Summary
Mitochondrial retrograde signaling is an important component of intracellular stress signaling in eukaryotes. UNCOUPLING PROTEIN (UCP)1 is an abundant plant inner-mitochondrial membrane protein with multiple functions including uncoupled respiration and amino-acid transport1,2 that influences broad abiotic stress responses. Although the mechanism(s) through which this retrograde function acts is unknown, overexpression of UCP1 activates expression of hypoxia (low oxygen)-associated nuclear genes.3,4 Here we show in Arabidopsis thaliana that UCP1 influences nuclear gene expression and physiological response by inhibiting the cytoplasmic PLANT CYSTEINE OXIDASE (PCO) branch of the PROTEOLYSIS (PRT)6 N-degron pathway, a major mechanism of oxygen and nitric oxide (NO) sensing.5 Overexpression of UCP1 (UCP1ox) resulted in the stabilization of an artificial PCO N-degron pathway substrate, and stability of this reporter protein was influenced by pharmacological interventions that control UCP1 activity. Hypoxia and salt-tolerant phenotypes observed in UCP1ox lines resembled those observed for the PRT6 N-recognin E3 ligase mutant prt6-1. Genetic analysis showed that UCP1 regulation of hypoxia responses required the activity of PCO N-degron pathway ETHYLENE RESPONSE FACTOR (ERF)VII substrates. Transcript expression analysis indicated that UCP1 regulation of hypoxia-related gene expression is a normal component of seedling development. Our results show that mitochondrial retrograde signaling represses the PCO N-degron pathway, enhancing substrate function, thus facilitating downstream stress responses. This work reveals a novel mechanism through which mitochondrial retrograde signaling influences nuclear response to hypoxia by inhibition of an ancient cytoplasmic pathway of eukaryotic oxygen sensing.
Keywords: mitochondria, retrograde signaling, oxygen sensing, N-degron pathway, UCP1, ERFVII, abiotic stress
Highlights
-
•
UCP1 inhibits the PCO branch of the PRT6 N-degron pathway
-
•
Inhibition leads to substrate stabilization and altered gene expression
-
•
Inhibition transduces UCP1 function during development and in response to stress
Barreto et al. uncover a retrograde signaling mechanism that links inner mitochondrial membrane protein UCP1 to cytoplasmic oxygen sensing. UCP1 activity is transduced via inhibition of the PCO N-degron pathway to influence gene expression. This mechanism integrates mitochondrial and nuclear functions during development and in response to stress.
Results and discussion
The inner mitochondrial membrane protein UCP1 inhibits the PCO N-degron pathway
Mitochondrial genomic DNA codes for less than 1% of its ∼2,000 proteins; therefore, mitochondria rely on the nuclear genome to remotely regulate their function.6 This nuclear-mitochondrion interaction requires inter-compartment signaling, which can be retrograde (mitochondria to nucleus) or anterograde (nucleus to mitochondria), acting to adjust organelle function during development and in response to environmental stresses.7 Major advances have been made in both animals and plants in defining different molecular mechanisms regulating mitochondrial retrograde signaling.6 Several extra-mitochondrial factors have been identified that play roles in retrograde and anterograde signaling for regulating the plant-specific ALTERNATIVE OXIDASE (AOX) and other mitochondrial proteins encoded by the nucleus. For example, it was shown in Arabidopsis thaliana that the endoplasmic reticulum-localized transcription factor ANAC017 is required for AOX1a activation.8 Unknown signals (presumed to be reactive oxygen species, ROS8) from dysfunctional mitochondria lead to protease cleavage of ANAC017, releasing an amino-terminal (Nt−) fragment that relocates to the nucleus to activate expression of components of the mitochondrial dysfunction stimulon,9 and ANAC017 was recently shown to be activated by submergence-associated oxidative stress10 and involved in submergence tolerance.11 In diverse flowering plant taxa, UCP1 overexpression (35S:UCP1ox) decreases the release of ROS by mitochondria, protecting plants from multiple biotic and abiotic stresses,4,12, 13, 14 and results in a broad alteration of the nuclear-derived transcriptome that interestingly includes activation of hypoxia (low oxygen)-responsive transcripts in Nicotiana tabacum.3 Pharmacological induction of mitochondrial dysfunction was also shown to result in upregulation of hypoxia gene expression in several studies demonstrating similarities between inhibition of mitochondrial electron transport chain components and response to hypoxia.15, 16, 17 These different datasets show that mitochondrial retrograde signaling enhances hypoxia-responsive gene expression in plants, though no mechanism has been described to account for this. As the mitochondrion is the major site of oxygen consumption in the cell, it might be expected that a relationship exists with cytoplasmic oxygen sensing, and links have been observed between the animal hypoxia-inducible factor (HIF) oxygen-sensing system and mitochondrial function.18
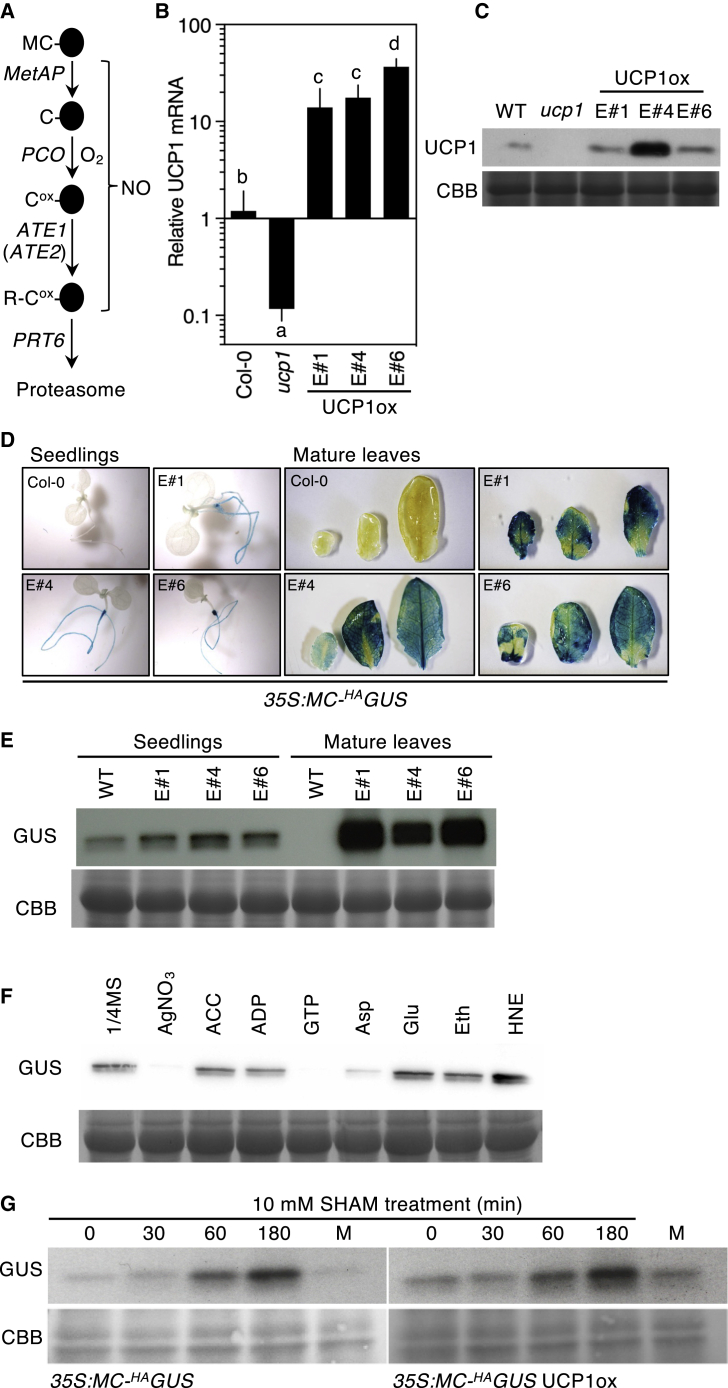
Plants have an oxygen-sensing mechanism that involves O2-dependent destruction of protein substrates with amino-terminal Cys− through the PLANT CYSTEINE OXIDASE (PCO) branch of the PRT6 N-degron pathway (hereafter referred to as the PCO N-degron pathway), and major substrates of the pathway are the group VII ETHYLENE RESPONSE FACTOR (ERFVII) transcription factors5 (Figure 1A). Under low-oxygen conditions, ERFVIIs are stabilized due to inhibition of PCO activity,19,20 resulting in induction of hypoxia-related gene expression. We investigated whether the previously observed constitutive induction of hypoxia gene expression by UCP1 overexpression3 was due to inhibition of PCO N-degron pathway activity in normoxia. An A. thaliana transgenic line, 35S:Met-Cys-HAGUS,21 constitutively expressing an artificial PCO N-degron pathway substrate (that following Met removal by MetAP [Figure 1A] becomes a substrate due to N-terminal Cys) was used to analyze the effect of UCP1 on PCO N-degron pathway activity. We transformed this line with a construct harboring UCP1 under the control of the 35S promoter (35S:UCP1, hereafter UCP1ox) (Figure S1). Out of 15 transgenic events, three that presented distinct enhanced levels of UCP1 RNA and protein (Figures 1B and 1C) were selected for further analysis. The accumulation of HAGUS was significantly increased in all UCP1ox lines, observed both as increased histochemical staining in mature leaves and increased protein abundance via western blotting in both seedlings and mature leaves (Figures 1D and 1E).
