Abstract
Background and Objectives
Although blood pressure (BP) control is considered the most effective measure to prevent functional decline after intracerebral hemorrhage (ICH), fewer than half of survivors achieve treatment goals. We hypothesized that long-term (i.e., prehemorrhage) hypertension severity may be a crucial factor in explaining poor BP control after ICH. We investigated changes in hypertension severity after vs before ICH using latent class analysis (LCA) and identified patient characteristics predictive of individuals' BP trajectories.
Methods
We analyzed data for ICH survivors enrolled in a study conducted at Massachusetts General Hospital (MGH) from 2002 to 2019 in Boston, a high-resource setting with near-universal medical insurance coverage. We captured BP measurements in the 12 months preceding and following the acute ICH hospitalization. Using LCA, we identified patient groups (classes) based on changes in hypertension severity over time in an unbiased manner. We then created multinomial logistic regression models to identify patient factors associated with these classes.
Results
Among 336 participants, the average age was 74.4 years, 166 (49%) were male, and 288 (86%) self-reported White race/ethnicity. LCA identified 3 patient classes, corresponding to minimal (n = 114, 34%), intermediate (n = 128, 38%), and substantial (n = 94, 28%) improvement in hypertension severity after vs before ICH. Survivors with undertreated (relative risk ratio [RRR] 0.05, 95% CI 0.01–0.23) or resistant (RRR 0.03, 95% CI 0.01–0.06) hypertension before ICH were less likely to experience substantial improvement afterwards. Residents of high-income neighborhoods were more likely to experience substantial improvement (RRR 1.14 per $10,000, 95% CI 1.02–1.28). Black, Hispanic, and Asian participants with uncontrolled hypertension before ICH were more likely to experience minimal improvement after hemorrhagic stroke (interaction p < 0.001).
Discussion
Most ICH survivors do not display consistent improvement in hypertension severity after hemorrhagic stroke. BP control after ICH is profoundly influenced by patient characteristics predating the hemorrhage, chiefly prestroke hypertension severity and socioeconomic status. Neighborhood income was associated with hypertension severity after ICH in a high-resource setting with near-universal health care coverage. These findings likely contribute to previously documented racial/ethnic disparities in BP control and clinical outcomes following ICH.
More than half of intracerebral hemorrhage (ICH) survivors experience recurrent stroke or functional decline within 5 years of the acute hemorrhage.1-4 Blood pressure (BP) control represents the cornerstone of secondary prevention after ICH, with lower BP associated with reduced risk of recurrent stroke, dementia, and functional decline.5,6 Fewer than half of ICH survivors achieve recommended treatment targets.2,7 Racial/ethnic disparities exist in hypertension control after an acute hemorrhagic stroke,8,9 but we have limited insight into biological and social factors associated with inadequate BP control among ICH survivors.
We hypothesized that information on BP measurements obtained before an ICH event may be analyzed alongside corresponding data after acute hemorrhage to define individual trajectories in hypertension severity. Differences in these trajectories could identify subgroups for development of novel targeted strategies, ultimately leading to improved BP control after ICH. We expected ICH survivors whose BP was elevated pre-ICH to be most likely to experience little to no change in hypertension severity afterwards. We also sought to identify biological and social factors accounting for changes in hypertension severity at the individual level. Because of the established racial/ethnic disparities in outcomes after ICH, we focused our approach through the lens of health equity in BP control among survivors.8
To pursue these objectives, we leveraged data from the single-center, ongoing, longitudinal study of ICH conducted at Massachusetts General Hospital (MGH). We analyzed temporal trajectories in hypertension severity after vs before ICH using an unbiased bioinformatic approach (latent class analysis [LCA]) allowing investigators to identify patient subgroups sharing similarities in disease risk factors or manifestations.10
Methods
Study Inclusion and Exclusion Criteria
We initially screened for inclusion all individuals age ≥18 years presenting to MGH between January 2002 and December 2019 with a new diagnosis of acute ICH.2 Individuals with ICH secondary to trauma, conversion of an ischemic infarct, rupture of a vascular malformation or aneurysm, or brain tumor were excluded. Based on previously reported findings, we approached and enrolled more than 99% of all consecutive ICH cases presenting to our institution. Because we focused on BP control in the 12 months before and after ICH, only individuals alive at 1 year from the acute ICH hospitalization were included. Survivors without at least 1 BP measurement in the 12 months before and after the acute ICH were excluded. Participant characteristics for those included in the final sample compared with those excluded due to missing BP measurements are shown in eTable 1 (links.lww.com/WNL/B806). We excluded all participants not discharged to a private residency, rehabilitation center, or nursing facility in Massachusetts. This criterion was applied because (1) we expected only participants residing in our hospital catchment area to be available for subsequent BP data capture2,5 and (2) interpretation of our findings would be informed by the availability of near-universal health care coverage for individuals residing in Massachusetts.11
Standard Protocol Approvals, Registrations, and Patient Consents
Study protocols were approved by the MGH institutional review board and written informed consent was obtained from all participants.
