Abstract
Background and Objectives
The goal of this work was to investigate the neuroimaging correlates of the Stages of Objective Memory Impairment (SOMI) system operationalized with the Free and Cued Selective Reminding Test (FCSRT), a widely used episodic memory measure.
Methods
The FCSRT begins with a study phase in which items (e.g., grapes) are identified in response to unique semantic cues (e.g., fruit) that are used in the test phase to prompt recall of items not retrieved by free recall. There are 3 test trials of the 16 items (maximum 48). Data from 4,484 cognitively unimpaired participants from the Anti-Amyloid Treatment in Asymptomatic Alzheimer's (A4) study were used. All participants had amyloid PET imaging, and a subset of 1,262 β-amyloid (Aβ)–positive had structural MRIs. We compared the Aβ mean cortical standardized uptake value ratio (SUVR) and volumetric measures of hippocampus, parahippocampal gyrus, entorhinal cortex, and inferior temporal cortex between the 5 SOMI stages.
Results
Participants had a mean age of 71.3 (SD 4.6) years; 40.6% were male; and 34.6% were APOE ε4 positive. Half had no memory impairment; the other half had retrieval deficits, storage limitations, or both. Analysis of covariance in the entire sample while controlling for age, sex, education, and APOE ε4 showed that individuals in higher SOMI stages had higher global amyloid SUVR (p < 0.001). Both SOMI-4 and -3 subgroups had higher amyloid SUVR than SOMI-0 and SOMI-1 subgroups. Individuals in higher SOMI stages had smaller hippocampal volume (p = 0.003), entorhinal cortex (p < 0.05), and inferior temporal lobes (p < 0.05), but there was no difference between parahippocampal gyrus volume of different SOMI stages. Pairwise comparison of SOMI subgroups showed that the SOMI-4, -3, and -2 subgroups had smaller hippocampal volume than the SOMI-0 and -1 subgroup. The SOMI-4 subgroup had significantly smaller entorhinal cortex and smaller inferior temporal lobe compared to all other groups.
Discussion
Presence of Alzheimer disease pathology is closely related to memory impairment according to SOMI stages in the cognitively unimpaired sample of A4. Results from structural MRIs suggest that memory storage impairment (SOMI-3 and -4) is present when there is widespread medial temporal lobe atrophy.
Trial Registration Information
ClinicalTrials.gov identifier: NCT02008357.
Classification of Evidence
This study provides Class I evidence that, in normal older individuals, higher stages of memory impairment assessed with FCSRT were associated with higher amyloid imaging burden and lower volume of hippocampus, entorhinal cortex, and inferior temporal lobes.
Performance on the Free and Cued Selective Reminding (FCSRT) defines what is sometimes called the core clinical phenotype of Alzheimer disease (AD), a memory disorder that cannot be remediated by effective encoding and retrieval processes.1,2 This phenotype is also called the amnestic syndrome of the hippocampal type because of its association with structural changes in medial temporal lobe, especially in the hippocampus and its subfields.3 Many studies have reported strong associations among AD biomarkers, including neuroimaging and CSF biomarkers of amyloid, tau, and neurodegeneration, and the 2 main FCSRT measures: free recall (FR) and total recall (TR), the sum of FR and cued recall.4-11
The FCSRT is effective in identifying prevalent dementia; predicting incident mild cognitive impairment (MCI), dementia, and AD; and distinguishing between AD and non-AD dementias in various longitudinal aging cohorts12-18 because the test controls the conditions of learning. In the study phase, participants are asked to identify items (e.g., grapes) in response to unique semantic cues (fruit); these cues are used in the test phase to prompt recall of items not retrieved by FR.19 In contrast to passively listening as items are presented in conventional word list learning tests, the FCSRT requires active cognitive engagement and deep semantic processing. By coordinating the conditions of encoding and retrieval with category cues, the FCSRT optimizes encoding specificity and maximizes recall.20
While impaired FR has predicted incident AD in several longitudinal aging cohorts,12,15,18,21 it is not optimized to identify β-amyloid (Aβ) status in cognitively normal individuals.22 To overcome this limitation and to identify participants at an earlier point in the preclinical AD trajectory, we developed the Stages of Objective Memory Impairment (SOMI) system, which provides practical cutoffs for classification of participants into 1 of 5 stages by both FR and TR scores (Table 1).23 Using Einstein Aging Study (EAS) data, we investigated whether the SOMI system is effective in identifying individuals at higher risk of incident AD. We showed that the first 3 SOMI stages (SOMI 0–2) typically precede clinical dementia by 5 to 8 years and refllect increasing retrieval difficulty, shown by declining FR in the context of intact TR. The next 2 SOMI stages (SOMI−3 and −4) precede clinical dementia by ≈1 to 3 years; in these stages, cuing fails to recover all of the items missed on FR. The advantage of SOMI over individual FR and TR scores is that it separates the measurement of retrieval impairment from memory storage impairment. Because these processes break down at different points in the predementia phase, the ability to distinguish between them allows an estimate of the participant's stage of illness along the AD continuum.
Table 1SOMI.
