Abstract
Background and Objectives
The objectives of this study were to develop and establish concurrent validity of a clinically relevant definition of poor cognitive outcome 1 year after mild traumatic brain injury (mTBI), to compare baseline characteristics across cognitive outcome groups, and to determine whether poor 1-year cognitive outcome can be predicted by routinely available baseline clinical variables.
Methods
Prospective cohort study included 656 participants ≥17 years of age presenting to level 1 trauma centers within 24 hours of mTBI (Glasgow Coma Scale score 13–15) and 156 demographically similar healthy controls enrolled in the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study. Poor 1-year cognitive outcome was defined as cognitive impairment (below the ninth percentile of normative data on ≥2 cognitive tests), cognitive decline (change score [1-year score minus best 2-week or 6-month score] exceeding the 90% reliable change index on ≥2 cognitive tests), or both. Associations of poor 1-year cognitive outcome with 1-year neurobehavioral outcomes were performed to establish concurrent validity. Baseline characteristics were compared across cognitive outcome groups, and backward elimination logistic regression was used to build a prediction model.
Results
Mean age of participants with mTBI was 40.2 years; 36.6% were female; 76.6% were White. Poor 1-year cognitive outcome was associated with worse 1-year functional outcome, more neurobehavioral symptoms, greater psychological distress, and lower satisfaction with life (all p < 0.05), establishing concurrent validity. At 1 year, 13.5% of participants with mTBI had a poor cognitive outcome vs 4.5% of controls (p = 0.003). In univariable analyses, poor 1-year cognitive outcome was associated with non-White race, lower education, lower income, lack of health insurance, hyperglycemia, preinjury depression, and greater injury severity (all p < 0.05). The final multivariable prediction model included education, health insurance, preinjury depression, hyperglycemia, and Rotterdam CT score ≥3 and achieved an area under the curve of 0.69 (95% CI 0.62–0.75) for the prediction of a poor 1-year cognitive outcome, with each variable associated with >2-fold increased odds of poor 1-year cognitive outcome.
Discussion
Poor 1-year cognitive outcome is common, affecting 13.5% of patients with mTBI vs 4.5% of controls. These results highlight the need for better understanding of mechanisms underlying poor cognitive outcome after mTBI to inform interventions to optimize cognitive recovery.
Mild traumatic brain injury (mTBI) affects tens of millions of individuals across the world annually1,2 and is an emerging risk factor for dementia.3-6 Research now focuses on unraveling mechanisms of this long-term adverse cognitive outcome, but far less attention has focused on characterizing shorter-term cognitive outcomes (i.e., within 1 year) after mTBI. Evidence from a systematic review suggests that approximately half of individuals with mTBI experience cognitive impairment on neuropsychological assessment at 3, 6, or ≥12 months after injury,7 refuting the long-held belief that the vast majority of patients with mTBI experience complete recovery by 3 months after injury.8 However, the studies included in this meta-analysis were relatively small (N < 300), and methods for defining cognitive impairment were heterogeneous. Specifically, cognitive impairment was often based on statistically significant but not clinically meaningful cutoffs, potentially overestimating poor cognitive outcomes. In addition, there is emerging evidence for a phenotype of early postrecovery cognitive decline within 1 year9 or 5 years10,11 of moderate to severe traumatic brain injury (TBI), which may be influenced by the intensity of cognitive rehabilitation in the acute rehabilitation period.10 These declining individuals may then be at high risk for continued decline and dementia. It is unknown whether early postrecovery cognitive decline may occur after mTBI.
To inform early cognitive rehabilitation and to optimize postinjury cognition, intervention trials of patients with acute mTBI with short-term cognition endpoints that are enriched with individuals at highest risk for poor cognitive outcomes are needed. However, there is currently no consensus in the field as to how to define cognition endpoints for clinical trials of mTBI.12 Prior studies10,13 have been limited by their focus on either cognitive impairment or cognitive decline; both are reasonably considered poor outcomes, but studies that focus on only 1 outcome definition are liable to overestimate the prevalence of good cognitive outcomes. Defining poor cognitive outcome as cognitive impairment alone could lead some patients with clinically relevant decline, yet shy of the impairment threshold, to be categorized as having a good cognitive outcome; similarly, defining poor cognitive outcome as cognitive decline alone would lead some severely chronically impaired patients to be categorized as good cognitive outcome. This limitation of the existing literature highlights the need for the development of a post-TBI cognitive outcome definition incorporating clinically relevant, patient-centered definitions of both cognitive impairment and cognitive decline.
With the use of data from the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study, a multicenter study of 1-year outcomes among patients presenting to level 1 trauma centers with acute TBI, the objectives of this analysis were (1) to create a clinically relevant definition of poor cognitive outcome at 1 year after mTBI, incorporating both cognitive impairment and cognitive decline; (2) to establish the concurrent validity of this definition; (3) to establish the prevalence of poor 1-year cognitive outcome and to characterize baseline characteristics among patients with good vs poor cognitive outcome at 1 year; and (4) to determine whether poor 1-year cognitive outcome can be predicted from baseline clinical variables that are routinely available in an acute trauma/emergency department (ED) setting. The overall goal is to directly inform future studies of patients at high risk for poor short-term cognitive outcome after mTBI to identify mechanisms and to design early interventions.
Methods
Study Population and Study Design
The TRACK-TBI study is a prospective multicenter study that enrolled patients presenting to the EDs of 18 US level 1 trauma centers within 24 hours of TBI who underwent a head CT as part of routine clinical care.14 The study also enrolled healthy controls who were recruited from friends and family members of enrolled patients with mTBI and who did not have a history of TBI within the past year. Participants were followed up for outcomes at up to 3 in-person visits occurring at 2 weeks, 6 months, and 1 year after injury/enrollment. A subset of indices assessed at the in-person visits could be performed by telephone when participants were unable to attend in-person visits, including the Glasgow Outcome Scale Extended TBI Version (GOSE-TBI) and the Galveston Orientation and Amnesia Test (GOAT) (telephone administration of other outcome measures used in the present analysis was not part of the approved study protocol). The present analysis focused on the TRACK-TBI phase 1 cohort comprising participants ≥17 years of age enrolled at the 11 then-existing sites between February 26, 2014, and May 4, 2016.
Standard Protocol Approvals, Registrations, and Patient Consents
The TRACK-TBI study was approved by the institutional review board of each enrolling institution, and all participants or their legally authorized representatives completed written informed consent.