Enrollment and Longitudinal Follow-up
Study staff collected demographic, social, and medical history information via in-person interview of patients (or reliable informants).2,5 Participants or informants also provided self-identified race and ethnicity, choosing from categories recommended by the NIH for use in research studies.8 We analyzed admission (i.e., first available) CT scans to determine ICH location and hematoma volume according to a previously validated methodology.1
All neuroimaging was analyzed blinded to clinical information. Participants were then interviewed by dedicated study staff (blinded to baseline and neuroimaging information) at 3, 6, and 12 months after ICH.2 We augmented patient-provided information via review of electronic health records (EHRs) using an enterprise data warehouse (EDW), a dedicated, secure data storage and retrieval platform for affiliated institutions in our health care delivery network.12 All participants in our study received care at clinical settings contributing data to the EDW. Additional information on social determinants of health (SDOH), medication exposures, and laboratory results were obtained in this manner. We attempted to address data missingness via additional manual review of EHR; random sampling imputation was used to fill in values that remained missing. We captured income information at the neighborhood (zip code) level using a validated, publicly available database.13 We obtained information on health insurance coverage status (insured vs uninsured) and coverage source (private sector, state or federal, mixed private and state/federal) from the EHR.
BP Data Capture
We queried the EDW to obtain BP values from patient encounters in our health care system (Mass General Brigham). BP measurements obtained on dates corresponding to inpatient stays were excluded, assuming unrelated medical conditions may have unduly altered BP homeostasis. We also excluded BP obtained on the day prior to admission and the day after discharge. EHRs were then manually reviewed by a board-certified physician to gather additional BP measurements not electronically available in the data warehouse. We prespecified the following time intervals for BP data capture: (1) 12 months before ICH; (2) from day of discharge from initial ICH hospitalization to 3 months later; (3) 3–6 months after discharge; (4) 6–12 months after discharge. Within each time period, we calculated median systolic BP (SBP) and diastolic BP (DBP) measurements from all available values, as described previously.2
Definition of Variables
Age at index ICH was analyzed as a continuous variable. Neighborhood median yearly income was analyzed as a continuous variable, calibrating for $10,000 increments. Race and ethnicity categories were collapsed to determine self-reported race/ethnicity as one of the following: White (i.e., non-Hispanic White), Asian (non-Hispanic Asian), Black or African American (non-Hispanic Black or African American), Hispanic or Latino (Hispanic of any race), or other (non-Hispanic other race, or unknown race and ethnicity). We defined use of English as preferred language or interpreter services, veterancy, United States as country of origin, and college education as dichotomous yes/no variables. Employment status, religious affiliation, marital status, and health care insurance coverage were analyzed as categorical variables. We defined chronic kidney disease as estimated glomerular filtration rate <60 (calculated using the first creatinine value obtained after admission for index ICH). ICH volume was analyzed as a continuous variable, calibrating for 10 mL increments.2 We defined lobar ICH as selective involvement of the cortex and subcortical white matter. Hemorrhages involving the thalami, basal ganglia, cerebellum, and brainstem (with or without concomitant involvement of cortex and subcortical white matter) were defined as nonlobar.1 We utilized the 2017 American College of Cardiology/American Heart Association guidelines to quantify hypertension severity before and after ICH, as follows: (1) normal BP (SBP <120 mm Hg and DBP <80 mm Hg); (2) elevated BP (SBP 120–129 mm Hg and DBP <80 mm Hg); (3) hypertension stage 1 (SBP 130–139 mm Hg or DBP 80–89 mm Hg); (4) hypertension stage 2 (SBP ≥140 mm Hg or DBP ≥90 mm Hg).14 Number of antihypertensive medications prescribed before ICH was analyzed as an ordinal variable indicating use of 0, 1, 2, or 3 or more. We then defined pre-ICH hypertension treatment status as follows: (1) not hypertensive: normal or elevated BP without use of antihypertensive medications; (2) controlled hypertension: normal or elevated BP on medication treatment; (3) undertreated hypertension: hypertension stage 1 or 2 using <3 antihypertensive agents; (4) treatment-resistant hypertension: hypertension stage 1 or 2 using ≥3 antihypertensive agents.
