Abstract
Purpose
In 2017, the first guidelines for fertility preservation in cancer patients were published in Japan. However, the impact of the guidelines remains unknown. Therefore, the authors conducted a nationwide survey on cryopreservation procedures in the period from shortly before to after publication of the guidelines (2016–2019) and compared the results with our previous survey (2011–2015). The authors also surveyed reproductive specialists’ awareness of the guidelines and implementation problems.
Methods
The authors sent a questionnaire to 618 assisted reproductive technology facilities certified by the Japanese Society of Obstetrics and Gynecology.
Results
The authors received responses from 395 institutions (63.8%). Among them, 144 institutions conducted cryopreservation for cancer patients (vs. 126 in 2011–2015) and performed 2537 embryo or oocyte and 178 ovarian tissue cryopreservation procedures (vs. 1085 and 122, respectively). Compared with the previous period, indications were more varied and protocols for controlled ovarian stimulation were more standardized. Reproductive specialists’ interest in oncofertility was high, but many reported three main difficulties: selecting a treatment method, storing samples in the long term, and securing the necessary human resources.
Conclusions
The practice of fertility preservation in cancer patients in Japan has been considerably affected by the first Japanese guidelines.
Keywords: assisted reproductive technology; breast cancer; childhood, adolescent, and young adult (CAYA); fertility preservation; oncofertility
We conducted a nationwide survey on cryopreservation procedures in the period from shortly before to after publication of the JSCO Clinical Practice Guidelines 2017 (2016–2019) and compared the results with our previous survey (2011–2015). In addition, reproductive specialists’ awareness of the guidelines and implementation problems were also surveyed. The present study shows that the practice of fertility preservation in cancer patients in Japan has considerably affected by the first Japanese guidelines.

1. INTRODUCTION
Cancer treatment with surgery, chemotherapy, or radiotherapy may cause severe damage to reproductive function. As cancer treatment outcomes improve, increasing attention is being paid to patients’ quality of life, including fertility, after cancer treatment. In addition, advances in assisted reproductive technology (ART) have improved pregnancy outcomes with cryopreserved materials, including embryos and oocytes. Thus, the demand for fertility preservation in childhood, adolescent, and young adult (CAYA) cancer patients has increased.
Since the American Society of Clinical Oncology (ASCO) first published recommendations on fertility preservation in cancer patients in 2006, awareness of oncofertility has become widespread. 1 The Japan Society of Fertility Preservation (JSFP) was established in 2012, and oncofertility treatment subsequently become increasingly popular in Japan. However, data on fertility preservation in cancer patients in Japan were unavailable because the country had no national registration system. Therefore, in 2016, we conducted the first nationwide survey to obtain data on oncofertility in Japan. For the survey, we sent a questionnaire to 613 ART institutions certified by the Japan Society of Obstetrics and Gynecology (JSOG) that asked about their experience in performing cryopreservation in cancer patients between January 2011 and December 2015. 2 The results showed that more than 1000 embryo or oocyte cryopreservation procedures and more than 100 ovarian tissue cryopreservation procedures had been conducted in the period of interest and that the number of cryopreservation procedures was increasing. We also found that age limits and indication restrictions for cryopreservation and protocols for controlled ovarian stimulation (COS) varied widely among institutions. At that time, guidelines and recommendations had been published by ASCO in the US 1 , 3 and FertiPROTEKT in German‐speaking countries 4 and by the International Society for Fertility Preservation (ISFP), 5 , 6 , 7 but none had been published in Japan. Our data suggested that there was an urgent need to establish guidelines to standardize fertility preservation procedures in our country. Therefore, the Japan Society of Clinical Oncology (JSCO) developed and published the JSCO Clinical Practice Guidelines 2017 for Fertility Preservation in Childhood, Adolescent, and Young Adult Cancer Patients. 8 , 9 In 2019, our research group then published a manual, the “Clinical Practice Manual for Fertility Preservation in Cancer Patients,” to assist reproductive specialists in complying with the guidelines. 10
The objective of the present study was to examine the possible effects of the JSCO 2017 guidelines on the practice of fertility preservation in female cancer patients in Japan. To do so, we distributed a questionnaire to JSOG‐certified ART institutions that asked about their experience with performing cryopreservation in cancer patients between January 2016 and December 2019, and then we compared the results with those of our previous survey. 2 In addition, to identify the issues surrounding cryopreservation that are faced by reproductive specialists in their clinical practice, we also asked about the specialists’ awareness of the guidelines and the associated manual and any difficulties they encounter regarding cryopreservation.
