Abstract
Clinical strains of Stenotrophomonas maltophilia are often highly resistant to multiple antibiotics, although the mechanisms of resistance are generally poorly understood. Multidrug resistant (MDR) strains were readily selected by plating a sensitive reference strain of the organism individually onto a variety of antibiotics, including tetracycline, chloramphenicol, ciprofloxacin, and norfloxacin. Tetracycline-selected MDR strains typically showed cross-resistance to erythromycin and fluoroquinolones and, in some instances, aminoglycosides. MDR mutants selected with the other agents generally displayed resistance to chloramphenicol and fluoroquinolones only, although two MDR strains (e.g., K1385) were also resistant to erythromycin and hypersusceptible to aminoglycosides. Many of the MDR strains expressed either moderate or high levels of a novel outer membrane protein (OMP) of ca. 50 kDa molecular mass, a phenotype typical of MDR strains of Pseudomonas aeruginosa hyperexpressing drug efflux systems. Indeed, the 50-kDa OMP of these S. maltophilia MDR strains reacted with antibody to OprM, the outer membrane component of the MexAB-OprM MDR efflux system of P. aeruginosa. Similarly, a ca. 110-kDa cytoplasmic membrane protein of these MDR strains also reacted with antibody to the MexB component of the P. aeruginosa pump. The outer and cytoplasmic membranes of several clinical S. maltophilia strains also reacted with the anti-OprM and anti-MexB antibodies. N-terminal amino acid sequencing of a cyanogen bromide-generated peptide of the 50-kDa OMP of MDR strain K1385, dubbed SmeM (Stenotrophomonas multidrug efflux), revealed it to be very similar to a number of outer membrane multidrug efflux components of P. aeruginosa and Pseudomonas putida. Deletion of the L1 and L2 β-lactamase genes confirmed that these enzymes were responsible for the bulk of the β-lactam resistance of K1385 and its parent. Still, overexpression of the MDR efflux mechanism in an L1- and L2-deficient derivative of K1385 did yield a modest increase in resistance to a few β-lactams. These data are consistent with the MDR efflux mechanism(s) playing a role in the multidrug resistance of S. maltophilia.
Stenotrophomonas maltophilia is an important nosocomial pathogen associated with infections of compromised individuals, including those with cystic fibrosis and underlying malignancies (3, 4, 6, 23, 27, 31, 45). Generally associated with infections of the respiratory tract (22), the organism is also a cause of bacteremia (15), endocarditis (11, 28), and urinary tract infections (44). S. maltophilia is intrinsically resistant to multiple antibiotics and disinfectants (37, 43), and clinical isolates often display high-level multidrug resistance (43). Multidrug resistant (MDR) strains are also readily selected from susceptible S. maltophilia in the laboratory (1, 16). Not surprisingly, then, a major predisposing factor for S. maltophilia infection is prior antibiotic usage (3, 7, 27, 42). Unfortunately, the intrinsic resistance of the organism and the ready selection of high-level MDR isolates in clinical strains pose a major problem vis-à-vis antistenotrophomomal chemotherapy (43).
Recent evidence indicates that antibiotic efflux may be a contributing factor to the intrinsic and acquired multidrug resistance of S. maltophilia (1). Indeed, antibiotic efflux mechanisms are increasingly recognized as a major factor in the intrinsic and acquired resistance of a number of significant human pathogens, including Pseudomonas aeruginosa and Burkholderia cepacia (29). Thus, effective antibiotic therapy of S. maltophilia infections may require the targeting of efflux mechanisms, in order to render the organism more susceptible to available antimicrobial agents. In the present report, we describe the isolation of a number of MDR strains of S. maltophilia which express homologues of known MDR efflux systems, consistent with the involvement of efflux mechanisms in acquired multidrug resistance. Moreover, a study of clinical isolates also confirms that efflux mechanisms likely contribute to the multidrug resistance of many of these.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Two strains of S. maltophilia, ATCC 13637 and ULA-511 (8), were used as parental wild-type strains in the isolation of various MDR strains. Escherichia coli strains DH5α (2) and S17-1 (39) have been described. Luria-Bertani (LB) broth (Luria broth base; Difco) and agar (LB broth containing 15% [wt/vol] agar [BDH]) were used as the growth media throughout. Bacterial cells were cultivated at 30 or 37°C as indicated. Plasmids pMON01 (38), pBluescript II SK(+) (Stratagene, La Jolla, Calif.), pTZ19U (Bio-Rad Laboratories, Hercules, Calif.), and pEX18Tc (12) have been described and were maintained in E. coli with appropriate antibiotic selection (pBluescript II SK[+] and pTZ19U, 100 μg of ampicillin per ml; pEX18Tc, 10 μg of tetracycline per ml; and pMON01, 30 μg of chloramphenicol per ml).
Antimicrobial agents.
Most antibiotics used were purchased from Sigma-Aldrich Canada Ltd. (Oakville, Ontario, Canada). Others were obtained from the following sources: panipenem and R-83201 (a carbapenem compound) from Sankyo Co., Ltd. (Tokyo, Japan); meropenem from Zeneca Ltd. (Macclesfield Cheshire, United Kingdom); Unasyn (sulbactam-ampicillin) from Pfizer Italinana (Latina, Italy); aztreonam from ICN Biomedicals Inc. (Aurora, Ohio); pirazmonam and cefepime from the Squibb Institute (Princeton, N.J.), cefpirome from Roussel UCLAF (Paris, France), and nitrocefin (Glaxo) from Becton Dickinson and Company (Cockeysville, Md.).
Selections of multiple antibiotic-resistant strains.
Selection of multiple antibiotic-resistant mutants was carried out by plating 50 μl of an overnight culture of ATCC 13637 or ULA-511 onto antibiotic-containing LB agar and by incubating for 24 to 48 h at 30 or 37°C. Antibiotics used included ciprofloxacin (at 1, 4, and 8 μg/ml), norfloxacin (at 30 μg/ml), tetracycline (at 20 and 30 μg/ml), and chloramphenicol (at 20 μg/ml). Resistant colonies were subsequently tested for cross-resistance to additional antibiotics, and those exhibiting resistance to at least two structurally unrelated antibiotics were saved for further study.
Antimicrobial susceptibility assay.
Susceptibility testing was carried out using a twofold serial dilution of the antibiotics in LB broth, with an inoculum of 5 × 105 cells/ml. Data were reported as MICs, which reflected the lowest concentration of antibiotic-inhibiting visible cell growth after an overnight incubation at 37 or 30°C.
Membrane preparation and SDS-polyacrylamide gel electrophoresis.
