Abstract
Vaccination is a highly effective preventive measure against COVID‐19. However, complementary treatments are needed to better control the disease. Fermented vegetables and spices, agonists of the antioxidant transcription factor nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2) and TRPA1/V1 channels (Transient Receptor Potential Ankyrin 1 and Vanillin 1), may help in the control of COVID‐19. Some preliminary clinical trials suggest that curcumin (spice) can prevent some of the COVID‐19 symptoms. Before any conclusion can be drawn and these treatments recommended for COVID‐19, the data warrant confirmation. In particular, the benefits of the foods need to be assessed in more patients, through research studies and large trials employing a double‐blind, placebo‐controlled design.
Keywords: broccoli, COVID‐19, curcumin, Nrf2, TRP channel
1. INTRODUCTION
Vaccination is a highly effective preventive measure against COVID‐19. However, it does not completely block the virus transmission and, although it reduces the severity of COVID‐19, it does not avoid all hospitalisations. Moreover, its effects decrease with time. Complementary treatments are therefore needed. Several expensive treatments are available, including monoclonal antibodies, but they are not readily available for most of the affected patients, particularly in developing countries. Drug repurposing could be cost‐effective, but, to date, no such treatment has been approved.
Several investigators have proposed that nutrients may play a role in preventing or controlling acute respiratory tract infections 1 or COVID‐19. 2 COVID‐19 may also be associated with a loss of biodiversity 3 and gut microbiota changes. 3
The COVID‐19 pandemic has opened fraught questions of equity on how the world has handled the pandemic. In addition to strategies providing universal access to vaccination, there is an urgent need for the global availability of simple and affordable treatments to complement vaccination.
2. THE UPDATED ANTI‐OXIDANT NRF2‐TRANSIENT RECEPTOR POTENTIAL CHANNEL HYPOTHESIS
It has been hypothesised that diet may partly explain large country variations in COVID‐19 death rates. 4 , 5 Some countries with low COVID‐19 death rates have a common habit of eating large quantities of fermented vegetables (such as cabbage) and various spices. The short duration of the Spring 2021 COVID‐19 outbreak in India due to the VOC‐δ (https://coronavirus.jhu.edu/map.html) may be related to diet.
One mechanism of COVID‐19 appears to be oxygen stress acting in synergy with transient receptor potential (TRP) channels. 6 Fermented vegetables and spices are agonists of the antioxidant transcription factor nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2), and spices are TRPA1/V1 (Transient Receptor Potential Ankyrin 1 and Vanillin 1) agonists. 7 Mechanisms related to oxygen stress may explain many of the COVID‐19 symptoms as well as its severity. 8 , 9 The potential clinical relevance of Nrf2 and TPRA1/V1 therapies has been shown in a proof‐of‐concept paper including three clinical cases. 10 Patients experienced a very rapid (minutes) improvement of some of the symptoms for a few hours. Cough and respiratory symptoms were improved better than loss of smell and taste. Induced‐cough challenges showed that broccoli capsules were effective within 10 min, whereas TRPA1/V1 agonists were effective within 1–2 min. Interestingly, paracetamol consistently increased the duration of action of curcumin and broccoli seeds, possibly through its TRPA1 activity. A synergy between Nrf2 and TRPA1/TRPV1 foods may explain the role of diet. Spicy foods are likely to desensitise TRP channels and act in synergy with exogenous anti‐oxidants that activate the Nrf2 pathway (Figure 1). 6 The proof‐of‐concept studies suggest that some nutrients may be of interest in several stages of COVID‐19 and may partly cover an unmet medical need. Since the publication of the proof‐of‐concept, several small clinical studies have been reported.
FIGURE 1.