2. MATERIALS AND METHODS
2.1. Data collection
Questionnaires were distributed to 618 ART institutions certified by the JSOG. The questionnaire consisted of two parts. The first part used the same format as our previous survey 2 and asked for information on cryopreservation procedures in cancer patients between January 2016 and December 2019, such as the number of cases, indications, age, and cryopreservation methods. In the second part, we asked reproductive specialists at all the JSOG‐certified ART institutions to respond to a set of questions designed to identify the problems the specialists currently faced and examine their awareness of the guidelines and the associated manual, regardless of whether their institution had performed cryopreservation in cancer patients in the designated period. At institutions that had performed or planned to start performing cryopreservation in cancer patients, we asked the reproductive specialists for information on aspects that may cause problems, including determining the indication, selecting protocols, cooperating with oncologists and other reproductive specialists, and securing the necessary human resources. The remaining institutions were asked why they had not performed or were not planning to perform cryopreservation in cancer patients. The present survey was conducted as part of the Japanese Ministry of Health, Labour and Welfare Research project (JPMH19DA1004) “Comprehensive research for support and dissemination of fertility preservation with medical indications.”
3. RESULTS
3.1. Number of institutions that perform cryopreservation for cancer patients and the institutions’ awareness of the guideline and manual
We received 395 replies (63.9% of the 618 certified ART institutions). Among them, 144 (36.5%) of the institutions conducted cryopreservation for cancer patients in 2016–2019, which was a higher number than in the previous study period (126). Of the institutions that did not perform cryopreservation (n = 251), 67 (27.1%) were planning to start offering cryopreservation to cancer patients, which was almost the same proportion as in our previous survey (25.9%). Thus, a total of 179 (45.3%) institutions, excluding the five non‐responders, neither performed nor were planning to perform cryopreservation. All the institutions had high awareness of the guidelines (91.9% of all institutions) and the guideline manual (87.6% of all institutions).
3.2. Overall cases of cryopreservation for cancer patients
In the period 2016–2019, 144 institutions performed 2537 embryo or oocyte cryopreservation procedures without concomitant ovarian tissue cryopreservation for cancer patients and 178 ovarian tissue cryopreservation procedures with concomitant embryo or oocyte cryopreservation (Table 1). The number of procedures was higher than those in the previous study period (1085 and 122, respectively). As in the previous period, most of the institutions provided cryopreservation to fewer than ten patients in 2016–2019 (Figure 1).
TABLE 1.
Number of cryopreservation procedures in cancer patients performed according to the type of cryopreserved material at Japanese Society of Obstetrics and Gynecology‐certified assisted reproductive technology institutions that responded to the survey
| Cryopreserved material | Procedures, n | |
|---|---|---|
| 2011–2015 (5 years) | 2016–2019 (4 years) | |
| Embryos only | 527 | 1246 |
| Oocytes only | 458 | 1222 |
| Embryos +oocytes | 100 | 69 |
| Total (embryos, oocytes) | 1085 | 2537 |
| Ovarian tissue | 97 | 97 |
| Ovarian tissue +oocytes | 21 | 37 |
| Ovarian tissue +embryos | 3 | 8 |
| Ovarian tissue +embryos + oocytes | 1 | 36 |
| Total (ovarian tissue) | 122 | 178 |
| Total (all materials) | 1207 | 2715 |
FIGURE 1.
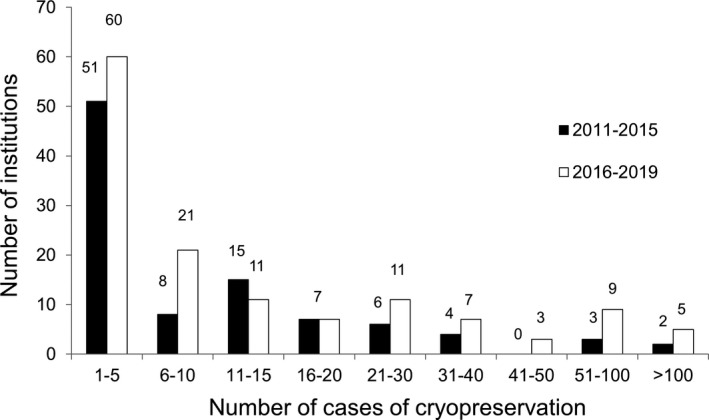
Number of cancer patients undergoing cryopreservation per institution. Although the overall number of cases increased in 2016–2019 from the period assessed in the previous survey (2011–2015), the number of cases per institution was still small
The main indications for cryopreservation were breast cancer (69.5%) and hematologic malignancy (17.8%), which was similar to the previous survey (68.8% and 19.6%, respectively) (Figure 2). Noteworthy is that the range of indications for cryopreservation was broader in the present survey (2016–2019) than in the previous one, which did not identify urological, oral, and skin cancer; thymic and post‐mediastinum tumor; and several benign diseases, such as autoimmune disease and chronic Epstein‐Barr virus infection. 2 Moreover, although both surveys identified bone and soft tissue tumors as an indication for cryopreservation, the 3.2‐fold change in the number of cases from the previous to the present survey (13 vs. 42, respectively) was larger than the 2.2‐fold increase in the total number of cases (1207 vs. 2715, respectively).