Defined by FR and TR Score Ranges and Years to Diagnosis on the pFCSRT + IR
We have recently shown that participants whose picture version of the FCSRT that includes Immediate Recall (pFCSRT + IR) performance classified them as SOMI-2 (moderate retrieval impairment) were at higher risk of incident AD (personal communication, Drs. Lynn Kuo and Qi Qi, November 31, 2021). Of 1,508 participants from the Baltimore Longitudinal Study of Aging (BLSA) who were free of dementia at baseline, 85 individuals developed AD over an average of >8 years of follow-up. Using bayesian joint modeling and all observed assessments, we found that the diagnostic accuracy of SOMI (83%-86%) was superior to that of FR alone (72%) or the sum of FR + TR (71%) in identifying incident AD after 3, 5, and 7 years of follow-up, demonstrating the advantage of SOMI over individual FR and TR scores.
The SOMI system could be useful for screening in clinical trials if performance was associated with the antemortem biomarkers of amyloid, tau, and neurodegeneration used in the National Institute on Aging–Alzheimer’s Association Research Framework.24 Because the pFCSRT + IR is currently used in the Anti-Amyloid Treatment in Asymptomatic Alzheimer's (A4) study25 and prerandomization data are publicly available, we were able to classify participants into SOMI stages. This allowed us to examine the association of SOMI with PET amyloid imaging and neurodegeneration using volumetric MRI. We predicted that participants in higher stages (SOMI-3 and -4) will have both higher global amyloid deposition and greater atrophy of hippocampal subregions than participants with no or mild impairment (SOMI-0 and -1).
Methods
Participants
A4 is a multicenter clinical trial being conducted in the United States, Canada, Australia, and Japan. Details of the screening process have been described previously.18 In brief, participants eligible for screening were 65 to 85 years of age, were assessed to be cognitively normal, were living independently, and had a study partner who would be able to provide information on daily life cognitive function on an annual basis. Participants with very low (≤1.5 SD below norms) Logical Memory Delayed Recall (LMDR-IIa) scores were excluded to eliminate individuals with MCI. Participant with very high (>1.5 SD above norms) LMDR-IIa scores were excluded after screening visit 1 to enhance enrollment of participants at higher risk of imminent cognitive decline associated with AD pathology. Participants with a Clinical Dementia Rating score of 0, Mini-Mental State Examination score of 25 to 30, and LMDR-IIa score of 6 to 18 were eligible to proceed to florbetapir PET imaging.
FCSRT Assessment
In the study phase of pFCSRT + IR, participants were asked to search a card containing 4 line drawings (e.g., grapes) for an item that goes with a unique category cue (e.g., fruit). A stimulus card is included in the supplemental material (eFigure 1, links.lww.com/WNL/B804). After all 4 items were identified, immediate cued recall of just those 4 items was tested. The study phase was repeated for all 16 drawings. The test phase consisted of 3 trials of FR each followed by cued recall for items not retrieved by FR (maximum score 48). Participants were stratified into different SOMI subgroups with the use of the score ranges of FR and TR (sum of FR and cued recall) as shown in Table 1.
Amyloid PET Imaging
At screening, participants underwent amyloid PET imaging acquired with florbetapir F-18 and measured with a mean cortical standardized uptake value ratio (SUVR) with a whole cerebellar reference region.26 We used a quantitative SUVR threshold of ≥1.10 to define amyloid positivity (Aβ+).27
Volumetric MRI
Only a subset of participants who were Aβ+ underwent structural MRI at baseline visit. Therefore, volumetric MRI measures were available for 1,262 Aβ+ participants. Volumetric measures of different cortical and subcortical regions were calculated automatically for all participants with FreeSurfer 6.0.28 For the purpose of this study, volumetric measures were collapsed across hemispheres and adjusted for estimated total intracranial volume.
Statistical Analyses
Statistical analyses were completed with SPSS version 25 (SPSS Inc, Chicago, IL) and Python version 3.9 (Python Software Foundation, Fredericksburg, VA). Sample characteristic differences among SOMI groups were examined with χ2 tests for categorical variables and analyses of variance for continuous variables (2 sided, p < 0.05). We used analysis of covariance to compare biomarker values of SOMI groups accounting for age, sex, education, and APOE ε4 status. Post hoc pairwise comparisons with Sidak correction were performed to assess differences between groups defined by SOMI, and values of p > 0.005 were considered significant (α = 0.05, 10 group comparisons).
Data Availability
Anonymized data are publicly available to any qualified investigator through the LONI website (ida.loni.usc.edu/login.jsp?project=ADNI).
Results
Sample Characteristics
A total of 4,484 cognitively unimpaired adults were included in this study, and volumetric MRI was available for 1,262 Aβ+ participants. Participants had a mean age of 71.3 (SD 4.6) years, were 40.6% male, and had 16.6 years of education; 34.6% were APOE ε4 positive. The mean FR was 29.0 (SD 5.59) and TR was 47.4 (SD 0.9).
