Abstract
Purpose
To explore the histopathological findings of cesarean scar defect (CSD) and the immunological component in women with cesarean scar syndrome (CSS).
Methods
This retrospective study was conducted in a university hospital and a public hospital. A total of 63 patients with secondary infertility due to CSS who underwent laparoscopic resection of the CSD lesion were enrolled (CSS group), and 21 patients who underwent hysterectomy with a history of cesarean section were enrolled as control (non‐CSS group). We compared the differences in histopathological findings of CSD lesions by hematoxylin and eosin staining and immunohistochemistry for CD3, CD20, CD56, CD68, CD138, myeloperoxidase, and tryptase between the two groups.
Results
The frequency of presence of endometrium on the CSD surface was significantly lower (p = 0.0023) and that of adenomyosis was significantly higher (p = 0.0195) in the CSS group than in the non‐CSS group. The number of CD3‐, CD20‐, CD68‐, and tryptase‐positive cells was significantly lower in the CSS group than in the non‐CSS group; however, the number of CD138‐positive cells was significantly higher in the CSS group (p = 0.0042).
Conclusions
This study suggested that the absence of endometrium, presence of adenomyosis, and chronic inflammation in CSD contributes to secondary infertility due to CSS.
Keywords: cesarean section, endometriosis, endometrium, infertility, inflammation
1. INTRODUCTION
Cesarean section can lead to the formation of a cesarean scar defect (CSD) in the lower part of the uterus; these complications are also known as isthmocele, cesarean scar dehiscence, diverticulum, pouch formation, or niche. 1 , 2 , 3 , 4 , 5 CSD causes various symptoms, such as abnormal uterine bleeding, dysmenorrhea, chronic pelvic pain, and secondary infertility. The prevalence of these symptoms following cesarean section ranges from 19.4% to 88%. 6 Morris advocated these clinical symptoms as cesarean scar syndrome (CSS). 7 We also have previously reported various symptoms, including fluid retention (typical of CSD), on ultrasonography in women with CSS. 8
Morris et al. explored the pathological changes of the area around the CSD using a paraffin block obtained from a series of 51 hysterectomy specimens. 9 These changes comprised an “overhang” of congested endometrium above the scar recess (61%), moderate to marked lymphocytic infiltration (65%), capillary dilatation (65%), free red blood cells in the endometrial stroma of the scar (59%), and iatrogenic adenomyosis confined to the scar (28%). However, these findings were not observed in patients with infertility.
Donnez et al. reported pathological findings in women with CSS, including those with secondary infertility. 2 They demonstrated that fibrotic tissues and signs of endometriosis were detected in 78.9% and 21% cases, respectively. Furthermore, the muscular density of the residual thin myometrium has been reported to be significantly lower than that of healthy myometrium in women who had previously undergone cesarean section. 2
The uterine immune system, including various immune cell types, such as T‐lymphocytes, B‐lymphocytes, natural killer cells, macrophages, plasma cells, neutrophils, and mast cells, has been reported to demonstrate a valid association with infertility. 10 , 11 , 12 , 13 , 14 , 15 However, the immunological analysis of CSD with CSS, especially in patients with secondary infertility, has been insufficient. Therefore, we evaluated the immunopathological differences in CSD between patients with and without CSS.
2. MATERIALS AND METHOD
2.1. Patients
This study was conducted retrospectively. The participants were divided into two groups: those with secondary infertility due to CSS (CSS group) and those without any symptoms due to CSS (non‐CSS group). Patients with secondary infertility due to CSS who underwent laparoscopic resection of CSD lesions at the Toyama Central Prefectural Hospital from August 2008 to July 2019 were enrolled. The non‐CSS group included patients who underwent hysterectomy due to benign gynecological diseases, such as myoma uteri and ovarian tumor, at the Shiga University of Medical Science. The exclusion criteria were cases without a regular menstrual cycle, with malignancy, tumor around the lower uterine segment and uterine cavity, cervical disease, or suspected infection. The background details of all participants, such as age, gravidity, parity, and the number of cesarean sections, were collected from medical records. This study was approved by the Ethical Committee of Shiga University of Medical Science (approval number R2018‐166). Considering the retrospective study design, an opt‐out consent process was used to guarantee the opportunity for refusal to participate in this study.