Neuropsychological Test Battery
Participants underwent neuropsychological testing at up to 3 in-person cognitive assessments at 2 weeks, 6 months, and 1 year after injury. The neuropsychological test battery used in the TRACK-TBI study was selected to cover multiple cognitive domains and to be consistent with TBI Common Data Elements (CDEs). The battery included 5 scores, each capturing different aspects of cognitive function, which were derived from 3 different tests: (1) the Rey Auditory Verbal Learning Test (RAVLT) immediate recall score (sum of learning trials 1–5, score range 0–15 for each trial)15 and 20-minute delayed recall score (score range 0–15),15 (2) the Trail Making Test (TMT) Part A score (time to complete, maximum allotted time 100 seconds)16 and Part B score (time to complete, maximum allotted time 300 seconds),16 and (3) the Processing Speed Index (PSI) from the Wechsler Adult Intelligence Scale–4th Edition17 (WAIS-IV; a composite score reflecting performance on the Symbol Search subtest and the Coding subtest; age-corrected mean 100, SD 15).
Definition of Cognitive Impairment
To create a clinically relevant definition of impairment for each test score, we used demographically adjusted cutoffs, including age-specific Schmidt metanorms for the RAVLT immediate and delayed recall scores,15 Heaton norms for TMT-A and TMT-B scores (adjusted for sex, age, race, and education; scaled as T scores),16 and age-adjusted WAIS-IV PSI standard scores.17 The threshold for impairment for each score was set at the ninth percentile as follows18: RAVLT immediate and delayed recall age-specific Schmidt metanorms score ≤−1.33, TMT-A and TMT-B Heaton norm score ≤37, and WAIS-IV PSI age-adjusted standard score ≤79. While studies of cognitive aging typically use more conservative thresholds,19 there is wide variation in the field, and we chose the ninth percentile threshold to be consistent with prior TRACK-TBI studies.18
Cognitive impairment was defined as meeting criteria for impairment on at least 1 score (RAVLT immediate, RAVLT delayed, TMT-A, TMT-B, WAIS-PSI score) on ≥2 of 3 tests (RAVLT, TMT, or WAIS-IV) or if the participant scored ≤75 on the GOAT20 at 1 year (indicating inability to complete the full battery due to cognitive impairment); 2 participants in our cohort met the GOAT-based criteria for cognitive impairment (eTable 1, links.lww.com/WNL/B771).
Definition of Cognitive Decline
To create a clinically relevant definition of decline for each test score, which takes into account test measurement error, we used reliable change indices (RCIs).10,11 For each of the 5 cognitive test scores, the best score before 1 year (either the 2-week or 6-month score) was subtracted from the 1-year score to produce a change score. Change from best score was chosen to capture decline from the maximum level of cognitive recovery after injury, regardless of the timing of this maximum level of recovery, because recovery trajectories are known to be highly variable across individuals.21 To generate an RCI threshold specifically relevant to our TBI cohort, reliability for each cognitive test score was calculated from the stability coefficient (test-retest correlation) from healthy controls. Decline for each score was defined as having a change score exceeding the 90% RCI in the direction of worsened cognitive function.10 Cognitive decline was defined as meeting criteria for decline on at least 1 score (RAVLT immediate, RAVLT delayed, TMT-A, TMT-B, WAIS-PSI score) on ≥2 of 3 tests (RAVLT, TMT, or WAIS-IV) (eTable 1, links.lww.com/WNL/B771). It is important to note that our concise cognitive battery is less susceptible to false-positive classification as “cognitive decline” with the RCI method because the false-positive rate increases with longer test batteries.22
Definition of 1-Year Poor Cognitive Outcome
Our primary definition of poor 1-year cognitive outcome was cognitive impairment (≥1 score on ≥2 of 3 tests meeting criteria for impairment), cognitive decline (≥1 score on ≥2 of 3 tests meeting criteria for decline), or both. This cognitive outcome categorization for each patient with mTBI and control participant was visualized by first stratifying the cohort by 1-year cognitive impairment status and then plotting the RCI used to determine cognitive decline categorization for each patient (i.e., RCI from the test with the second greatest decline).
Because there is no expert consensus definition of poor 1-year cognitive outcome after mTBI, we additionally report the prevalence of poor 1-year cognitive outcome using 3 more liberal definitions: (1) cognitive impairment on ≥1, decline on ≥1 of the 5 scores, or both; (2) cognitive impairment on ≥2, decline on ≥2 of the 5 scores, or both; or (3) cognitive impairment on ≥1 score on ≥2, or decline on ≥1 score on ≥2 of the 3 different tests, both, or death before the 1-year follow-up (eTable 1, links.lww.com/WNL/B771). The third definition is relevant for the planning of clinical trials enriched with patients at high risk for poor short-term cognitive outcome after mTBI because this high-risk group would include individuals who die within 1 year of mTBI in an intention-to-treat analysis.
TBI CDE 1-Year Outcomes
To establish the concurrent validity of our 1-year poor cognitive outcome definition, we assessed associations with 4 TBI CDE outcomes that were measured at 1-year after injury. The GOSE-TBI23 is a self- or proxy-reported global measure of functional impairment due only to the TBI (i.e., not due to co-occurring polytrauma), with possible scores ranging from 1 (deceased) to 8 (upper good recovery). The Rivermead Post Concussion Symptoms Questionnaire24 is a measure of self-reported injury-related symptoms, with higher scores indicating more severe symptoms (score range 0–64). The 18-item Brief Symptom Inventory Global Severity Index (BSI-18-GSI)25 is a self-reported measure of psychological distress, with higher scores indicating greater psychological distress (T score range 36–81). The Satisfaction With Life Scale26 is a self-reported measure of general life satisfaction, with higher scores indicating greater life satisfaction (score range 5–35).
Baseline Variables
We assessed the following demographic and preexisting medical comorbidity baseline self-reported variables: age, sex (male, female), race (White, Black, other), ethnicity (Hispanic/Latino, non-Hispanic/Latino), education (less than high school, high school or equivalent, more than high school), employment status (full-time, part-time, retired, student, disabled/unemployed/not working), annual family income (<$35,000, $35,000–<$75,000, ≥$75,000, not reported), health insurance (private insurance, Medicare/Medicaid, uninsured), military service, hypertension, diabetes, prior TBI, depression, anxiety, headache/migraines, developmental disability, and illicit drug use. Laboratory variables included ED blood glucose (>200 mg/dL, ≤200 mg/dL, or not performed), ED blood alcohol level (≥80 mg/dL, <80 mg/dL, or not performed), and ED toxicology screen (positive, negative, or not performed).