Figure 1.
Genetic alteration of mitochondrial UCP1 abundance influences the stability of artificial PCO N-degron pathway substrate Cys-HAGUS
(A) Schematic representation of the PLANT CYSTEINE OXIDASE (PCO) N-degron pathway. Black ovals indicate proteins, amino terminal (Nt) amino acids are single letter codes, and ox indicates oxidized cysteine. MetAP, METHIONINE AMINO-PEPTIDASE; ATE, ARGINYL TRANSFERASE; PRT6, PROTEOLYSIS6; O2, oxygen; NO, nitric oxide.
(B and C) UCP1 RNA (B) and protein expression (C) compared to Col-0 35S:Met-Cys-HAGUS (WT) in three independent transgenic lines containing 35S:UCP1 (E#1, #4, and #6) and the ucp1 mutant.
(D and E) Histochemical visualization of GUS activity (D) and western blot analysis (E) of HAGUS protein abundance in whole seedlings or mature leaves.
(F) Western blot of HAGUS in UCP1ox leaf discs incubated for 30 min in the dark in ½ MS medium supplemented or not with AgNO3 (100 μM), ACC (10 μM), ADP (100 μM), GTP (100 μM), aspartate (5 mM), glutamate (5 mM), ethanol (Eth) (0.1%), or HNE (in 0.1% ethanol) (30 μM).
(G) Western blot analysis of HAGUS abundance following treatment with SHAM (10 mM). M indicates mock samples sprayed with 2% ethanol only. CBB, Coomassie Brilliant Blue loading control.
Error bars indicate SD; letters one-way ANOVA. See also Figure S1.
We investigated if known inducers or inhibitors of UCP1 activity might affect the UCP1ox-induced stabilization of HAGUS using line E#4 (henceforth UCP1ox). A marked decrease in HAGUS protein was observed when leaf discs were treated with GTP (a classical UCP1 inhibitor22), while no influence was found for ADP treatment (a stimulator of coupled respiration not linked to UCP1 activity) (Figure 1F). UCP1 activator 4-hydroxynonenal (HNE)14 increased HAGUS accumulation slightly compared to the ethanol control. We also tested if Asp and Glu, two amino acids proposed to be transported by UCP1,2 influence PCO N-degron pathway activity and observed relatively less HAGUS protein in Asp-treated UCP1ox, compared to Glu treatment. It was previously shown that ethylene enhances PHYTOGLOBIN (PGB)1 expression, which in turn reduces NO levels and stabilizes ERFVIIs.23 Interestingly, treatment with AgNO3 (an inhibitor of ethylene perception) strongly reduced HAGUS accumulation, whereas ACC (an ethylene precursor and stimulator of ethylene synthesis) had no effect. To investigate whether inhibition of the PCO N-degron pathway may be a general action of mitochondrial retrograde signaling, we analyzed the influence of seedling treatment with salicylhydroxamic acid (SHAM) (a relatively selective in vitro competitive inhibitor of AOX). We observed increased stabilization of HAGUS in both wild type (WT) and UCP1ox, suggesting an additive effect on stabilization (Figure 1G). In addition, we observed increased accumulation of the ERFVII HRE23xHA (from the transgene 35S:HRE23xHA24) when seedlings were treated with either SHAM or antimycin A (an inhibitor of cytochrome c reductase) (Figure S1C). These data suggest that alterations of the activities of discrete mitochondrial functions affect the activity of the cytoplasmic PCO N-degron pathway.
Overexpression of UCP1 enhances abiotic stress tolerance and hypoxia-related gene expression through ERFVII transcription factors
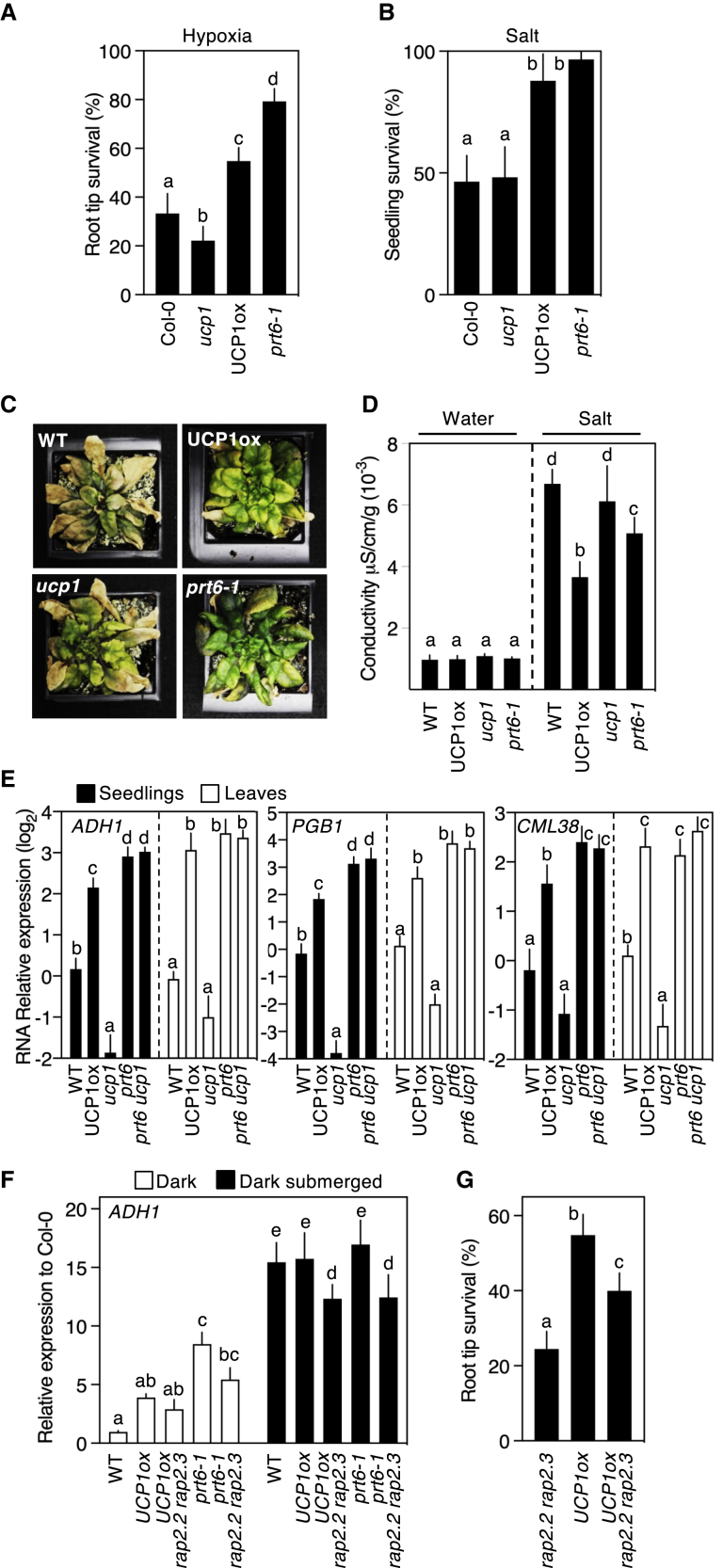
The PCO N-degron pathway has been shown to be a general sensor of plant stress.21,25,26 The prt6 mutant enhances tolerance to a number of abiotic and biotic stresses, whereas genetic removal of the ERFVII substrates of the PCO N-degron pathway increases susceptibility to those stresses.21,23,24,26, 27, 28, 29, 30, 31 We investigated whether genetic alteration of UCP1 activity (using UCP1ox and ucp1 mutant) would alter the response to abiotic stresses. Similar to prt6-1, UCP1ox seedlings showed increased tolerance to high salt, and seedling root meristems to hypoxia, compared to WT Col-0 accession, and root meristems of ucp1 were more sensitive to hypoxia stress (Figures 2A and 2B). At a later stage of development (starting at 21 days old), UCP1ox irrigated for 3 weeks with 150 mM NaCl exhibited enhanced salt tolerance compared to WT and ucp1. Conductivity, an indirect measurement of ion leakage, increased 7-fold in WT plants after 1 week irrigation with high salt compared to 4- and 5-fold increase in UCP1ox and prt6-1, respectively (Figures 2C and 2D). As hypoxia gene expression is increased in response to ERFVII stabilization in A. thaliana32,33 and UCP1 overexpression in N. tabacum,3 we analyzed, in A. thaliana, the influence of UCP1 on the expression of selected members of the “core 49” genes with conserved hypoxia induction:34 ALCOHOL DEHYDROGENASE (ADH)1; PGB1, recently shown to prime ethylene-mediated pre-adaptation to hypoxia;23 and CALMODULIN-LIKE (CML)38, a calcium ion sensor localizing to ribo-nucleoprotein complexes in hypoxia.35 In both seedlings and mature leaves, UCP1ox greatly increased transcript accumulation of the target genes, whereas this was strongly reduced in ucp1 in comparison to WT for all three genes (Figure 2E). As expected, expression was increased in prt6, at a similar level to prt6 ucp1, which indicates that PRT6 acts downstream of UCP1 in controlling hypoxia gene expression (because lack of PRT6 constitutively stabilizes ERFVIIs). Because UCP1 was previously shown to enhance AOX1 protein accumulation,12,36 we analyzed UCP1 influence on the expression of other mitochondrially located proteins encoded by nuclear genes. Interestingly, in both seedlings and mature leaves, expression of NAD(P)H DEHYDROGENASE B4 (NDB4) (previously shown to be a marker for mitochondrial retrograde signaling37) was induced in UCP1ox, prt6, and prt6 ucp1 but reduced in erfVII and ucp1 compared to Col-0 (Figure S2A). A similar expression profile was also observed for the nuclear-encoded mitochondrial inner membrane protein DICARBOXYLATE CARRIER (DIC)3 (important for the redox connection between mitochondria and cytoplasm38).