FIGURE 2.
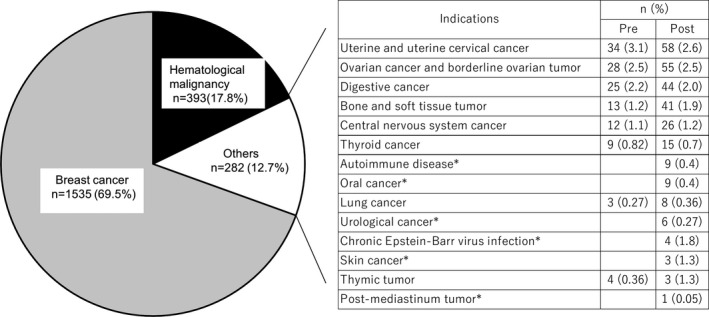
Indications for cryopreservation in cancer patients in the period 2016–2019 at 144 institutions, compared to those in the period 2011–2015 at 126 institutions. Compared with the previous survey (2011–2015), the range of diseases for which cryopreservation was performed has increased; the diseases that were not named as indications for cryopreservation in the previous survey (2011–2015) are marked with an asterisk. Pre, 2011–2015, i.e., before publication of the Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 for Fertility Preservation in Childhood, Adolescent, and Young Adult Cancer Patients 8 , 9 ; post, 2016–2019, i.e., from shortly before to after publication of the guidelines
The number of institutions that performed cryopreservation for the two leading indications, breast cancer and hematologic malignancy, and the number of patients with these malignancies increased by 1.5‐ to 2‐fold compared with the previous study period; that was in accordance with an increase in total numbers of cases and institutions that performed cryopreservation (Table 2). For these two indications, we found no difference in patient age at cryopreservation between the present and previous periods (Table 2).
TABLE 2.
Characteristics of cryopreservation for childhood, adolescent, and young adult patients with breast cancer and hematologic malignancy
| Institutions, n | Patients, n | Number of patients per institution, median (range) |
Patient age at cryopreservation, years, mean ± SD (range) |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Breast cancer | 72 | 116 | 760 | 1535 | 4 (1–127) | 6 (1–139) | 35.5 ± 3.52 (20–48) | 35.2 ± 2.56 (17–50) |
| Hematologic malignancy | 50 | 73 | 216 | 393 | 2 (1–66) | 3 (1–37) | 27.0 ± 5.86 (5–42) | 27.0 ± 5.17 (11–45) |
3.3. Embryo and oocyte cryopreservation
During the period 2016–2019, the types of cryopreservation performed were as follows: embryo only, 1246; oocyte only, 1222; and embryo and oocyte, 69 (Table 1). Seventy institutions (44.0%) and 63 institutions (52.5%) set age limits for embryo and oocyte cryopreservation, respectively (Table 3). These percentages were numerically lower than those in the previous period (51.5% and 63.0%, respectively), but the difference did not reach statistical significance (p = 0.2556 and p = 0.1345, respectively). The indications for embryo and oocyte cryopreservation were also restricted by 63 institutions (39.6%) and 47 institutions (39.1%), respectively. These proportions were almost same as the previous period (34.7% and 35.1%, respectively). However, in the current survey, more institutions than in the previous survey reported excluding diseases that required a hysterectomy from the indications for cryopreservation (41 vs. 16 for cryopreservation of embryos and 34 vs. 15 for cryopreservation of oocytes, respectively).
TABLE 3.
Number of institutions that set age and indication limits for cryopreservation in childhood, adolescent, and young adult cancer patients
| Institutions with age limits, n (%) | Institutions with indication restrictions, n (%) | Not permitted in case of a disease requiring a hysterectomy, n | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Embryos | 52 (51.5) | 70 (44.0) | 34 (34.7) | 63 (39.6) | 16 | 41 |
| Oocytes | 46 (63.0) | 63 (52.5) | 26 (35.1) | 47 (39.1) | 15 | 34 |
| Ovarian tissue | 19 (67.8) | 22 (48.9) | 15 (57.7) | 23 (51.1) | 10 | 11 |
In breast cancer patients, COS was performed for embryo cryopreservation in 99.1% (109/110) of institutions and for oocyte preservation in 97.5% (79/81) (Table 4). Furthermore, 83.5% (86/103) and 92.4% (73/79) of institutions used an aromatase inhibitor (AI) during COS to prevent an increase in serum estradiol levels. These proportions were significantly higher than those in the previous survey (embryo cryopreservation, 83.5% vs. 62.9%, p = 0.0022; and oocyte cryopreservation, 92.4% vs. 68.3%, p = 0.00037). In patients with hematologic malignancy, 100% (69/ 69) and 98.3% (59/60) of the institutions performed COS in embryo and oocyte cryopreservation, respectively. Among these institutions, 79.4% (54/ 68) and 91.5% (54/ 59) conducted random‐start COS to shorten the time until oocyte collection could be performed. These proportions were also significantly higher than those in the previous survey (embryo cryopreservation, 79.4% vs. 46.9%, p = 0.00038; and oocyte cryopreservation, 91.5% vs. 52.1%, p = 0.0000069).