The following TBI-related variables were assessed: Glasgow Coma Scale (GCS; 13, 14, 15), injury cause (motor vehicle crash [occupant], motorcycle crash, motor vehicle crash [cyclist/pedestrian], fall, assault, other), loss of consciousness (yes/suspected, no), alteration of consciousness (yes/suspected, no), posttraumatic amnesia (yes/suspected, no), hypotension in ED (defined as systolic blood pressure <90 mm Hg or diastolic blood pressure <60 mm Hg), hypoxia in the ED (defined as oxygen saturation <90%), ED disposition (intensive care unit, ward unit, discharge), head CT scan positive for acute intracranial injury (complicated mTBI; defined according to the TBI CDE Neuroimaging Working Group expert consensus recommendations27,28), and Rotterdam CT score (severity of intracranial trauma score with range 1–6 where 6 is most severe trauma, categorized as <3 vs ≥3).29
Statistical Analyses
All analyses were performed with propensity weighting to account for missing outcome data/study attrition.30-33 Propensity weights were derived from boosted logistic regression models predicting complete (versus noncomplete) 1-year cognitive outcome data from the following baseline variables: site, age, sex, race, ethnicity, education, employment status, insurance, ED disposition, GCS score, mental health history, cause of injury, initial CT results, alcohol use, and illicit drug use. Separate propensity weights were derived for controls using demographic variables only: site, age, sex, race, ethnicity, and education. Weights were proportional to the inverse probability of complete 1-year cognitive outcome data and were normed so that the sum equaled the number of cases with complete data on 1-year cognitive outcome. The use of propensity weights makes the estimates reported herein representative of the entire TRACK-TBI phase 1 cohort.
To establish concurrent validity of our 1-year cognitive outcome definition, we compared 1-year TBI CDE outcomes between the good and poor cognitive outcome groups using logistic regression (GOSE-TBI score <8 [incomplete recovery] vs GOSE-TBI score 8 [complete recovery]) and linear regression (Rivermead Post Concussion Symptoms Questionnaire, BSI-18-GSI, Satisfaction With Life Scale scores). We then compared propensity-weighted baseline characteristics across groups defined by 1-year cognitive outcome status using t tests for continuous variables and second-order Rao-Scott–corrected χ2 tests for categorical variables.
To determine whether poor 1-year cognitive outcome could be predicted with baseline clinical variables that are routinely available in an acute trauma/ED setting, we generated 2 weighted logistic regression prediction models: (1) we included all variables that differed between patients with mTBI with good vs poor cognitive outcome at a p < 0.2 level in univariable analyses; and (2) we applied backward stepwise regression (α = 0.05) to reduce the number of baseline variables included in the model. The resulting receiver operating characteristic curves were plotted, and the individual multivariable odds ratios of each included baseline predictor were calculated.
All reported p values were based on 2-sided tests, with values of p < 0.05 considered statistically significant. Stata SE version 15.0 (StataCorp, College Station, TX) and R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Australia) were used to perform statistical analyses.
Data Availability
Data requests can be made to the TRACK-TBI Study Executive Committee.34
Results
Study Population
We included all patients with mTBI (defined as admission GCS score 13–15) and healthy controls who had sufficient 1-year cognitive outcome data to permit classification of good vs poor cognitive outcome (i.e., completed at least 2 of 3 cognitive tests) (n = 656 mTBI, n = 156 controls; Figure 1). Overall, 18.0% of the phase 1 TRACK-TBI mTBI cohort was lost to follow-up at 1 year (no data available), and 1.4% died before 1 year (n = 16 with GOSE score 1 at 1 year). Of the phase 1 mTBI cohort who survived with at least some 1-year data (n = 1,136), 57.7% (n = 656) had sufficient cognitive outcomes data at 1 year to be included in our analysis. Compared to those without sufficient cognitive outcomes data at 1 year who were excluded from the present analysis, those who were included were more likely to be White and non-Hispanic/Latino, to have more than a high school education, to have history of headache/migraines, and to have had a TBI as a result of being a cyclist/pedestrian in a motor vehicle crash (all p < 0.05).
Figure 1. TRACK-TBI Participant Flow Diagram.
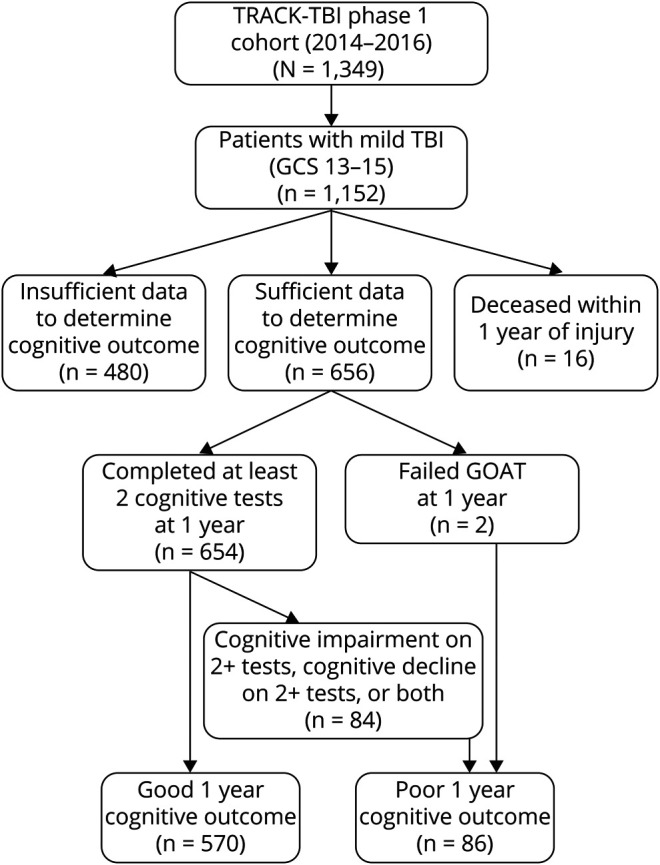
GCS = Glasgow Coma Scale; GOAT = Galveston Orientation and Amnesia Test; TBI = traumatic brain injury; TRACK-TBI = Transforming Research and Clinical Knowledge in Traumatic Brain Injury.
