Abstract
Background and Objectives
Sudomotor impairment has been recognized as a key feature in differentiating Parkinson disease (PD) and multiple system atrophy–parkinsonian type (MSA-P), with the latter characterized by diffuse anhidrosis in prospective study, including patients in late stage of disease. We aimed to evaluate morphologic and functional postganglionic sudomotor involvement in patients with newly diagnosed MSA-P and PD to identify possible biomarkers that might be of help in differentiating the 2 conditions in the early stage.
Methods
One hundred patients with parkinsonism within 2 years from onset of motor symptoms were included in the study. At the time of recruitment, questionnaires to assess nonmotor, autonomic, and small fiber symptoms were administered, and patients underwent postganglionic sudomotor function assessment by the dynamic sweat test and punch skin biopsy from the distal leg. Skin samples were processed for indirect immunofluorescence with a panel of antibodies, including noradrenergic and cholinergic markers. The density of intraepidermal, sudomotor, and pilomotor nerve fibers was measured on confocal images with dedicated software. A follow-up visit 12 months after recruitment was performed to confirm the diagnosis.
Results
We recruited 57 patients with PD (M/F 36/21, age 63.5 ± 9.4 years) and 43 patients with MSA-P (M/F 27/16, age 62.3 ± 9.0 years). Clinical scales and questionnaires showed a more severe clinical picture in patients with MSA-P compared to those with PD. Sweating output and intraepidermal, pilomotor, and sudomotor nerve densities, compared to controls, were lower in both groups but with a greater impairment in patients with MSA-P. Pilomotor and sudomotor nerve density correlated with sweating function and with nonmotor clinical symptoms. A composite sudomotor parameter defined as the arithmetic product of sweat production multiplied by the density of sudomotor fibers efficiently separated the 2 populations; the receiver operating characteristics curve showed an area under the curve of 0.83.
Discussion
Dynamic sweat test and the quantification of cutaneous autonomic nerves proved to be a sensitive morpho-functional approach to assess the postganglionic component of the sudomotor pathway, revealing a more severe involvement in MSA-P than in PD early in the disease course. This approach can be applied to differentiate the 2 conditions early.
Classification of Evidence
This study provides Class II evidence that postganglionic sudomotor morpho-functional assessment accurately distinguish patients with PD from patients with MSA-P.
The term parkinsonism defines several clinical conditions characterized mainly by motor symptoms such as rigidity, tremor, and bradykinesia. This term includes the most prevalent of such disorders, idiopathic Parkinson disease (PD), as well as rarer conditions with a different pathogenesis, prognosis, and response to pharmacologic treatment such as multiple system atrophy (MSA).1
Parkinsonisms affect ≈2% of people >65 years of age.2 Lack of available disease-modifying treatment leads over time to a severe disability, hence representing a heavy burden for health systems worldwide.3
During the disease course, the presence and extent of signs and symptoms and the development over time of more specific neuroimaging features can clarify the clinical picture and help to fulfill established diagnostic criteria to reach a diagnosis.1 However, the latter occurs only after years, when patients are less susceptible to being treated with disease-modifying drugs that could become soon available.
In addition, the response to pharmacologic therapy can be ambiguous and misleading.4 Therefore, an early differential diagnosis could present a challenging task for physicians, even if they are experts in movement disorders.5 This often leads to a diagnostic delay and to suboptimal therapeutic choices. For these reasons, early biomarkers able to help clinicians in the differential diagnosis among different forms of parkinsonism are highly demanded.
Autonomic dysfunction and symptoms are often the presenting feature in patients with parkinsonism and may date back many years before the motor symptoms become fully developed.6
Sudomotor dysfunction has been recognized as a key feature, involving both preganglionic and, recently documented, postganglionic dysfunction in MSA7 with a greater severity in MSA-parkinsonian type (MSA-P) compared to MSA-cerebellar type. However, these include retrospective studies or prospective studies involving only patients in advanced stage of disease,8,9 while there is a lack of studies on prospective evaluation of sudomotor dysfunction in the early stage of the diseases.
Therefore, we aimed to prospectively evaluate morphologic and functional autonomic sudomotor involvement in patients with early stage (within 2 years of motor onset) of MSA-P and PD to identify possible biomarkers able to accurately distinguish patients with PD from patients with MSA-P in the early stage of the disorder.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board (Fondazione G. Pascale No. “5/15 Maugeri”), and all patients included in the study signed a written informed consent.
Subjects with parkinsonism within 2 years from motor onset were prospectively recruited from 3 centers (University College London Queen Square, UK; Neurology Division “ICS Maugeri” IRCCS of Telese Terme, Italy; and Neurology Department, University of Naples Federico II, Italy) between November 2017 and December 2019 in a prospective longitudinal study including baseline assessment and 12-month follow-up visit to confirm diagnosis.