Figure 2.
Stress tolerance and gene expression are enhanced in UCP1ox plants through ERFVII substrates of the PCO N-degron pathway
(A) Root tip survival in response to hypoxia.
(B) Seedling survival following growth on media containing NaCl (125 mM).
(C) Images of plants irrigated with 150 mM NaCl for 3 weeks.
(D) Conductivity (electrolyte leakage) of mature leaves collected from plants watered with 150 mM NaCl or water for 1 week.
(E) Relative expression of transcripts for ADH1, PGB1, and CML38 in 7-day-old seedlings and mature leaves.
(F) Relative ADH1 transcript levels in control (1 h dark) and treated (1 h submerged+dark) seedlings for WT, mutants, and UCP1ox combinations.
(G) Root tip survival in response to hypoxia (Col-0 shown in A).
Error bars indicate SD; letters one-way ANOVA. See also Figure S2.
To demonstrate a direct link between UCP1 and potential downstream ERFVII activities, we analyzed gene expression and physiological tolerance to hypoxia in UCP1ox rap2.2 rap2.3, a combination that removes two of the three constitutively highly expressed (at the RNA level) ERFVIIs. After 1 h submergence in the dark (to simulate flooding stress), the expression of ADH1, PGB1, and CML38 was enhanced in all lines tested, but a significant reduced induction was observed in both UCP1ox rap2.2 rap2.3 versus UCP1ox and prt6-1 rap2.2 rap2.3 versus prt6-1 (Figures 2F and S2B). Hypoxia tolerance, measured as seedling root meristem survival,23 was also reduced in UCP1ox rap2.2 rap2.3 compared to UCP1ox, though was still greater than rap2.2 rap2.3 (Figure 2G). Taken together with the observation of the inhibition of the PCO N-degron pathway by UCP1 (Figure 1), these data indicate that UCP1 acts through ERFVIIs in regulating hypoxia gene expression and tolerance to hypoxia and salt stress.
UCP1 inhibition of the PCO/PRT6 N-degron pathway is a normal part of seedling development
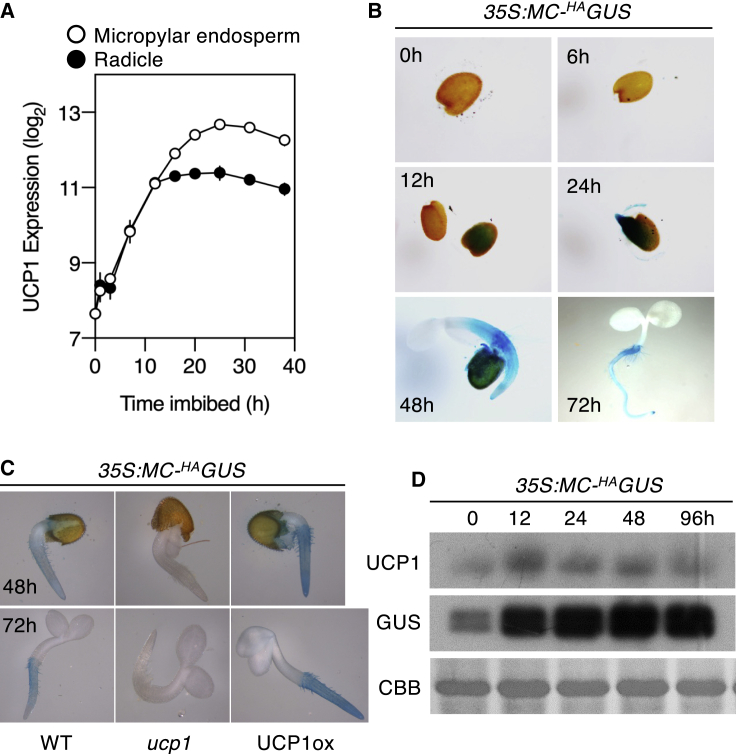
Transcripts of UCP1 are ubiquitously expressed across A. thaliana tissues but are upregulated under particular developmental conditions, particularly during germination (Figure S3A). We hypothesized that PCO N-degron pathway substrates may be stabilized under physiological and tissue/temporal conditions where UCP1 is relatively highly expressed. RNA expression of UCP1 increases strongly during seedling germination and establishment (Figure 3A; see imbibed seeds 24 h; Figure S3A), a feature that was strongly correlated with an increase in GUS activity in 35S:Met-Cys-HAGUS seedlings (Figure 3B). To determine if this pattern of HAGUS stabilization was a consequence of UCP1, we crossed 35S:Met-Cys-HAGUS to a ucp1 mutant with significantly reduced levels of UCP1 expression (Figure 1B). Remarkably, the ucp1 mutation abolished HAGUS accumulation in seedlings, despite equivalent levels of transgene expression (Figures 3C and S3B). Treatment with the proteasome inhibitor bortezomib increased HAGUS accumulation in the roots of ucp1, indicating that its absence in this background was due to elevated proteasomal degradation relative to WT. (Figure S3B). In addition, a correlation between increased UCP1 RNA and protein (as reported previously in several species39) and HAGUS protein accumulation was observed when mature plants were subjected to cold stress (Figures 3D and S3C). Cold-induced UCP1 expression may represent a mechanism for enhancing PCO N-degron pathway substrate accumulation in response to low temperatures, which we previously reported for the ERFVIIs and VERNALIZATION2.25 We also investigated whether UCP1 expression is altered during submergence, a condition that stabilizes the substrates of the PCO N-degron pathway due to reduced oxygen,24,40 and found that UCP1 protein accumulated during submergence in the dark whereas it decreased when plants were maintained only in the dark (Figure S3D). HAGUS was strongly stabilized under submergence in the dark after only 10 min.
Figure 3.
Expression of UCP1 during germination influences GUS accumulation
(A) Expression of UCP1 during germination and seedling establishment.41
(B) Histochemical staining of 35S:MC-HAGUS (hours imbibition in the light).
(C) Comparison of histochemical staining for 35S:MC-HAGUS in WT, ucp1, and UCP1ox backgrounds (hours imbibition in the light).
(D) Western blot analysis of HAGUS and UCP1 in mature leaves following transfer to 4°C in the light (h).
See also Figure S3.