Concurrent Validity
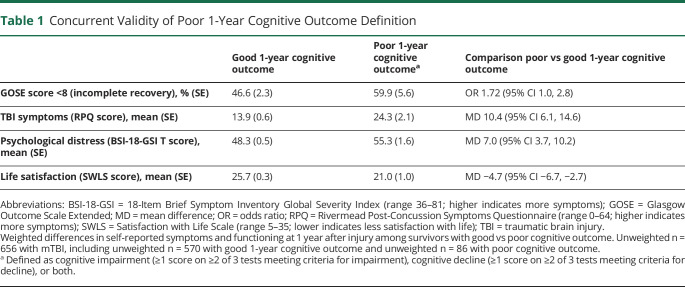
We first established the concurrent validity of our definition of poor 1-year cognitive outcome by investigating associations with 4 TBI CDE outcomes that were measured at 1 year after injury (Table 1). Compared with patients with mTBI with good cognitive outcome, those with poor cognitive outcome were more likely to have incomplete functional recovery (GOSE-TBI score <8), more mTBI-related symptoms, greater psychological distress, and less satisfaction with life at 1-year after TBI (all p < 0.05).
Table 1.
Concurrent Validity of Poor 1-Year Cognitive Outcome Definition
Prevalence of Poor 1-Year Cognitive Outcome
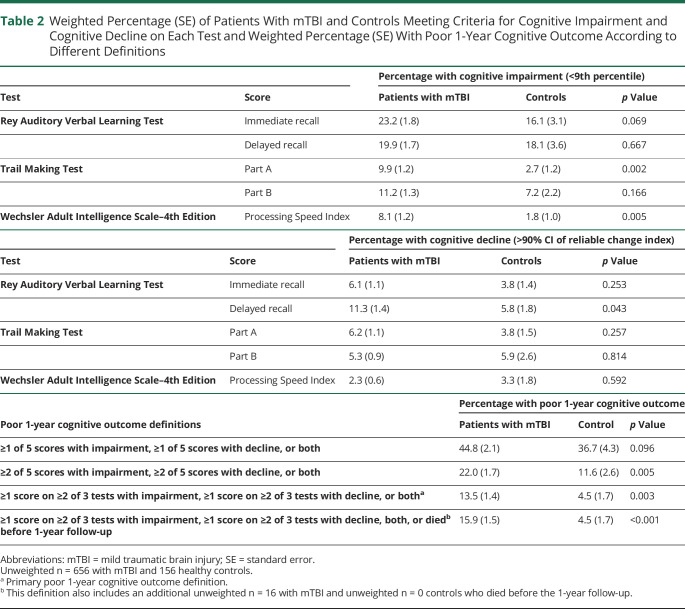
Healthy controls were similar to patients with mTBI on sex, race/ethnicity, and education (all p > 0.05) but were slightly younger than patients with TBI (37 years vs 40 years, p = 0.049). Overall, with the use of our primary definition, 13.5% of patients with mTBI had poor 1-year cognitive outcome, with 10.1% having cognitive impairment only, 1.6% having cognitive decline only, and 1.8% having both cognitive impairment and decline. Significantly fewer controls experienced poor 1-year cognitive outcome (4.5%, p = 0.003 vs mTBI), with 3.3% having cognitive impairment only, 0% having cognitive decline only, and 1.2% having both cognitive impairment and decline. Prevalence estimates for the 3 most stringent definitions of poor cognitive outcome ranged from 13.5% to 22.0% among patients with mTBI and from 4.5% to 11.6% among control participants. With the use of these stringent definitions, poor cognitive outcome was consistently significantly more common among patients with TBI vs controls (all p < 0.05). In contrast, the prevalence estimates for the least stringent definition were not significantly different between patients with TBI and controls (44.8% vs 36.7%, p = 0.096) (Table 2).
Table 2.
Weighted Percentage (SE) of Patients With mTBI and Controls Meeting Criteria for Cognitive Impairment and Cognitive Decline on Each Test and Weighted Percentage (SE) With Poor 1-Year Cognitive Outcome According to Different Definitions
Among patients with mTBI who were categorized as having cognitive impairment (i.e., impaired on at least 1 score on ≥2 of 3 tests), 57.6% were impaired in 2 of 3 tests, and 42.4% were impaired on all 3 tests in the battery. The most common scores to show impairment in patients with mTBI were the RAVLT immediate recall score (23.2%) and the RAVLT delayed recall score (19.9%). The scores with the greatest percent difference in impairment between patients with mTBI and controls were the TMT-A and the RAVLT immediate recall scores (Table 2). Among patients with mTBI who were categorized as having cognitive decline (i.e., decline on at least 1 score on ≥2 of 3 tests), 82.9% had decline on 2 tests, and 17.1% and had decline on all 3 tests. The most common score to show decline in patients with mTBI was the RAVLT delayed recall score (11.3%); the RAVLT delayed recall score was also the score with the greatest percent difference in decline between patients with mTBI and controls (Table 2).
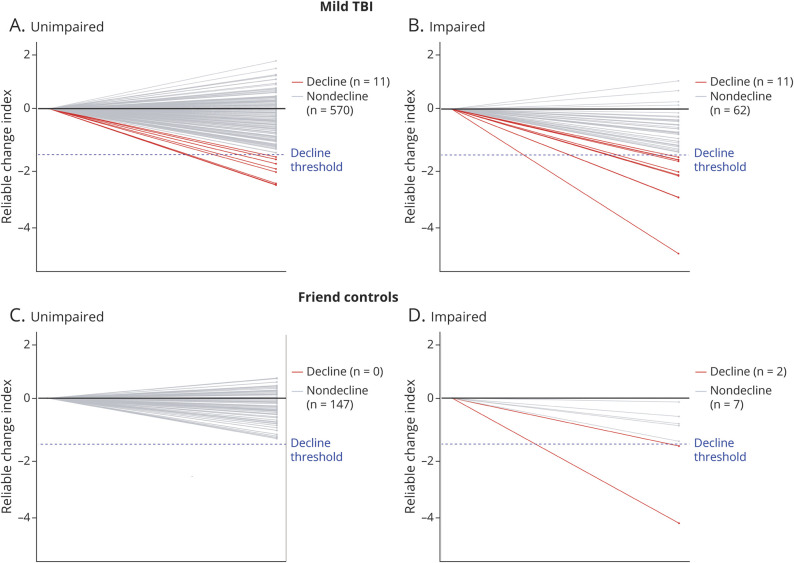
Figure 2 shows each individual patient's 1-year RCI score, which was used to define categorization of cognitive decline (i.e., the RCI from the test with the second greatest decline) after stratification by 1-year cognitive impairment status. Eleven patients with mTBI (and 0 controls) would have been miscategorized as having good cognitive outcome if cognitive decline had not been considered in the outcome definition (Figure 2, A and C).