Statistical Methods
We first applied latent class analysis to hypertension severity data at each time point, followed by multinomial logistic regression predicting class membership. We prespecified sensitivity analyses investigating interactions between self-reported race/ethnicity and other variables in the final model. LCA utilized as input individual participants' hypertension stage data at all prespecified time points (12 months before ICH; 3, 6, and 12 months after ICH), with the goal of identifying individual-specific trajectories in BP. The optimal number of classes was chosen according to Akaike information criterion. The LCA analyses returned for each study participant probability for membership in each class, and we assigned individuals to the class with the highest probability. We then conducted univariable analyses screening factors predicting assignment to LCA-derived patient classes. Continuous variables were compared using 1-way analysis of variance (ANOVA); categorical variables were compared using χ2 test or Fisher exact test (as appropriate). Predictors with univariable associations with p < 0.20 were included in a multinomial logistic regression model predicting class membership. We subsequently generated via backward elimination a minimal model including only variables with p < 0.05 comparisons between any of the classes, along with variables that improved overall model fit (ANOVA p < 0.05). We examined the final resulting model for multicollinearity by computing variance inflation factor (VIF) for all variables; none of the included variables met predefined criteria (VIF >5.0) for removal from analyses. We investigated interactions between self-reported race/ethnicity and other variables in the final multinomial model. Categorical variables were collapsed as necessary to ensure n ≥10 in each cell. Interaction terms with p < 0.05 between any 2 of the 3 classes or ANOVA p < 0.05 were considered significant. Analyses were performed using STATA v16.0 and R v4.0.2.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Study Participants and Patient Classes
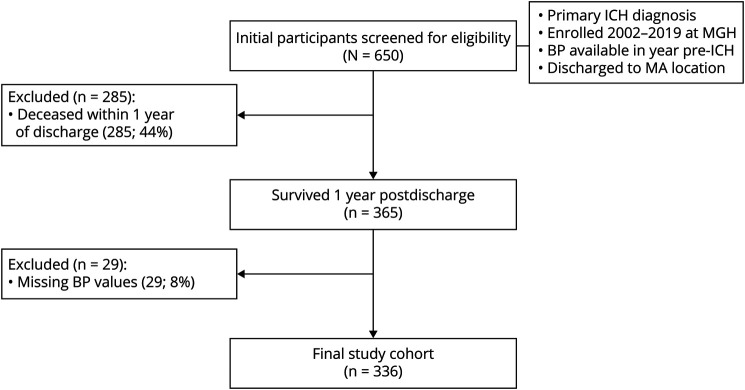
After application of inclusion and exclusion criteria to 650 potentially eligible ICH cases (Figure 1), we included 336 events in all subsequent analyses (Table 1). Among excluded cases, 285/314 (91%) were excluded due to mortality before 1 year from discharge from index ICH hospitalization. We excluded 29/314 (9%) participants due to missing BP measurements during follow-up. We present comparisons between individuals excluded due to missing BP values and participants in the final study group in eTable 1 (links.lww.com/WNL/B806). We analyzed a total of 2,238 BP measurements in the 12 months before ICH and 1,882, 1,275, and 1,458 BP measurements at 3, 6, and 12 months after ICH, respectively.
Figure 1. Study Participants and Inclusion/Exclusion Criteria.
BP = blood pressure; ICH = intracerebral hemorrhage; MGH = Massachusetts General Hospital.
Table 1.