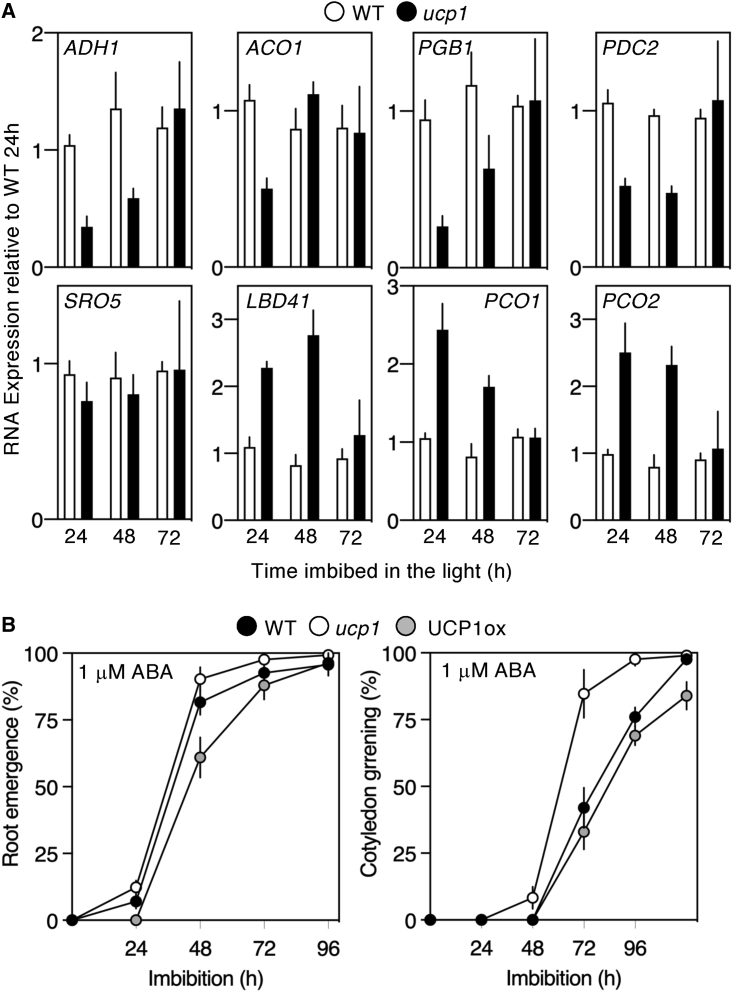
To analyze the role of UCP1 in influencing gene expression during germination, we examined differential RNA expression between WT (Col-0) and ucp1 for known hypoxia-related genes comparing pre-chilled seeds following transfer to the light for 24, 48, and 72 h (Figure 4A). Interestingly, the transcripts for several genes, including ADH1, PGB1, ACO1, and PDC2, showed a similar lower expression pattern in ucp1 compared to WT at the beginning of the time course, increasing toward the end. Other hypoxia-related transcripts34 either showed no difference in expression or increased expression (e.g., PCO1 and 2). These data indicate that UCP1 is required for expression of some, but not all, hypoxia-related genes during early germination. The importance of fermentative metabolism, in particular of ADH1, was recently demonstrated for plants growing under normal non-hypoxic conditions,42 and increased levels of PGB1 were shown to reduce NO levels and enhance stabilization of Cys-2 N-degron substrates.23 Increased hypoxia-related gene expression in seedlings of Col-0 and ucp1 following submergence was similar, indicating that inhibition of the PCO N-degron pathway by hypoxia is downstream of UCP1 function (Figure S4A) because reduced environmental oxygen levels stabilize pathway substrates due to inhibition of PCO activity,19,20 regardless of upstream regulation. This requirement may contribute to ecologically relevant abiotic stress tolerance during this early developmental period.43 In support of this hypothesis, the ucp1 mutant exhibited more rapid establishment than WT on media plates containing abscisic acid (1 μM ABA), resembling responses observed in ERFVII mutants,21,28 while in the UCP1ox line establishment was delayed, and no differences in germination were observed on media without ABA (Figures 4B and S4B).
Figure 4.
Influence of UCP1 on gene expression and physiology of germination
(A) Relative expression of hypoxia-related transcripts in WT and ucp1 during germination.
(B) Germination (root emergence) and establishment (cotyledon greening) of WT, ucp1, and UCP1ox on media containing 1 μM ABA.
Error bars indicate SD. See also Figure S4.
It has previously been shown that chemically or genetically induced mitochondrial dysfunction increased hypoxia-associated gene expression, suggesting the existence of a novel pathway of plant mitochondrial retrograde signaling.17 In the present work we describe a mechanism linking the activity of mitochondrial inner-membrane protein UCP1 to inhibition of the PCO N-degron pathway, which controls gene expression under hypoxia and abiotic stress tolerance, and show that this is a normal feature of plant development. Our observations that chemical treatment with SHAM and antimycin A also inhibit the PCO N-degron pathway are indicative of a more general mechanism of mitochondrial retrograde signaling. It was previously shown that AOX protein is reduced in the ucp1 mutant36 and increased in UCP1ox tobacco leaves,3 highlighting possible interactions between different pathways. In addition to this, we observed a similar UCP1-regulated expression of transcripts for nuclear-encoded mitochondrial marker proteins that was regulated through PRT6. The PRT6/UBR N-degron pathway evolved early in eukaryotes,44 and the fact that mammals possess a PCO counterpart, ADO,45 raises the possibility that modulation of the animal ADO N-degron pathway could also act as part of a mechanism of mitochondrial retrograde signaling. The regulation of hypoxia responses by UCPs is not restricted to plants; for example, the lack of UCP1 in mouse brown adipose tissue is sufficient to decrease cold-inducible hypoxia through downregulation of hypoxia-inducible factor 1A (HIF-1A).46,47 Likewise, the activity of human UCP2 is strongly associated with HIF-1A stabilization in distinct cancer cells.48 It has been described recently that during germination, seed response to mitochondrial ROS production and, subsequently, ethylene induces a mitochondrial retrograde response.49 Our results build on that report as we show that during germination UCP1 regulates PCO N-degron pathway function.
It remains unknown how UCP1 influences mitochondrial signaling, especially because of the multiple functions that have been assigned to the protein.1,2 It is possible that it can work as an uncoupler that limits ROS production by preventing excessive reduction levels of electron transport components, in addition to a recently identified function as a transporter. How the mitochondrial activity of UCP1 leads to cytoplasmic stabilization of Cys-2 N-degron substrates is also unclear. Although signals for chloroplast retrograde signaling are known,6 none so far have been identified for plant mitochondrial retrograde signaling. Of the known requirements for activity of the PCO N-degron pathway (Figure 1A),5 it is unlikely that UCP1 activity influences cellular oxygen levels. Moreover, NO levels increase in UCP1ox lines, rather than decrease, which would be required to inhibit the pathway21,28 (Figure S4C). Comparison of mitochondrially induced cytoplasmic changes with factors influencing the kinetic properties of enzymes in the PCO N-degron pathway may help to focus identification of important features.17,19 Interestingly, PCO1 and PCO2 transcripts were upregulated in the ucp1 mutant and downregulated in UCP1ox (Figure S4D), suggesting that UCP1-mediated changed PCO activity may result in altered pathway activity, resulting in the observed changed stability of HAGUS.