Figure 2. Cognitive Decline Status.
Cognitive decline status among participants with mild traumatic brain injury (TBI) (A, without cognitive impairment; B, with cognitive impairment) and controls (C, without cognitive impairment; D, with cognitive impairment). Plots shows each individual patient's 1-year reliable change index (RCI) score that was used to define categorization of cognitive decline (i.e., the RCI from the test with the second greatest decline) after stratification by 1-year cognitive impairment status.
Predictors of Poor 1-Year Cognitive Outcome
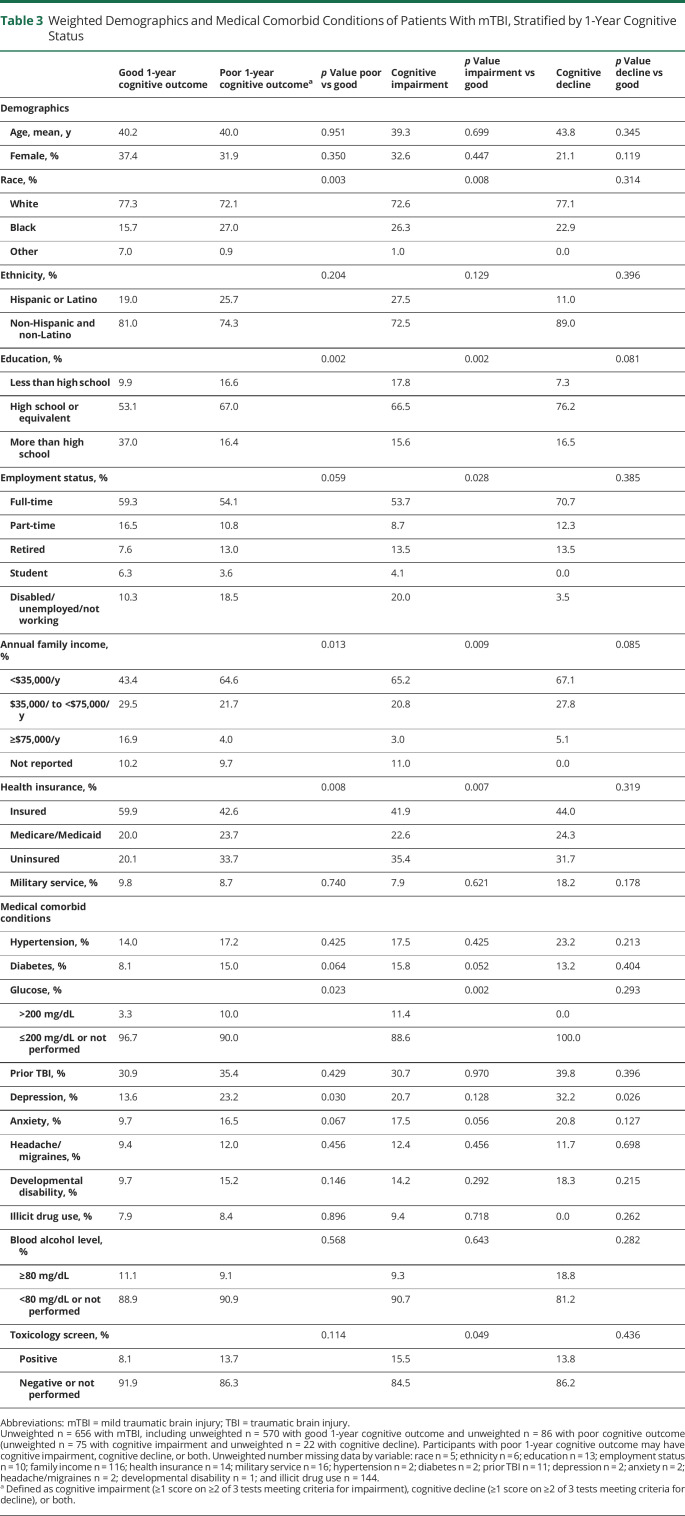
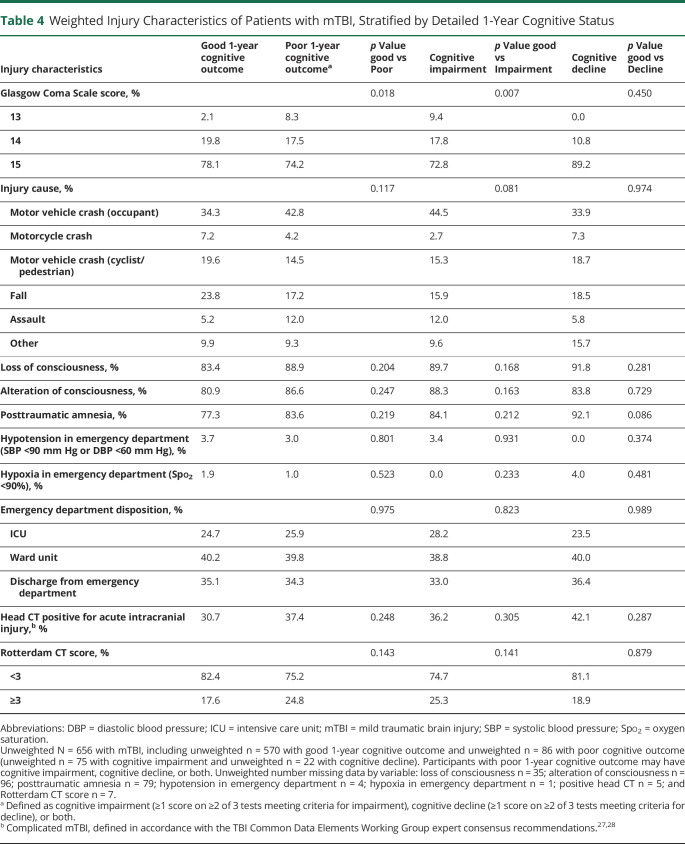
Overall, participants with mTBI were 40.2 years of age, 36.6% were female, 76.6% were White, and 19.9% were Hispanic/Latino. Compared with participants with good cognitive outcome, participants with poor cognitive outcome were more likely to be of non-White race, to have a high school education or less, to have lower annual family income, to be uninsured, and to have higher glucose on presentation, a history of depression, and lower GCS score (all p < 0.05) (Tables 3 and 4). Similar patterns were observed when patients with cognitive impairment and cognitive decline were compared to those with good cognitive outcome, although participants with cognitive impairment were also significantly more likely to be disabled, unemployed, or not working and to have a positive toxicology screen (both p < 0.05).