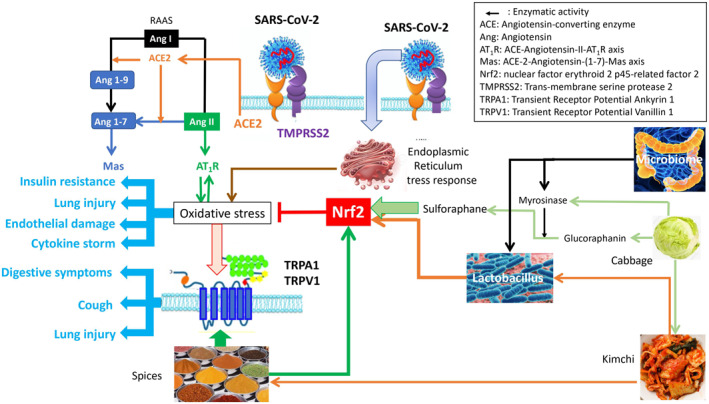
Putative mechanisms of Nrf2‐TRP agonists in the management of COVID‐19
3. CLINICAL STUDIES
3.1. Sulforaphane and Nrf2
The activation of Nrf2 has been proposed as a strategy against COVID‐19. 11 , 12 Sulforaphane is the most potent natural activator of Nrf2. In vitro, sulforaphane inhibits the expression of IL‐6 and IL‐8 by a bronchial epithelial cell line exposed to the SARS‐CoV‐3 spike protein. 13 Sulforaphane exhibits in vitro and in vivo antiviral activity against pandemic SARS‐CoV‐2 and seasonal HCoV‐OC43 coronaviruses. 14 This study led to a patent filed by the Johns Hopkins University on a stabilised synthetic sulforaphane compound SFX‐01. 15 A phase II study by Evgen Pharma was initiated using SFX‐01 to reduce acute respiratory distress syndrome (ARDS) associated with COVID‐19. Negative interim results on 133 patients with pneumonia led to the discontinuation of the study, 16 suggesting that acting only on Nrf2 may be insufficient for the control of severe COVID‐19.
A trial in process (https://clinicaltrials.gov/ct2/show/NCT04421391) is assessing whether QuadraMune®, a food supplement containing sulforaphane, can prevent SARS‐CoV‐2 infection.
3.2. Curcumin
Curcumin has anti‐viral and immunomodulatory effects 17 and inhibits SARS‐CoV‐2 in vitro. 18 , 19
A randomised clinical trial including 140 patients was carried out with curcumin (1050 mg) and piperine (5 mg) as an adjuvant therapy for COVID‐19. 20 Patients with mild, moderate and severe symptoms who received a curcumin/piperine treatment (N = 70) showed inconstant early symptomatic recovery (fever, cough, sore throat, and breathlessness), less deterioration, fewer red flag signs, better ability to maintain oxygen saturation above 94% on room air, and better clinical outcomes compared to patients of the control group (N = 70).
A double‐blind, placebo‐controlled study was carried out in Iran on 40 COVID‐19 patients. 160 mg of Nano‐curcumin was administered daily for 14 days. 21 To facilitate the application of curcumin and improve its stability and solubility, it was formulated with the aid of nanotechnology in Nano‐micelles that have interesting anti‐microbial properties. 22 All of the COVID‐19 subjects in both groups received Betaferon for 5 days, Bromhexine every 8 h, and Atrovastatin daily. In a Per Protocol evaluation, fever, cough, dyspnoea (few patients) and chest radiographs were improved by Nano‐curcumin. After treatment with Nano‐curcumin, IL‐6 and IL‐1β gene expression and secretion were significantly decreased, but IL‐18 mRNA and TNF‐α were unchanged.
The efficacy of the oral Nano‐curcumin formulation in the management of mild to moderate outpatient COVID‐19 was investigated by a randomised triple‐blind, placebo‐controlled clinical trial. 23 COVID‐19 patients from an outpatient setting who fulfilled the inclusion criteria were randomly allocated to the treatment (N = 30) group or to the placebo (N = 30) group. Patients of the treatment group received the oral nanocurcumin formulation (Sinacurcumin soft gel which contains 40 mg of curcuminoids as nanomicelles) and two soft gels twice a day after food for 2 weeks. All symptoms except sore throat resolved faster in the treatment group, and the difference was significant for chills, cough, as well as smell and taste disturbances. The CRP serum level was lower in the treatment group at the end of 2 weeks and the lymphocyte count was significantly higher. No substantial adverse reaction was reported in the treatment group.