A mechanism of retrograde signaling linking ANAC017 with submergence has been described,10,11 and although different from the mechanism described here, it will be important to understand its possible crosstalk with UCP1-mediated signaling that functions during normoxia. Together with the already known roles of the PCO N-degron pathway in oxygen and NO sensing, this work shows that the pathway forms part of a mitochondrial retrograde signaling mechanism linking oxygen consumption (in the mitochondrion) with oxygen sensing (in the cytoplasm) with importance for both normal development and responses to environmental stress.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Monoclonal Anti-HA antibody produced in mouse | Sigma-Aldrich | H3663; RRID:AB_262051 |
| Goat anti-Mouse IgG1 Secondary Antibody | ThermoFisher | PA1-74421; RRID:AB_10988195 |
| Anti-UCP - uncoupling protein Antibody | Agrisera | AS12 1850; RRID:AB_2904553 |
| Secondary Antibody: (goat) anti-rabbit IgG HRP conjugate | Invitrogen | G21234; RRID:AB_2536530 |
| Antibody detection kit: Pierce ECL Western Blotting Substrate | ThermoFisher | 32106 |
| Anti-β-Glucuronidase Antibody | Sigma-Aldrich | G5420; RRID:AB_477020 |
| Anti-Rabbit-HRP conjugate | Sigma-Aldrich | A0545; RRID:AB_257896 |
| Chemicals, peptides, and recombinant proteins | ||
| Murashige and Skoog (MS) medium | Sigma-Aldrich | M5524 |
| (±)-Abscisic acid | Sigma-Aldrich | A1049 |
| DAF-2 DA (4,5-diaminofluorescein diacetate) | Sigma-Aldrich | D2813-1MG |
| X-Gluc solution (5-bromo-4-chloro-3-indolyl-beta-Dglucuronic acid, cyclohexylammonium salt) | X-GLUC Direct | X-Gluc |
| 4-hydroxy Nonenal (HNE) | Cayman | 32100 |
| L-Aspartic acid | Sigma-Aldrich | A9256 |
| L-Glutamic acid | Sigma-Aldrich | G1251 |
| Salicylhydroxamic acid (SHAM) | Sigma-Aldrich | S607 |
| DAF-2 DA (4,5-diaminofluorescein diacetate) | Sigma-Aldrich | D2813-1MG |
| Experimental models: Organisms/strains | ||
| Arabidopsis thaliana Col-0 | NASC | N1092 |
| Arabidopsis thaliana ucp1 | 36 | NASC: N811222, SAIL242_A09C |
| Arabidopsis thaliana 35S:UCP1 in 35S:Met-Cys-HAGUS | This study | N/A |
| Arabidopsis thaliana ucp1 in 35S:Met-Cys-HAGUS | This study | N/A |
| Arabidopsis thaliana rap2.2 rap2.3, prt6 rap2.2 rap2.3 | 28 | rap2.2 rap2.3, prt6 rap2.2 rap2.3 |
| Arabidopsis thaliana 35S:UCP1 in rap2.2 rap2.3 | This study | 35S:UCP1 in rap2.2 rap2.3 |
| Arabidopsis thaliana prt6-1 | 30 | NASC: N9873, SAIL 1278_H11 |
| Arabidopsis thaliana ucp1 prt6-1 | This study | ucp1 prt6 |
| Arabidopsis thaliana 35S:HRE23xHA | 24 | 35S:HRE23xHA |
| Oligonucleotides | ||
| qPCR_UCP1_F: ATTCTGGAGCGCTGAATGCT | N/A | N/A |
| qPCR_UCP1_R: ATCCTGCTCCCAGTCCAGAT | N/A | N/A |
| qPCR_ADH1_F: CCCGGGGTTGTGGAAAAGTA | N/A | N/A |
| qPCR_ADH1_R: CCCATGGTGATGATGCAACG | N/A | N/A |
| qPCR_PGB1_F: AGGGAAAGTTACGGTGAGGG | N/A | N/A |
| qPCR_PGB1_R: TGCATACTTGGCCACCTCAA | N/A | N/A |
| qPCR_CML38_F: AAGCCCTTTCCCCTATTTCTCA | N/A | N/A |
| qPCR_CML38_R: CTCCGGCTGAATCTTCCCTC | N/A | N/A |
| qPCR_ACO1_F: ACCTCAGATGCAGATTGGGAAAGC | N/A | N/A |
| qPCR_ACO1_R: CCATCGTCTTGCTGAGTTCCTCTG | N/A | N/A |
| qPCR_PDC2_F: CCCCAAATCCGCAGTAGAGT | N/A | N/A |
| qPCR_PDC2_R: CCTCAAGGGGACACACATTT | N/A | N/A |
| qPCR_SRO5_F: CTTGGACCTCAAGTTCTTTTC | N/A | N/A |
| qPCR_SRO5_R: CGCAGCTTCCAGATTCAGAG | N/A | N/A |
| qPCR_LBD41_F: TGAAGCGCAAGCTAACGCA | N/A | N/A |
| qPCR_LBD41_R: ATCCCAGGACGAAGGTGATTG | N/A | N/A |
| qPCR_PCO1_F: ATTGGGTGGTTGATGCTCCAATG | N/A | N/A |
| qPCR_PCO1_R: ATGCATGTTCCCGCCATCTTC | N/A | N/A |
| qPCR_PCO2_F: CTTCGAGCCGTTTTGGATGA | N/A | N/A |
| qPCR_PCO2_R: ACGTCACTAACGGAGATCGTCC | N/A | N/A |
| qPCR_DIC3_F: AATCTTCCCGTGAAACCTTAC | N/A | N/A |
| qPCR_DIC3_R: AAGGAAATGCTGCCGATGAG | N/A | N/A |
| qPCR_NDB4_F: GCCAGTGGCTTTGGTACTC | N/A | N/A |
| qPCR_NDB4_R: CTGCTAGAGTCACGGCCAA | N/A | N/A |
| Genotyping_UCP1_F: GACGAAGATGTGAAGTAGACC | N/A | N/A |
| Genotyping_UCP1_R: CAAAGAGAAGATACATGTTG | N/A | N/A |
| Genotyping_RAP2.2_F: ATGACAACATTGGGATGCAAC | N/A | N/A |
| Genotyping_RAP2.2_R: TTTCTTGGCATATGCTGAACC | N/A | N/A |
| Genotyping_RAP2.3_F: ATGTGTGGCGGTGCTATTATT | N/A | N/A |
| Genotyping_RAP2.3_R: TTACTCATACGACGCAATGAC | N/A | N/A |
| Genotyping_RAP2.12_F: CTCAGCTGTCTTGAACGTTCC | N/A | N/A |
| Genotyping_RAP2.12_R: TGGCTACTCCTGAATGCAAAC | N/A | N/A |
| Genotyping_HRE1_F: ACCGCGGGTTAAAATCTAGTG | N/A | N/A |
| Genotyping_HRE1_R: TTCAGCTGTGTTGAAAGTCCC | N/A | N/A |
| Genotyping_HRE2_F: TTGCAAAAGGTTATAGAGCACAC | N/A | N/A |
| Genotyping_HRE2_R: CGACGGTGTTTAGTGTGTTTG | N/A | N/A |
| Genotyping_PRT6_F: GGAGTTTTCTATGTCCAGTGAGAGTTT | N/A | N/A |
| Genotyping_PRT6_R: GTCTCCAATGACACGTTCACTTGTCT | N/A | N/A |
| Recombinant DNA | ||
| PGWB14:UCP1 | This study | 35S:UCP1 |
| Software and algorithms | ||
| ImageJ | NIH – public domain | https://imagej.nih.gov/ij/download.html |
| Prism Graphpad v.8 | Prism | Graphpad |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Michael J. Holdsworth (michael.holdsworth@nottingham.ac.uk).
Materials availability
Materials generated in this study are available from the Lead Contact upon request. Distribution of transgenic lines is governed by the appropriate material transfer agreements (MTAs) and availability of seed material is dependent on provision of appropriate import permits acquired by the receiver.
Experimental model and subject details
A. thaliana genetic materials including ucp1, rap2.2 rap2.3, 35S:MC-HAGUS erfVII, and prt6-1 were described before,27,28,30,36 except for UCP1ox lines (35S:UCP1) that were generated in this study. All mutants and transgenics are in the Col-0 accession (Wild Type, WT). For general growth plants were incubated in growth rooms under 120 μmol m-2 S-1 12 h light at 21°C 12 h dark at 15°C in a mixture of Levington M3 compost (57%), Medium grade 2-5 mm vermiculite (29%) and Medium grade 2-5 mm perlite (14%). Growth conditions for specific experiments are given below in the Analysis of plant growth and response to stresses section.
Method details
Plant transformation
The A. thaliana UCP1 coding sequence was amplified from Col-0 cDNA, recombined into pCR8GW (Invitrogen, Carlsbad, CA, USA), and then into pGWB14.50 The construct was introduced into Agrobacterium tumefaciens (strain GV3101) that then used to transform A. thaliana 35S:Met-Cys-HAGUS21 using standard protocols.51 Three transgenic events overexpressing UCP1 in the 35S:Met-Cys-HAGUS background, hereafter named as UCP1ox E#1, E#4 and E#6, were selected based on the UCP1 expression level for further analysis. Oligonucleotide primers used for genotyping are shown in the key resources table.
Analysis of plant growth and response to stresses
Unless indicated, Petri plates containing half-strength Murashige and Skoog (MS) medium (pH 5.7) supplemented with 1% (w/v) agar were used in all assays.52
ABA
Seeds were sown on Petri plates supplemented with abscisic acid (1 μM ABA), and incubated in the dark for 96 h at 4°C. The plates were transferred to a growth chamber under a long-day photoperiod (16 h L, 8 h D).52 Radicle emergence and greening of the cotyledons were scored.