Table 3.
Weighted Demographics and Medical Comorbid Conditions of Patients With mTBI, Stratified by 1-Year Cognitive Status
Table 4.
Weighted Injury Characteristics of Patients with mTBI, Stratified by Detailed 1-Year Cognitive Status
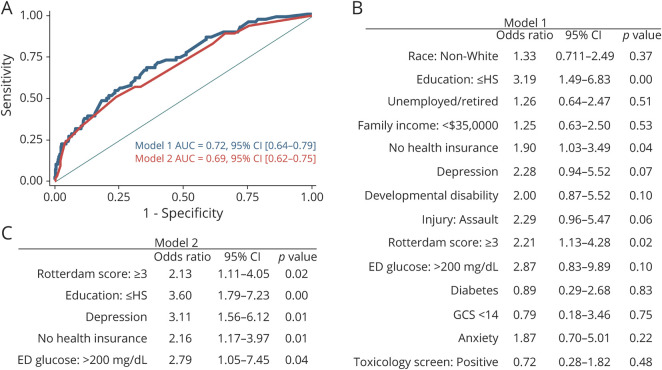
Weighted logistic regression models included baseline characteristics and injury-related variables that differed at a p < 0.2 level in univariable analyses comparing patients with mTBI with good and those with poor 1-year cognitive outcome. These models achieved fair discrimination of good vs poor outcomes (area under the curve 0.72, 95% CI 0.64–0.79) (Figure 3). After backward stepwise regression, the final prediction model included baseline education, health insurance, preinjury history of depression, hyperglycemia, and Rotterdam CT score and achieved an area under the curve of 0.69 (95% CI 0.62–0.75), with each variable being associated with >2-fold increased odds of poor 1-year cognitive outcome.
Figure 3. Prediction Models of Poor 1-Year Cognitive Recovery* Among Patients With mTBI.
(A) Receiver operating characteristic curves for models 1 and 2. (B) Model 1 including baseline characteristics that differed between good and poor 1-year cognitive outcome at the p < 0.2 level (Tables 2 and 3). (C) Model 2 including baseline characteristics after backward stepwise regression using α = 0.05. *Defined as cognitive impairment (≥1 score on ≥2 of 3 tests meeting criteria for impairment), cognitive decline (≥1 score on ≥2 of 3 tests meeting criteria for decline), or both. For the prediction models, baseline characteristics were entered as binary variables with the following reference values as follows: White race, more than a high school (HS) education, working full-time/part-time/students, income  $35,000, health insurance, no depression, no developmental disability, nonassault injury, Rotterdam score <3, emergency department (ED) glucose <200 mg/dL or not done, no diabetes, Glasgow Coma Scale (GCS) score ≥14, no anxiety, and negative toxicology screen. AUC = area under the curve; mTBI = mild traumatic brain injury.
$35,000, health insurance, no depression, no developmental disability, nonassault injury, Rotterdam score <3, emergency department (ED) glucose <200 mg/dL or not done, no diabetes, Glasgow Coma Scale (GCS) score ≥14, no anxiety, and negative toxicology screen. AUC = area under the curve; mTBI = mild traumatic brain injury.
Discussion
In this large multisite cohort study of patients presenting acutely to level 1 trauma centers with mTBI (GCS score 13–15), we developed and established the concurrent validity of a clinically meaningful definition of poor 1-year cognitive outcome, incorporating both cognitive impairment and cognitive decline. We found that poor cognitive outcome at 1 year after injury is common, affecting 13.5% (range 13.5%–44.8%, depending on definition used) of patients with mTBI compared with 4.5% (range 4.5%–36.7% depending on definition used) of controls. Our results suggest that a better understanding of mechanisms underlying poor cognitive outcome after mTBI is needed to identify high-risk patients who can be targeted for future multimodal intervention studies focused on optimization of cognitive outcomes after mTBI.
We provide evidence to support our primary definition of poor cognitive outcome; we demonstrate the added value of considering both impairment and decline to avoid erroneously categorizing patients with clinically meaningful decline or who are chronically impaired as having a good cognitive outcome. We provide evidence to suggest that it is not advisable to rely on only a single score (our least stringent definition) to define poor cognitive outcome because this will not discriminate individuals with TBI-related cognitive outcome from controls. Our definition further prevents misclassification by using individually tailored cutoffs that are based on demographically corrected normative data and clinically meaningful reliable change to define poor cognitive outcome at the individual participant level as opposed to simply comparing group-level mean scores.7 Our estimate of poor 1-year cognitive outcome (13.5%) using our primary definition is substantially lower than the estimate reported in a recent meta-analysis of 45 studies (nearly 50%). In this prior meta-analysis, studies reporting only group-level means were included as having a 100% prevalence of poor cognitive outcome if the group-level mean for participants with TBI was significantly lower than the group level mean for controls, an approach that would be expected to dramatically overestimate poor cognitive outcome at the individual level.7
In the TRACK-TBI cohort, participants with a poor cognitive outcome 1 year after TBI were more likely to be of non-White race and of lower socioeconomic status.35,36 In the dementia field, a growing body of literature suggests that racial differences in dementia risk may be attributable, at least in part, to disparities in education, health insurance, and other socioeconomic factors,37 as well as to disparities in vascular risk factors such as diabetes38 and in psychiatric health factors such as depression.39,40 Indeed, in our population, we also saw a higher prevalence of hyperglycemia and depression among participants with poor 1-year cognitive outcome. Several studies have found racial disparities in global functional recovery after TBI that were partly explained by differences in socioeconomic status.41,42 It remains less clear whether racial differences in cognitive outcome after TBI may also be partly explained by socioeconomic, vascular, or psychiatric risk factors. Future studies investigating racial differences in post-TBI cognitive outcomes will also need to consider the potential impact of institutional racism and racial disparities in treatment.43
A surprising finding was that older age was not identified as a predictor of poor cognitive outcome.44 This finding highlights the value of using age-adjusted normative cutoffs for cognitive impairment.45 This finding also likely reflects the somewhat healthier-than-average composition of the older adults enrolled in TRACK-TBI, which excluded individuals with dementia or disabling medical conditions. Additional studies of cognitive outcome in more representative samples of older adults are needed. However, our findings suggest that premorbidly healthy older adult survivors of mTBI may achieve equally good cognitive recovery compared to younger adult survivors of mTBI.