Diagnosis of PD or MSA-P was made according the fulfillment of established diagnostic criteria.10,11 We included patients with probable MSA-P confirmed at follow-up and according to consensus criteria.11 Patients who did not fulfill the diagnostic criteria for either of the diseases at follow-up were excluded from the present analysis. Patients with a known peripheral neuropathy as documented by clinical history and abnormalities of neurophysiologic tests or with conditions potentially affecting the peripheral nervous system such as glucose intolerance, dysendocrinopathies, vitamin E, vitamin B12, and folic acid deficiency, hepatic or renal failure, HIV, or connective tissue disorders were excluded from the study.
At recruitment, patients were required not to have been started on l-dopa treatment to avoid possible iatrogenic impairment.12 At the baseline visit, all patients underwent neurologic examination, levodopa challenge, and brain MRI. Patient underwent bedside assessment of blood pressure in resting supine position and blood pressure changes within 3 minutes of standing. Autonomic symptoms and sudomotor functional data were obtained at the baseline visit, along with clinical scales and punch skin biopsy as described below. Analysis of all skin biopsies was performed at 1 center (Neurology Division “ICS Maugeri” IRCCS of Telese Terme, Italy).
Morphologic and functional sensory and autonomic findings were compared with data extracted from our age- and sex-stratified normative dataset of controls that included 200 healthy volunteers.
Clinical Scales
For all patients, the severity of motor impairment was assessed with the clinician-scored motor evaluation (part III) of the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)13 and Hoehn and Yahr14 (H&Y) staging score. H&Y stage was used to calculate disease progression rate as the ratio between disease duration and H&Y stage. For patients with a diagnosis of MSA-P, the Unified Multiple System Atrophy Rating Scale15 also was administered. To assess the presence of nonmotor symptoms and, in particular, those related to autonomic dysfunction and the involvement of small fibers, all patients completed the Nonmotor Symptoms Scale (NMSS),16 the Composite Autonomic Symptom Score (COMPASS)-31 questionnaire,17 and the Small Fiber Neuropathy Symptoms Inventory Questionnaire (SFN-SIQ).18
Sudomotor Function Testing
All patients underwent a functional assessment of sudomotor function using the dynamic sweat test (DST).19 Briefly, 10 minutes after the administration of 1% pilocarpine by iontophoresis and after the preparation of the skin with iodine alcohol, the sudomotor response was assessed by measuring over time the imprint of sweat drops through a transparent tape treated with starch powder. The density per 1 cm2 of activated sweat glands, the sweat produced per minute per 1 cm2, and the average sweat volume produced by each sweat gland were recorded. The test was performed at lateral distal leg bilaterally.
A neurophysiologic assessment of the sudomotor pathway was obtained with the sympathetic skin response (SSR) in a subgroup of patients. SSR was recorded at the palm of the hand and sole of the foot with surface electrodes after random electric stimuli were delivered at the wrist along the median nerve.12
Morphology Study
At the baseline visit, all patients performed a 3-mm punch skin biopsy from the lateral distal leg bilaterally in the same area where sudomotor function was tested. To maximize the sampling of arrector pili muscles and sweat glands, skin biopsies were centered on a hair follicle outlet. Skin samples were immunostained for indirect immunofluorescence according to standard procedures20 using a large panel of antibodies, including primary antibodies against the pan neuronal marker protein gene product 9.5, collagen type IV, vasoactive intestinal peptide as a marker of cholinergic fibers, and dopamine-β-hydroxylase as a marker of noradrenergic fibers. Structures marked with primary antibodies were visualized using species-specific secondary antibodies coupled with Cy2 and Cy3 fluorophores. The complete list of antibodies with source and dilution is reported in eTable 1. Digital confocal images were acquired for analysis with a nonlaser confocal system (Apotome2 Zeiss, Jena, Germany, EU). Intraepidermal nerve fiber (IENF) density was measured according to current guidelines.21 Quantification of pilomotor and sudomotor nerves with pan neuronal and selective cholinergic and noradrenergic markers was performed following previously described procedures that were validated by comparison with unbiased stereologic methodologies.22,23 Briefly, for pilomotor nerve quantification, arrector pili muscle segments, parallel to the focal plane, were acquired with a 20× objective. The single optical section having the highest number of fibers running for at least 100 µm parallel to the major axis of the muscle was selected from the Z stack. A line was then traced perpendicular to the major axis of the muscle and intercepting the highest number of fibers. Pilomotor nerve fiber density was calculated as number of intercepts per muscle width in millimeters (fibers per millimeter). For each skin biopsy and for each staining, pilomotor nerve fiber density was expressed as the average of the measurements performed in all muscles suitable for quantification. For sudomotor nerve fiber quantification, Neurolucida 360 software (Microbrightfield Bioscience, Williston, VT) identified the voxels with the highest probability of being nerve-associated within the 3-dimensiona; confocal image stack of the gland innervation acquired with a 20× objective. The software then traced the nerve trajectory that was morphologically compatible with the nerve structure (it was set to trace only continuous, linear structures at least 10 µm in length so to exclude non–nerve-related fluorescence). After the 3-dimensional tracing, the total length was recorded and later converted to a length density (nanometer per cubic micrometers) after calculation of the volume of the imaged gland by tracing its contour on each optical section of the confocal image. All measurements were performed by the same operator blinded to the diagnosis of the participant.