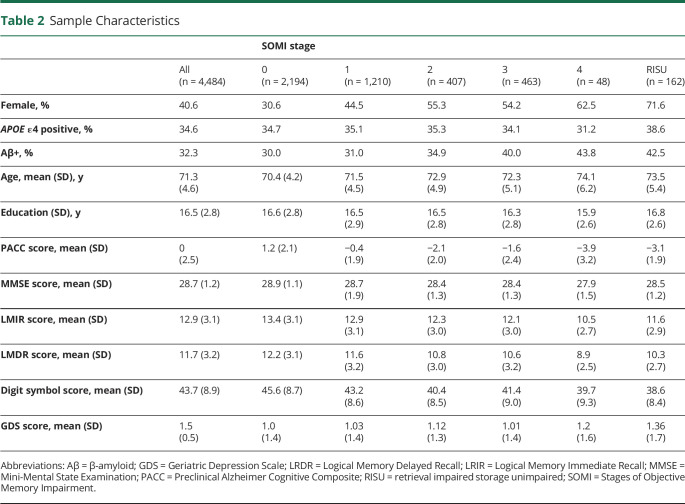
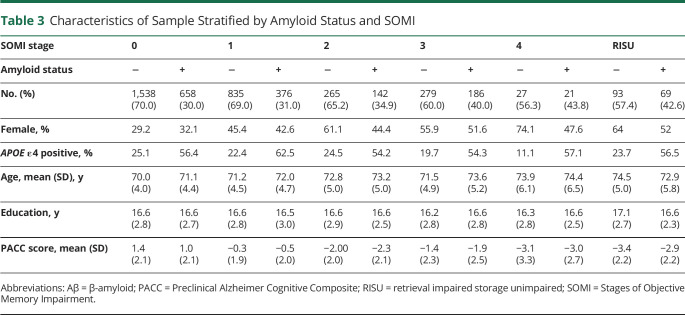
Table 2 summarizes sample characteristics and classification of participants into SOMI stages. Participants in higher SOMI stages were older (F = 44.7, p < 0.001) and more likely to be female (F = 54.0, p < 0.001). SOMI groups did not differ in education or relative frequency of APOE ε4 status. A total of 162 (3.6%) individuals could not be classified by the SOMI system because retrieval was impaired but storage was unimpaired. Compared with Aβ− individuals classified as in SOMI-0 or SOMI-1, those categorized as in SOMI-3 or SOMI-4 were older (p < 0.001 for all comparisons) and more likely to be female (p < 0.001 for all), but they did not differ in education. Table 3 summarizes the characteristics of Aβ− and Aβ+ individuals according to SOMI stages.
Table 2.
Sample Characteristics
Table 3.
Characteristics of Sample Stratified by Amyloid Status and SOMI
Differences in Amyloid Levels
Analysis of covariance in the entire sample showed that individuals in higher SOMI stages had higher global amyloid SUVR (F = 8.4, p < 0.001). Specifically, those in SOMI-4 had higher amyloid SUVR than those in SOMI-0, -1, and -2 (p < 0.001 for all) Individuals in SOMI-3 had higher amyloid SUVR than those in SOMI-0 (p < 0.001) and SOMI-1 (p = 0.003). There was no difference between amyloid SUVR of individuals in SOMI-0 and SOMI-1. (Figure 1A). We stratified the sample into Aβ− and Aβ+ subgroups to evaluate subthreshold and suprathreshold amyloid SUVR differences between the SOMI stages. At subthreshold levels (Aβ−), amyloid SUVR was not significantly different between SOMI stages. At suprathreshold levels (Aβ+), those in SOMI-4 had higher amyloid SUVR than individuals in SOMI-0 (p < 0.001) and SOMI-1 (p =0.002), and those in SOMI-2 and SOMI-3 stage had higher amyloid SUVR than those in SOMI-0 (p = 0.002 for both) (Figure 1B).
Figure 1. Boxplot Comparing Amyloid PET SUVR Across Different SOMI Subgroups for the Whole Population (A) and Sample Stratified According to Aβ Status (B).
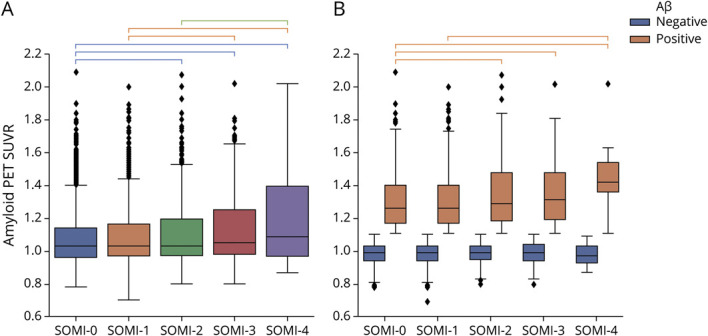
Groups significantly different from each other are connected to each other with brackets (p < 0.005). Aβ = β-amyloid; SOMI = Stages of Objective Memory Impairment; SUVR = standardized uptake value ratio.
Differences in Volumetric MRI Measures
Univariate analysis accounting for age, sex, education, and APOE ε4 showed that individuals in higher SOMI stages had smaller hippocampal volume (F = 3.66, p = 0.003) (Figure 2). Pairwise comparison showed that those in SOMI-0 had the largest hippocampal volume compared to all other groups (SOMI-1 p = 0.002; SOMI-2, -3, and -4 p < 0.001 for all). Individuals in SOMI-1 also had larger hippocampal volume than those in SOMI-3 (p < .001), and SOMI-4 (p < 0.001). There was no difference in hippocampal volume between individuals in SOMI-2 and SOMI-3 (p = 0.202), SOMI-2 and SOMI-4 (p = 0.026), or SOMI-3 and SOMI-4 (p = 0.102). Addition of amyloid SUVR to the univariate analysis as a covariate attenuated the differences; however, the difference between SOMI subgroups remained significant (F = 3.24, p = 0.007).
Figure 2. Volume of Different Regions by SOMI Stage.