An open, non‐randomised clinical trial assessed the efficacy of Nano‐curcumin in the management of 21 mild‐to‐moderate hospitalised COVID‐19 patients. 24 By comparison with the control groups, most of the symptoms—including fever and chills, tachypnoea, myalgia and cough—resolved significantly faster in the curcumin group. Moreover, SaO2 was significantly higher in the treatment group after 2, 4, 7 and 14 days of follow‐up, as was the lymphocyte count after 7 and 14 days. The duration of supplemental O2 use and hospitalisation was also meaningfully shorter in the treatment group.
A trial in progress (https://clinicaltrials.gov/ct2/show/NCT05008003) is evaluating the combination of vitamin D, curcumin and quercetin on the early symptoms. The active comparator will be the standard of care.
4. SAFETY
4.1. Nrf2‐containing foods and supplements
Cruciferous vegetables such as broccoli are very healthy. No side effects (apart from some diarrhoea) have been reported using the regular dietary intake, except in certain patients with thyroid diseases. 25 During the COVID‐19 pandemic, there were no reports of negative broccoli interaction.
It is considered that the daily intake of broccoli sprouts is around 30–50 g. Comparisons between sulforaphane and phenolic compounds of broccoli seeds and sprouts have been made. It appears that seeds contain 50%–70% of sprout compounds. 26 In another study, broccoli seeds contained more glucoraphanin than sprouts (around 1.5‐fold). 27 The glucoraphanin level in the broccoli seeds is largely determined by plant genotype, although the environment in which the plants are grown (e.g. location, year, drought, pollution and disease pressure) also plays a clear and significant role. 28 Thus, it may be assumed that seeds contain between 0.5 and 1.5 g of bioactive components per gram of sprouts. A study published on ClinicalTrials.gov (ID: NCT03390855) showed that a daily consumption of 30 g of raw (not cooked), fresh broccoli sprouts during 10 weeks (70 days) was safe. 29 The daily dose of broccoli seed capsules arbitrarily proposed in food supplements ranges from 300 to 1000 mg (less than 5% of the daily intake of broccoli sprouts).
Kimchi, a traditional Korean fermented food, usually contains cabbage and/or radish (Nrf2), garlic, red pepper, ginger and other spices (TRPA1/V1). Fermentation by lactic acid bacilli increases the health benefits of Kimchi (Nrf2). 30 Koreans consume around 60 g of Kimchi per day 31 and there have been no reports associating Kimchi with side effects in COVID‐19 patients.
In clinical studies, doses of up to 800 μmol daily of glucoraphanine have been reported without safety concerns. 29 , 30 , 31
There have not been any reports of a negative interaction between broccoli or kimchi and COVID‐19.
Although there is no recommendation for pharmacologic doses issued in Europe or the United States for both broccoli and sulforaphane, broccoli may, at a very high dose, have some pharmacologic effects such as (i) the antagonism of aryl hydrocarbon receptors modulating the CYP1 (Cytochrome P450, family 1, subfamily A, polypeptide 1) family of cytochromes P450 32 or (ii) a direct effect of CYP1. 33 These effects must be studied carefully before performing trials at high doses. Moreover, in broccoli seeds, there are many other compounds that may have pharmacologic properties when ingested at high doses. 34
4.2. Curcumin
Curcumin and black pepper are routinely used in the Asian cuisine at relatively high doses, and capsules are available in pharmacies or biofood shops. Curcumin has a long‐established safety record. 35 According to the JECFA (Joint United Nations and World Health Organization Expert Committee on Food Additives) and EFSA (European Food Safety Authority) reports, the Allowable Daily Intake (ADI) value of curcumin is up to 3 mg/kg body weight based on the No‐Observed‐Adverse‐Effect Level (NOAEL). 35 , 36 , 37 In the EFSA re‐evaluation of 2014, 37 the dose of curcumin dietary food supplements, supplied in a solid form including capsules, should be 300 mg per day. Several trials on healthy subjects have supported the safety and efficacy of curcumin. Despite this well‐established safety, some negative side effects have been reported. Seven subjects receiving 500–12,000 mg in a dose–response study and followed for 72 h experienced diarrhoea, headache, rash and yellow stool. 38 In another study, some subjects receiving 0.45–3.6 g/day of curcumin for one to 4 months reported nausea and diarrhoea as well as an increase in serum alkaline phosphatase and lactate dehydrogenase contents. 39
Curcumin was found to have some effect on coagulation, in particular on platelets, but these effects were considered as beneficial in haemostasis, anticoagulation and fibrinolysis. 40
A warning was issued for curcumin by the Anses (Agence nationale de sécurité sanitaire, alimentation, environnement, travail, France), the French agency for food safety, as it may impact SARS‐CoV‐2 infection. 41 The Anses recommends the use of curcuma only in the frame of a clinical trial.