We defined a morpho-functional composite sudomotor parameter (CSP) as the arithmetic product of sweat production in microliters per square centimeter multiplied by the density of sudomotor fibers in micrometer per cubic millimeter as marked with the pan neuronal marker protein gene product 9.5 in each subject.
Statistical Analysis
All data are presented as mean ± SD. The Kolmogorov-Smirnov test was used to assess the normal distribution of all parameters. Differences among patients and controls and between patients subgroups were evaluated by the analysis of variance with Bonferroni correction.
Pearson and Spearman tests were used as appropriate to assess the correlation between clinical, morphologic, and demographic data.
We used the receiving operating characteristics curves to discriminate groups using CSP. We considered a value of p < 0.05 significant. Statistical analysis was performed with STATA/SE software (version 14.1, StataCorp, College Station, TX).
Data Availability
Anonymized data will be shared on request by a qualified investigator.
Results
One hundred patients with a provisional diagnosis of PD (60 patients) or MSA-P (40 patients) were recruited. For the purpose of the current study, we presented findings on morpho-functional sudomotor and clinical assessment on 100 patients (age 63.1 ± 9.2 years; M/F 63/37) with a diagnosis of PD (57 patients) or MSA-P (43 patients) as a preliminary report of a larger project (PE-2013-02359028) aiming to assess sensory and autonomic markers in the early diagnosis of parkinsonism.
At the follow-up visit (1 year from baseline visit), diagnosis was confirmed in 97 of 100 patients. Three patients diagnosed with PD at baseline visit were later diagnosed with MSA-P at follow-up. Final patient groups included 57 with PD and 43 with probable MSA-P according to the criteria.11
Standing tests performed at bedside examination showed orthostatic hypotension in 28% (16 of 57) of patients with PD (PD-OH).24 Thirty-one of 43 patients with MSA-P (72%) showed orthostatic decrease of blood pressure within 3 minutes of standing by at least 30 mm Hg systolic or 15 mm Hg diastolic, meeting the criteria for orthostatic hypotension according the consensus criteria for MSA.11 Urinary symptoms were present in all patients with MSA-P, with a subgroup of patients (28 of 43, 65%) presenting with severe genitourinary dysfunction, including urinary incontinence and erectile failure. Seventeen of 57 patients with PD (30%) presented mild urinary and sexual symptoms.
Patients with MSA-P and those with PD were not different regarding age and disease duration. There was no correlation between disease duration and any of the sudomotor function and morphology outcome measures described below, although this was expected because disease duration was similar for all patients according to the inclusion criteria.
Motor impairment, as measured by MDS-UPDRS motor score, was significantly higher in MSA-P compared to PD. In addition, the rate of disease progression (disease duration in years per H&Y stage) was higher in MSA-P compared to PD. Both NMSS score and COMPASS-31 total score were higher in patients with MSA-P compared to those with PD. SFN-SIQ total score was not different in the 2 groups, although some of the subscores such as micturition problems, skin sensitivity and facial flushing indicated a more severe impairment in MSA-P. The questionnaire scores, patients stratified by disease severity, and demographic data are reported in Table 1. Analytical data with the scale subscores are reported in eTable 2.
Table 1.
Demographic and Clinical Data
Autonomic Sudomotor Function Testing
No side-to-side differences were observed in both groups of patients for autonomic sudomotor functional and morphologic measures, so we included the average value from the 2 sides as representative.
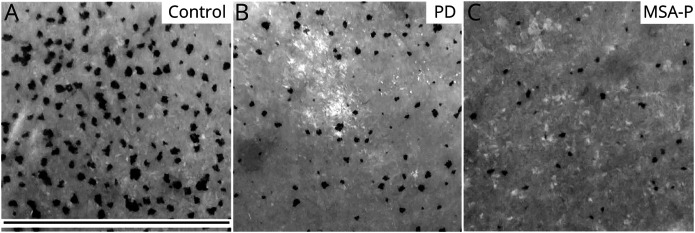
Sudomotor function, as assessed by the DST, was impaired, compared to controls, in both groups of patients, but all parameters were lower in MSA-P compared to PD (Figure 1). Moreover, in analysis of the results obtained in each patient with respect to the fifth percentile cutoff of normal values, 90% of patients with MSA-P and 59% of patients with PD showed a reduced sweat drop density, 50% of those with MSA-P and 17% of patients with PD showed a reduced sweat output per gland, and 76% of patients with MSA-P and 49% of those with PD showed a reduced sweat output per area.