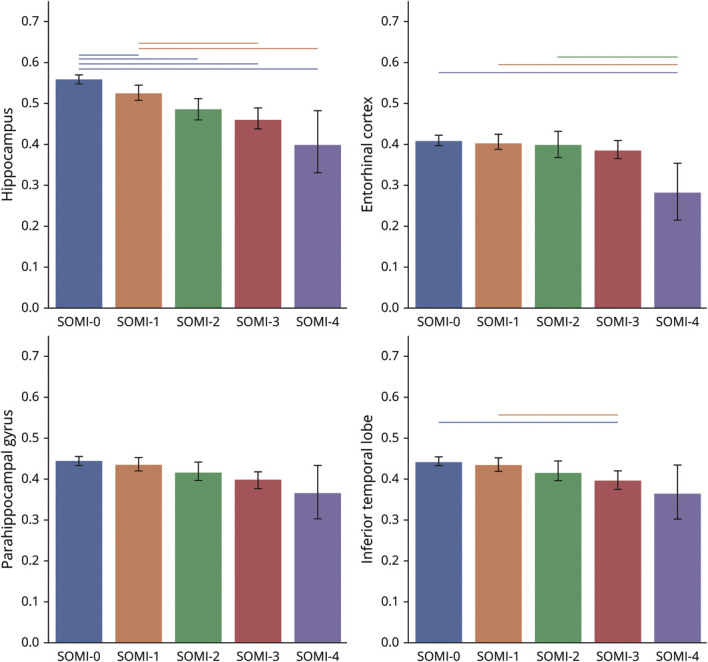
Volumetric measures are normalized and rescaled to 0 to 1 to improve comparability. Groups significantly different from each other (p < 0.005) are connected to each other with lines. SOMI = Stages of Objective Memory Impairment.
There was no significant difference between parahippocampal gyrus volume between SOMI subgroups. Those in higher SOMI stages had smaller entorhinal cortex (F = 2.25, p = 0.047) and inferior temporal lobes (F = 2.39, p = 0.036). Post hoc analysis showed that those in SOMI-4 had smaller entorhinal cortex than individuals in SOMI-0 (p < 0.001), SOMI-1 (p = 0.002), and SOMI-2 (p = 0.005), but there was no difference in entorhinal cortex volume among other subgroups. In addition, post hoc analysis showed that participants in SOMI-3 had smaller inferior temporal lobes than participants in SOMI-0 (p < 0.001) and SOMI-1 (p = 0.004). Addition of amyloid SUVR to the univariate analysis as a covariate did not affect significant differences observed between subgroups.
Classification of Evidence
This study provides Class I evidence that, in normal older individuals, higher stages of memory impairment assessed with FCSRT were associated with higher amyloid imaging burden and lower volume of hippocampus, entorhinal cortex, and inferior temporal lobes.
Discussion
The SOMI system distinguishes retrieval impairment observed in preclinical AD in the context of intact memory storage from memory storage impairment that occurs later in the AD continuum. We used FR and TR scores on pFCSRT + IR to classify participants from the A4 study into SOMI stages. The sample was screened to have a Clinical Dementia Rating global score of 0, a Mini-Mental State Examination score of 25 to 30, and an LMDR-IIa score of 6 to 18. Even in this sample, extensively screened to identify and eliminate participants with cognitive impairment, including memory impairment based on delayed story recall, 511 of 4,484 (11.4%) were in SOMI-3 or -4, indicating that they had both memory retrieval and storage impairment based on the FCSRT. This indicates that the FCSRT detects memory impairment in an ostensibly cognitively normal group.
We examined the association of SOMI stage with global amyloid levels. In the entire cohort, as SOMI stage increased, the proportion of participants who were Aβ+ also increased. Among Aβ+ individuals, those in higher SOMI stages had higher global amyloid levels. Individuals in SOMI-3 and -4 (those with storage and retrieval deficits) had significantly higher amyloid levels compared to individuals in earlier SOMI stages (those with unimpaired memory or mildly impaired retrieval). These findings suggest that individuals with memory impairment detected by SOMI in this “cognitively normal” group have a biological substrate for cognitive impairment; higher levels of amyloid are associated with concurrent storage and retrieval deficits.
The relationship between SOMI stage and atrophy of medial temporal lobe subregions complements the relationship between SOMI and global amyloid levels. Only Aβ+ individuals underwent structural MRI at baseline, providing volumetric measures of the hippocampus, parahippocampal gyrus, entorhinal cortex, and inferior temporal cortex. The relationship of volumetric measure to SOMI stage differed among measures: (1) progressing from 1 SOMI stage to the next was associated with increasing levels of hippocampal atrophy; (2) individuals with impairment of memory storage (SOMI-3 and -4) displayed greater atrophy of the inferior temporal cortex compared to those with no storage impairment; and (3) atrophy of the entorhinal cortex was present only in individuals in SOMI-4 compared to those in all other stages who did not differ.
Our results indicate that even among individuals screened for cognitive impairment, hippocampal atrophy is evident in Aβ+ subgroups with retrieval but not storage impairment. Prior studies suggest that hippocampal volume atrophy might be the earliest sign of neurodegeneration due to AD.29,30 The development of memory storage impairment was associated with atrophy of inferior temporal cortex, but volume loss in entorhinal cortex occurred only with advanced storage issues in SOMI-4.