Bacterial cells were grown in 30 ml of LB broth to the exponential phase of the growth and then collected by centrifugation (15 min at room temperature; 5,000 × g). Cell pellets were washed once with 20 ml of sodium phosphate buffer (50 mM, pH 7.2), resuspended in 3 ml of the same buffer, and stored on ice. Following breakage of cells by sonication (six 30-s pulses at 50% power with a Vibra Cell sonicator (Sonics and Materials Inc., Danbury, Conn.), unbroken cells were removed by centrifugation (6,000 × g in a Beckman TL100.3 rotor for 10 min) and the cell envelopes were pelleted by centrifugation (260,000 × g in the TL100.3 rotor for 30 min). The cell envelope-containing pellets were washed once with 2 ml of sodium phosphate buffer (50 mM, pH 7.2) and subsequently resuspended in 0.5 ml of the same buffer. Following treatment of the cell envelopes with 1.5% (wt/vol) N-lauroylsarcosine (sarkosyl) for 30 min at room temperature, the sarkosyl-soluble cytoplasmic membrane-containing and sarkosyl-insoluble outer membrane-containing fractions were recovered by ultracentrifugation (Beckman TL100.3 rotor at 80,000 rpm for 30 min) (9). The membrane protein composition was subsequently analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (20) using 11% (wt/vol) polyacrylamide in the running gel.
Western immunoblotting.
Western immunoblotting of membrane proteins resolved on SDS-polyacrylamide gels was carried out as described (40) using antibodies raised to the P. aeruginosa MDR efflux proteins MexB (40), OprM (47), OprJ (33), and OprN (14).
Purification of outer membrane protein SmeM.
Outer membranes (as sarkosyl-insoluble cell envelopes) of S. maltophilia MDR strain K1385 were prepared from 1 liter of cells cultured to log phase in LB broth at 30°C following disruption of cells using a French pressure cell (36). Outer membranes were washed once with 20 ml of sodium phosphate buffer (50 mM, pH 7.2) and resuspended in 5 ml of the same buffer. An equal volume of sample loading buffer (125 mM Tris-HCl [pH 6.6], 4% [wt/vol] SDS, 20% [wt/vol] glycerol, 1.4 M 2-mercaptoethanol, 0.001% [wt/vol] bromphenol blue) was added to the resuspended outer membranes, which were subsequently heated at 100°C for 5 min before being loaded on a preparative SDS-polyacrylamide gel (11% [wt/vol]; 1.5 mm thick). Following staining of the gel with 0.05% (wt/vol) Coomassie brilliant blue R250 (in H2O), the gel was destained with H2O, the ca. 50-kDa outer membrane protein band was excised, and the protein was electroeluted as described (36). The eluted SmeM protein was concentrated in a Centricon-10 concentrator (Amicon, Inc., Beverly, Mass.) and washed several times with H2O before being recovered in a final volume of 500 μl. The purity of the purified protein was confirmed by SDS-polyacrylamide gel electrophoresis.
Cyanogen bromide cleavage and N-terminal amino acid sequencing.
Ten micrograms of purified SmeM in a final volume of 500 μl was treated with 50 mM cyanogen bromide (CNBr) in 70% (vol/vol) formic acid (in distilled water [dH2O]) at 25°C for 20 h. The reaction was diluted 1:9 with dH2O and lyophilized, and the sample was resuspended in 5 ml of dH2O before being lyophilized again. This was repeated a second time, after which the CNBr-treated protein was analyzed on a SDS-polyacrylamide gel (15% [wt/vol] acrylamide in the running gel) that was prepared 24 h prior to use. The cathode running buffer was supplemented with 1 mM thioglycolytic acid in order to reduce N-terminal blockage of the cleaved polypeptide fragments (25). Following electrophoresis, the proteolytic fragments resolved by SDS-polyacrylamide gel electrophoresis were transferred onto an Immobilon-P membrane (Millipore Corp., Bedford, Mass.) at a constant current of 150 mA and 4°C for 2.5 h. The electroblotted protein was then stained with Coomassie blue R250 (0.25% [wt/vol] in a solution of 45% [vol/vol] methanol and 10% [vol/vol] acetic acid) for 1 min at room temperature and washed three times for 3 min each with 200 ml of dH2O and 30 s with 50 ml of methanol. The stained fragments were subsequently excised, and N-terminal protein microsequencing (Edman degradation) was carried out by the National Research Council, Ottawa, Ontario, Canada.
Construction of L1 and L2 β-lactamase-deficient mutants.
Elimination of the blaS-encoded L1 enzyme in S. maltophilia was carried out by introduction of a deletion into the blaS gene present on plasmid pMON01. Two primers, sml1xz (5′-ATATGGATCCGGTGGCGTGGTTATGG-3′; BamHI site underlined) and sml2xz (5′-TAAAGTCGACCTGAAGGCCGC; SalI site underlined), and Vent polymerase (New England Biolabs, Mississauga, Ontario, Canada) were used to amplify a fragment of ca. 1.5 kb carrying the intact blaS gene from plasmid pMON01. The PCR mixture contained 10 ng of pMON01, 40 pmol of each primer, 200 M (each) deoxynucleoside triphosphate, 2 mM MgSO4, 10% (wt/vol) dimethylsulfoxide, and 2 U of Vent DNA polymerase in 1× thermo reaction buffer and was heated for 2 min at 94°C followed by 30 cycles of 1 min at 94°C, 1 min at 52°C, and 1.5 min at 72°C. The 1.5-kb PCR product was purified using the Qiaquick PCR purification kit (Qiagen Inc., Mississauga, Ontario, Canada), digested with BamHI and SalI, and subcloned into pBluescript II SK(+) to yield plasmid pLZ392. Subsequent digestion of pLZ392 with SmaI and EcoNI excised a ca. 120-bp fragment from within the blaS coding region, and the plasmid was then purified free of this fragment using Prep-a-gene (Bio-Rad) following agarose gel electrophoresis and excision of the plasmid band. The 5′ overhang left by EcoNI digestion was filled in using Klenow DNA polymerase (NEB) and the vector recircularized (minus the 120 bp of blaS) by ligation to yield pLZ393. The blaS deletion was subcloned as a BamHI-SalI fragment into the gene replacement vector, pEX18Tc, producing pLZ394. Following introduction of this vector into E. coli S17-1, it was mobilized into S. maltophilia strains ULA-511 and K1385 via conjugation as described (34). Transconjugants carrying pLZ394 in the chromosome were selected on LB agar containing tetracycline (40 μg/ml for ULA-511; 80 μg/ml for K1385) and norfloxacin (10 μg/ml; for counterselection). Transconjugants were then streaked onto LB agar containing 10% (wt/vol) sucrose, and sucrose-resistant colonies arising after overnight incubation at 37°C were screened for the presence of the blaS deletion. Initially, this was assessed by examining the imipenem susceptibility of putative mutants (i.e., strains susceptible to 20 μg of imipenem per ml were likely blaS mutants). Confirmation of the BlaS− status of putative mutants was achieved by analyzing β-lactamase profiles using isoelectric focusing and by demonstrating increased susceptibility to multiple carbapenems and loss of Zn2+-dependent EDTA-inhibitable β-lactamase activity (see below).