Figure 1. Sweating Output in PD and MSA-P Compared to Control.
Sweating output assessed by the dynamic sweat test (DST) shows a more severe reduction of activated sweat glands in a patient with multiple system atrophy with parkinsonism (MSA-P) (C) compared to a patient with Parkinson disease (PD) (B). (A) Control subject. Black scale bar is 1.5 cm.
SSR was obtained in 56 patients (10 with MSA-P and 46 with PD). SSR amplitude was reduced in patients compared to controls.12 Moreover, we found a lower SSR amplitude at the hand in MSA-P compared to PD. A complete absence of response was observed in 30% of individuals with MSA-P and 4% of patients with PD.
Quantitative measures of autonomic sudomotor function testing are reported in Table 2.
Table 2.
Morphologic and Functional Findings
Morphology Study
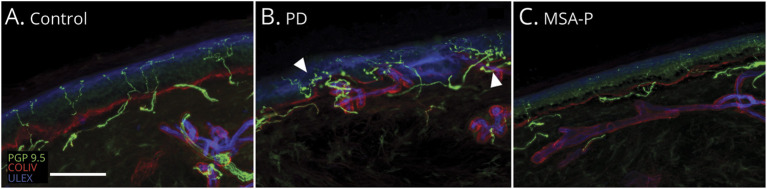
A total of 300 skin samples (200 from patients, 100 from controls) were examined. Arrector pili muscles were absent in 2 MSA-P and 2 PD skin samples. Sweat glands were always present in the skin of patients with PD and absent in 2 participants with MSA-P. Qualitative morphologic analysis of skin samples showed consistent derangement of somatic and autonomic cutaneous innervation in patients compared to controls (Figures 2–4). In both patients with MSA-P and patients with PD, IENFs were irregularly distributed along the epidermis with long tracts devoid of fibers. Branching and clusters of fibers, interpretable as regenerative attempts, were more frequently observed in patients with PD compared to patients with MSA-P (Figure 2). Subepidermal plexus was generally poorer in MSA-P. The innervation of dermal adnexa was scattered and irregular in both groups. Overall, sweat gland structure was more severely deranged in those with MSA-P compared to patients with PD (Figure 3), with loss of the compact globular shape of the sweat gland structure and inclusion of lipoid tissue regardless of the density of residual sudomotor nerves (eFigure 1).
Figure 2. Epidermal Nerve Fiber Confocal Images Showing Epidermal Denervation in Patients With PD (B) and MSA (C) Compared to control (A).
Loss of intraepidermal nerve fiber is more severe in multiple system atrophy with parkinsonism (MSA-P) (C) compared with Parkinson disease (PD) (B), in which branching and clusters (arrowheads in B) can also be observed as regenerative attempts. White scale bar is 100 µm.
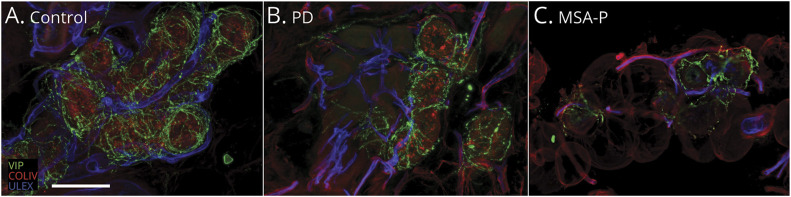
Figure 3. Autonomic Sudomotor Nerve Fiber Confocal Images Showing Sweat Gland Autonomic Denervation in Patients With PD (B) and MSA-P (C) Compared to Controls (A).
Cholinergic (vasoactive intestinal peptide [VIP]) sudomotor nerve fibers are more severely affected in multiple system atrophy with parkinsonism (MSA-P) (C) compared to Parkinson disease (PD) (B). White scale bar is 100 µm. COLIV = collagen IV; ULEX = ulex europaeus agglutinin 1.
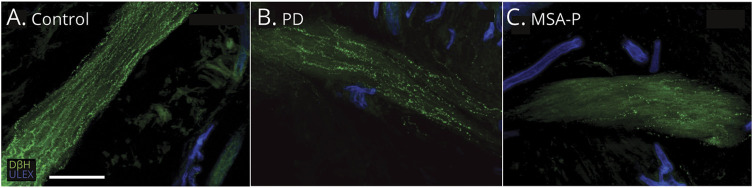
Figure 4. Autonomic Pilomotor Nerve Fiber Confocal Images Showing Arrector Pili Muscle Autonomic Denervation in Patients With PD (B) and MSA-P (C) Compared to Controls (A).