Prior studies among individuals with moderate to severe TBI provide evidence that early intensive rehabilitation can modify cognitive trajectories.10,46 More research is needed to determine the role of cognitive rehabilitation among patients with mTBI at risk for poor 1-year cognitive outcomes and how to predict who falls into this risk category.10,46 Our final prediction model for poor 1-year cognitive outcome included both preexisting/modifiable factors such as education, health insurance, and depression and the injury-related/nonmodifiable factor of Rotterdam CT score (other injury-related factors such as loss of consciousness were not included, suggesting that significant injury on CT is the strongest injury-related predictor of 1-year cognitive outcome). This prediction model achieved fair discrimination between good and poor 1-year cognitive outcomes, indicating that significant heterogeneity in outcome remains and highlighting the need for future studies aimed at identifying novel predictors and mechanisms of poor cognitive outcome 1 year after mTBI.
In our cohort, patients with mTBI were most likely to meet criteria for impairment and decline based on performance on the RAVLT, and the greatest percent difference in impairment and decline between patients with mTBI and controls was also seen on the RAVLT (as well as on the TMT-A for impairment). This suggests that the domains of memory and processing speed are most affected at 1 year after mTBI. The literature on associations of TBI with poor performance in specific cognitive domains is heterogeneous, with studies reporting poor functioning in memory, attention, processing speed, and executive function, among others.47,48 It is possible that different tests may be more sensitive to cognitive performance after TBI or that different domains of cognition may be affected more by TBI at different points in the recovery process; future studies incorporating multidomain neuropsychological assessment and investigating trajectories of cognitive performance over time are needed.
Certain limitations should be taken into consideration in the interpretation of our results. First, although we hypothesize that this methodologic approach to define poor 1-year cognitive outcome can be applied to any mTBI population, our specific results may not generalize to other non–trauma center–based mTBI cohorts with less severe injuries. Second, although our definition of cognitive impairment was based on demographically adjusted normative data, the normative populations for each test were different, and while all norms adjusted for age, only the norms for the TMT test also were adjusted for other demographics. Furthermore, because patients with TBI were enrolled at the time of TBI, we do not have a measure of preinjury cognitive status. Third, as in many studies of TBI,30 our outcome data are limited by study attrition and resultant missing data. In studies of TBI, there are 2 main reasons for missing data, which would result in biasing the results in different directions: (1) low-functioning individuals may not be able to perform the assessment, and (2) high-functioning individuals may have little interest in returning for follow-up assessments.31 We used propensity weighting31,33 in our main analyses to account for the missing outcome data; this method upweights individuals who are similar to the participants with missing data, thereby accounting for multiple reasons for missing data. It is important to note that cognitive data were more likely to be missing than GOSE data, likely because cognitive tests were required to be obtained in person and could not be completed by an informant. It will be important for future studies to consider both the incorporation of telephone cognitive assessments to decrease missing data49 and the inclusion of statistical methods to account for missing data, as we did here.31,33
Our study also has several strengths. First, the TRACK-TBI study is a large, multisite study with repeated measures of cognition over the first year after injury. Second, we created and established the concurrent validity of a patient-centered cognitive endpoint definition that incorporates clinically relevant cutoffs and avoids erroneously classifying declining patients as having a good cognitive outcome if they do not meet criteria for impairment. Our definition also avoids misclassifying chronically impaired patients as having a good cognitive outcome if they do not meet criteria for decline. We additionally looked at multiple definitions of poor 1-year cognitive outcome, which can be used to inform avenues of future research designed to unravel potentially modifiable predictors and biological mechanisms of poor short-term cognitive outcome after mTBI.
We found that poor 1-year cognitive outcome is common and is seen in 13.5% of patients with mTBI presenting to trauma centers in the United States compared with 4.5% of controls. Our study highlights the importance of the use of a more nuanced definition of poor 1-year cognitive outcome, incorporating longitudinal cognitive assessment so that both cognitive decline and cognitive impairment can be used to classify cognitive outcomes more accurately after mTBI and to better understand distinct subsets of post-mTBI cognitive outcomes. Further work is needed to continue to identify biopsychosocial mechanisms of post-mTBI cognitive recovery, to better identify persons at highest risk for poor 1-year cognitive outcomes, and to develop multidomain interventions to optimize recovery and prevent future decline and dementia in mTBI populations.
Glossary
- BSI-18-GSI
18-item Brief Symptom Inventory Global Severity Index
- CDE
Common Data Element
- ED
emergency department
- GCS
Glasgow Coma Scale
- GOAT
Galveston Orientation and Amnesia Test
- GOSE-TBI
Glasgow Outcome Scale Extended TBI Version
- mTBI
mild TBI
- PSI
Processing Speed Index
- RAVLT
Rey Auditory Verbal Learning Test
- RCI
reliable change index
- TBI
traumatic brain injury
- TMT
Trail Making Test
- TRACK-TBI
Transforming Research and Clinical Knowledge in TBI
- WAIS-IV
Wechsler Adult Intelligence Scale–4th Edition
Appendix 1. Authors
Appendix 2. Coinvestigators
Footnotes
Podcast: NPub.org/Podcast9812
Study Funding
The TRACK-TBI study was funded by NIH/National Institute of Neurological Disorders and Stroke grants RC2NS069409 and U01NS086090 and by the Department of Defense grant W81XWH-14-0176. This work was supported by Department of Defense grant W81XWH-18-1-0514 to Dr. Raquel Gardner. Drs. Schneider, Jain, and Gardner were supported by Department of Defense grant W81XWH-21-1-0590.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.James SL, Theadom A, Ellenbogen RG, Bannick MS; GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66(pt B):75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One. 2013;8(5):e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordström P, Michaëlsson K, Gustafson Y, Nordström A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol. 2014;75(3):374-381. [DOI] [PubMed] [Google Scholar]
- 5.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71(12):1490-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, Yaffe K. Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol. 2018;75(9):1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG. Mild traumatic brain injury (mTBI) and chronic cognitive impairment: a scoping review. PLoS One. 2017;12(4):e0174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll LJ, Cassidy JD, Holm L, Kraus J, Coronado VG; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury.Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004(43 suppl):113-125. [DOI] [PubMed] [Google Scholar]