To knock out the gene for the L2 enzyme, two primers, sml3xz (5′-ACTTGTCGACTGGGGAGGGCTTCAATAATC-3′; SalI site underlined) and sml4xz (5′-AGTAGGATCCGATCGGGCAAGCCATTTCT-3′; BamHI site underlined), were designed based on the nucleotide sequence of the L2 β-lactamase gene (GenBank accession number Y08562) (46) and used in PCR to amplify a DNA fragment of ca. 1 kb from the genomic DNA of S. maltophilia ULA-511 and ATCC 13637. The reaction mixture was formulated as above, and the reaction was carried out as described for the blaS gene, with the exception that the 72°C incubation was 1 min in duration. The PCR fragment carrying the L2 β-lactamase gene was cloned into plasmid pTZ19U (Bio-Rad) following digestion with SalI and BamHI, yielding plasmid pLZ405. Digestion of pLZ405 with StyI and subsequent treatment with T4 DNA polymerase and religation removed four nucleotides from within the L2 β-lactamase gene (bp 558 to 561). The disrupted gene was then cloned into pEX18Tc on a SalI-BamHI fragment yielding pLZ416, and the plasmid was introduced into E. coli S17-1 by transformation. The vector was mobilized as described above into S. maltophilia strains ULA-511 and K1385 and the blaS mutants of each (i.e., K1445 and K1446) with initial selection on tetracycline and norfloxacin and subsequent selection on sucrose. Sucrose-resistant colonies were then screened for the L2 β-lactamase deficiency using a qualitative β-lactamase assay with nitrocefin as substrate (for L2 β-lactamase-deficient mutants) (see below) or an inability to grow on LB agar containing 2,000 μg of ampicillin per ml (for L1− L2− double mutants). The β-lactamase deficiency of these strains was ultimately confirmed using a quantitative β-lactamase assay and isoelectric focusing (IEF) (see below).
β-Lactamase assays.
Extraction and assay of β-lactamase activity was carried out as described previously (19) using nitrocefin and imipenem as substrates (final concentrations of 100 μM). Hydrolysis of the β-lactams was assessed either visually (qualitative assay), where the shift in color of the nitrocefin from yellow to pink after 5 min was indicative of hydrolysis and, thus, β-lactamase activity, or spectrophotometrically (nitrocefin, λ = 485 nm; imipenem, λ = 299 nm) (quantitative assay). In some experiments, ZnCl2 (0.1 mM) and Na2-EDTA (1 mM) were added to assess their influence on activity.
IEF.
β-Lactamases extracted as above were prepared in piperazine-N,N′-bis (2-ethanesulfonic acid) (25 mM, pH 7.0) and subjected to IEF as described (26) using a slab of IEF gels comprised of 5% (wt/vol) acrylamide, 3% (wt/vol) bisacrylamide, 2% (wt/vol) ampholytes (Bio-Lyte pH 3-10; Bio-Rad), 5% (wt/vol) glycerol, 0.005 mg of riboflavin per ml, 0.01 mg of ammonium persulfate per ml, and 0.1% (vol/vol) N,N,N′,N′-tetramethylethylenediamine. The running buffers included 20 mM concentrations (each) of L-lysine and L-arginine as catholyte and 7 mM phosphoric acid as anolyte. β-Lactamases were visualized using nitrocefin (1 mM).
RESULTS AND DISCUSSION
In vitro selection of MDR S. maltophilia.
S. maltophilia ATCC 13637 is an antibiotic susceptible American Type Culture Collection (ATCC) type strain and was used in the in vitro isolation of MDR mutants. S. maltophilia ULA-511, though less susceptible to several antibiotics than ATCC 13637 (Table 1), is generally regarded as a reference strain (32) and, thus, was also used to isolate MDR strains. Following plating then of S. maltophilia strains ATCC 13637 and ULA-511 onto LB agar supplemented with tetracycline, chloramphenicol, ciprofloxacin, or norfloxacin, many resistant colonies were obtained and several of these displayed resistance to additional antibiotics (Table 1). Most MDR derivatives showed substantial increases in resistance to chloramphenicol and ciprofloxacin (with the exception of those selected on tetracycline, which showed a slight, if any, change in chloramphenicol resistance), with only modest, if any, increases in tetracycline resistance (Table 1). Only the tetracycline-selected MDR derivatives of ATCC 13637 strains also showed an increase in resistance to erythromycin, while most of the MDR derivatives of ULA-511 were resistant to this antibiotic. These patterns of resistance were reminiscent of previously described quinolone-selected quinolone- and chloramphenicol-resistant and quinolone-, chloramphenicol-, and doxycycline-resistant mutants of S. maltophilia (16), although these mutants displayed only two- to threefold changes in MICs, and resistance to agents such as erythromycin was not tested.
TABLE 1.
In vitro selection of MDR strains of S. maltophilia
| Straina | Selectionb (μg/ml) | MIC (μg/ml) ofc
|
||||
|---|---|---|---|---|---|---|
| TET | CAM | CIP | NOR | ERY | ||
| ATCC 13637 | 2 | 4 | 1 | 4 | 128 | |
| K1430 | CAM (20) | 4 | 64 | 16 | 128 | 128 |
| K1431 | CAM (20) | 8 | >128 | 16 | 128 | 128 |
| K1432 | TET (30) | 8 | 8 | 16 | 16 | 1,024 |
| K1433 | TET (30) | 8 | 8 | 4 | 16 | 1,024 |
| K1434 | NOR (30) | 4 | 64 | 16 | 64 | 128 |
| K1435 | NOR (30) | 8 | 128 | 16 | 128 | 128 |
| K1436 | CIP (1) | 4 | 64 | 16 | 128 | 128 |
| K1437 | CIP (8) | 4 | 64 | 16 | 128 | 128 |
| ULA-511 | 8 | 8 | 4 | 16 | 512 | |
| K1438 | CAM (20) | 8 | 64 | 32 | 128 | 512 |
| K1439 | CAM (20) | 16 | 64 | >32 | 128 | 1,024 |
| K1440 | NOR (30) | 16 | 8 | 16 | 64 | 1,024 |
| K1385 | CIP (4) | 16 | 64 | 32 | 128 | 2,048 |
| K1441 | CIP (8) | 16 | 32 | 32 | 128 | 2,048 |
| K1442 | CIP (4) | 16 | 16 | 32 | 256 | 2,048 |
MDR strains K1430 to K1437 were derived from strain ATCC 13637, and strains K1385 and K1438 to K1442 were derived from strain ULA-511.