Noradrenergic (dopamine β-hydroxylase [DβH]) pilomotor nerves are more severely affected in multiple system atrophy with parkinsonism (MSA-P) (C) compared to Parkinson disease (PD) (B). White scale bar is 100 µm. ULEX = ulex europaeus agglutinin 1.
Moreover, the average volume of sweat glands in patients with MSA-P was significantly lower compared to patients with PD and in both groups of patients compared to controls. The nerve density of a total of 1,220 sweat glands (from 41 with MSA-P and 57 with PD) and 1,980 pilomotor muscles (from 41 with MSA-P and 55 with PD) was assessed on confocal digital images. We found a reduced density of IENF and pilomotor and sudomotor fibers compared to controls in both patients with PD and patients with MSA-P. IENF density and pilomotor and sudomotor fiber density were lower in individuals with MSA-P compared to those with PD. Abnormalities of sensory and autonomic nerves were already evident in 4 patients with MSA-P who showed normal DST.
Cutaneous sensory and autonomic nerve densities of patients with PD, individuals with MSA-P, and controls are summarized in Table 2.
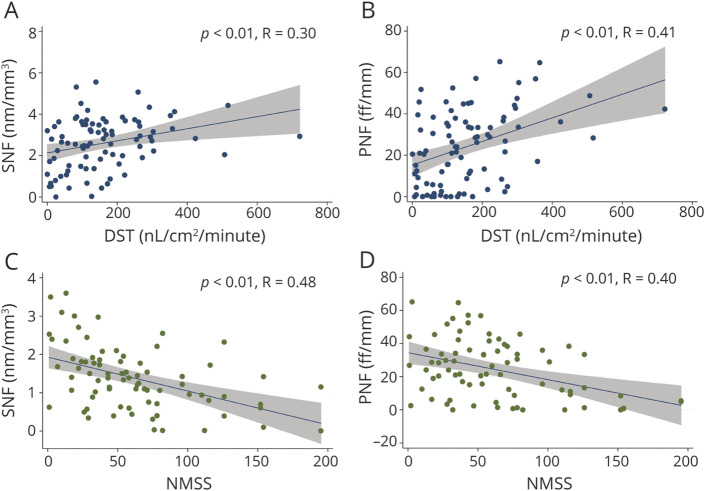
Pilomotor and sudomotor nerve density correlated with activated sweat gland density and sweat production per area and with nonmotor clinical symptoms (NMSS and COMPASS-31 scores) (Figure 5 and eFigure 2). Sweat gland volume correlated with sweat production per area.
Figure 5. Correlations of Autonomic Nerve Density With Functional and Clinical Findings (A–D).
DST = dynamic sweat test; NMSS = Nonmotor Symptoms Scale; PNF = pilomotor nerve fibers; SNF = sudomotor nerve fibers.
CSP score was significantly lower in patients with MSA-P compared to those with PD (222.6 ± 251.8 in MSA-P vs 611.3 ± 484.5 in PD; p < 0.001) and efficiently separated the 2 populations, with the receiving operating characteristics curve showing an area under the curve of 0.83 (eFigure 2) with a sensitivity of 60.4% and a specificity of 87.5% using a CSP cutoff of 436. CSP was also significantly different between patients with MSA-P with orthostatic hypotension and those with PD-OH (416.7 ± 219.4; p < 0.01) and differentiated the 2 populations with an area under the curve of 0.81.
Classification of Evidence
This study provides Class II evidence that postganglionic sudomotor morpho-functional assessment accurately distinguishes individuals with PD from those with MSA-P.
Discussion
We assessed autonomic postganglionic sudomotor function in prospectively enrolled patients with PD or MSA-P with recent onset of motor symptoms to identify potential early biomarkers able to differentiate the 2 conditions. The patient groups were similar in age and disease duration, but they differed in the severity of both motor and nonmotor impairment. The latter has been described in a study looking at patients with longer disease duration,9 but it is of interest that this is confirmed at the early stage of the disorder.
We found morphologic and functional abnormalities of postganglionic sudomotor pathway in both patient groups compared to controls with a more severe impairment in patients with MSA-P compared with patients with PD. In particular, we found a cutaneous denervation with a parallel loss of sensory and autonomic (pilomotor and sudomotor) nerves, suggesting that the degeneration of the 2 nerve populations must proceed at the same rate. Moreover, autonomic nerve loss correlated with sweating function impairment (as assessed by DST) and with autonomic symptoms (as assessed by COMPASS-31), while it did not correlate with disease duration. The latter indicates a prominent postganglionic involvement since the early stage of the diseases, independently of disease severity and not just as a result of transsynaptic degeneration in long-standing disease, as previously hypothesized.8 Longitudinal studies including follow-up skin biopsy might elucidate the possible progression of small fiber impairment.
