associated proteins inhibit assembly of the cell division machinery (pink) at mid-cell while the Min proteins (green) inhibit FtsZ assembly at cell poles. Segregation of replicated chromosomes provides nucleoid and Min protein free space for assembly of FtsZ and the division machinery at mid-cell.
B. Ready-Set-Go. Black dotted line indicates section of cell shown in next three images. The transition between the initiation and elongation stages of DNA replication [depicted here by duplication of the origin of replication (yellow)] reveals a specific site for FtsZ assembly at mid-cell (Ready). Open arrow indicates point in cell cycle at which future division site is established (Set). Closed arrow indicates initial steps in formation of the mature division machinery (Go).
C. Nucleoid organization model. Replication-initiation dependent (Spo0J) and independent (NOC) changes in nucleoid structure (lighter areas near mid-cell in the second, third, and last images) facilitate assembly of FtsZ and the division machinery at mid-cell.
Despite the compelling nature of such a model, there is significant data indicating that the Min system and NO are insufficient to explain the precision of FtsZ ring placement. Importantly, FtsZ assembles at mid-cell with high fidelity in the absence of both Min and NO in B. subtilis and E. coli (Wu and Errington, 2004; Bernhardt and de Boer, 2005). In B. subtilis, min and noc are neither necessary nor sufficient for assembly of FtsZ at midcell. B. subtilis ezrA- mutants form misplaced polar FtsZ rings despite their presence (Haeusser et al., 2004), and outgrown spores of min- noc- double mutants still preferentially support medial FtsZ assembly (Rodrigues and Harry, 2012).
Recent work from the Harry lab (Moriya et al., 2010; Rodrigues and Harry, 2012) and the Dekker & Männik labs (Männik et al., 2012; Bailey et al., 2014) has also suggested that a simplistic model based solely on Min and NO was insufficient, despite its compelling nature. The Harry Lab proposed a model in which successful initiation of DNA replication licenses FtsZ assembly at midcell (Moriya et al., 2010). Notably, in newborn cells the origin of replication is located at midcell and moves towards quarter positions subsequent to the initiation of DNA replication. According to this model, which they called “Ready-Set-Go”, the transition from initiation to the elongation phase of DNA replication (“Ready”) positively marks mid-cell for FtsZ assembly (“Set”), so that upon completion of replication and segregation FtsZ will assemble at midcell (“Go”) (Figure 1B). However, the molecular mechanism underlying this potentiation of a medial site to set it for FtsZ assembly remained obscure.
In this issue, Hajduk et al. (Hajduk et al., 2019) reexamine not only NO but also their own Ready-Set-Go hypothesis to synthesize a model where Noc functions in B. subtilis alongside another DNA-binding protein, Spo0J, to prevent FtsZ assembly during the early stages of DNA replication. Importantly, they show that Spo0J achieves this both through its direct impact on chromosome structure and indirectly through recruitment of SMC proteins that organize chromosome morphology.
Negotiating the Links between Nucleoid Morphology and FtsZ assembly
The basis for the Ready-Set-Go was the observation the initiation phase of DNA replication, but not elongation, was sufficient to ensure medial localization of FtsZ. Blocking initiation results in cells with a cytokinetic ring positioned immediately adjacent to a centrally localized, and compact, nucleoid. Permitting initiation, but preventing subsequent steps required for the elongation phase of replication, permits the formation of a medial FtsZ ring positioned over a bilobed or diffuse nucleoid. It was this latter observation—that the position of the FtsZ ring strongly correlated with nucleoid morphology—that prompted the Harry lab to investigate the contribution of ParB homolog Spo0J to medial FtsZ assembly.
Genetic analysis pointed to an important role for Spo0J in preventing premature FtsZ assembly over unreplicated DNA. Using the heat-sensitive dna-1 variant of the essential DNA replication initiation protein, DnaB, Hajduk et al. examined FtsZ ring positioning in the presence or absence of Spo0J. Consistent with previous work (Moriya et al., 2010), inhibiting replication initiation by shifting dna-1 mutants to non-permissive conditions (48°C) reduced the frequency of medial FtsZ rings from ~90% to ~10%. Instead of mid-cell, FtsZ rings were predominantly off-center, immediately adjacent to the centrally-positioned nucleoid. The defect was partially ameliorated in dna-1 spo0J double mutants, where 40% of cells exhibited medial FtsZ rings at 48°C. Changes in medial FtsZ ring frequency were also accompanied by an alteration of nucleoid morphology. Centrally positioned FtsZ rings were typically associated with bilobed or diffuse nucleoids in dna-1 spo0J cells.
Not to be left out, Hajduk et al. determined that Noc, the protein proposed to work in conjunction with the Min system in earlier models, also contributed to medial FtsZ localization. Deletion of noc increased the frequency of medial FtsZ rings in dna-1 cells at non-permissive condition, both alone and in combination with a spo0J null mutation. Intriguingly, Noc is itself a ParB homolog, raising the possibility of a general role for this family of proteins in division site selection in other bacteria.