S. maltophilia strains ATCC 13637 and ULA-511 were plated on the indicated antibiotics (concentrations in parentheses), and resistant colonies were assessed for resistance to other agents as described in Materials and Methods.
TET, tetracycline; CAM, chloramphenicol; CIP, ciprofloxacin; NOR, norfloxacin; ERY, erythromycin.
Although chloramphenicol and norfloxacin selection of MDR strains of S. maltophilia has yet to be described, tetracycline selection of an MDR strain (D457R) has been reported (1). This strain, like most of the MDR strains described here, showed substantial changes in resistance to ciprofloxacin, norfloxacin, and chloramphenicol but only a modest (2.7-fold) increase in resistance to tetracycline (1). Strains ATCC 13637 and ULA-511 are highly resistant to novobiocin (MICs, >1,024 μg/ml) and β-lactams (MICs, >256 μg/ml for most β-lactams tested), precluding any measurable increase in resistance to these agents in the MDR strains.
Interestingly, differences in aminoglycoside resistance were apparent in several MDR strains. One class of MDR strain, represented by K1439 (ULA-511 derived) and K1443 (ATCC 13637 derived), exhibited little or no change in aminoglycoside resistance (Table 2). These strains are thus comparable to the aforementioned tetracycline-selected MDR strain, D457R, which also showed no significant change in aminoglycoside resistance (1). In contrast, a second class of MDR mutant, represented by K1433 (ATCC 13637 derived), displayed significantly increased resistance to aminoglycosides (8- to 32-fold) (Table 2). This is the first report of cross-resistance to quinolones and aminoglycosides in this organism. Finally, a third class of MDR strain (e.g., K1385; ULA-511 derived) displayed increased susceptibility to aminoglycosides, with a two- to eightfold decrease in MIC for six aminoglycosides (Table 2). Antibiotic hypersusceptibility has also been seen in nfxB-type MDR strains of P. aeruginosa, where hyperexpression of the MexCD-OprJ MDR efflux system correlates with increased susceptibility to most β-lactams (24). Apparently, this results from a concomitant decrease in expression of the MexAB-OprM MDR efflux system in nfxB strains (10). This latter efflux system (but not MexCD-OprJ) generally accommodates β-lactams and provides resistance to specific β-lactams in wild-type cells (17–19), such that the decreased MexAB-OprM expression seen in nfxB strains leads to a decrease in β-lactam resistance. Thus, it is possible that expression of the MDR efflux system likely responsible for the multidrug resistance of K1385 (see below) may lead to decreased expression of another efflux system which accommodates aminoglycosides.
TABLE 2.
Aminoglycoside susceptibility of MDR S. maltophilia
| Straina | MIC (μg/ml) ofb
|
|||||
|---|---|---|---|---|---|---|
| GEN | AMI | TOB | KAN | NEO | STR | |
| ATCC 13637 | 8 | 16 | 256 | 8 | 32 | 32 |
| K1433 | 256 | 512 | 2,048 | 256 | >2,048 | 1,024 |
| K1443 | 16 | 16 | 128 | 8 | 32 | 64 |
| ULA-511 | 512 | 512 | 2,048 | 1,024 | >2,048 | 256 |
| K1385 | 32 | 64 | 256 | 128 | 1,024 | 64 |
| K1438 | 256 | 256 | 1,024 | 512 | 2,048 | 256 |
| K1439 | 256 | 128 | 512 | 512 | 2,048 | 128 |
MDR strains K1433 and K1443 were derived from ATCC 13637, while K1438, K1385, and K1439 were derived from ULA-511.
GEN, gentamicin; AMI, amikacin; TOB, tobramycin; KAN, kanamycin; NEO, neomycin; STR, streptomycin.
Association of efflux proteins with multidrug resistance in S. maltophilia.
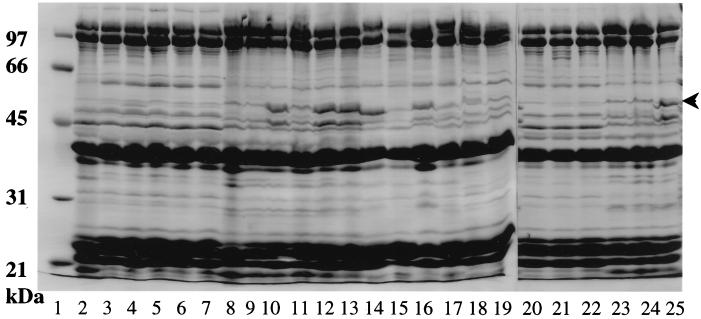
Multidrug resistance in P. aeruginosa is correlated with enhanced expression of outer membrane proteins of ca. 50 kDa molecular mass. These proteins are the outer membrane components of multidrug efflux systems that are up-regulated in MDR strains as a result of mutations, usually in regulatory genes. To assess then the possible involvement of efflux in the multidrug resistance of S. maltophilia, outer membranes were prepared from several MDR strains and examined on SDS-polyacrylamide gels. All examples of ATCC 13637-derived MDR strains showed a modest increase in expression of a ca. 50-kDa protein (representative examples are shown in Fig. 1, lanes 3 to 7 and 21 to 22), a phenotype much like that of the aforementioned D457R (1). In contrast, MDR strains of ULA-511 either failed to express any novel outer membrane proteins (e.g., K1140 and K1438 of Fig. 1, lanes 9 and 24, respectively) or produced fairly substantial levels of a ca. 50-kDa protein (e.g., K1441 and K1385 of Fig. 1, lanes 10 and 25, respectively). This latter phenotype has not previously been described in S. maltophilia.
FIG. 1.