Participant Characteristics
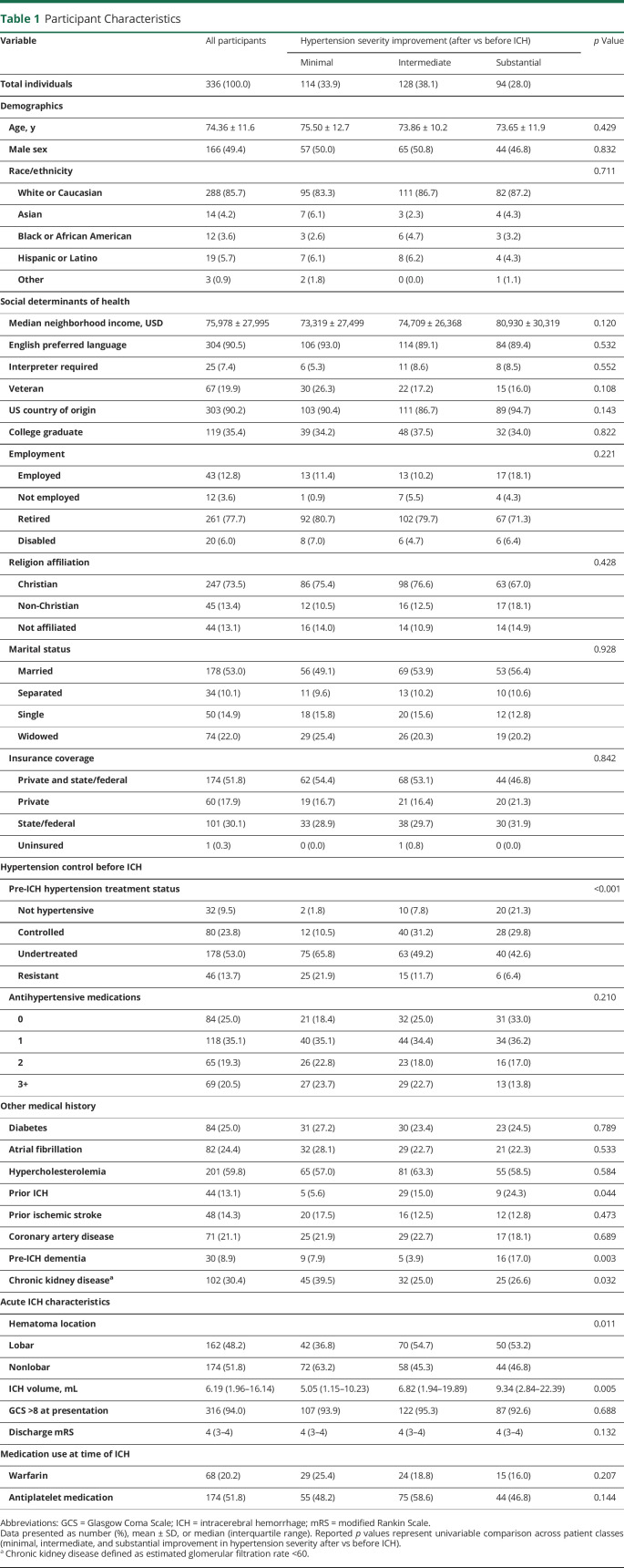
We performed LCA of hypertension severity (defined as normal BP, elevated BP, stage 1 or stage 2) in the 12 months before and after hemorrhagic stroke to define patient groups with similar trajectories after vs before ICH. We identified a model with 3 classes as best fit for the data provided. We present information on hypertension severity in each patient class in the 12 months before and after ICH in Figure 2, A–C. Upon visual inspection (Figure 2, D–F), we identified differences in hypertension severity changes after vs before ICH across LCA-defined patient classes, and identified them accordingly as minimal (n = 114), intermediate (n = 128), and substantial (n = 94) improvement.
Figure 2. Hypertension Severity After vs Before ICH.
(A–C) Stacked bar charts representing prevalence of hypertension (HTN) severity stages before intracerebral hemorrhage (ICH) (12 months preceding) and afterwards (3, 6, and 12 months after discharge from stroke hospitalization). Data presented as absolute number of patients in each category and percentage. (D–F) Sankey diagrams of changes in hypertension severity staging before vs after ICH, subdivided by patient classes identified by latent class analysis.