2.2. Immunohistochemistry
All paraffin‐embedded blocks were sliced into 4‐µm‐thick sections using a sliding microtome. The sections were mounted on slides after floating on a 42°C water bath. After deparaffinization, the antigens were retrieved in an antigen retrieval reagent (Immunosaver, Nissin EM, Tokyo, Japan) at 98°C for 30 min. Thereafter, they were immersed in 3% hydrogen peroxide for 10 min to block endogenous peroxidase. The sections were covered with a blocking solution for 20 min to suppress non‐specific antibody binding, followed by exposure to primary antibodies in a humidity chamber box overnight at 4 °C. Regarding the primary antibodies, rabbit anti‐tryptase was obtained from Cell Signaling Technology (mAb#19523) and diluted at 1:500; mouse anti‐CD3 (#413241), anti‐CD20 (#422441), anti‐CD68 (#413791), anti‐CD138 (#413881), and anti‐myeloperoxidase (MPO) (#418261) were obtained from Nichirei Biosciences Inc. (Histofine Simple Stain MAX‐PO(M) kit series). Monoclonal mouse anti‐CD56 (#M7304) was obtained from DAKO and diluted at 1:300. After washing with phosphate‐buffered saline, these slides were incubated with horseradish peroxidase‐conjugated secondary antibody (MAX‐PO; Nichirei Biosciences Inc., Tokyo, Japan) for 30 min at room temperature and developed with diaminobenzidine (VECTOR SK‐4100; VECTASTAIN, Vector Laboratories, CA, USA). The slides were counterstained with Mayer's hematoxylin solution, followed by dehydration and mounting.
2.3. Histological evaluation
After hematoxylin and eosin staining using one of the 4‐µm‐thick continuous section series, we confirmed the region of CSD in all cases. Thereafter, we evaluated the presence of a continuous endometrium from the uterine cavity on the CSD and ectopic endometriosis (adenomyosis) in the myometrium under the CSD using hematoxylin and eosin‐stained sections. Positive cells in the immunohistochemical staining sections were counted in the area within 2500 µm from the uterine cavity side of the CSD under high‐power field (HPF), and the total number of positive cells in five random HPFs was recorded. Sections were evaluated using a microscope (OLYMPUS IX83, Tokyo Japan). First, a tile scan for the sections was obtained under a low‐power field using software (cellSens dimension; OLYMPUS, Tokyo, Japan) and detected the counting area. The counting area included adenomyosis in the myometrium but excluded the endometrium because there was little endometrium on the surface of the myometrium in the CSS group.
2.4. Statistical analysis
All data were analyzed using GraphPad Prism ver.7 (GraphPad Software, Inc., San Diego, CA, USA). The D’Agostino‐Pearson test was used to evaluate data distribution. Normally distributed data are presented as mean ± standard deviation. Non‐normally distributed data are presented as median (interquartile range). Comparisons between the CSS and the control groups were performed using an unpaired two‐tailed t‐test or the Mann‐Whitney U test for parametric and non‐parametric data, respectively. Categorical data were compared using Fisher's exact test. Differences with a p‐value of < 0.05 in all cases were considered statistically significant.
3. RESULTS
Eighty‐four participants were enrolled in this study, including 63 and 21 patients with and without CSS, respectively; we compared the microscopic findings of the two groups. The background details of patients in each group are presented in Table 1. There were significant differences in age, gravidity, parity, and the number of cesarean sections between the groups.
TABLE 1.
Background details of the patients in this study
| CSS group | non‐CSS group | p‐value | |
|---|---|---|---|
| Number | 63 | 21 | |
| Age (years) | 35.9 ± 3.3 | 43.6 ± 3.3 | <0.05 |
| Gravidity, median (IQR) | 1 (1–2) | 2 (2–3) | <0.05 |
| Parity, median (IQR) | 1 (1–1) | 2 (1–2.5) | <0.05 |
|
The number of C/S, Median (IQR) |
1 (1–1) | 2 (1–2) | <0.05 |
Abbreviations: CSS, cesarean scar syndrome; C/S, cesarean section; IQR, interquartile range.
The frequency of presence of endometrium on the CSD surface was significantly lower in the CSS group than in the non‐CSS group (p = 0.0023) (Table 2, Figure 1). Moreover, significant differences were noted in terms of the presence of adenomyosis between the groups (p = 0.0195) (Table 2, Figure 1).