Significantly, the authors again saw an increase in bilobed and diffuse nucleoids in the noc spo0J double mutants. They also observed that these cells accumulated FtsZ at mid-cell during exponential growth. It therefore appears that Noc and Spo0J potentiate changes in chromosome structure and/or organization that deter FtsZ assembly at mid-cell prior to replication initiation. Not surprisingly, premature FtsZ ring formation results in an increased frequency of the invaginating septum “guillotining” unsegregated DNA. Fluorescent tagging of specific chromosomal regions showed that loss of interactions between chromosomal arms underlie the alteration in replication-blocked nucleoid morphology in noc spoOJ double mutants as well.
Taking a Mulligan on Some Models
Although it integrates ideas from previous models for the regulation of FtsZ ring placement, this new model—which we have taken the liberty of naming “Chromosome-Architecture Driven Division (CADD) model (Figure 1C) supersedes both the Harry lab’s own “Ready-Set-Go” hypothesis as well as the idea that Noc and Min function synergistically to corral FtsZ at mid-cell. Importantly, this model of how cells calculate mid-cell shares consistency with observations from other labs. The Rudner Lab previously deduced the existence of a Noc-independent form of NO in B. subtilis arising from unidentified factors changing chromosome structure (Bernard et al., 2010). As mentioned above, work from the Dekker lab has also ruled out a strict nucleoid occlusion model in E. coli. (Männik et al., 2012). Whether or not a similar system is at play in E. coli, which encodes both its own Min system and the NO-associated SlmA, but does not encode a functional equivalent of spo0J, is an open question.
Intriguingly, the Veening Lab reported a ParB- and SMC-mediated mechanism for Streptococcus pneumoniae chromosome segregation influencing proper FtsZ ring placement (van Raaphorst et al., 2017). Lacking both Min and homologs of either Noc or SlmA, alterations in chromosome structure in S. pneumoniae exerts its affects by altering localization of a third protein MapZ, that ultimately helps guide FtsZ assembly (Fleurie et al., 2014).
Altogether these findings raise the still outstanding question of whether there still is some unidentified “mark” at midcell that serves as a mechanism to recruit FtsZ in rod-shaped species like B. subtilis and E. coli, or if negative regulation is sufficient to achieve high fidelity localization. These questions, as well as those that will inevitably follow them, await further study. The Harry lab’s willingness to overthrow their own model in light of new data, highlights the vital importance of revision in science, and bodes well for the future of the field of bacterial cell division in particular.
References:
- Bailey MW, Bisicchia P, Warren BT, Sherratt DJ, and Männik J (2014) Evidence for divisome localization mechanisms independent of the Min system and SlmA in Escherichia coli. PLoS Genet 10: e1004504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Marquis KA, and Rudner DZ (2010) Nucleoid occlusion prevents cell division during replication fork arrest in Bacillus subtilis. Mol Microbiol 78: 866–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt TG, and de Boer PAJ (2005) SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol Cell 18: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleurie A, Lesterlin C, Manuse S, Zhao C, Cluzel C, Lavergne J-P, et al. (2014) MapZ marks the division sites and positions FtsZ rings in Streptococcus pneumoniae. Nature 516: 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusser DP, and Margolin W (2016) Splitsville: structural and functional insights into the dynamic bacterial Z ring. Nat Rev Microbiol 14: 305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusser DP, Schwartz RL, Smith AM, Oates ME, and Levin PA (2004) EzrA prevents aberrant cell division by modulating assembly of the cytoskeletal protein FtsZ. Mol Microbiol 52: 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduk IV, Mann R, Rodrigues CDA, and Harry EJ (2019) The ParB homologs, Spo0J and Noc, together prevent premature midcell Z ring assembly when the early stages of replication are blocked in Bacillus subtilis. Mol Microbiol, In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männik J, Wu F, Hol FJH, Bisicchia P, Sherratt DJ, Keymer JE, and Dekker C (2012) Robustness and accuracy of cell division in Escherichia coli in diverse cell shapes. Proc Natl Acad Sci USA 109: 6957–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan LG, Liew ATF, Bottomley AL, and Harry EJ (2014) Division site positioning in bacteria: one size does not fit all. Front Microbiol 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya S, Rashid RA, Rodrigues CDA, and Harry EJ (2010) Influence of the nucleoid and the early stages of DNA replication on positioning the division site in Bacillus subtilis. Mol Microbiol 76: 634–647. [DOI] [PubMed] [Google Scholar]
- Rodrigues CDA, and Harry EJ (2012) The Min system and nucleoid occlusion are not required for identifying the division site in Bacillus subtilis but ensure its efficient utilization. PLoS Genet 8: e1002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett VW, and Margolin W (2015) The Min system and other nucleoid-independent regulators of Z ring positioning. Front Microbiol 6: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba FJ (1982) On the precision and accuracy achieved by Escherichia coli cells at fission about their middle. Arch Microbiol 131: 55–59. [DOI] [PubMed] [Google Scholar]
- van Raaphorst R, Kjos M, and Veening J-W (2017) Chromosome segregation drives division site selection in Streptococcus pneumoniae. Proc Natl Acad Sci USA 114: E5959–E5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, and Errington J (2004) Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell 117: 915–925. [DOI] [PubMed] [Google Scholar]
- Yu XC, and Margolin W (1999) FtsZ ring clusters in min and partition mutants: role of both the Min system and the nucleoid in regulating FtsZ ring localization. Mol Microbiol 32: 315–326. [DOI] [PubMed] [Google Scholar]