DST and the quantification of cutaneous autonomic nerves proved to be a sensitive morpho-functional approach to assess postganglionic component of the sudomotor pathway, revealing a more severe involvement in MSA-P than in PD early in the disease course. In particular, the reduced density of activated sweat glands is the consequence of sudomotor nerve loss that must induce a loss of m3 receptors located on the gland surface, making the sweat gland unresponsive to pilocarpine stimulation. Compared with the normal fifth percentile cutoff, we found a lower activation of sweat glands in 90% of patients with MSA-P and 59% of patients with PD. In addition to the higher loss of sudomotor nerves in MSA-P compared to PD, this may be explained by the derangement and the reduced volume of the glandular structure that was very relevant in MSA-P. Moreover, 4 patients with MSA presented abnormalities of cutaneous innervation but normal sweat drop density evaluated with DST, indicating the presence of degeneration at the time when function study might fail to capture abnormalities of sudomotor function.
We found a lower SSR amplitude and a lack of response in a higher percentage of patients with MSA-P compared to patients with PD. This may be in agreement with a more severe involvement of both central and peripheral components of the sudomotor pathway in MSA.
We described CSP, combining morphologic and functional sudomotor measures, that is able to differentiate MSA-P from PD with an excellent specificity and good sensitivity. CSP was also able to differentiate MSA-P from the PD-OH subgroup. Longitudinal studies are needed to validate this score in monitoring disease progression.
Earlier literature reports that the autonomic involvement is primarily postganglionic in PD and preganglionic in MSA.25 However, a postganglionic involvement has been demonstrated in patients with MSA by our group and others.8,23 Recently, a postganglionic sudomotor impairment has been demonstrated in the 62% of patients with MSA using the quantitative sudomotor axon reflex test (QSART), with a correlation with glucose hypometabolism of basal ganglia or cerebellum on fluorodeoxyglucose-PET and with disease severity.26 This finding is in line with previous studies reporting abnormal QSART in 59% of patients with MSA mostly linked to the more advanced stage of the disease.8 Compared to QSART, DST explores only the direct activation of sweat glands, not providing information on the neural network responsible for the axon-reflex response. However, it provides the possibility of assessing the number of activated sweat glands in the stimulated area, a parameter that is missing with QSART. For these technical differences, in our prospective study, DST was able to detect sudomotor abnormalities in the majority of the patients with MSA-P and in most of the patients with PD.
Our finding may appear to disagree with several reports describing a more severe loss of cutaneous nerves in PD compared to MSA. However, this may be due to the different characteristics of the selected patients because our cohort included only patients with idiopathic PD in the early stage of the disease, while previous studies have included mainly patients with PD who were older27 and with associated orthostatic hypotension.28 In addition, discrepancies with previous reports29 may arise from differences in the techniques of nerve visualization and analysis.
Autonomic dysfunction characterizes MSA since its onset and it is an essential criterion required even for the lowest degree of diagnostic certainty (MSA possible).11
Although PD has for many years been considered a pure motor condition, autonomic involvement has been reported since the earliest stages of disease,30 and it has been reported to correlate with postural instability.31 The description of the retrograde progression of synuclein aggregate accumulation starting from autonomic structures and spreading later on to motor structures of the CNS further support the early autonomic involvement in PD.32
PD and MSA share a common neuropathologic hallmark, the pathologic accumulation of misfolded α-synuclein that leads to neuron dysfunction and death in synucleinopathies.33 While in PD the misfolded α-synuclein accumulates in neurons, in MSA, the pathologic accumulation occurs in oligodendroglia. Therefore, the glial cytoplasmatic inclusions are the hallmarks of MSA. In addition to brain involvement, α-synuclein aggregates have been observed in caudal segments of spinal cord in Lewy body disorders,34-36 including lamina I and II of the dorsal horn; lamina VII, IX, and X; but also the sacral dorsal roots, Lissauer tract, and especially the lateral collateral region. Synuclein aggregates were found also in sympathetic ganglia of 11 of 26 patients with MSA.37 These findings might explain the broad spectrum of pathology in these disorders, including postganglionic autonomic involvement and the sensory denervation.
In recent years, several authors reported the presence of synuclein aggregates in cutaneous nerves in PD and MSA using skin biopsy although with a different pattern of α-synuclein distribution. In particular, such aggregates have been reported to be relatively more prevalent in the autonomic innervation of dermal adnexa of PD compared to MSA.38,39 However, the relationship between the extent of synuclein aggregates in the skin and disease severity in terms of clinical impairment and nerve degeneration still needs to be clarified. In fact, no such relationship has been found so far, and from a physiopathologic standpoint, the presence of synuclein aggregates may be regarded as an event that precedes nerve degeneration and is affected by the severity and rate of progression of the disorder. We have not evaluated the α-synuclein accumulation in this current preliminary study.