Outer membrane protein profiles of MDR S. maltophilia from in vitro selection and clinical sources. Lanes: 2 and 20, parent strain ATCC 13637; 3, K1434; 4, K1431; 5, K1443; 6, K1451; 7, K1452; 8 and 23, parent strain ULA-511; 9, K1440; 10, K1441; 11, K1439; 12, K1442; 13, K1453; 14, K1014; 15, K1017; 16, K1018; 17, K1021; 18, K1023; 19, K1025; 21, K1430; 22, K1436; 24, K1438; and 25, K1385. K1434, K1431, K1443, K1451, K1452, K1430, and K1436 are ATCC 13637-derived MDR strains. K1440, K1441, K1439, K1442, K1453, K1438, and K1385 are ULA-511-derived MDR strains. Clinical MDR isolates include K1014, K1017, K1018, K1021, K1023, and K1025 (lanes 14 to 19). The position of the ca. 50-kDa protein whose expression increases in the MDR strains is indicated by an arrow. Molecular mass standards are shown on the left (lane 1). Outer membranes were prepared from cells cultured at 30°C, and 50 μg of protein was loaded in each instance.
Western immunoblotting with antibodies to the outer membrane efflux component OprM of P. aeruginosa demonstrated that a cross-reactive protein of ca. 50 kDa molecular mass was present in those ULA-511-derived MDR strains expressing substantial levels of a 50-kDa protein (e.g., K1385, K1441, K1442, and K1453; Fig. 2A). ULA-511-derived MDR strains which failed to demonstrate enhanced expression of an outer membrane protein (K1440 and K1439; Fig. 2A), the ATCC 13637-derived MDR strains, and the parent strains ATCC 13637 and ULA-511 all failed to react with the antiserum or reacted very poorly (Fig. 2A and data not shown). Similarly, a ca. 50-kDa outer membrane protein of strains K1385, K1441, K1442, and K1453 also reacted with antibodies to OprJ and OprN (data not shown), the respective components of the MexCD-OprJ and MexEF-OprN MDR efflux systems of P. aeruginosa.
FIG. 2.
Western immunoblots of outer (A) and inner (B) membrane proteins of in vitro-selected MDR (lanes 2 to 7) and clinical (lanes 8 to 13) isolates of S. maltophilia developed with antibodies to P. aeruginosa efflux proteins OprM (A) and MexB (B). Lanes: 1, parent strain ULA-511; 2, K1385; 3, K1440; 4, K1441; 5, K1439; 6, K1442; 7, K1453; 8, K1014; 9, K1017; 10, K1018; 11, K1021; 12, K1023; and 13, K1025. Lanes 2 to 7 are ULA-511-derived MDR strains, and lanes 8 to 13 are clinical MDR isolates. Equivalent amounts of protein (as confirmed by Coomassie staining of a duplicate gel) were loaded in each lane. Molecular mass standards are indicated on the left.
To confirm the likely association of the cross-reactive 50-kDa outer membrane proteins of S. maltophilia with an antibiotic efflux system, the 50-kDa protein of K1385 (here dubbed SmeM for Stenotrophomonas multidrug efflux) was purified (Fig. 3, lanes 4 and 6), and CNBr-generated fragments were subjected to N-terminal amino acid sequencing. The sequence obtained from one of these (19 kDa) (Fig. 3, lane 7) very closely matched an internal amino acid sequence of a number of outer membrane MDR efflux proteins of P. aeruginosa and Pseudomonas putida (Fig. 4), including OprM (20 matches out of 24 amino acids) (35), OprJ (18 matches out of 24) (33), and SrpC (21 matches out of 24) (13). Thus, K1385, at least, is hyperexpressing a homologue of these efflux proteins. MDR strains K1385, K1441, K1442, and K1017 also expressed an inner membrane protein which reacted with antibodies to MexB (Fig. 2B), the inner membrane component of the MexAB-OprM MDR efflux system of P. aeruginosa. It is likely, therefore, that the multidrug resistance of these strains results from overexpression of a homologue of this efflux system in S. maltophilia.
FIG. 3.
Purification and cyanogen bromide cleavage of the 50-kDa outer membrane protein SmeM. Outer membranes of S. maltophilia MDR strain K1385 (lane 3) were prepared from cells cultured at 30°C, and the 50-kDa protein (SmeM) which is overexpressed in this strain (cf. parent strain ULA-511, lane 2) was electroeluted and subjected to CNBr cleavage as described in Materials and Methods. The purified protein is shown in lanes 4 (indicated by an arrow) and 6, and the CNBr cleavage products are shown in lane 7. A weak band of ca. 30 kDa (in lane 7, indicated by a filled arrowhead) was N terminally blocked, while a ca. 19-kDa band (in lane 7, indicated by an open arrowhead) yielded amino acid sequence as presented in Fig. 4. Molecular mass standards are shown in lanes 1 and 5 (97, 66, 46, 31, 21, and 14 kDa from top to bottom). The left panel (lanes 1 to 4) shows a 11% (wt/vol) polyacrylamide gel, and the right panel (lanes 5 to 7) shows a 15% (wt/vol) gel.
FIG. 4.
Alignment of a partial sequence of SmeM with Pseudomonas outer membrane MDR efflux proteins (OprM and OprJ of P. aeruginosa and SrpC of P. putida). Residues conserved in all proteins are highlighted with an asterisk, while highly conserved residues are indicated by a dot. The numbers to the right of the amino acid sequences indicate the positions of the first to the last amino acids of the depicted sequences within the proteins. The amino acid sequence of SmeM was derived from N-terminal sequencing of a 19-kDa CNBr fragment of the purified protein.
The nature of the multidrug resistance of the other strains, including those reminiscent of the previously described D457R, remains undetermined, despite previously unsupported suggestions that it was due to efflux (1). Despite the obvious presence of an efflux mechanism in many of the MDR strains described here, no differences in tetracycline accumulation were observable in wild-type and MDR mutants of S. maltophilia and accumulation levels increased in both strains upon carbonyl cyanide m-chlorophenylhydrazone treatment (data not shown). Similarly, accumulation of radiolabeled ciprofloxacin was indistinguishable in K1385 and ULA-511, and treatment with carbonyl cyanide m-chlorophenylhydrazone increases accumulation levels in both strains and to comparable levels (data not shown). Previous studies of the MDR strain D475 also failed to show differences in drug accumulation between parent and mutant (1).
Role of efflux in the multidrug resistance of clinical isolates of S. maltophilia.