- 9.Ruff M, Young D, Guatille T, Marshall LF. Verbal learning deficits following severe head injury: heterogeneity in recovery over 1 year. J Neurosurg. 1991;75:50-58. [Google Scholar]
- 10.Till C, Colella B, Verwegen J, Green RE. Postrecovery cognitive decline in adults with traumatic brain injury. Arch Phys Med Rehabil. 2008;89(12 suppl):S25-S34. [DOI] [PubMed] [Google Scholar]
- 11.Millis SR, Rosenthal M, Novack TA, et al. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001;16(4):343-355. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg ND, Crane PK, Dams-O'Connor K, et al. Developing a cognition endpoint for traumatic brain injury clinical trials. J Neurotrauma. 2017;34(2):363-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser EE, Downing MG, Biernacki K, McKenzie DP, Ponsford JL. Cognitive reserve and age predict cognitive recovery after mild to severe traumatic brain injury. J Neurotrauma. 2019;36(19):2753-2761. [DOI] [PubMed] [Google Scholar]
- 14.Nelson LD, Temkin NR, Dikmen S, et al. Recovery after mild traumatic brain injury in patients presenting to US level I trauma centers: a Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study. JAMA Neurol. 2019;76(9):1049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rey A. L’examen clinique en psychologie [the Clinical Psychological Examination]. Presses Universitaires de France; 1964. [Google Scholar]
- 16.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271-276. [Google Scholar]
- 17.Wechsler D. Wechsler Adult Intelligence Scale (WAIS) Manual. 4th ed. Psychological Corp; 2008. [Google Scholar]
- 18.Nelson LD, Ranson J, Ferguson AR, et al. Validating multidimensional outcome assessment using the TBI common data elements: an analysis of the TRACK-TBI pilot sample. J Neurotrauma 2017;34(22):3158-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider AL, Sharrett AR, Gottesman RF, et al. Normative data for 8 neuropsychological tests in older blacks and whites from the Atherosclerosis Risk in Communities (ARIC) study. Alzheimer Dis Assoc Disord. 2015;29:32-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin HS, O'Donnell VM, Grossman RG. The Galveston Orientation and Amnesia Test: a practical scale to assess cognition after head injury. J Nerv Ment Dis. 1979;167(11):675-684. [DOI] [PubMed] [Google Scholar]
- 21.Gardner RC, Cheng J, Ferguson AR, et al. Divergent six month functional recovery trajectories and predictors after traumatic brain injury: novel insights from the Citicoline Brain Injury Treatment Trial study. J Neurotrauma. 2019;36(17):2521-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson LD. False-positive rates of reliable change indices for concussion test batteries: a Monte Carlo simulation. J Athl Train. 2015;50(12):1319-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44(4):285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587-592. [DOI] [PubMed] [Google Scholar]
- 25.Derogatis L. Brief Symptom Inventory 18 (BSI-18): Administration, Scoring, and Procedures Manual. Pearson; 2001. [Google Scholar]
- 26.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71-75. [DOI] [PubMed] [Google Scholar]
- 27.Duhaime AC, Gean AD, Haacke EM, et al. Common Data Elements in radiologic imaging of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1661-1666. [DOI] [PubMed] [Google Scholar]
- 28.Haacke EM, Duhaime AC, Gean AD, et al. Common Data Elements in radiologic imaging of traumatic brain injury. J Magn Reson Imaging. 2010;32(3):516-543. [DOI] [PubMed] [Google Scholar]
- 29.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173-1182. [DOI] [PubMed] [Google Scholar]
- 30.Richter S, Stevenson S, Newman T, et al. Handling of missing outcome data in traumatic brain injury research: a systematic review. J Neurotrauma. 2019;36(19):2743-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielson JL, Cooper SR, Seabury SA, et al. Statistical guidelines for handling missing data in traumatic brain injury clinical research. J Neurotrauma. 2021;38(18):2530-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403-425. [DOI] [PubMed] [Google Scholar]
- 33.Gottesman RF, Rawlings AM, Sharrett AR, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC Neurocognitive Study. Am J Epidemiol. 2014;179(8):956-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.University of California, San Francisco. TRACK-TBI. Accessed January 25, 2022. tracktbi.ucsf.edu/collaboration-opportunities
- 35.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72-83. [DOI] [PubMed] [Google Scholar]
- 36.Kivimäki M, Batty GD, Pentti J, et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020;5(3):e140-e149. [DOI] [PubMed] [Google Scholar]
- 37.Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arvanitakis Z, Bennett DA, Wilson RS, Barnes LL. Diabetes and cognitive systems in older black and white persons. Alzheimer Dis Assoc Disord. 2010;24:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattarai JJ, Oehlert ME, Multon KD, Sumerall SW. Dementia and cognitive impairment among U.S. Veterans with a history of MDD or PTSD: a retrospective cohort study based on sex and race. J Aging Health. 2019;31(8):1398-1422. [DOI] [PubMed] [Google Scholar]
- 40.Hooker K, Phibbs S, Irvin VL, et al. Depression among older adults in the United States by disaggregated race and ethnicity. Gerontologist. 2019;59(5):886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shafi S, Marquez de la Plata C, Diaz-Arrastia R, et al. Racial disparities in long-term functional outcome after traumatic brain injury. J Trauma. 2007;63(6):1263-1270. [DOI] [PubMed] [Google Scholar]
- 42.Gary KW, Arango-Lasprilla JC, Stevens LF. Do racial/ethnic differences exist in post-injury outcomes after TBI? A comprehensive review of the literature. Brain Inj. 2009;23:775-789. [DOI] [PubMed] [Google Scholar]
- 43.Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. 2000;21(4):75-90. [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison-Felix C, Kolakowsky-Hayner SA, Hammond FM, et al. Mortality after surviving traumatic brain injury: risks based on age groups. J Head Trauma Rehabil. 2012;27:E45-E56. [DOI] [PubMed] [Google Scholar]
- 45.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christensen BK, Colella B, Inness E, et al. Recovery of cognitive function after traumatic brain injury: a multilevel modeling analysis of Canadian outcomes. Arch Phys Med Rehabil. 2008;89(12 suppl):S3-S15. [DOI] [PubMed] [Google Scholar]
- 47.Wood RL. Accelerated cognitive aging following severe traumatic brain injury: a review. Brain Inj. 2017;31(10):1270-1278. [DOI] [PubMed] [Google Scholar]
- 48.Kaup AR, Peltz C, Kenney K, Kramer JH, Diaz-Arrastia R, Yaffe K. Neuropsychological profile of lifetime traumatic brain injury in older veterans. J Int Neuropsychol Soc. 2017;23(1):56-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson LD, Barber JK, Temkin NR, et al. Validity of the Brief test of adult cognition by telephone in level 1 trauma center patients six months post-traumatic brain injury: a TRACK-TBI study. J Neurotrauma. 2020;38(8):1048-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data requests can be made to the TRACK-TBI Study Executive Committee.34