We expect that the relationship between SOMI stage and MRI volumetrics would likely be weaker in a sample not screened for amyloid positivity. This is a limitation on generalizability of our results imposed by the design of A4 study. Volumetric data are currently available only for the amyloid-positive individuals in the A4 study, but a parallel study (Longitudinal Evaluation of Amyloid Risk and Neurodegeneration [LEARN] cohort) is following up a smaller sample (N = 600) of amyloid-negative individuals from the A4 study, and we are hoping to conduct a follow-up study using data from that sample.
SOMI stages displayed a similar relationship to hippocampal volume and tau PET imaging of the entorhinal and inferior temporal cortices among participants in the Harvard Aging Brain Study (HABS).31 Mean hippocampal volume for individuals in SOMI-3 and -4 combined was smaller than for individuals in SOMI-0, -1, and -2. Mean inferior temporal tau in SOMI-3 and -4 was higher compared to SOMI-0 and 1, and mean entorhinal tau in SOMI-3 and -4 was higher compared to SOMI-0. Together, these results indicate that memory storage impairment is present when there is widespread medial temporal lobe pathology.
In this study, the base rate of amyloid positivity among all participants was 32%. Rates of amyloid positivity were 30% for stage 0, 31% for stage 1, 35% for stage 2, 40% for stage 3, and 44% for stage 4. A sensitivity analysis indicated that the SOMI system can add incremental value to using a single FR and TR cutoff for identifying amyloid-positive individuals. The optimal Youden index–based cutoff point for prediction of Aβ positivity for FR was 30 (Aβ+ = 35.2% for FR ≤ 30 vs Aβ+ = 28.2 for FR > 30) and for TR was 47 (Aβ+ = 35.7 for TR ≤ 47 and Aβ+ = 29.5 for TR = 48). Individuals in SOMI-3 and SOMI-4 stages have higher rates of amyloid positivity (40.0% and 43.8%, respectively). While SOMI stage does not eliminate the need for amyloid screening, it provides a strategy for increasing the yield of amyloid positivity among those receiving biomarker assessment.
These data pertain to a study of an amyloid-targeted therapy. For trials that target other pathologic mechanisms (e.g., tau), when data become available, we would assess rates of tau positivity by SOMI stage to optimize a sampling design. However, as indicated by the many failed trials of AD, presence of target pathology (i.e., amyloid) and even the successful removal of it from brain (e.g., aducanumab trials) do not guarantee trial success. Using sensitive clinical tests such as SOMI and Preclinical Alzheimer Cognitive Composite (PACC), which are proven to be effective tests for detecting early and late cognitive decline during the preclinical stages of AD, in combination with biomarkers can lead to improved trial design.
A recent study from the HABS showed that Aβ+ cognitively normal individuals demonstrated longitudinal decline on all individual PACC components and all PACC variations.32 Differences between the Aβ+ and Aβ− groups emerged earlier when FCSRT FR was included in the PACC. FR alone or combined with TR was the only individual component of the PACC to show differences between the Aβ+ group who progressed to Clinical Dementia Rating score of 0.5 and those that remained stable. These findings support the notion that using the FSCRT-derived SOMI system can improve our ability to detect early and late cognitive decline during the preclinical stages of AD, which may prove advantageous in the design of prevention trials.
In the context of clinical trials, we envision that SOMI could be used as a criterion for prioritizing enrollment of participants. The optimal criterion for sample enrichment based on SOMI depends on the design of trial, its duration, and its objectives. One approach could be using SOMI staging for selecting participants who are more likely to be amyloid positive before evaluation with expensive or invasive biomarkers such as PET scans or CSF-based biomarkers. SOMI-3 and -4 groups are more likely to be amyloid positive; However, selecting individuals in SOMI-3 and -4 for biomarker evaluation has its own drawbacks. While this approach would increase biomarker positivity rate and decrease the cost of biomarker assessment before enrollment, it would also require cognitive screening of far more individuals with the FCSRT, which could potentially slow down recruitment and limit the generalizability of findings to those who are in SOMI-3 and -4 at the time of enrollment.
An alternative approach might be to enroll individuals who are in SOMI-1 or greater. While this approach has minimal value in the enrichment of a sample with amyloid-positive individuals before obtaining amyloid PET, from our previous studies, this group is expected to show faster cognitive decline and incident dementia in a shorter time frame, which is more appropriate for trials.21,23 In the EAS, we showed that among 142 participants who developed AD over 10 years, average time to diagnosis was 7 years if FR was intact >30 (SOMI-0), 5 years if SOMI-1, 4 years if SOMI-2, and 2 years if SOMI-3 and -4.23 The trajectory of FR decline in the preclinical AD continuum of the BLSA participants was consistent with the SOMI prediction. FR declined gradually, beginning at 7 years, until there was a doubling of the rate of FR decline ≈4 years later.21 From these results, we expect that in a trial in which time to dementia is the endpoint, the SOMI system would add important information that could be used to match the characteristics of the eligible sample to the design and duration of the study. Given the long duration of progression to dementia in the SOMI-0 group, the expected rate of memory decline is likely too slow to demonstrate treatment benefits in any of the current clinical trials.
The SOMI system could also inform clinical trial design as an outcome measure. SOMI-3 is highly associated with AD neuropathology defined by current guidelines33 and neurofibrillary tangle pathology defined by Braak and Braak.34 Compared to participants with no memory impairment (SOMI-0), participants classified into SOMI-3 and -4 stages were 4 times more likely to have positive AD neuropathology and were nearly 6 times as likely to have more advanced Braak neurofibrillary tangle pathology. Using conversion from earlier stages to SOMI-3 as an outcome in conjunction with biomarkers could be an effective method to reduce the duration of active treatment in the trials.