Salt
Seeds were sown on Petri plates on half-strength MS medium pH 5.7 containing 1% (w/v) agar, stratified for 96 h at 4°C and grown in vertical position in constant light conditions for 3 days, when seedlings were transferred to plates containing half-strength MS supplemented with 150 mM NaCl for 7 days. Seedlings were then transferred back to fresh half-strength MS medium plates and after 5 days seedling survival was scored. For experiments performed at adult stage, plants were grown under neutral-day photoperiod (12 h L: 12 h D) and treatment started 3 weeks after germination of seeds sowed directly on soil.21 Plants were watered with NaCl solution (150 mM) or water twice over 10 days in the case of plants used for cell damage assay. Irrigation with NaCl solution continued for 30 days in total before pictures were taken. Salt accumulation in the soil was avoided by allowing excess irrigation water to drain out of the pots.
Cold
Seeds were sown on half-strength MS medium, pH 5.7 containing 1% (w/v) agar, cold-treated for 96 h at 4°C, grown in vertical plates in constant light conditions for 12 days. The seedlings were transferred to growth-rooms in a long-day photoperiod (16 hL, 8 hD). Cold treatment was carried out using plants grown under long-day photoperiod for 21 days, after which they were transferred to a growth room with the same light intensity, but at 4°C. Plants were kept for 0, 12, 24, 48 and 96 h at 4°C, and the leaves were collected and frozen in liquid nitrogen.
Submergence
Seeds were sown on half-strength MS medium, pH 5.7 containing 1% (w/v) agar, stratified for 96 h at 4°C and grown in vertical plates at constant light conditions for 7 days. Approximately 50 seedlings were submerged in H2O in an Eppendorf tube for 0, 10, 30, 60 and 120 min in the dark. Seedlings were removed from the tube and snap frozen in liquid nitrogen. The same number of seedlings were maintained in a humidity chamber for 0, 10, 30, 60 and 120 min in the dark as controls.
Hypoxia
Seeds were sown on quarter-strength MS medium, pH 5.7 containing 1% (w/v) agar, stratified for 96 h at 4°C and grown in vertical plates at short day conditions for 4 days. The opened plates were placed in a dark sealed chamber with constant flow of N2 gas (4L/min) for 4 h, then closed and returned to the growth chamber. After 4 days the primary root tips were scored based on continued growth (survived).23 Control untreated plates were kept in an opened dark chamber at normoxia for the same amount of time as treated plants.
Chemical treatment of leaf discs
Seeds were sown on half-strength MS medium, pH 5.7 containing 1% (w/v) agar, stratified for 96 h at 4°C and grown in vertical plates at neutral day conditions for 7 days. Then the seedlings were transferred to a soil and grown under neutral day conditions for an additional 3 weeks. Leaf discs were excised using a hole punch and incubated in quarter-strength MS medium, pH 5.7 separately supplemented or not with ADP (100 μM), GTP (100 μM), aspartate (5 mM), glutamate (5 mM), ethanol (0.1% v/v), HNE (in 0.1% ethanol v/v) (30 μM). Leaf discs were vacuum infiltrated for 5 min and incubated in the dark for 30 min. Then leaf discs were removed from the medium, washed three times in 100 mM sodium phosphate buffer (pH 7.0), the excess water was quickly removed in a paper towel and the discs frozen in liquid nitrogen.
SHAM and Antimycin A treatments
Transgenic 35S:MC-HAGUS or 35S:HRE23xHA seedlings were grown in a Weiss Technik fitotron SGC 120 biological chamber under long day conditions (16 h light/ 8 h dark). For SHAM treatment, seedlings were grown in Levington F2 compost, vermiculite, and perlite mix (4:2:1 v/v) After 4 weeks, rosettes were randomized in trays and sprayed with solutions containing either 2% (v/v) ethanol, or 2% ethanol supplemented with 10 mM salicylhydroxamic acid (Sigma; S607). Leaves were then harvested and flash frozen in liquid nitrogen at time points indicated. For antimycin A treatment, seed were surface sterilized using 10% bleach and sown on square plates containing ½ MS medium pH 5.7 with 1% (w:v) agar. Ten d after germination, plates were then sprayed with solutions containing either 2% ethanol or 2% ethanol supplemented with 50 μM Antimycin A (Sigma; A8674). Whole seedlings were then harvested and flash frozen at the time points indicated.
Gene expression analyses
qRT-PCR
Total RNA was isolated from mature leaves (from 28 d-old plants grown in soil) or seedlings (age indicated for each experiment) using a RNAeasy Plant Mini Kit (QIAGEN) or a Nucleospin TriPrep (Macherey-Nagel) depending on the subsequent application. The RNA was used for first-strand cDNA synthesis using a Revertaid First Strand cDNA Synthesis kit (Fermentas, Waltham, MA, USA) according to the manufacturer’s protocol. Real-time PCR was performed using the ABI PRISM 7500 system (Applied Biosystems, Foster City, CA, USA) with SYBR Green dye (Applied Biosystems). The reactions were performed at least in technical triplicate with three biological replicates and the results were expressed relative to the expression levels of a housekeeping gene in each sample using the 2–ΔΔC T method. Oligonucleotide primers are shown in the key resources table.
Measurement of ion leakage
Cell damage was determined by measuring ion leakage as described.21 Leaf disks of 0.6 cm2 from 24 leaves were excised using a hole punch. Disks were rinsed briefly with water and floated on 5 mL of double distilled water for 6 h at room temperature. The conductivity of the water was measured using a conductivity meter (SevenGo, Mettler-Toledo, Columbus, OH, USA).
NO detection by fluorescence microscopy
Endogenous NO levels were measured as previously described53 by immersing 9-day-old seedlings in 10 mM Tris-HCl (pH 7.4) containing 10 mM DAF-2 DA (4,5-diaminofluorescein diacetate, Sigma-Aldrich). Seedlings were shaken gently for 15 min in the dark, and subsequently washed for 20 min in 10 mM Tris-HCl (pH 7.4). The seedlings were visualized using a Leica DM5000B fluorescence microscope with excitation at 488 nm and emission 520 nm. NO intensity was determined by selecting equal areas of the same root zone and analyzing with Fiji software.28 The tissue autofluorescence was subtracted from all of the measured samples.
Analysis of protein abundance by western blotting, and GUS enzyme activity
Protein extracts were prepared by grinding the tissue to a fine powder in liquid nitrogen and extracted using a buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 10% (v/v) glycerol, 0.05% IGEPAL and 1x complete protease inhibitor cocktail (Roche, Basel, CH).24 Total protein content in samples was quantified by Bradford protocol against a BSA standard curve. Total protein was separated by SDS–PAGE and transferred to nitrocellulose membrane (Immune-Blot PVDF, Biorad, Hercules, CA, USA) by electroblotting. Page Ruler Pre-Stained Protein Ladder (Invitrogen, Carlsbad, CA, USA) was loaded as a reference for protein size. Membranes were probed with anti-HA (Sigma-Aldrich, St. Louis, MO, USA), 1:2,500 (v/v) dilution; anti-actin (Agrisera, Vannas, SWE), 1:5,000; anti-UCP1 (Agrisera, Vannas, SWE), 1:2,000 and anti-ADH (Agrisera, Vannas, SWE), 1:2500 primary antibodies. The primary antibodies were detected using the following secondary antibodies: (goat) anti-rabbit IgG HRP conjugate (Invitrogen, Carlsbad, USA) at 1:10,000 dilution or (goat) anti-mouse IgG HRP conjugate (Invitrogen, Carlsbad, USA) at 1:10,000 dilution. Signal was detected using Pierce ECL Western Blotting Substrate (ThermoFisher, Waltham, MA, USA). GUS histochemical staining was carried out by incubating tissues in 100 mM phosphate buffer pH 7.0, containing potassium ferrycyanide (1 mM), potassium ferrocyanide (1 mM), Triton X-100 (0.1% v/v) and X-Gluc (1 mM). The samples were incubated at 37°C and photographed.
Western blot analysis of SHAM effects
Plant tissues were ground using a pestle and mortar in liquid N2 and proteins were extracted using 150 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 2 mM EGTA, 5% (v/v) glycerol, 10 mM ditriothreitol (DTT), 0.5% Triton X-100 and protease inhibitor cocktail (Sigma-Aldrich; S8830) at a ratio of 1:3 w:v. Samples were run on 10% v:v poly-acrylamide gels and transferred to PVDF membranes overnight at 4°C. Membranes were blocked using 5% w:v milk (Marvel) in TBST, and probed with anti-β-glucuronidase (1:2000 dilution) (Sigma; G5420) and anti-Rabbit-HRP (1:2000 dilution) (Sigma; A0545). Signal was then detected using PierceTM ECL Western Blotting Substrate (Thermo Scientific; 32106)
Quantification and statistical analysis
All experiments were performed at least three times. Vertical lines represent standard deviation in all graphs. Statistical comparisons were conducted with RStudio v4.0.3 software. All collected data was evaluated for normality by the Shapiro-Wilk test and for homogeneity of variances by Levene’s test. Data transformation was applied when necessary. For statistical comparisons, the one-or two-way Analysis of Variance (ANOVA) with Tukey’s multiple comparisons test was used, employing the following factors when applied: genotype, treatment or tissue and the interaction. Significant differences (alpha < 0.05) are denoted with different letters. A t test was applied for Figure S2A because a large variance in gene expression was observed due to the discrepant profile of genotypes, hampering genotype differentiation by multiple comparison tests.