5. NEED TO SCALE UP THE RESEARCH ON NRF2‐TPRA1 CLINICAL THERAPIES
It is important to find simple, cheap and safe complementary treatments for COVID‐19. The results of these few clinical studies cannot yet be taken as formal evidence since the dietary patterns in India or Iran differ from those in Europe, and the sample size is small. Moreover, the proof‐of‐concept study in a very limited number of patients has led to the hypothesis that combined Nrf2‐TRPA1 foods may be beneficial for some COVID‐19 symptoms and that there is a synergy between Nrf2 and TRPA1 agonists. The study has suggested that there could be a very rapid onset of symptom relief.
Before any conclusion can be drawn and these treatments recommended for COVID‐19, the data warrant confirmation. In particular, the benefits of the foods need to be assessed in more patients, through research studies and large trials employing a double‐blind, placebo‐controlled design (Table 1).
TABLE 1.
Research needs and clinical research priorities
| The mechanisms of action of the compounds tested should be investigated using proper experimental studies. |
| Higher priority clinical studies |
| • Impact on hospitalised patients: A relatively small‐scale POC study could check whether the nutrients can improve cough and oxygen saturation (SaO2) within minutes in hospitalised patients, complementing the standard of care. Depending on the results, a DB‐PC‐RCT could assess the efficacy of these nutrients on the severity of symptoms, need for ICU and duration of hospitalisations (High priority). |
| • Prevention and control of COVID‐19 in older people living in home care services: Many people with severe outcomes and death are elderly and live in these settings. 46 – 48 It would be of paramount importance to test whether these safe compounds can reduce the severity of COVID‐19 in infected people as well as the transmission to the other residents when there is an outbreak in a setting (high priority). |
| • Reduction of symptoms in long COVID‐19: Many patients suffer from long COVID‐19 symptoms with an impact on social life and work (high priority, relatively easy to be carried out). |
| Lower priority clinical studies |
| • Prevention of severe COVID‐19 symptoms in COVID‐19 patients: A large‐scale study is needed to assess whether patients who developed mild (and/or early) COVID‐19 may be prevented from developing severe COVID‐19 symptoms and/or hospitalisations (medium priority). |
| • Prevention of COVID‐19 symptoms in asymptomatic SARS‐CoV‐2‐infected people: A large‐scale study is needed to assess whether asymptomatic SARS‐CoV‐2‐infected people may be protected from developing COVID‐19 symptoms and deteriorating to severe COVID‐19 symptoms (low priority as this would be extremely difficult to do). |
| Exploratory |
| • Prevention of SARS‐CoV‐2 infection: Nrf2 was found to be involved in SARS‐CoV‐2 infection. 49 It is possible that the long‐term use of large quantities of Nrf2 agonists such as curcuma or kimchi may prevent infection (exploratory). |
In countries where large amounts of spices are eaten, the consumption of fermented vegetables is also high. This is the case for cassava in Africa or many fermented vegetables in Asia. Different types of fermented foods and spices are widely consumed in eastern Asian countries. Among them, kimchi is the most popular Korean traditional food. In such countries, it is possible that another form of TRP desensitisation, ‘tachyphylaxis’, may be important. This is the reduction or the disappearance of symptoms in response to repeated applications of agonists. 42 , 43 , 44
SARS‐CoV‐2 variants of concern (VOCs) may have a different impact on cells of the respiratory tract, and the SARS‐CoV‐2 B.1.1.529 Omicron variant may cause attenuated lung disease. 45 The hypothesis should therefore be tested with all VOCs.