The strength of our study is the enrollment of individuals with early-stage α-synucleinopathy within a cohort of drug-naive patients prospectively evaluated with validated sudomotor testing and cutaneous morphologic study.
A limitation of this study is the definition of disease onset by the manifestation of the first motor dysfunction. This might have underestimated disease duration, in particular in patients with early autonomic and nonmotor features preceding motor symptoms and signs. Moreover, as per the inclusion criteria, all patients were recruited at a very early stage of disease and were followed up for a period of 12 months. Thus, we cannot rule out the possibility that the reported absence of correlation between disease duration and morpho-functional parameters can be related to the short period of observation, according to the study design.
We demonstrated reduced sudomotor function, IENF, and pilomotor and sudomotor fiber density in the early stage of α-synucleinopathies. Such impairment showed a greater severity in patients with MSA-P compared with those with PD and correlated with clinical scales. A novel composite morpho-functional sudomotor score is a helpful biomarker in differentiating MSA-P and PD in the early stage of the disease with a sensitivity of 60.4% and a specificity of 87.5%. The sensitivity and specificity of the test were confirmed in comparisons of patients with MSA-P and PD-OH.
Our data support the hypothesis that the assessment of sudomotor system may provide suitable targets in the search for early biomarkers able to differentiate MSA vs PD.
Glossary
- COMPASS-31
Composite Autonomic Symptom Score
- CSP
composite sudomotor parameter
- DST
dynamic sweat test
- H&Y
Hoehn and Yahr
- IENF
intraepidermal nerve fiber
- MDS-UPDRS
Movement Disorders Society Unified Parkinson’s Disease Rating Scale
- MSA
multiple system atrophy
- MSA-P
MSA-parkinsonian type
- NMSS
Nonmotor Symptoms Scale
- PD
Parkinson disease
- PD-OH
PD with orthostatic hypotension
- QSART
quantitative sudomotor axon reflex test
- SFN-SIQ
Small Fiber Neuropathy Symptoms Inventory Questionnaire
- SSR
sympathetic skin response
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This study was financed by Italian Ministry of Health “Ricerca Finalizzata 2013,” project code PE-2013-02359028. Dr. Valeria Iodice is supported by National Institute for Health Research University College London Hospitals Biomedical Research Centre. Dr. Shiwen Koay was supported by the Guarantors of Brain Entry Fellowship.
Disclosures
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol. 2021;20(5):385-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Campenhausen S, Bornschein B, Wick R, et al. Prevalence and incidence of Parkinson's disease in Europe. Eur Neuropsychopharmacol. 2005;15(4):473-490. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stankovic I, Petrovic I, Pekmezovic T, et al. Longitudinal assessment of autonomic dysfunction in early Parkinson's disease. Parkinsonism Relat Disord. 2019;66:74-79. [DOI] [PubMed] [Google Scholar]
- 5.Osaki Y, Morita Y, Fukumoto M, Akagi N, Yoshida S, Doi Y. Cross-sectional and longitudinal studies of three-dimensional stereotactic surface projection SPECT analysis in Parkinson's disease. Mov Disord. 2009;24(10):1475-1480. [DOI] [PubMed] [Google Scholar]
- 6.Todorova A, Jenner P, Ray Chaudhuri K. Non-motor Parkinson's: integral to motor Parkinson's, yet often neglected. Pract Neurol. 2014;14(5):310-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellecchia MT, Stankovic I, Fanciulli A, et al. Can autonomic testing and imaging contribute to the early diagnosis of multiple system atrophy? A systematic review and recommendations by the Movement Disorder Society Multiple System Atrophy Study Group. Mov Disord Clin Pract. 2020;7(7):750-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coon EA, Fealey RD, Sletten DM, et al. Anhidrosis in multiple system atrophy involves pre- and postganglionic sudomotor dysfunction. Mov Disord. 2017;32(3):397-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipp A, Sandroni P, Ahlskog JE, et al. Prospective differentiation of multiple system atrophy from Parkinson disease, with and without autonomic failure. Arch Neurol. 2009;66(6):742-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30(12):1591-1601. [DOI] [PubMed] [Google Scholar]
- 11.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolano M, Provitera V, Manganelli F, et al. Loss of cutaneous large and small fibers in naive and l-dopa-treated PD patients. Neurology. 2017;89(8):776-784. [DOI] [PubMed] [Google Scholar]