It should be noted that we do not believe that the FCSRT is capturing something unique. Other memory tests are also effective at predicting incident MCI and dementia.35,36 However, the ability to distinguish measures of storage and retrieval and the control of cognitive processes may provide particular benefits. Many studies by our group and others indicate that FCSRT is a sensitive cognitive test for detecting signs of memory impairment in both storage and retrieval in prodromal stages of AD and other dementia.15-18 SOMI staging provides a practical system (and cutoffs for classification) that can be used as part of the design of future interventional studies.
Another approach to identifying persons in the earliest stages of AD includes using at least 2 tests of a single domain or single tests of multiple domains in addition to specific memory parameters (e.g., learning slope).37,38 This approach has theoretical advantages and disadvantages over our approach, which relies on a single cognitive test. We expect there to be overlap between early and late MCI and SOMI stages. These approaches have not been compared head to head in terms of simplicity, ease of use, and predictive validity for biomarkers or cognitive decline.
There are limitations of this study worth noting. Of the participants, 3.4% could not be classified into a SOMI stage because their retrieval was impaired but memory storage was unimpaired. We plan to study characteristics of this unique group in our future studies. In the setting of clinical trials, in which screening with the FCSRT precedes biomarker assessment, excluding unclassified participants until we better understand them may improve outcomes by decreasing the number of participants who do not display the core clinical memory AD phenotype. The A4 study is a prevention trial; therefore, all participants who had PET scans were cognitively normal at baseline. Excluding participants with very high LMDR-IIa criteria (>1.SD) from the A4 trial slightly affects the generalizability of our study. However, even after the exclusion of these participants, the sample includes a large range of cognitive abilities among individuals considered normal according to the current gold standard diagnostic criteria. Another limitation is the high educational level of the cohort. We are hoping to follow up this study in a more diverse cohort in the future to confirm its generalizability to other samples.
In this study, we used data from a very large sample of older adults. While using large datasets is generally considered an advantage in epidemiologic studies, it also poses a statistical challenge. In large samples, analysis power is substantially increased. This might lead to an exaggerated tendency to reject the null hypotheses while differences are not clinically or biologically meaningful. However, our results could be considered robust because they are hypothesis driven and consistent with literature and prior studies in other samples with diverse populations and different sample sizes.4,6-8,10,17 Last, the relationship between SOMI stage and MRI volumetrics would likely be weaker in a sample not screened for amyloid positivity.
Even in highly selected cognitively normal older adults, the presence of AD pathology is closely related to memory impairment based on SOMI stages. We envision that SOMI could be used for enrichment of future clinical trials.
Acknowledgment
The A4 study is a secondary prevention trial in preclinical AD, aiming to slow cognitive decline associated with brain amyloid accumulation in clinically normal older individuals. The A4 study is funded by a public-private-philanthropic partnership, including funding from the NIH-National Institute on Aging, Eli Lilly and Co, Alzheimer's Association, Accelerating Medicines Partnership, GHR Foundation, an anonymous foundation, and additional private donors, with in-kind support from Avid and Cogstate. The companion observational LEARN study is funded by the Alzheimer's Association and GHR Foundation. The A4 and LEARN studies are led by Dr. Reisa Sperling at Brigham and Women's Hospital, Harvard Medical School, and Dr. Paul Aisen at the Alzheimer's Therapeutic Research Institute (ATRI), University of Southern California. The A4 and LEARN Studies are coordinated by ATRI at the University of Southern California, and the data are made available through the Laboratory for Neuro Imaging at the University of Southern California. The participants screened for the A4 study provided permission to share their deidentified data to advance the quest to find a successful treatment for AD. The authors acknowledge the dedication of all the participants, the site personnel, and all of the partnership team members who continue to make the A4 and LEARN studies possible. The complete A4 Study Team list is available at a4study.org/a4-study-team.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- A4
Anti-Amyloid Treatment in Asymptomatic Alzheimer's
- BLSA
Baltimore Longitudinal Study of Aging
- EAS
Einstein Aging Study
- FCSRT
Free and Cued Selective Reminding
- FR
free recall
- HABS
Harvard Aging Brain Study
- LEARN
Longitudinal Evaluation of Amyloid Risk and Neurodegeneration
- LMDR-IIa
Logical Memory Delayed Recall
- MCI
mild cognitive impairment
- PACC
Preclinical Alzheimer Cognitive Composite
- pFCSRT + IR
picture version of the FCSRT that includes Immediate Recall
- SOMI
Stages of Objective Memory Impairment
- SUVR
standardized uptake value ratio
- TR
total recall
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