6. CONCLUSION
Without any doubt, in all infectious diseases, vaccination is the most important prophylactic treatment. However, in the case of infection, other treatment options are needed. Complementary treatments will never replace vaccination but can help to better control COVID‐19 and might even reduce SARS‐CoV‐2 infection. Complementary treatments with plant‐derived nutrients can help to better control COVID‐19 and might even reduce SARS‐CoV‐2 infection. These treatments would certainly not replace any appropriate treatment but do have the potential to reduce morbidity and shorten time of sick leave. These nutrients have the advantage of being available and affordable globally. Curcumin as a TRPA1/V1 agonist and Nrf2 agonists may represent prototypes for other nutrients. They certainly merit further investigation in various aspects of COVID‐19.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Jean Bousquet: Writing – original draft; Writing – review & editing. Tari Haahtela: Writing – review & editing; Hubert Blain: Writing – review & editing. Wienczylslawa Czarlewski: Writing – review & editing; Torsten Zuberbier: Writing – review & editing. Anna Bedbrook: Writing a– review & editing. Alvaro Cruz: Writing – review & editing). Joao Fonseca: Writing – review & editing. Ludger Klimek: Writing – review & editing. Piotr Kuna: Writing – review & editing. Boleslaw Samolinski: Writing – review & editing. Arunas Valiulis: Writing – review & editing. Antoine Lemaire: Writing a – review & editing. Josep M. Anto: Writing – review & editing.
ACKNOWLEDGEMENT
Open access funding enabled and organized by Projekt DEAL.
Bousquet J, Haahtela T, Blain H, et al. Available and affordable complementary treatments for COVID‐19: from hypothesis to pilot studies and the need for implementation. Clin Transl Allergy. 2022;e12127. 10.1002/clt2.12127
REFERENCES
- 1. Vlieg‐Boerstra B, de Jong N, Meyer R, et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: a systematic review and meta‐analysis. Allergy. 2021. [DOI] [PubMed] [Google Scholar]
- 2. Yedjou CG, Njiki S, Enow J, et al. Pharmacological effects of selected medicinal plants and vitamins against COVID‐19. J Food Nutr. 2021;7(2):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jabczyk M, Nowak J, Hudzik B, Zubelewicz‐Szkodzinska B. Diet, probiotics and their impact on the gut microbiota during the COVID‐19 pandemic. Nutrients. 2021;13(9):3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bousquet J, Anto JM, Iaccarino G, et al. Is diet partly responsible for differences in COVID‐19 death rates between and within countries? Clin Transl Allergy. 2020;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bousquet J, Anto JM, Czarlewski W, et al. Cabbage and fermented vegetables: from death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID‐19. Allergy. 2021;76(3):735–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bousquet J, Czarlewski W, Zuberbier T, et al. Potential interplay between Nrf2, TRPA1, and TRPV1 in nutrients for the control of COVID‐19. Int Arch Allergy Immunol. 2021:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bousquet J, Czarlewski W, Zuberbier T, et al. Spices to control COVID‐19 symptoms: yes, but not only. Int Arch Allergy Immunol. 2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liviero F, Campisi M, Mason P, Pavanello S. Transient receptor potential vanilloid subtype 1: potential role in infection, susceptibility, symptoms and treatment of COVID‐19. Front Med. 2021;8:753819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jaffal SM, Abbas MA. TRP channels in COVID‐19 disease: potential targets for prevention and treatment. Chem Biol Interact. 2021;345:109567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bousquet J, Le Moing V, Blain H, et al. Efficacy of broccoli and glucoraphanin in COVID‐19: from hypothesis to proof‐of‐concept with three experimental clinical cases. World Allergy Organ J. 2021;14(1):100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuadrado A, Pajares M, Benito C, et al. Can activation of NRF2 be a strategy against COVID‐19? Trends Pharmacol Sci. 2020;41(9):598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bousquet J, Cristol JP, Czarlewski W, et al. Nrf2‐interacting nutrients and COVID‐19: time for research to develop adaptation strategies. Clin Transl Allergy. 2020;10(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gasparello J, Finotti A, Gambari R. Tackling the COVID‐19 “cytokine storm” with microRNA mimics directly targeting the 3'UTR of pro‐inflammatory mRNAs. Med Hypotheses. 2021;146:110415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ordonez AA, Bullen CK, Villabona‐Rueda AF, et al. Sulforaphane exhibits in vitro and in vivo antiviral activity against pandemic SARS‐CoV‐2 and seasonal HCoV‐OC43 coronaviruses. bioRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brando L, Ordonez A, Yoken R, Jain S. Use of sulforaphane to inhibit coronaviruses such as SARS‐CoV‐2 and to treat coronavirus diseases such as COVID19. Patent C16492. 2021.