Clinical strains of S. maltophilia are often highly resistant to multiple antibiotics (43). Examination of several clinical strains obtained from the Kingston General Hospital Microbiology Lab confirmed this (Table 3, compare clinical strains with the ATCC 13637 reference strain). Some of these (e.g., K1014, K1018, and K1021) produced elevated levels of a ca. 50-kDa outer membrane protein (Fig. 1, lanes 14 and 16 to 19), and Western immunoblotting confirmed the presence of an OprM cross-reactive protein in these strains (Fig. 2A, lanes 8 and 10 to 13). Thus, it is likely that clinical strains also express MDR efflux systems and that these contribute to the clinically relevant multidrug resistance of these strains.
TABLE 3.
Multidrug resistance of clinical isolates of S. maltophilia
| Straina | MIC (μg/ml) ofb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| TET | CAM | CIP | NOR | ERY | GEN | AMI | KAN | |
| ATCC 13637 | 2 | 4 | 1 | 4 | 128 | 8 | 16 | 8 |
| K1013 (2) | 8 | 16 | 16 | — | — | — | — | — |
| K1014 (4) | 16 | 32 | 32 | 256 | 2,048 | 256 | 512 | 512 |
| K1015 (6) | 16 | 8 | 8 | — | — | — | — | — |
| K1016 (8) | 8 | 16 | 8 | — | — | — | — | — |
| K1017 (10) | 16 | 16 | 32 | 64 | 1,024 | 256 | 256 | 256 |
| K1018 (12) | 8 | 32 | 32 | 128 | 1,024 | 16 | 64 | 256 |
| K1019 (14) | 8 | 16 | 16 | — | — | — | — | — |
| K1020 (16) | 8 | 32 | 16 | — | — | — | — | — |
| K1021 (18) | 16 | 32 | 32 | 128 | 1,024 | 64 | 128 | 256 |
| K1022 (20) | 4 | 16 | 4 | — | — | — | — | — |
| K1023 (22) | 16 | >128 | >128 | >256 | 1,024 | 256 | 512 | 512 |
| K1024 (24) | 4 | 16 | 4 | — | — | — | — | — |
| K1025 (11558) | 8 | 64 | 32 | 32 | 1,024 | >1,024 | >1,024 | >1,024 |
| K1027 (16098) | 4 | 8 | 4 | — | — | — | — | — |
| K1028 (16196) | 4 | 16 | 8 | — | — | — | — | — |
β-Lactamase-independent resistance to β-lactams in MDR strains of S. maltophilia.
MDR efflux systems such as MexAB-OprM (18, 41), AcrAB (21, 30), and to a very limited extent, MexCD-OprJ (10, 41) accommodate β-lactam antibiotics, and their expression is thus associated with enhanced resistance to members of this class of antibiotic. The high intrinsic resistance of S. maltophilia to virtually all classes of β-lactams, including penicillins, cephalosporins, and carbapenems, however, precluded any assessment of the contribution of the MDR phenotype (or the associated MDR efflux system) itself to β-lactam resistance. The intrinsic resistance to β-lactams is apparently due to two enzymes, termed L1 (5) and L2 (46), which are metal-dependent class B and serine class A enzymes, respectively. To assess the ability, then, of the MexAB-OprM-like efflux system in, e.g., K1385, to accommodate β-lactams and thus contribute to β-lactam resistance, it was necessary to eliminate the L1 and L2 β-lactamase genes. As seen in Table 4, elimination of the L1 enzyme (confirmed by IEF) eliminated the imipenem hydrolytic capability of ULA-511 and K1385, although the ability to hydrolyze nitrocefin was retained. Although loss of L1 markedly enhanced the susceptibility of the ULA-511 and K1385 derivatives to carbapenems (Table 5), no changes in MIC were seen for other β-lactams and no differences were observable between the L1− derivative of the wild type and the L1− derivative of the MDR strain. Thus, expression of the MexAB-OprM-like efflux system in K1385 is not correlated with any change in resistance to carbapenems. Elimination of the L2 enzyme (also confirmed by IEF), as expected, failed to impact on imipenem hydrolysis in either ULA-511 (see strain K1447, Table 4) or K1385 (see strain K1448, Table 4), although most of the nitrocefin hydrolytic capability was lost (Table 4). The remaining nitrocefin hydrolytic activity was EDTA sensitive (Table 4), indicating that it was attributable to the metal-dependent L1 enzyme. Loss of L2 had no impact on resistance to carbapenems or penicillins but did decrease resistance to some of the cephalosporins and monobactams (Table 5). Again, however, no differences were seen between the L2 mutant of ULA-511 (K1447) and the L2 mutant of K1385 (K1448) (Table 5). Elimination of both L1 and L2 (confirmed by IEF) virtually eradicated hydrolysis of both imipenem and nitrocefin (see K1449 and K1450, Table 4), and the loss of these enzymes rendered the ULA-511 and K1385 derivatives exquisitely sensitive to virtually all β-lactams (with the exception of cloxacillin). Moreover, in the absence of these β-lactamases, modest resistance to a few β-lactams, including piperacillin, cefepime, cefpirome, and aztreonam, was seen in the MDR derivative relative to the ULA-511 derivative (Table 5). These data suggest that the putative efflux mechanism operating in K1385 accommodates a limited number of β-lactams, in addition to quinolones, chloramphenicol, and erythromycin (and perhaps tetracycline). This is reminiscent of the MexCD-OprJ efflux system expressed in nfxB MDR strains (10, 41). Efforts are currently underway to clone the corresponding MDR genes from K1385.
TABLE 4.
β-Lactamase activity of S. maltophilia L1 and L2 β-lactamase-deficient mutants
| Strain | β-Lactamase phenotype | Assay buffera | Hydrolysis rate ofb
|
|
|---|---|---|---|---|
| Imipenem | Nitrocefin | |||
| ULA-511 | Parent (L1+ L2+) | KPi-ZnCl2 | 10.58 | 46.57 |
| KPi-Na2-EDTA | 3.27 | 24.73 | ||
| K1385 | MDR (L1+ L2+) | KPi-ZnCl2 | 12.71 | 59.04 |
| KPi-Na2-EDTA | 2.83 | 43.68 | ||
| K1445 | ULA-511 ΔL1 | KPi-ZnCl2 | 0.00 | 40.53 |
| KPi-Na2-EDTA | 0.00 | ND | ||
| K1446 | K1385 ΔL1 | KPi-ZnCl2 | 0.00 | 41.45 |
| KPi-Na2-EDTA | 0.00 | ND | ||
| K1447 | ULA-511 ΔL2 | KPi-ZnCl2 | 10.94 | 5.19 |
| KPi-Na2-EDTA | 1.72 | 0.02 | ||
| K1448 | K1385 ΔL2 | KPi-ZnCl2 | 8.14 | 4.21 |
| KPi-Na2-EDTA | 2.31 | 0.01 | ||
| K1449 | ULA-511 ΔL1 ΔL2 | KPi-ZnCl2 | 0.00 | 0.02 |
| KPi-Na2-EDTA | 0.00 | 0.04 | ||
| K1450 | K1385 ΔL1 ΔL2 | KPi-ZnCl2 | 0.00 | 0.03 |
| KPi-Na2-EDTA | 0.00 | 0.02 | ||
Assays were carried out in 50 mM potassium phosphate buffer (KPi), pH 7.2, in the presence of 0.1 mM ZnCl2 or 1 mM Na2-EDTA.