Acknowledgments
P.B. was a FAPESP (2014/17634-5, 2015/24881-1, and 2017/22745-9) and CAPES (88887.572598/2020-00) postdoc research fellow. This work was supported by the Biotechnology and Biological Sciences Research Council (grant nos. BB/R002428/1 and BB/S005293/1) to M.J.H. P.A. was supported by the FAPESP grant 2016/23218-0 through the Genomics for Climate Change Research Center (GCCRC). I.G.M. is a CNPq productivity research fellow. D.J.G. and R.O. were supported by the European Research Council (ERC Starting Grant 715441-GasPlaNt).
Author contributions
P.B., M.J.H., and P.A. conceived the project and designed experiments. P.B., C.D., R.O., G.S., J.V., and M.J.H. performed the experiments. P.B., C.D., J.Y., D.J.G., I.G.M., M.J.H., and P.A. analyzed the data. M.J.H., P.B., and P.A. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: February 2, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2022.01.037.
Contributor Information
Michael J. Holdsworth, Email: michael.holdsworth@nottingham.ac.uk.
Paulo Arruda, Email: parruda@unicamp.br.
Supplemental information
Data and code availability
This study did not generate any unique datasets or code.
References
- 1.Ježek P., Holendová B., Garlid K.D., Jabůrek M. Mitochondrial uncoupling proteins: subtle regulators of cellular redox signaling. Antioxid. Redox Signal. 2018;29:667–714. doi: 10.1089/ars.2017.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monné M., Daddabbo L., Gagneul D., Obata T., Hielscher B., Palmieri L., Miniero D.V., Fernie A.R., Weber A.P.M., Palmieri F. Uncoupling proteins 1 and 2 (UCP1 and UCP2) from Arabidopsis thaliana are mitochondrial transporters of aspartate, glutamate, and dicarboxylates. J. Biol. Chem. 2018;293:4213–4227. doi: 10.1074/jbc.RA117.000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreto P., Okura V., Pena I.A., Maia R., Maia I.G., Arruda P. Overexpression of mitochondrial uncoupling protein 1 (UCP1) induces a hypoxic response in Nicotiana tabacum leaves. J. Exp. Bot. 2016;67:301–313. doi: 10.1093/jxb/erv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreto P., Yassitepe J.E.C.T., Wilson Z.A., Arruda P. Mitochondrial uncoupling protein 1 overexpression increases yield in Nicotiana tabacum under drought stress by improving source and sink metabolism. Front. Plant Sci. 2017;8:1836. doi: 10.3389/fpls.2017.01836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holdsworth M.J., Vicente J., Sharma G., Abbas M., Zubrycka A. The plant N-degron pathways of ubiquitin-mediated proteolysis. J. Integr. Plant Biol. 2020;62:70–89. doi: 10.1111/jipb.12882. [DOI] [PubMed] [Google Scholar]
- 6.Pfannschmidt T., Terry M.J., Van Aken O., Quiros P.M. Retrograde signals from endosymbiotic organelles: a common control principle in eukaryotic cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020;375:20190396. doi: 10.1098/rstb.2019.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford T., Lehotai N., Strand Å. The role of retrograde signals during plant stress responses. J. Exp. Bot. 2018;69:2783–2795. doi: 10.1093/jxb/erx481. [DOI] [PubMed] [Google Scholar]
- 8.Ng S., Ivanova A., Duncan O., Law S.R., Van Aken O., De Clercq I., Wang Y., Carrie C., Xu L., Kmiec B., et al. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell. 2013;25:3450–3471. doi: 10.1105/tpc.113.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng X., Li L., De Clercq I., Narsai R., Xu Y., Hartmann A., Claros D.L., Custovic E., Lewsey M.G., Whelan J., Berkowitz O. ANAC017 coordinates organellar functions and stress responses by reprogramming retrograde signaling. Plant Physiol. 2019;180:634–653. doi: 10.1104/pp.18.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bui L.T., Shukla V., Giorgi F.M., Trivellini A., Perata P., Licausi F., Giuntoli B. Differential submergence tolerance between juvenile and adult Arabidopsis plants involves the ANAC017 transcription factor. Plant J. 2020;104:979–994. doi: 10.1111/tpj.14975. [DOI] [PubMed] [Google Scholar]
- 11.Meng X., Li L., Narsai R., De Clercq I., Whelan J., Berkowitz O. Mitochondrial signalling is critical for acclimation and adaptation to flooding in Arabidopsis thaliana. Plant J. 2020;103:227–247. doi: 10.1111/tpj.14724. [DOI] [PubMed] [Google Scholar]
- 12.Barreto P., Okura V.K., Neshich I.A.P., Maia Ide.G., Arruda P. Overexpression of UCP1 in tobacco induces mitochondrial biogenesis and amplifies a broad stress response. BMC Plant Biol. 2014;14:144. doi: 10.1186/1471-2229-14-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S., Liu A., Zhang S., Li C., Chang R., Liu D., Ahammed G.J., Lin X. Overexpression of mitochondrial uncoupling protein conferred resistance to heat stress and Botrytis cinerea infection in tomato. Plant Physiol. Biochem. 2013;73:245–253. doi: 10.1016/j.plaphy.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Smith A.M.O., Ratcliffe R.G., Sweetlove L.J. Activation and function of mitochondrial uncoupling protein in plants. J. Biol. Chem. 2004;279:51944–51952. doi: 10.1074/jbc.M408920200. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt R.R., Fulda M., Paul M.V., Anders M., Plum F., Weits D.A., Kosmacz M., Larson T.R., Graham I.A., Beemster G.T.S., et al. Low-oxygen response is triggered by an ATP-dependent shift in oleoyl-CoA in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2018;115:E12101–E12110. doi: 10.1073/pnas.1809429115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner S., Steinbeck J., Fuchs P., Lichtenauer S., Elsässer M., Schippers J.H.M., Nietzel T., Ruberti C., Van Aken O., Meyer A.J., et al. Multiparametric real-time sensing of cytosolic physiology links hypoxia responses to mitochondrial electron transport. New Phytol. 2019;224:1668–1684. doi: 10.1111/nph.16093. [DOI] [PubMed] [Google Scholar]
- 17.Wagner S., Van Aken O., Elsässer M., Schwarzländer M. Mitochondrial energy signaling and its role in the low-oxygen stress response of plants. Plant Physiol. 2018;176:1156–1170. doi: 10.1104/pp.17.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas L.W., Ashcroft M. Exploring the molecular interface between hypoxia-inducible factor signalling and mitochondria. Cell. Mol. Life Sci. 2019;76:1759–1777. doi: 10.1007/s00018-019-03039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White M.D., Kamps J.J.A.G., East S., Taylor Kearney L.J., Flashman E. The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors. J. Biol. Chem. 2018;293:11786–11795. doi: 10.1074/jbc.RA118.003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White M.D., Klecker M., Hopkinson R.J., Weits D.A., Mueller C., Naumann C., O’Neill R., Wickens J., Yang J., Brooks-Bartlett J.C., et al. Plant cysteine oxidases are dioxygenases that directly enable arginyl transferase-catalysed arginylation of N-end rule targets. Nat. Commun. 2017;8:14690. doi: 10.1038/ncomms14690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vicente J., Mendiondo G.M., Movahedi M., Peirats-Llobet M., Juan Y.T., Shen Y.Y., Dambire C., Smart K., Rodriguez P.L., Charng Y.Y., et al. The Cys-Arg/N-end rule pathway is a general sensor of abiotic stress in flowering plants. Curr. Biol. 2017;27:3183–3190.e4. doi: 10.1016/j.cub.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vercesi A.E., Borecký J., Maia Ide.G., Arruda P., Cuccovia I.M., Chaimovich H. Plant uncoupling mitochondrial proteins. Annu. Rev. Plant Biol. 2006;57:383–404. doi: 10.1146/annurev.arplant.57.032905.105335. [DOI] [PubMed] [Google Scholar]