TABLE 4.
Ovarian stimulation methods in childhood, adolescent, and young adult patients with breast cancer and hematologic malignancy
| (a) Breast cancer | ||||||
|---|---|---|---|---|---|---|
| Institutions, n | COS, n (%) | AI, n (%) | ||||
| Pre | Post | Pre | Post | Pre | Post | |
| Embryos | 87 | 110 | 82/87 (94.0) | 109/110 (99.1) | 51/81 (62.9) | 86/103 (83.5)* |
| Oocytes | 64 | 81 | 61/64 (95.3) | 79/81 (97.5) | 41/60 (68.3) | 73/79 (92.4)* |
| (b) Hematologic malignancy | ||||||
|---|---|---|---|---|---|---|
| Institutions, n | COS, n (%) | Random‐start COS, n (%) | ||||
| Pre | Post | Pre | Post | Pre | Post | |
| Embryos | 53 | 69 | 52/53 (98.1) | 69/69 (100) | 23/49 (46.9) | 54/68 (79.4)* |
| Oocytes | 50 | 60 | 49/50 (98.0) | 59/60 (98.3) | 24/46 (52.1) | 54/59 (91.5)* |
Pre, 2011–2015, i.e., before publication of the Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 for Fertility Preservation in Childhood, Adolescent, and Young Adult Cancer Patients 8 , 9 ; post, 2016–2019, i.e., from shortly before to after publication of the guidelines. *p < 0.05 vs. ratio during 2011–2015 (pre).
Abbreviations: AI, aromatase inhibitor; COS, controlled ovarian stimulation.
3.4. Embryo transfer
Embryo transfer (ET) was performed by 93 (76.9%) institutions after embryo cryopreservation and by 20 (22.5%) after oocyte cryopreservation (Table 5). For embryo cryopreservation, these percentages were statistically higher than those in the previous survey (p = 0.0083), and for oocyte cryopreservation, they were numerically higher (p = 0.2290). A total of 402 and 38 patients underwent ET with cryopreserved embryos and oocytes, respectively, which was more than twice the number in the previous survey (Table 5).
TABLE 5.
Number of embryo transfers and ovarian tissue transplant at Japanese Society of Obstetrics and Gynecology‐certified assisted reproductive technology institutions that responded to the survey
| (a) ET | ||||||
|---|---|---|---|---|---|---|
| Institutions that performed ET, n (%) | Patients that underwent ET, n | Institutions with pregnancy outcomes, n (%) | ||||
| Pre | Post | Pre | Post | Pre | Post | |
| Embryos | 59 (59.5) | 93 (76.9)* | 167 | 402 | 42 (71.2) | 73 (78.5) |
| Oocytes | 11 (14.9) | 20 (22.5) | 15 | 38 | 7 (63.6) | 8 (40.0)* |
| (b) Ovarian tissue transplant | ||||||
|---|---|---|---|---|---|---|
| Institutions that performed transplant, n (%) | Patients that underwent transplant, n | Institutions with pregnancy outcomes, n (%) | ||||
| Pre | Post | Pre | Post | Pre | Post | |
| Ovarian tissue | 4 (14.8) | 6 (31.6) | 7 | 8 | 0 (0) | 1 (16.7) |
Pre, 2011–2015, i.e., before publication of the Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 for Fertility Preservation in Childhood, Adolescent, and Young Adult Cancer Patients 8 , 9 ; post, 2016–2019, i.e., from shortly before to after publication of the guidelines. *p < 0.05 vs. the percentage during the period of the previous survey, i.e., 2011 to 2015 (pre).
Abbreviation: ET, embryo transfer.
3.5. Ovarian tissue cryopreservation
During the period 2016–2019, the following numbers of procedures were performed: ovarian tissue cryopreservation, 97; ovarian tissue and oocyte cryopreservation, 37; ovarian tissue and embryo cryopreservation, 8; and ovarian tissue, embryo, and oocyte cryopreservation, 36 (Table 1). The number of ovarian tissue cryopreservation procedures performed concomitantly with embryo and/or oocyte cryopreservation increased by 1.5‐fold compared with the previous study period. Twenty‐two institutions (48.9%) set age limits for ovarian tissue cryopreservation, and 23 institutions (51.1%) set restrictions for the indications. These percentages were numerically lower than those in the previous survey (67.8% and 57.7%, respectively), but the difference did not reach statistical significance (p = 0.1451 and p = 0.6213, respectively). The number of institutions that excluded diseases requiring hysterectomy from the permitted indications was almost the same as in the previous survey (11 vs. 10, Table 3). Eight patients in six institutions underwent transplant of cryopreserved ovarian tissue (Table 5). One institution reported that two patients became pregnant naturally after ovarian tissue transplant, whereas no such pregnancy was reported in the previous study period.