Hydrolysis rate is presented as micromoles of substrate (imipenem or nitrocefin) hydrolyzed per milligram of protein per minute. ND, not determined.
TABLE 5.
β-Lactam susceptibility of β-lactamase-deficient and MDR strains of S. maltophilia
| Antibiotic | MIC (μg/ml) for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| K1199 (parent) | K1385 (MDR) | K1445 (ULA-511 ΔL1) | K1446 (K1385 ΔL1) | K1447 (ULA-511 ΔL2) | K1448 (K1385 ΔL2) | K1449 (ULA-511 ΔL1 ΔL2) | K1450 (K1385 ΔL1 ΔL2) | |
| Imipenem | 512 | 512 | 4 | 4 | 512 | 512 | 0.25 | 0.5 |
| Meropenem | 1,024 | 512 | 1 | 2 | 512 | 512 | 0.25 | 0.5 |
| Panipenem | >1,024 | >1,024 | 64 | 64 | >1,024 | >1,024 | 4 | 8 |
| R-83201 | 512 | 256 | 1 | 1 | 512 | 512 | 0.25 | 0.25 |
| Penicillin G | >1,024 | >1,024 | 1,024 | 512 | >1,024 | >1,024 | 1 | 1 |
| Ampicillin | >1,024 | >1,024 | 1,024 | 1,024 | >1,024 | >1,024 | 0.25 | 0.25 |
| Unasyn | 1,024 | 512 | 64 | 64 | 1,024 | 512 | 1 | 1 |
| Amoxicillin | >1,024 | >1,024 | 1024 | 512 | >1,024 | 1,024 | 0.5 | 0.25 |
| Cloxacillin | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 | 256 | 512 |
| Carbenicillin | >1,024 | >1,024 | 1,024 | 1,024 | 512 | 256 | 2 | 2 |
| Piperacillin | >1,024 | >1,024 | 512 | 256 | >1,024 | >1,024 | 8 | 32 |
| Ticarcillin | >1,024 | >1,024 | 1,024 | 1,024 | 256 | 128–256 | 2 | 2 |
| Cefsulodin | >1,024 | >1,024 | >1,024 | >1,024 | 4 | 4 | 4 | 4 |
| Cefotaxime | 512 | 256 | 256 | 128–256 | 128 | 128 | 2 | 2 |
| Cefoperazone | >1,024 | 256 | 512 | 256 | 256 | 256 | 4 | 8 |
| Ceftriaxone | 512 | 512 | 256 | 256 | 256 | 256 | 2 | 2 |
| Ceftazidime | 256 | 256 | 128 | 128 | 256 | 256 | 2 | 2 |
| Cefepime | 128 | 64 | 128 | 64 | 8 | 8 | 1 | 8 |
| Cefpirome | 512 | 256 | 256 | 256 | 64 | 64 | 2 | 8 |
| Aztreonam | >1,024 | >1,024 | >1,024 | >1,024 | 4 | 4 | 2 | 8 |
| Pirazmonam | 8 | 1 | 4 | 2 | 1 | 0.5 | 1 | 0.5 |
| Ciprofloxacina | 4 | 32 | 4 | 32 | 4 | 32 | 4 | 32 |
Included to show that resistance to non-β-lactams was not affected by loss of the β-lactamases.
ACKNOWLEDGMENTS
We thank R.E.W. Hancock and K. Coleman for strains and R. Levesque for plasmid pMON01.
This work was supported by funding from the Canadian Bacterial Diseases Network (a consortium of the Centres of Excellence Program). K.P. is the Canadian Cystic Fibrosis Foundation (CCFF) Martha Morton Scholar. X.-Z. L. is supported by a studentship from the CCFF.
REFERENCES
- 1.Alonso A, Martinez J L. Multiple antibiotic resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1997;41:1140–1142. doi: 10.1128/aac.41.5.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 3.Ballestero S, Virseda I, Escobar H, Suarez L, Baquero F. Stenotrophomonas maltophilia in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 1995;14:728–729. doi: 10.1007/BF01690887. [DOI] [PubMed] [Google Scholar]
- 4.Burns J L, Emerson J, Stapp J R, Yim D L, Krzewinski J, Louden L, Ramsey B W, Clausen C R. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 5.Crowder M W, Walsh T R, Banovic L, Petit M, Spencer J. Overexpression, purification, and characterization of the cloned metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1998;42:921–926. doi: 10.1128/aac.42.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denton M, Todd N J, Kerr K G, Hawkey P M, Littlewood J M. Molecular epidemiology of Stenotrophomonas maltophilia isolated from clinical specimens from patients with cystic fibrosis and associated environmental samples. J Clin Microbiol. 1998;36:1953–1958. doi: 10.1128/jcm.36.7.1953-1958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denton M, Todd N J, Littlewood J M. Role of anti-pseudomonal antibiotics in the emergence of Stenotrophomonas maltophilia in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 1996;15:402–405. doi: 10.1007/BF01690098. [DOI] [PubMed] [Google Scholar]
- 8.Felici A, Amicosante G, Oratore A, Strom R, Ledent P, Joris B, Fanuel L, Frere J-M. An overview of the kinetic parameters of class B β-lactamases. Biochem J. 1993;291:151–155. doi: 10.1042/bj2910151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotoh N, Tsujimoto H, Tsuda M, Okamoto K, Nomura A, Wada T, Nakahashi M, Nishino T. Characterization of the MexC-MexD-OprJ multidrug efflux system in ΔmexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1938–1943. doi: 10.1128/aac.42.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez Rodero F, Masia M M, Cortes J, Ortiz de la Tabla V, Mainar V, Vilar A. Endocarditis caused by Stenotrophomonas maltophilia: case report and review. Clin Infect Dis. 1996;23:1261–1265. doi: 10.1093/clinids/23.6.1261. [DOI] [PubMed] [Google Scholar]
- 12.Hoang T T, Karkhoff-Schweizer R R, Kutchma A J, Schweizer H P. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 13.Kieboom J, Dennis J J, de Bont J A M, Zylstra G J. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem. 1998;273:85–91. doi: 10.1074/jbc.273.1.85. [DOI] [PubMed] [Google Scholar]
- 14.Kohler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 15.Krcmery V, Jr, Spanik S, Krupova I, Trupl J, Kunova A, Smid M, Pichnova E. Bacteremia due to multiresistant gram-negative bacilli in neutropenic cancer patients: a case controlled study. J Chemother. 1998;10:320–325. doi: 10.1179/joc.1998.10.4.320. [DOI] [PubMed] [Google Scholar]