TABLE 2.
Comparison of the presence of endometrium on the CSD surface and adenomyosis in CSD
| CSS group | non‐CSS group | p‐value | |
|---|---|---|---|
| Number | 63 | 21 | |
| Presence of endometrium | 14 (22.2%) | 13 (61.9%) | 0.0023 |
| Presence of adenomyosis | 27 (42.9%) | 3 (14.3%) | 0.0195 |
Fisher's exact test was conducted.
Abbreviations: CSD, cesarean scar defect; CSS, cesarean scar syndrome.
FIGURE 1.
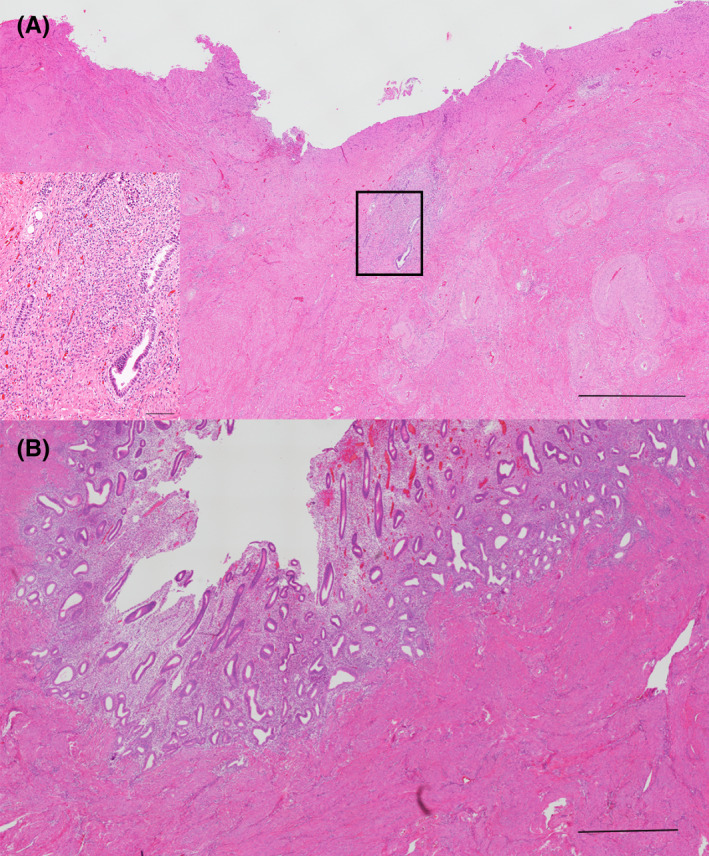
Hematoxylin and eosin staining for CSD in the CSS and non‐CSS groups. (A) Microscopic findings in the CSS group. There is no endometrium on the CSD surface; however, adenomyosis is observed under the CSD (scale bar: 1000 µm). The enlarged image of the squared part is shown in the lower left corner (scale bar: 200 µm). (B) The endometrium covered the CSD surface in the non‐CSS group. Macroscopic findings are shown in the upper right corner (scale bar: 1000 µm). CSD, cesarean scar defect; CSS, cesarean scar syndrome
In the CSS group, the total number of CD3‐, CD20‐, CD56‐, CD68‐, CD138‐, MPO‐, and tryptase‐positive cells in five random HPFs was 8.8 ± 2.0, 2.0 ± 0.7, 0.3 ± 0.1, 2.1 ± 0.5, 2.0 ± 0.5, 6.9 ± 1.1, and 2.9 ± 0.5 (mean ± standard error), respectively. In contrast, in the non‐CSS group, the total number was 27.3 ± 3.5, 4.5 ± 1.5, 0.3 ± 0.1, 4.7 ± 0.9, 0.2 ± 0.1, 3.7 ± 1.0, and 7.6 ± 1.2 (mean ± standard error), respectively (Figure 2, Figure S1). According to the statistical analysis, the total number of CD3‐, CD20‐, CD68‐, and tryptase‐positive cells was significantly lower in the CSS group than in the non‐CSS group (p < 0.0001, p = 0.0015, p = 0.0006, p < 0.0001, respectively). However, the number of CD138‐positive cells was significantly higher in the CSS group (p = 0.0042).
FIGURE 2.