3.6. Reproductive specialists’ awareness of cryopreservation for cancer patients
We asked the reproductive specialists at institutions that performed or planned to start performing cryopreservation for cancer patients about the respective issues they experienced (Figure 3). As shown in the figure, 95.2% of the reproductive specialists often or sometimes experienced difficulties in setting age limits and/or restricting the indication, and 94.3%, in selecting a protocol for COS. Regarding the storage of samples, 86.2% were anxious about long‐term storage, and 83.3%, about the possibility of unused samples accumulating. Performing cryopreservation for cancer patients requires cooperation with oncologists and sometimes with other reproductive specialists; 71.4% of the reproductive specialists responded that they were able to smoothly cooperate with oncologists, and 66.8%, that it was easy to introduce patients to other reproductive specialists. In only 28.6% of the institutions were psychologists involved in providing information to patients. Information was provided by doctors and nurses in 52.9% of the institutions and by doctors only in 18.5% of the institutions. Most of the reproductive specialists (90.9%) agreed that they had difficulties in securing the necessary human resources to support patients’ decision‐making process, including doctors, nurses, and psychologists, and 51.2% agreed that they had difficulties accessing information on subsidies.
FIGURE 3.
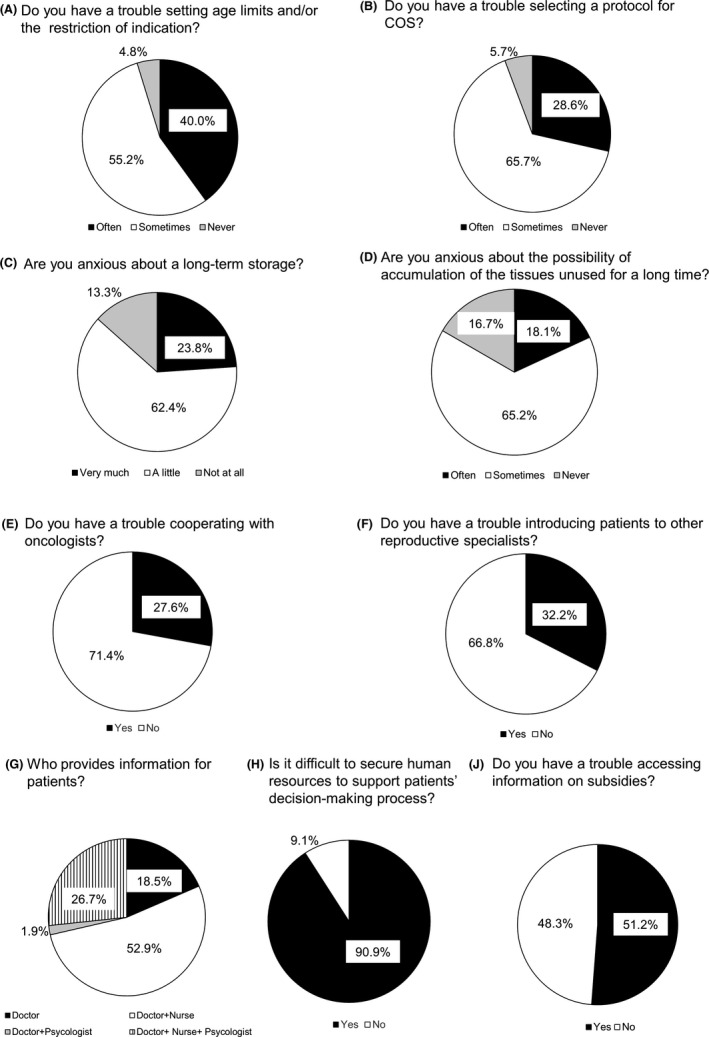
Results of the survey of reproductive specialists’ awareness of cryopreservation for cancer patients at 211 institutions that performed or planned to start performing fertility preservation in cancer patients. We asked reproductive specialists at institutions that are performing or planning to start performing fertility preservation in cancer patients (144 and 67 institutions, respectively) nine questions about awareness of cryopreservation for cancer patients
The remainder of the institutions were asked why they did not perform or plan to start performing cryopreservation for cancer patients (Figure 4). The main reason was anxiety about long‐term storage (52.5%), and the second most common reason was that there was no need to perform cryopreservation for cancer patients because another ART clinic in the neighborhood was performing it (20.7%). More institutions tended to be anxious about the cooperation with oncologists (11.7%) and burden of obtaining informed consent (9.5%) than about the protocol or indications (3.4%).