R.B.L. receives research support from the following sources unrelated to this manuscript: 5U10 NS077308 (PI), R21 AG056920 (investigator), 1RF1 AG057531 (site principal investigator [PI]), RF1 AG054548 (investigator), 1RO1 AG048642 (investigator), R56 AG057548 (investigator), U01062370 (investigator), RO1 AG060933 (investigator), RO1 AG062622 (investigator), and 1UG3FD006795 (multiple PI [mPI]), 1U24NS113847 (investigator). R.A.S. receives research support from National Institute on Aging: P01 AG036694; R01 AG054029; R01 AG063689; R01AG053798; U24AG057437; K24 AG035007; Alzheimer's Association: LEARN-15-338729; and clinical trial research support from Eli Lilly and Co and Eisai. P.S.A. receives research support from the National Institute on Aging, the Alzheimer's Association, and the Foundation for NIH and has research agreements with Lilly and Eisai. A.E. receives research support from National Institute on Aging K23 AG063993, 2PO1 AG003949, the Alzheimer's Association (2019-AACSF-641329), and Cure Alzheimer's Fund
Disclosure
E. Grober receives a small royalty for commercial use of the FCSRT + IR. R.B. Lipton receives research support from the following sources unrelated to this manuscript: 5U10 NS077308 (PI), R21 AG056920 (investigator), 1RF1 AG057531 (site PI), RF1 AG054548 (investigator), 1RO1 AG048642 (investigator), R56 AG057548 (investigator), U01062370 (investigator), RO1 AG060933 (investigator), RO1 AG062622 (investigator), 1UG3FD006795 (mPI), and 1U24NS113847 (investigator). He holds stock options in eNeura Therapeutics and Biohaven Holdings; serves as consultant or advisory board member for or has received honoraria from Abbvie (Allergan), American Academy of Neurology, American Headache Society, Amgen, Avanir, Biohaven, Biovision, Boston Scientific, Dr. Reddy's (Promius), Electrocore, Eli Lilly, eNeura Therapeutics, Equinox, GlaxoSmithKline, Grifols, Lundbeck (Alder), Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, and Vedanta; and receives royalties from Wolff's Headache 7th and 8th Edition, Oxford Press University, 2009, Wiley and Informa. R.A. Sperling receives research support from National Institute on Aging: P01 AG036694; R01 AG054029; R01 AG063689; R01AG053798; U24AG057437; and K24 AG035007; Alzheimer's Association: LEARN-15-338729; clinical trial research support from Eli Lilly and Co and Eisai, and consulting fees from AC Immune, Acumen, Genentech, Janssen, Cytox, Prothena, Renew, Alnylam, Neuraly, and Neurocentria. P.S. Aisen receives research support from the National Institute on Aging, the Alzheimer's Association, and the Foundation for NIH; has research agreements with Lilly and Eisai; and receives consulting fees from Merck, Biogen, Abbvie, Roche, Rainbow Medical, Immunobrain Checkpoint, and Shionogi. A.E. Veroff receives consulting fees from Janssen and a small royalty for commercial use of some FCSRT materials. A. Ezzati serves as consultant or advisory board member for and has received honoraria from Eisai and PCORI Health Care Horizon Scanning Team. K.V. Papp has received funding from the Alzheimer's Association and the NIH and has served as a paid consultant for Biogen Idec and Digital Cognition Technologies. D.M. Rentz receives consulting and scientific advisory board fees from Digital Cognition Technologies, Biogen Idec, and Neurotrack. K.A. Johnson receives research support from the National Institute on Aging: P01 AG036694, R01 AG054029, R01 AG063689; R01AG053798, U24AG057437, R01AG054076, and R01AG049607; National Institute on Deafness and Other Communication Disorders: R01DC014296; Alzheimer's Association: LEARN-15-338729; clinical trial research support from Eli Lilly and Co, Cerveau, and Eisai; and received consulting fees from AC Immune, AZTherapies, Genentech, Janssen, Takeda, and Novartis. Go to Neurology.org/N for full disclosures.
References
- 1.Dubois B, Feldman HH, Jacova C, et al. . Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614-629. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B, Epelbaum S, Nyasse F, et al. . Cognitive and neuroimaging features and brain beta-amyloidosis in individuals at risk of Alzheimer's disease (INSIGHT-preAD): a longitudinal observational study. Lancet Neurol. 2018;17(4):335-346. [DOI] [PubMed] [Google Scholar]
- 3.Sarazin M, Chauvire V, Gerardin E, et al. . The amnestic syndrome of hippocampal type in Alzheimer's Disease: an MRI study. J Alzheimer's Dis. 2010;22:285-294. [DOI] [PubMed] [Google Scholar]
- 4.Hanseeuw BJ, Betensky RA, Jacobs HIL, et al. . Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 2019;76(8):915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insel PS, Donohue MC, Sperling R, Hansson O, Mattsson-Carlgren N. The A4 study: β-amyloid and cognition in 4432 cognitively unimpaired adults. Ann Clin Transl Neurol. 2020;7(5):776-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J, Gabelle A, Dorey A, et al. . Initial memory deficit profiles in patients with a cerebrospinal fluid Alzheimer's disease signature. J Alzheimers Dis. 2014;41(4):1109-1116. [DOI] [PubMed] [Google Scholar]
- 7.Wagner M, Wolf S, Reischies FM, et al. . Biomarker validation of a cued recall memory deficit in prodromal Alzheimer disease. Neurology. 2012;78(6):379-386. [DOI] [PubMed] [Google Scholar]
- 8.Slachevsky A, Barraza P, Hornberger M, et al. . Neuroanatomical comparison of the "word" and “picture” versions of the Free and Cued Selective Reminding Test in Alzheimer's disease. J Alzheimers Dis. 2018;61(2):589-600. [DOI] [PubMed] [Google Scholar]
- 9.Bernard CC. PCC characteristics at rest in 10-year memory decliners. Neurobiol Aging. 2015;36(10):2812-2820. [DOI] [PubMed] [Google Scholar]