- 16. Evgen Pharma Halts Trial as SFX‐01 Drug Fails to Help Covid Patients . 2021. https://wwwmorningstarcouk/uk/news/AN_1625827864539143700/evgen‐pharma‐halts‐trial‐as‐sfx‐01‐drug‐fails‐to‐help‐covid‐patientsaspx [Google Scholar]
- 17. Thimmulappa RK, Mudnakudu‐Nagaraju KK, Shivamallu C, et al. Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID‐19. Heliyon. 2021;7(2):e06350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bormann M, Alt M, Schipper L, et al. Turmeric root and its bioactive ingredient curcumin effectively neutralize SARS‐CoV‐2 in vitro. Viruses. 2021;13(10):1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marin‐Palma D, Tabares‐Guevara JH, Zapata‐Cardona MI, et al. Curcumin inhibits in vitro SARS‐CoV‐2 infection in Vero E6 cells through multiple antiviral mechanisms. Molecules. 2021;26(22):6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pawar KS, Mastud RN, Pawar SK, et al. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID‐19: a randomized clinical trial. Front Pharmacol. 2021;12:669362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valizadeh H, Abdolmohammadi‐Vahid S, Danshina S, et al. Nano‐curcumin therapy, a promising method in modulating inflammatory cytokines in COVID‐19 patients. Int Immunopharmacol. 2020;89(Pt B):107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trigo‐Gutierrez JK, Vega‐Chacon Y, Soares AB, Mima EGO. Antimicrobial activity of curcumin in nanoformulations: a comprehensive review. Int J Mol Sci. 2021;22(13):7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmadi R, Salari S, Sharifi MD, et al. Oral nano‐curcumin formulation efficacy in the management of mild to moderate outpatient COVID‐19: a randomized triple‐blind placebo‐controlled clinical trial. Food Sci Nutr. 2021;9(8):4068–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saber‐Moghaddam N, Salari S, Hejazi S, et al. Oral nano‐curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease‐19 patients: an open label nonrandomized clinical trial. Phytother Res. 2021. [DOI] [PubMed] [Google Scholar]
- 25. Felker P, Bunch R, Leung AM. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr Rev. 2016;74(4):248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lv J, Bao S, Liu T, et al. Sulforaphane delays diabetes‐induced retinal photoreceptor cell degeneration. Cell Tissue Res. 2020;382(3):477–486. [DOI] [PubMed] [Google Scholar]
- 27. Yagishita Y, Fahey JW, Dinkova‐Kostova AT, Kensler TW. Broccoli or sulforaphane: is it the source or dose that matters? Molecules. 2019;24(19):3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farnham M, Stephenson K, Fahey J. Glucoraphanin level in broccoli seed is largely determined by genotype. Hortscience. 2005;40:50–53. [Google Scholar]
- 29. Lopez‐Chillon MT, Carazo‐Diaz C, Prieto‐Merino D, Zafrilla P, Moreno DA, Villano D. Effects of long‐term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin Nutr. 2019;38(2):745–752. [DOI] [PubMed] [Google Scholar]
- 30. Park K, Ju J. Kimchi and health benefits. In: KY Park DK, Lee KW, Park S, eds. Korean Functional Foods. Composition, Processing and Health Benefits. CRC Press. 2018:43–77. [Google Scholar]