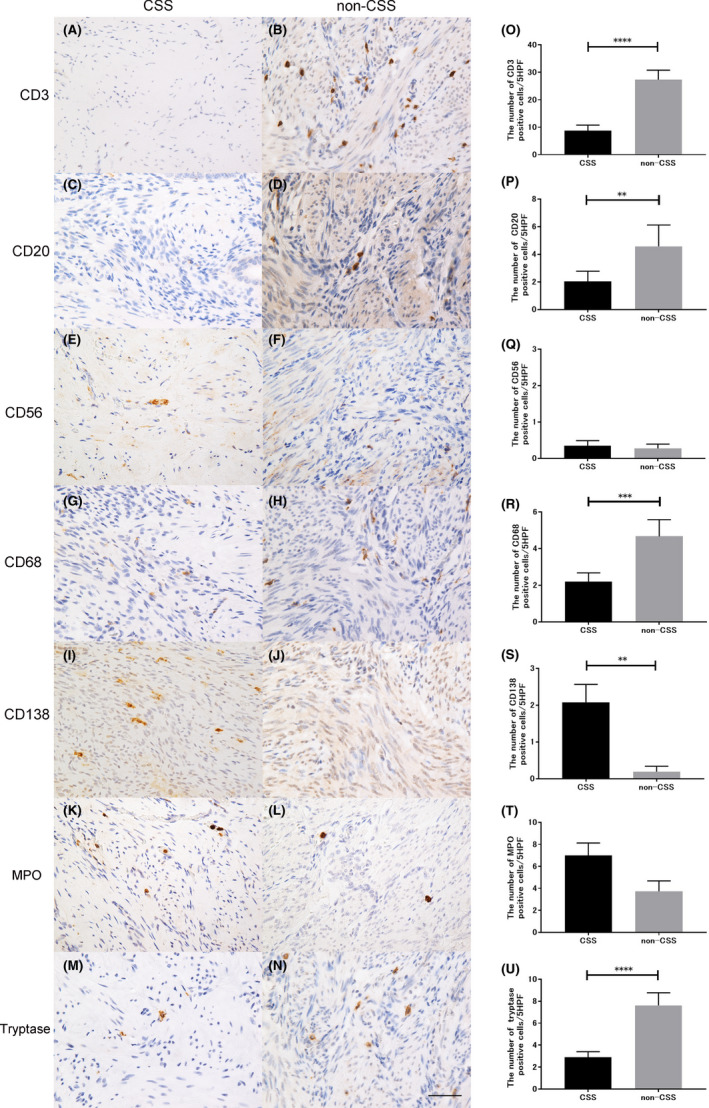
Immunohistological evaluation of the CSD lesions in the CSS and non‐CSS groups. (A–N) Representative figures of immunohistochemistry for CD3‐ (A, B), CD20‐ (C, D), CD56‐ (E, F), CD68‐ (G, H), CD138‐ (I, J), myeloperoxidase‐ (K, L), and tryptase‐positive (M, N) cells in the CSD lesions in both groups (scale bar: 50 µm). (O–U) Immunopathological comparison between the CSS and the non‐CSS groups. CSD, cesarean scar defect; CSS, cesarean scar syndrome
4. DISCUSSION
To the best of our knowledge, this study is the first to report the immunopathology of CSD in women with CSS, particularly regarding infertility. This microscopic analysis of CSD in women revealed the lack of endometrium and the presence of adenomyosis in women with CSS. Furthermore, the expression of acute inflammatory markers under CSD was lower in the CSS group than in the non‐CSS group. However, the expression of chronic inflammatory marker was higher in the CSS group. These immunological imbalances suggested that CSS had a character of chronic inflammation rather than acute inflammation.