FIGURE 4.
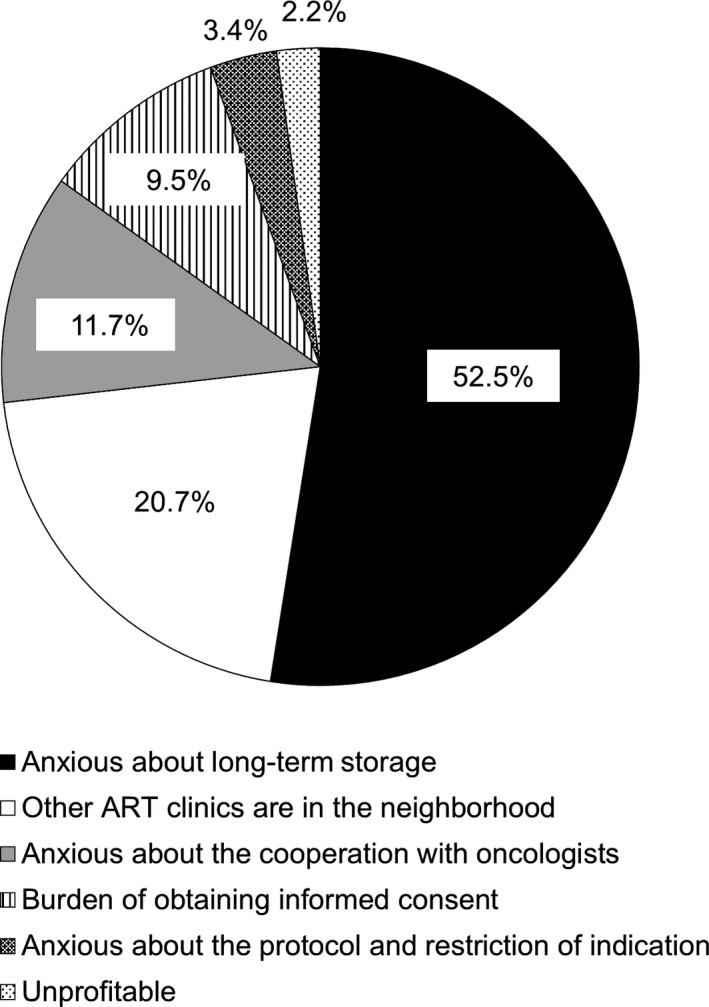
The reasons given by the institutions that do not perform cryopreservation (n = 179) for why they do not perform or plan to perform cryopreservation in cancer patients. ART, assisted reproductive technology
4. DISCUSSION
In this survey, a questionnaire was sent to 618 JSOG‐certified ART institutions to inquire about the implementation of fertility preservation therapy for cancer patients during the 4‐year period from 2016 to 2019. One hundred and forty‐four institutions performed fertility preservation during the period, which had increased from 126 in the previous study period (2011–2015). The number of institutions that were planning to start performing cryopreservation for cancer patients was the same as in the previous study. Although this survey was conducted in 2020, specialists’ awareness of the guidelines and the associated manual, which were published in 2017 and 2019, respectively, was high. These results show that the demand for fertility preservation in cancer patients is increasing and that specialists’ interest in this practice is high. Furthermore, not only the number of patients undergoing cryopreservation but also the range of diseases had increased, indicating that fertility preservation has become recognized among oncologists in a wider range of specialties. In addition, the number of patients with bone and soft tissue tumors who underwent cryopreservation had increased over 3‐fold compared with the previous study period, indicating that recognition of cryopreservation by oncologists in this field has increased. Taken together, these data suggest that not only the opportunities for cancer patients to undergo fertility preservation but also the awareness of providers, in particular oncologists, have increased. Awareness of the concept of oncofertility has been increasing not only among Japanese health care providers in the fields of oncology and reproductive medicine but also among the general population in Japan. In response to this trend, in April 2021 the national public subsidy system started providing subsidies for fertility preservation for medical indications, a development that is not only very welcomed for patients in need but also a major step forward in promoting the advancement of oncofertility in our country.