- 13.Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738-750. [DOI] [PubMed] [Google Scholar]
- 14.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427-442. [DOI] [PubMed] [Google Scholar]
- 15.Wenning GK, Tison F, Seppi K, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord. 2004;19(12):1391-1402. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri KR, Martinez-Martin P, Brown RG, et al. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord. 2007;22(13):1901-1911. [DOI] [PubMed] [Google Scholar]
- 17.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. Compass 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87(12):1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakkers M, Merkies IS, Lauria G, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology. 2009;73(14):1142-1148. [DOI] [PubMed] [Google Scholar]
- 19.Provitera V, Nolano M, Caporaso G, Stancanelli A, Santoro L, Kennedy WR. Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology. 2010;74(1):50-56. [DOI] [PubMed] [Google Scholar]
- 20.Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain. 2008;131(pt 7):1903-1911. [DOI] [PubMed] [Google Scholar]
- 21.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17(3):903-912, e944-909. [DOI] [PubMed] [Google Scholar]
- 22.Nolano M, Provitera V, Caporaso G, Stancanelli A, Vitale DF, Santoro L. Quantification of pilomotor nerves: a new tool to evaluate autonomic involvement in diabetes. Neurology. 2010;75(12):1089-1097. [DOI] [PubMed] [Google Scholar]
- 23.Provitera V, Nolano M, Caporaso G, et al. Postganglionic sudomotor denervation in patients with multiple system atrophy. Neurology. 2014;82(24):2223-2229. [DOI] [PubMed] [Google Scholar]
- 24.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69-72. [DOI] [PubMed] [Google Scholar]
- 25.Orimo S, Ozawa E, Oka T, et al. Different histopathology accounting for a decrease in myocardial MIBG uptake in PD and MSA. Neurology. 2001;57(6):1140-1141. [DOI] [PubMed] [Google Scholar]
- 26.Kim SW, Chung SJ, Lee S, et al. Postganglionic sudomotor dysfunction and brain glucose hypometabolism in patients with multiple system atrophy. J Parkinsons Dis. 2021;11(3):1247-1256. [DOI] [PubMed] [Google Scholar]
- 27.Giannoccaro MP, Donadio V, Giannini G, et al. Comparison of 123I-MIBG scintigraphy and phosphorylated alpha-synuclein skin deposits in synucleinopathies. Parkinsonism Relat Disord. 2020;81:48-53. [DOI] [PubMed] [Google Scholar]
- 28.Donadio V, Incensi A, Rizzo G, et al. Skin biopsy may help to distinguish multiple system atrophy-parkinsonism from Parkinson's disease with orthostatic hypotension. Mov Disord. 2020;35(9):1649-1657. [DOI] [PubMed] [Google Scholar]
- 29.Brumberg J, Kuzkina A, Lapa C, et al. Dermal and cardiac autonomic fiber involvement in Parkinson's disease and multiple system atrophy. Neurobiol Dis. 2021;153:105332. [DOI] [PubMed] [Google Scholar]
- 30.Cersosimo MG, Benarroch EE. Autonomic involvement in Parkinson's disease: pathology, pathophysiology, clinical features and possible peripheral biomarkers. J Neurol Sci. 2012;313(1-2):57-63. [DOI] [PubMed] [Google Scholar]
- 31.You S, Kim HA, Lee H. Association of postural instability with autonomic dysfunction in early Parkinson's disease. J Clin Med. 2020;9(11):3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1)21-134. [DOI] [PubMed] [Google Scholar]
- 33.George S, Rey NL, Reichenbach N, Steiner JA, Brundin P. Alpha-synuclein: the long distance runner. Brain Pathol. 2013;23(3):350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson's disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113(4):421-429. [DOI] [PubMed] [Google Scholar]
- 35.Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson's disease. Acta Neuropathol. 2012;124(5):643-664. [DOI] [PubMed] [Google Scholar]
- 36.Nardone R, Holler Y, Brigo F, et al. Spinal cord involvement in Lewy body-related alpha-synucleinopathies. J Spinal Cord Med. 2020;43(6):832-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sone M, Yoshida M, Hashizume Y, Hishikawa N, Sobue G. Alpha-synuclein-immunoreactive structure formation is enhanced in sympathetic ganglia of patients with multiple system atrophy. Acta Neuropathol. 2005;110(1):19-26. [DOI] [PubMed] [Google Scholar]
- 38.Doppler K, Weis J, Karl K, et al. Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Mov Disord. 2015;30(12):1688-1692. [DOI] [PubMed] [Google Scholar]
- 39.Donadio V, Incensi A, El-Agnaf O, et al. Skin alpha-synuclein deposits differ in clinical variants of synucleinopathy: an in vivo study. Sci Rep. 2018;8(1):14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on request by a qualified investigator.