As Morris already reported the presence of infiltrating lymphocytes in the CSD area, we also anticipated high inflammation in the CSD area. 9 However, there was little inflammatory cell infiltration in women with CSS, and the findings were similar to those of fibrosis, as reported by Donnez. 2 Although acute inflammatory markers were not detected in the CSD area, chronic inflammatory markers, such as CD138, were observed in the CSD area. CD138 is the marker of plasma cells and is used for the diagnosis of chronic endometritis (CE). 15 CE is distinct from acute endometritis (AE) owing to infection caused by conditions, such as pelvic inflammatory disease. 15 Although AE is diagnosed when a large number of neutrophils are detected in the endometrial stroma, CE is diagnosed when CD138‐positive cells are observed in the endometrial stroma. In cases of CE, proinflammatory cytokines, such as interleukin‐6, interleukin‐1β, and tumor necrosis factor α, are increased in menstrual effluents. 16 These proinflammatory cytokines are considered one of the causes of infertility. CE has been reported to affect the pregnancy and live birth rates. 17 Since CD138‐positive cells were frequently observed in adenomyosis in this study, proinflammatory cytokines may have been generated from the CSD area. The exudation of these cytokines may lead to an accumulation of fluid in the lower uterine segment, impeding pregnancy.
The absence of the endometrium on the CSD surface is consistent with hysteroscopic findings for CSD. Similar to our previous report, hysteroscopic findings demonstrated dendric vessels on the CSD surface and the absence of the endometrium. 18 , 19 , 20 In contrast, the endometrium could be observed on the CSD surface in the non‐CSS group. This difference may contribute to infertility in the CSS group; however, the reason for the absence of the endometrium on the CSD surface remains unclear. Impaired blood flow to the myometrium caused by the suture because of the cesarean section may lead to poor generation of the endometrium on the cesarean scar.
Adenomyosis under CSD was observed in both the CSS and the non‐CSS groups; however, the frequency of the presence of adenomyosis was higher in the CSS group. The actual frequency of adenomyosis may be higher than that reported in our present study as well as previous studies because these examinations were not based on all continuous sections of the paraffin blocks. Adenomyosis may be related to dysmenorrhea in women with CSS. Although the causes of the absence of endometrium and the presence of adenomyosis are unknown, we considered that suturing at the cesarean section may be a related factor. Accidental inclusion of the endometrium into the myometrium at the cesarean section may be relevant to the development of adenomyosis.
There are several limitations to this study. First, the method of suturing at the cesarean sections was unknown. Therefore, it remains unclear whether the CSD generated by the cesarean section will lead to the development of CSS. We believe that the key point is the method of suturing the myometrium during the cesarean section. The second limitation was the settings of the control group. An ideal control is a patient who is relatively young, healthy, and fertile; has a history of cesarean section; and has undergone hysterectomy. However, hysterectomy has an ethical issue. Therefore, the patient with hysterectomy due to a benign disorder with a history of cesarean section was considered as the control in this study. There were unavoidable differences in the backgrounds of the patients between the infertile and the hysterectomy groups.
In conclusion, this study suggested that the absence of endometrium and the presence of adenomyosis and chronic inflammation in CSD may be contributing factors for infertility. Further studies are needed regarding the differences between women who develop CSS after cesarean section and those who do not.
CONFLICTS OF INTEREST
Asuka Higuchi, Shunichiro Tsuji, Yuri Nobuta, Akiko Nakamura, Daisuke Katsura, Tsukura Amano, Fuminori Kimura, Satoshi Tanimura, and Takashi Murakami declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
ST, ST, and TM contributed to the conception and design; AH, ST, YN, and AN contributed to the acquisition of data; AH and ST analyzed the data and drafted the manuscript; ST, YN, DK, TA, and FK substantively revised it; TM made final approval of the version. All authors read and approved the final manuscript.
HUMAN RIGHTS STATEMENT AND INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki declaration of 1964 and its later amendments. This research was approved by the Ethics Committee of Shiga University of Medical Science (approval number R2018‐166). The information on conducting the study was made public, and the opportunity for refusal was guaranteed as much as possible by opt‐out, as suggested by the Ethics Committee.
ETHICS APPROVAL
This research was approved by the Ethics Committee of Shiga University of Medical Science (approval number R2018‐166).
Supporting information
Fig S1
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Higuchi A, Tsuji S, Nobuta Y, et al. Histopathological evaluation of cesarean scar defect in women with cesarean scar syndrome. Reprod Med Biol. 2022;21:e12431. doi: 10.1002/rmb2.12431
Funding information
This study was supported by the Grants‐in‐Aid for Scientific Research (KAKENHI; 20K09616). The grant provided financial support for the preparation of the article, such as English language editing services and Open Access Publication Fee
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, ST, upon reasonable request.