The number of cryopreserved embryos and oocytes more than doubled in the period 2016 to 2019 compared with the previous period. Furthermore, the treatment of cancer patients seems to become more standardized after publication of the guidelines and manual. For example, more institutions than in the previous study period excluded diseases that required treatment by hysterectomy from the indications for cryopreservation of embryos and oocytes because, after hysterectomy, patients could have a baby only by surrogate motherhood, which is currently not allowed in Japan. Furthermore, the protocols used for COS were more standardized in patients with breast cancer and hematologic malignancy. In breast cancer, elevated estradiol levels during COS are a matter of concern; therefore, the guidelines and the associated manual recommend the use of AI. The current survey shows that the use of AI in cryopreservation of embryos and oocytes has increased significantly. In addition, physicians treating patients with hematologic malignancy generally are unable to allow enough time for COS before starting treatment, so the guidelines and the associated manual recommend random‐start COS. After publication of the guidelines, significantly more institutions used random‐start COS for embryo and oocyte cryopreservation. In addition to the high level of awareness of the guidelines and manual, these changes suggest that treatment methods, which varied among institutions at the time of the previous survey, have become more standardized.
Regarding the use of cryopreserved materials, the cycle number of ETs increased about 2‐fold. The proportion of institutions that performed ET with cryopreserved embryos increased significantly compared with the previous survey, although the proportion of institutions that performed ET with cryopreserved oocytes was still low. The number of patients who underwent transplant of cryopreserved ovarian tissue was also still small; however, in the current survey, one institution reported that two patients became pregnant after cryopreservation of ovarian tissue whereas no institutions reported any such pregnancies in the previous study.
In this study, we also conducted a survey of reproductive specialists at the JSOG‐certified ART institutions to inquire about fertility preservation in cancer patients. Previous studies surveyed oncologists in the United States and France about their attitudes toward fertility preservation in cancer patients. 11 , 12 They found that oncologists were not aware of the need for fertility preservation, had limited knowledge about it, and did not collaborate with reproductive specialists. As for reproductive specialists, an international survey of in vitro fertilization specialists was published in 2019. 13 The providers reported a lack of collaboration with oncologists, which was in accordance with the results of the survey in oncologists. 11 , 12
The results of the second part of the questionnaire, which asked reproductive specialists about problems they faced in the context of cryopreservation, revealed three main difficulties in our country: selecting a treatment method, storing samples in the long term, and securing the necessary human resources. More than 90% of the reproductive specialists at institutions that performed or planned to start performing fertility preservation for cancer patients had trouble selecting a protocol for COS and setting age limits and/or restricting the indications. Because of the small number of cases of fertility preservation for cancer patients per institution in Japan (the majority of the institutions performed the procedure in fewer than 10 patients in the 4‐year study period), the difficulty in selecting a treatment method may be due to a lack of experience and knowledge. The present survey suggests that the guidelines and the associated manual have had a profound impact on reproductive specialists’ practice, indicating that both documents should be revised and amended on the basis of the latest evidence and that methods to convey the respective knowledge should be developed, such as an e‐leaning system.
Concern about long‐term storage was cited as a problem by reproductive specialists at almost 90% of the institutions that performed or planned to start performing fertility preservation for cancer patients and was also the most common reason given for not performing it (cited by just over half of the institutions). In Japan, about 20 000 cancer patients aged 15–39 years are newly diagnosed annually. 14 Advances in cancer treatment have improved 5‐year survival rates, making it necessary for physicians to consider patients’ future fertility to improve their quality of life. Tissues from childhood cancer patients in particular may need to be stored for decades. The longer the storage period, the more likely it is that the institution where the cryopreserved materials are stored will have difficulties contacting patients because of changes in the patients’ circumstances. For ethical reasons, simply disposing of the frozen materials is difficult, even if the renewal period has passed. Furthermore, for several reasons the likelihood that frozen materials will not be used is higher in the field of oncofertility than in the field of general infertility treatment; for example, patients may not wish to have a baby, even though oocytes, embryos, and/or ovarian tissues have been frozen in preparation for future pregnancies, or they may have a baby without using frozen materials. As a result, we can assume that frozen materials may accumulate without being used and without any contact with the patients. Even if institutions manage to keep in touch with their patients, the current situation in Japan where private clinics are responsible for most of the ART cycles in the country makes it unrealistic to expect that an individual clinic will be responsible for storing frozen materials in the long term, perhaps for decades. It is necessary to discuss the possibility of consolidating the institutions for storage; at discussion, caution should be taken to ensure the safety during storage and the transparency of handling of frozen materials after storage is completed.
To support patients’ decision‐making process about fertility preservation, it is essential that information is provided not only by doctors and nurses but also by navigators, clinical psychologists, pharmacists, and social care workers who specialize in oncofertility. In this survey, information was provided by doctors and nurses in just over half of the institutions and by only doctors in almost 20% of the institutions; psychologists were involved in providing information in almost a third of institutions. However, the current survey did not ask about the involvement of professional groups other than these three, so this topic will require further evaluation in future research. In addition, reproduction specialists reported that 90% of the institutions had difficulty in securing the necessary human resources to support patients’ decision‐making process. Considering the recent increase in demand for oncofertility, development of human resources is an urgent issue. To improve the current shortage of trained staff in Japan, the JSFP began accrediting certified patient navigators in 2020 with the aim to develop leaders in this field across occupations. The increase in the number of health care providers with knowledge and experience in oncofertility will help solve the problem of ensuring that sufficient human resources are available.