- 16.Lesco Bornet M, Pierre J, Sarkis Karam D, Lubera S, Bergogne E. Susceptibility of Xanthamonas maltophilia to six quinolones and study of outer membrane proteins in resistant mutants selected in vitro. Antimicrob Agents Chemother. 1992;36:669–671. doi: 10.1128/aac.36.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X-Z, Ma D, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to β-lactam resistance. Antimicrob Agents Chemother. 1994;38:1742–1752. doi: 10.1128/aac.38.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X-Z, Zhang L, Srikumar R, Poole K. β-Lactamase inhibitors are substrates of the multidrug efflux pumps of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:399–403. doi: 10.1128/aac.42.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugtenberg B, Mrijers J, Peters R, van der Hoek P, van Alphen L. Electrophoretic resolution of the major outer membrane protein of Escherichia coli K12 into four bands. FEBS Lett. 1975;58:254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- 21.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993;175:6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maningo E, Watanakunakorn C. Xanthamonas maltophilia and Pseudomonas cepacia in lower respiratory tracts of patients in critical care units. J Infect. 1995;31:89–92. doi: 10.1016/s0163-4453(95)91985-6. [DOI] [PubMed] [Google Scholar]
- 23.Marshall W F, Keating M R, Anhalt J P, Steckelberg J M. Xanthamonas maltophilia: an emerging nosocomial pathogen. Mayo Clin Proc. 1989;64:1097–1104. doi: 10.1016/s0025-6196(12)64979-9. [DOI] [PubMed] [Google Scholar]
- 24.Masuda N, Gotoh N, Ohya S, Nishino T. Quantitative correlation between susceptibility and OprJ production in NfxB mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:909–913. doi: 10.1128/aac.40.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsudaira P T. A practical guide to protein and peptide purification for microsequencing. San Diego, Calif: Academic Press, Inc.; 1989. [Google Scholar]
- 26.Matthew M, Harris A M, Marshall M, Ross G W. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 27.Muder R R, Harris A P, Muller S. Bacteremia due to Stenotrophomonas (Xanthamonas) maltophilia: a prospective, multicenter study of 91 episodes. Clin Infect Dis. 1996;22:508–512. doi: 10.1093/clinids/22.3.508. [DOI] [PubMed] [Google Scholar]
- 28.Munter R G, Yinnon A M, Schlesinger Y, Hershko C. Infective endocarditis due to Stenotrophomonas (Xanthamonas) maltophilia. Eur J Clin Microbiol Infect Dis. 1998;17:353–356. doi: 10.1007/BF01709460. [DOI] [PubMed] [Google Scholar]
- 29.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikaido H, Basina M, Nguyen V, Rosenberg E Y. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those β-lactam antibiotics containing lipophilic side chains. J Bacteriol. 1998;180:4686–4692. doi: 10.1128/jb.180.17.4686-4692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadakis K A, Vartivarian S E, Vassilaki M E, Anaissie E J. Stenotrophomonas maltophilia meningitis. Report of two cases and review of the literature. J Neurosurg. 1997;87:106–108. doi: 10.3171/jns.1997.87.1.0106. [DOI] [PubMed] [Google Scholar]
- 32.Payne D J, Cramp R, Batson J H, Neal J, Knowles D. Rapid identification of metallo- and serine β-lactamases. Antimicrob Agents Chemother. 1994;38:991–996. doi: 10.1128/aac.38.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J-I, Li X-Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB multidrug resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 34.Poole K, Heinrichs D E, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 35.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole K, Neshat S, Krebes K, Heinrichs D E. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J Bacteriol. 1993;175:4597–4604. doi: 10.1128/jb.175.15.4597-4604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn J P. Clinical problems posed by multiresistant nonfermenting gram-negative pathogens. Clin Infect Dis. 1998;27:S117–S124. doi: 10.1086/514912. [DOI] [PubMed] [Google Scholar]
- 38.Sanschagrin F, Dufresne J, Levesque R C. Molecular heterogeneity of the L-1 metallo-β-lactamase family from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1998;42:1245–1248. doi: 10.1128/aac.42.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon R, Priefer U, Puehler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology. 1983;1:784–791. [Google Scholar]
- 40.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srikumar R, Li X-Z, Poole K. The inner membrane efflux components are responsible for the β-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol. 1997;179:7875–7881. doi: 10.1128/jb.179.24.7875-7881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Couwenberghe C J, Farver T B, Cohen S H. Risk factors associated with isolation of Stenotrophomonas (Xanthamonas) maltophilia in clinical specimens. Infect Control Hosp Epidemiol. 1997;18:316–321. doi: 10.1086/647618. [DOI] [PubMed] [Google Scholar]
- 43.Vartivarian S E, Anaissie E, Bodey G, Sprigg H, Rolston K. A changing pattern of susceptibility of Xanthamonas maltophilia to antimicrobial agents: implications for therapy. Antimicrob Agents Chemother. 1994;38:624–627. doi: 10.1128/aac.38.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vartivarian S E, Papadakis K A, Anaissie E J. Stenotrophomonas (Xanthamonas) maltophilia urinary tract infections: a disease that is usually severe and complicated. Arch Intern Med. 1996;156:433–435. [PubMed] [Google Scholar]
- 45.Vartivarian S E, Papadakis K A, Palacios J A, Manning J T, Jr, Anaissie E J. Mucocutaneous and soft tissue infections caused by Xanthamonas maltophilia. Ann Intern Med. 1994;121:969–973. doi: 10.7326/0003-4819-121-12-199412150-00011. [DOI] [PubMed] [Google Scholar]
- 46.Walsh T R, MacGowan A P, Bennett P M. Sequence analysis and enzyme kinetics of the L2 serine β-lactamase from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1997;41:1460–1464. doi: 10.1128/aac.41.7.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Q, Li X-Z, Srikumar R, Poole K. Contribution of the outer membrane efflux protein OprM to antibiotic resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1682–1688. doi: 10.1128/aac.42.7.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]