REFERENCES
- 1. Florio P, Filippeschi M, Moncini I, Marra E, Franchini M, Gubbini G. Hysteroscopic treatment of the cesarean‐induced isthmocele in restoring infertility. Curr Opin Obstet Gynecol. 2012;24:180‐186. [DOI] [PubMed] [Google Scholar]
- 2. Donnez O, Donnez J, Orellana R, Dolmans MM. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil Steril. 2017;107(1):289–296.e2. [DOI] [PubMed] [Google Scholar]
- 3. Van Horenbeeck A, Temmerman M, Dhont M. Cesarean scar dehiscence and irregular uterine bleeding. Obstet Gynecol. 2003;102:1137‐1139. [PubMed] [Google Scholar]
- 4. Luo L, Niu G, Wang Q, Xie HZ, Yao SZ. Vaginal repair of cesarean section scar diverticula. J Minim Invasive Gynecol. 2012;19:454‐458. [DOI] [PubMed] [Google Scholar]
- 5. Bij de Vaate AJ, Brölmann HA, van der Voet LF, van der Slikke JW, Veersema S, Huirne JA. Ultrasound evaluation of the cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet Gynecol. 2011;37:93‐99. [DOI] [PubMed] [Google Scholar]
- 6. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562‐572. [DOI] [PubMed] [Google Scholar]
- 7. Morris H. Cesarean scar syndrome. S Afr Med J. 1996;86:1558. [PubMed] [Google Scholar]
- 8. Tsuji S, Murakami T, Kimura F, et al. Management of secondary infertility following cesarean section: report from the Subcommittee of the Reproductive Endocrinology Committee of the Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res. 2015;41:1305‐1312. [DOI] [PubMed] [Google Scholar]
- 9. Morris H. Surgical pathology of the lower uterine segment caesarean section scar: is the scar a source of clinical symptoms? Int J Gynecol Pathol. 1995;14:16‐20. [DOI] [PubMed] [Google Scholar]
- 10. Bulmer JN. Immune aspects of pathology of the placental bed contributing to pregnancy pathology. Baillieres Clin Obstet Gynaecol. 1992;6:461‐488. [DOI] [PubMed] [Google Scholar]
- 11. Robertson SA. Control of the immunological environment of the uterus. Rev Reprod. 2000;5:164‐174. [DOI] [PubMed] [Google Scholar]
- 12. Menzies FM, Shepherd MC, Nibbs RJ, Nelson SM. The role of mast cells and their mediators in reproduction, pregnancy and labour. Hum Reprod Update. 2011;17:383‐396. [DOI] [PubMed] [Google Scholar]
- 13. Tremellen KP, Russell P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: adenomyosis and macrophages. J Reprod Immunol. 2012;93:58‐63. [DOI] [PubMed] [Google Scholar]
- 14. Franasiak JM, Scott RT. Contribution of immunology to implantation failure of euploid embryos. Fertil Steril. 2017;107:1279‐1283. [DOI] [PubMed] [Google Scholar]
- 15. Kimura F, Takebayashi A, Ishida M, et al. Review: chronic endometritis and its effect on reproduction. J Obstet Gynaecol Res. 2019;45:951‐960. [DOI] [PubMed] [Google Scholar]
- 16. Tortorella C, Piazzolla G, Matteo M, et al. Interleukin‐6, interleukin‐1β, and tumor necrosis factor α in menstrual effluents as biomarkers of chronic endometritis. Fertil Steril. 2014;101:242‐247. [DOI] [PubMed] [Google Scholar]
- 17. Hirata K, Kimura F, Nakamura A, et al. Histological diagnostic criterion for chronic endometritis based on the clinical outcome. BMC Womens Health. 2021;21:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsuji S, Kimura F, Yamanaka A, et al. Impact of hysteroscopic surgery for isthmocele associated with cesarean scar syndrome. J Obstet Gynaecol Res. 2018;44:43‐48. [DOI] [PubMed] [Google Scholar]
- 19. Tsuji S, Takahashi A, Higuchi A, et al. Pregnancy outcomes after hysteroscopic surgery in women with cesarean scar syndrome. PLoS One. 2020;15:e0243421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanimura S, Funamoto H, Hosono T, et al. New diagnostic criteria and operative strategy for cesarean scar syndrome: endoscopic repair for secondary infertility caused by cesarean scar defect. J Obstet Gynaecol Res. 2015;41:1363‐1369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, ST, upon reasonable request.