- 10.Zammit AR, Ezzati A, Zimmerman ME, Lipton RB, Lipton ML, Katz MJ. Roles of hippocampal subfields in verbal and visual episodic memory. Behav Brain Res. 2017;317:157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzati A, Katz MJ, Zammit AR, et al. . Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia. 2016;93(Pt B):380-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derby CA, Burns LC, Wang C, et al. . Screening for predementia AD: time-dependent operating characteristics of episodic memory tests. Neurology. 2013;80(14):1307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz MJ, Lipton RB, Hall CB, et al. . Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in Blacks and Whites: a report from the Einstein Aging Study. Alzheimer Dis Associated Disord. 2012;26(4):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarazin M, Berr C, De Rotrou J, et al. . Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69(19):1859-1867. [DOI] [PubMed] [Google Scholar]
- 15.Di Stefano F, Epelbaum S, Coley N, et al. . Prediction of Alzheimer's disease dementia: data from the GuidAge prevention trial. J Alzheimers Dis. 2015;48(3):793-804. [DOI] [PubMed] [Google Scholar]
- 16.Lemos R, Maroco J, Simoes MR, Santiago B, Tomas J, Santana I. The Free and Cued Selective Reminding Test for predicting progression to Alzheimer's disease in patients with mild cognitive impairment: a prospective longitudinal study. J Neuropsychol. 2017;11(1):40-55. [DOI] [PubMed] [Google Scholar]
- 17.Teichmann M, Epelbaum S, Samri D, et al. . Free and Cued Selective Reminding Test: accuracy for the differential diagnosis of Alzheimer's and neurodegenerative diseases: a large-scale biomarker-characterized monocenter cohort study (ClinAD). Alzheimers Dement. 2017;13(8):913-923. [DOI] [PubMed] [Google Scholar]
- 18.Auriacombe S, Helmer C, Amieva H, Berr C, Dubois B, Dartigues JF. Validity of the Free and Cued Selective Reminding Test in predicting dementia: the 3C study. Neurology. 2010;74(22):1760-1767. [DOI] [PubMed] [Google Scholar]
- 19.Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3(1):13-36. [Google Scholar]
- 20.Tulving E. Elements of Episodic Memory. Oxford University Press; 1983. [Google Scholar]
- 21.Grober E, An Y, Lipton RB, Kawas C, Resnick SM. Timing of onset and rate of decline in learning and retention in the pre-dementia phase of Alzheimer's disease. J Int Neuropsychol Soc. 2019;25(7):699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papp KV, Rentz DM, Mormino EC, et al. . Cued memory decline in biomarker-defined preclinical Alzheimer disease. Neurology. 2017;88(15):1431-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grober E, Veroff AE, Lipton RB. Temporal unfolding of declining episodic memory on the Free and Cued Selective Reminding Test in the predementia phase of Alzheimer's disease: implications for clinical trials. Alzheimers Dement. 2018;10:161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR Jr, Bennett DA, Blennow K, et al. . NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperling RA, Donohue MC, Raman R, et al. . Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol. 2020;77(6):735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pontecorvo MJ, Arora AK, Devine M, et al. . Quantitation of PET signal as an adjunct to visual interpretation of florbetapir imaging. Eur J Nucl Med Mol Imaging. 2017;44(5):825-837. [DOI] [PubMed] [Google Scholar]
- 27.Johnson KA, Sperling RA, Gidicsin CM, et al. . Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9(5):S72-S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karas G, Sluimer J, Goekoop R, et al. . Amnestic mild cognitive impairment: structural MR imaging findings predictive of conversion to Alzheimer disease. AJNR Am J Neuroradiol. 2008;29(5):944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanpitukpongse TP, Mazurowski MA, Ikhena J, Petrella JR. Predictive utility of marketed volumetric software tools in subjects at risk for Alzheimer disease: do regions outside the hippocampus matter?. AJNR Am J Neuroradiol. 2017;38(3):546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grober E, Papp K, Rentz D, et al. . Neuroimaging correlates of Stages of Objective Memory Impairment (SOMI) system. Alzheimers Dement. 2021;13(1):e12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mormino EC, Papp KV, Rentz DM, et al. . Early and late change on the preclinical Alzheimer's cognitive composite in clinically normal older individuals with elevated amyloid β. Alzheimers Dement. 2017;13(9):1004-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyman BT, Phelps CH, Beach TG, et al. . National Institute on Aging–Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer Dement. 2012;8(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. [DOI] [PubMed] [Google Scholar]
- 35.Chang Y-L, Bondi MW, Fennema-Notestine C, et al. . Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to Alzheimer's disease. Neuropsychologia. 2010;48(5):1237-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duke Han S, Nguyen CP, Stricker NH, Nation DA. Detectable neuropsychological differences in early preclinical Alzheimer's disease: a meta-analysis. Neuropsychol Rev. 2017;27(4):305-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas KR, Edmonds EC, Eppig J, Salmon DP, Bondi MW. Using neuropsychological process scores to identify subtle cognitive decline and predict progression to mild cognitive impairment. J Alzheimers Dis. 2018;64(1):195-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas KR, Bangen KJ, Weigand AJ, et al. . Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology. 2020;94(4):e397-e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are publicly available to any qualified investigator through the LONI website (ida.loni.usc.edu/login.jsp?project=ADNI).