This study compares the current situation before and after the publication of the guidelines, but the period covered is different. This time, the period was 4 years, which is shorter than the previous 5 years. Therefore, it is not an accurate comparison, but the number of cases has clearly increased since the previous survey even during the 4 years. Furthermore, we surveyed only reproductive specialists, and by conducting a survey of oncologists in the future, we will be able to understand the current status of oncofertility from both directions. Based on the results, we will be able to further improve fertility preservation of cancer patients.
In conclusion, the present survey shows that demand and interest in fertility preservation for cancer patients are increasing in Japan and that the JCSO guidelines and the associated manual seems to have had a profound impact on the practice of oncofertility among reproductive specialists. Furthermore, it is speculated that the practice among oncologists has been affected as well. The present survey also identifies several issues that reproductive specialists face in this context and thus confirms the importance of continuous efforts by health care providers to promote oncofertility. Lastly, it highlights the need for discussions throughout society about the future of oncofertility in our country.
CONFLICT OF INTEREST
Yoshinobu Maeda received honoraria from Kyowa‐Kirin, Bristol‐Myers Squibb, Chugai, Phizer, Celgene, Novartis, and Takeda, and research grants from Nippon Shinyaku and Chugai. The other authors declare that they have no conflict of interest.
ETHICAL APPROVAL
The research protocol of this study was approved by the institutional review board of The University of Tokyo (approval No. 2019294NI).
HUMAN RIGHTS STATEMENTS AND INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments.
ANIMAL RIGHTS
No experiments using animals were performed.
ACKNOWLEDGMENTS
The authors thank Yumiko Muramatsu (The University of Tokyo) for her assistance with organizing the questionnaires and collecting the data. We thank also all the clinicians who participated in this study. The study was supported by Ministry of Health, Labour and Welfare Grant Number JPMH19DA1004 to Y.O.
Kunitomi C, Harada M, Sanada Y, et al. The possible effects of the Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 on the practice of fertility preservation in female cancer patients in Japan. Reprod Med Biol. 2022;21:e12453. doi: 10.1002/rmb2.12453
REFERENCES
- 1. ASCO recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917‐2931. [DOI] [PubMed] [Google Scholar]
- 2. Sanada Y, Harada M, Kunitomi C, et al. A Japanese nationwide survey on the cryopreservation of embryos, oocytes and ovarian tissue for cancer patients. J Obstet Gynaecol Res. 2019;45:2021‐2028. [DOI] [PubMed] [Google Scholar]
- 3. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500‐2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women–a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin's lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284:427‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ISFP Practice Committee , Kim SS, Donnez J, et al. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J Assist Reprod Genet. 2012;29:465‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt KT, Andersen CY, ISFP Practice Committee . Recommendations for fertility preservation in patients with lymphomas. J Assist Reprod Genet. 2012;29:473‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klemp JR, Kim SS, ISFP Practice Committee . Fertility preservation in young women with breast cancer. J Assist Reprod Genet. 2012;29:469‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harada M, Kimura F, Takai Y, et al. Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 for fertility preservation in childhood, adolescent, and young adult cancer patients: part 1. Int J Clin Oncol. 2022;27:265‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tozawa A, Kimura F, Takai Y, et al. Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 for fertility preservation in childhood, adolescent, and young adult cancer patients: part 2. Int J Clin Oncol. 2022;27:281‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The research group ‘Development of the infrastructure of oncofertility in Japan’ in Practical Research for Innovative Cancer Control by the Japan Agency for Medical Research and Development. Clinical Practice Manual for Fertility Preservation in Cancer Patients. Kanehara; 2019. (in Japanese). [Google Scholar]
- 11. Forman EJ, Anders CK, Behara MA. A nationwide survey of oncologists regarding treatment‐related infertility and fertility preservation in female cancer patients. Fertil Steril. 2010;94:1652‐1656. [DOI] [PubMed] [Google Scholar]
- 12. Sallem A, Shore J, Ray‐Coquard I, et al. Fertility preservation in women with cancer: a national study about French oncologists’ awareness, experience, and feeling. J Assist Reprod Genet. 2018;35:1843‐1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shoham G, Levy‐Toledano R, Leong M, Wissman A, Yaron Y, Shoham Z. Oncofertility: insights from IVF specialists‐ a worldwide web‐based survey analysis. J Assist Reprod Genet. 2019;36:1013‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inoue I, Nakamura F, Matsumoto K, Takimoto T, Higashi T. Cancer in adolescents and young adults: National incidence and characteristics in Japan. Cancer Epidemiol. 2017;51:74‐80. [DOI] [PubMed] [Google Scholar]