- 31. Tak YJ, Lee JG, Yi YH, et al. Association of handgrip strength with dietary intake in the Korean population: findings based on the Seventh Korea National Health and Nutrition Examination Survey (KNHANES VII‐1), 2016. Nutrients. 2018;10(9):1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdull Razis AF, Noor NM. Naturally‐occurring glucosinolates, glucoraphanin and glucoerucin, are antagonists to aryl hydrocarbon receptor as their chemopreventive potency. Asian Pac J Cancer Prev. 2015;16(14):5801–5805. [DOI] [PubMed] [Google Scholar]
- 33. Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factor‐erythroid 2‐related factor‐2. Nutrients. 2017;9(3):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chartoumpekis DV, Ziros PG, Chen JG, Groopman JD, Kensler TW, Sykiotis GP. Broccoli sprout beverage is safe for thyroid hormonal and autoimmune status: results of a 12‐week randomized trial. Food Chem Toxicol. 2019;126:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods. 2017;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kocaadam B, Sanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57(13):2889–2895. [DOI] [PubMed] [Google Scholar]
- 37. European Food Safety Authority . Refined exposure assessment for curcumin (E 100). EFSA J. 2014;12(10). https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2014.3876 [Google Scholar]
- 38. Lao CD, Ruffin MTt, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. [DOI] [PubMed] [Google Scholar]
- 40. Keihanian F, Saeidinia A, Bagheri RK, Johnston TP, Sahebkar A. Curcumin, hemostasis, thrombosis, and coagulation. J Cell Physiol. 2018;233(6):4497–4511. [DOI] [PubMed] [Google Scholar]
- 41. Risques liés à la consommation de compléments alimentaires contenant des plantes pouvant interférer avec la réponse immunitaire et inflammatorie associée à l’infecftion par le SARS‐CoV‐2. anses, sisine n°2020‐SA‐0045 . 2020. https://www.anses.fr/fr/system/files/NUT2020SA0045.pdf [Google Scholar]
- 42. Touska F, Marsakova L, Teisinger J, Vlachova V. A “cute” desensitization of TRPV1. Curr Pharm Biotechnol. 2011;12(1):122–129. [DOI] [PubMed] [Google Scholar]
- 43. Tian Q, Hu J, Xie C, et al. Recovery from tachyphylaxis of TRPV1 coincides with recycling to the surface membrane. Proc Natl Acad Sci U S A. 2019;116(11):5170–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanz‐Salvador L, Andres‐Borderia A, Ferrer‐Montiel A, Planells‐Cases R. Agonist‐ and Ca2+‐dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J Biol Chem. 2012;287(23):19462–19471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Diamond M, Halfmann P, Maemura T, et al. The SARS‐CoV‐2 B.1.1.529 Omicron virus causes attenuated infection and disease in mice and hamsters. Res Sq. 2021. [Google Scholar]
- 46. Blain H, Rolland Y, Benetos A, et al. Atypical clinical presentation of COVID‐19 infection in residents of a long‐term care facility. Eur Geriatr Med. 2020;11(6):1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blain H, Rolland Y, Schols J, et al. Interim EuGMS guidance to prepare European long‐term care facilities for COVID‐19. Eur Geriatr Med. 2020;11(6):899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blain H, Rolland Y, Tuaillon E, et al. Efficacy of a test‐retest strategy in residents and health care personnel of a nursing home facing a COVID‐19 outbreak. J Am Med Dir Assoc. 2020;21(7):933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Olagnier D, Farahani E, Thyrsted J, et al. SARS‐CoV2‐mediated suppression of NRF2‐signaling reveals potent antiviral and anti‐inflammatory activity of 4‐octyl‐itaconate and dimethyl fumarate. Nat Commun. 2020;11(1):4938. [DOI] [PMC free article] [PubMed] [Google Scholar]