- 23.Hartman S., Liu Z., van Veen H., Vicente J., Reinen E., Martopawiro S., Zhang H., van Dongen N., Bosman F., Bassel G.W., et al. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019;10:4020. doi: 10.1038/s41467-019-12045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbs D.J., Lee S.C., Isa N.M., Gramuglia S., Fukao T., Bassel G.W., Correia C.S., Corbineau F., Theodoulou F.L., Bailey-Serres J., Holdsworth M.J. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature. 2011;479:415–418. doi: 10.1038/nature10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbs D.J., Tedds H.M., Labandera A.M., Bailey M., White M.D., Hartman S., Sprigg C., Mogg S.L., Osborne R., Dambire C., et al. Oxygen-dependent proteolysis regulates the stability of angiosperm polycomb repressive complex 2 subunit VERNALIZATION 2. Nat. Commun. 2018;9:5438. doi: 10.1038/s41467-018-07875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vicente J., Mendiondo G.M., Pauwels J., Pastor V., Izquierdo Y., Naumann C., Movahedi M., Rooney D., Gibbs D.J., Smart K., et al. Distinct branches of the N-end rule pathway modulate the plant immune response. New Phytol. 2019;221:988–1000. doi: 10.1111/nph.15387. [DOI] [PubMed] [Google Scholar]
- 27.Abbas M., Berckhan S., Rooney D.J., Gibbs D.J., Vicente Conde J., Sousa Correia C., Bassel G.W., Marín-de la Rosa N., León J., Alabadí D., et al. Oxygen sensing coordinates photomorphogenesis to facilitate seedling survival. Curr. Biol. 2015;25:1483–1488. doi: 10.1016/j.cub.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbs D.J., Md Isa N., Movahedi M., Lozano-Juste J., Mendiondo G.M., Berckhan S., Marín-de la Rosa N., Vicente Conde J., Sousa Correia C., Pearce S.P., et al. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol. Cell. 2014;53:369–379. doi: 10.1016/j.molcel.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gravot A., Richard G., Lime T., Lemarié S., Jubault M., Lariagon C., Lemoine J., Vicente J., Robert-Seilaniantz A., Holdsworth M.J., Manzanares-Dauleux M.J. Hypoxia response in Arabidopsis roots infected by Plasmodiophora brassicae supports the development of clubroot. BMC Plant Biol. 2016;16:251. doi: 10.1186/s12870-016-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holman T.J., Jones P.D., Russell L., Medhurst A., Ubeda Tomás S., Talloji P., Marquez J., Schmuths H., Tung S.A., Taylor I., et al. The N-end rule pathway promotes seed germination and establishment through removal of ABA sensitivity in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2009;106:4549–4554. doi: 10.1073/pnas.0810280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendiondo G.M., Gibbs D.J., Szurman-Zubrzycka M., Korn A., Marquez J., Szarejko I., Maluszynski M., King J., Axcell B., Smart K., et al. Enhanced waterlogging tolerance in barley by manipulation of expression of the N-end rule pathway E3 ligase PROTEOLYSIS6. Plant Biotechnol. J. 2016;14:40–50. doi: 10.1111/pbi.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey-Serres J., Fukao T., Gibbs D.J., Holdsworth M.J., Lee S.C., Licausi F., Perata P., Voesenek L.A., van Dongen J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012;17:129–138. doi: 10.1016/j.tplants.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 33.van Dongen J.T., Licausi F. Oxygen sensing and signaling. Annu. Rev. Plant Biol. 2015;66:345–367. doi: 10.1146/annurev-arplant-043014-114813. [DOI] [PubMed] [Google Scholar]
- 34.Mustroph A., Lee S.C., Oosumi T., Zanetti M.E., Yang H., Ma K., Yaghoubi-Masihi A., Fukao T., Bailey-Serres J. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010;152:1484–1500. doi: 10.1104/pp.109.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lokdarshi A., Conner W.C., McClintock C., Li T., Roberts D.M. Arabidopsis CML38, a calcium sensor that localizes to ribonucleoprotein complexes under hypoxia stress. Plant Physiol. 2016;170:1046–1059. doi: 10.1104/pp.15.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweetlove L.J., Lytovchenko A., Morgan M., Nunes-Nesi A., Taylor N.L., Baxter C.J., Eickmeier I., Fernie A.R. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc. Natl. Acad. Sci. USA. 2006;103:19587–19592. doi: 10.1073/pnas.0607751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Clercq I., Vermeirssen V., Van Aken O., Vandepoele K., Murcha M.W., Law S.R., Inzé A., Ng S., Ivanova A., Rombaut D., et al. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell. 2013;25:3472–3490. doi: 10.1105/tpc.113.117168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmieri L., Picault N., Arrigoni R., Besin E., Palmieri F., Hodges M. Molecular identification of three Arabidopsis thaliana mitochondrial dicarboxylate carrier isoforms: organ distribution, bacterial expression, reconstitution into liposomes and functional characterization. Biochem. J. 2008;410:621–629. doi: 10.1042/BJ20070867. [DOI] [PubMed] [Google Scholar]
- 39.Barreto P., Couñago R.M., Arruda P. Mitochondrial uncoupling protein-dependent signaling in plant bioenergetics and stress response. Mitochondrion. 2020;53:109–120. doi: 10.1016/j.mito.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Licausi F., Kosmacz M., Weits D.A., Giuntoli B., Giorgi F.M., Voesenek L.A.C.J., Perata P., van Dongen J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature. 2011;479:419–422. doi: 10.1038/nature10536. [DOI] [PubMed] [Google Scholar]
- 41.Dekkers B.J.W., Pearce S., van Bolderen-Veldkamp R.P., Marshall A., Widera P., Gilbert J., Drost H.G., Bassel G.W., Müller K., King J.R., et al. Transcriptional dynamics of two seed compartments with opposing roles in Arabidopsis seed germination. Plant Physiol. 2013;163:205–215. doi: 10.1104/pp.113.223511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ventura I., Brunello L., Iacopino S., Valeri M.C., Novi G., Dornbusch T., Perata P., Loreti E. Arabidopsis phenotyping reveals the importance of alcohol dehydrogenase and pyruvate decarboxylase for aerobic plant growth. Sci. Rep. 2020;10:16669. doi: 10.1038/s41598-020-73704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green P.T., Harms K.E., Connell J.H. Nonrandom, diversifying processes are disproportionately strong in the smallest size classes of a tropical forest. Proc. Natl. Acad. Sci. USA. 2014;111:18649–18654. doi: 10.1073/pnas.1321892112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holdsworth M.J., Gibbs D.J. Comparative biology of oxygen sensing in plants and animals. Curr. Biol. 2020;30:R362–R369. doi: 10.1016/j.cub.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 45.Masson N., Keeley T.P., Giuntoli B., White M.D., Puerta M.L., Perata P., Hopkinson R.J., Flashman E., Licausi F., Ratcliffe P.J. Conserved N-terminal cysteine dioxygenases transduce responses to hypoxia in animals and plants. Science. 2019;365:65–69. doi: 10.1126/science.aaw0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue Y., Petrovic N., Cao R., Larsson O., Lim S., Chen S., Feldmann H.M., Liang Z., Zhu Z., Nedergaard J., et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Basse A.L., Isidor M.S., Winther S., Skjoldborg N.B., Murholm M., Andersen E.S., Pedersen S.B., Wolfrum C., Quistorff B., Hansen J.B. Regulation of glycolysis in brown adipocytes by HIF-1α. Sci. Rep. 2017;7:4052. doi: 10.1038/s41598-017-04246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baffy G. Mitochondrial uncoupling in cancer cells: liabilities and opportunities. Biochim. Biophys. Acta. Bioenerg. 2017;1858:655–664. doi: 10.1016/j.bbabio.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Jurdak R., Launay-Avon A., Paysant-Le Roux C., Bailly C. Retrograde signalling from the mitochondria to the nucleus translates the positive effect of ethylene on dormancy breaking of Arabidopsis thaliana seeds. New Phytol. 2021;229:2192–2205. doi: 10.1111/nph.16985. [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- 51.Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 52.Dietrich D., Schmuths H., De Marcos Lousa C., Baldwin J.M., Baldwin S.A., Baker A., Theodoulou F.L., Holdsworth M.J. Mutations in the Arabidopsis peroxisomal ABC transporter COMATOSE allow differentiation between multiple functions in planta: insights from an allelic series. Mol. Biol. Cell. 2009;20:530–543. doi: 10.1091/mbc.E08-07-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any unique datasets or code.