Hypertension Severity After vs Before ICH
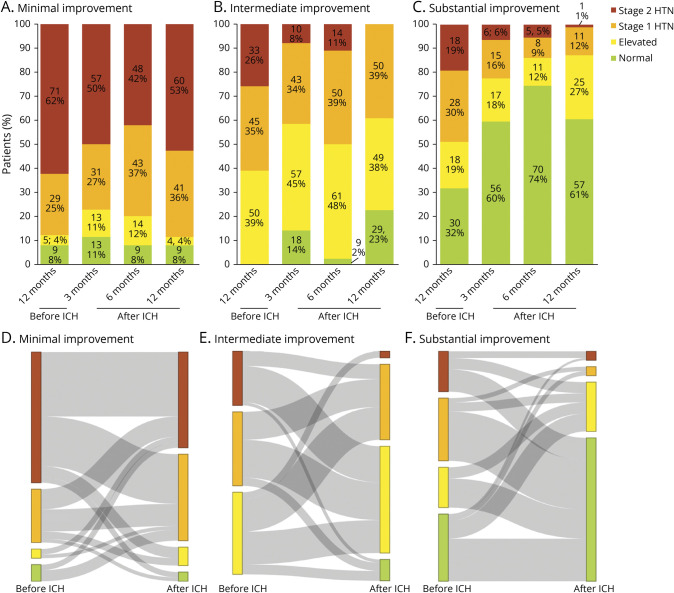
We sought to confirm that LCA-derived classes indicated different trajectories in hypertension severity change after vs before ICH (Figure 3). Among all participants, 163 (49%) displayed lower hypertension severity after ICH when compared to before ICH. Decrease in hypertension severity after ICH was experienced by 42/114 (37%) individuals in the minimal improvement class, 67/128 (52%) individuals in the moderate improvement class, and 54/94 (57%) individuals in the substantial improvement class. Among participants experiencing lower hypertension severity after ICH, the average reduction was 1.3 severity stages in the minimal improvement class, 1.5 severity stages in the moderate improvement class, and 1.9 severity stages in the substantial improvement class. Overall class assignment was significantly associated with changes in hypertension severity (by at least 1 stage) before vs after ICH (p < 0.001 for comparison).
Figure 3. Changes in Hypertension Severity After vs Before ICH.
(A–C) Stacked bar charts representing changes in hypertension staging before vs after intracerebral hemorrhage (ICH), subdivided by patient classes identified by latent class analysis. Individuals with positive values experienced worsening of hypertension control (with the number indicating how many stages higher after ICH); individuals with negative values experienced improvement in hypertension control (with the number indicating how many stages lower after ICH). Individuals assigned a value of zero experienced identical hypertension severity before vs after ICH. Data presented as absolute number of patients in each category and percentage.
Factors Associated With Changes in Hypertension Severity After vs Before ICH
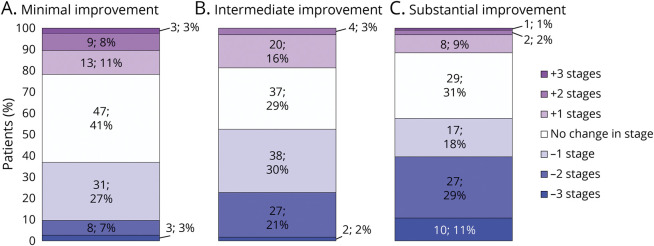
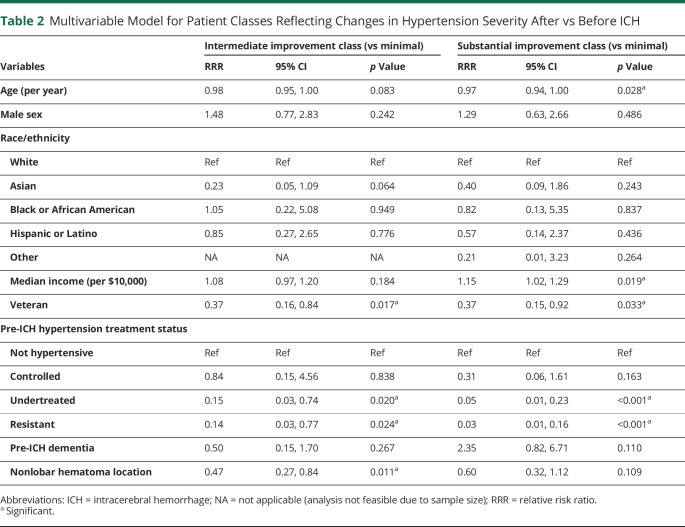
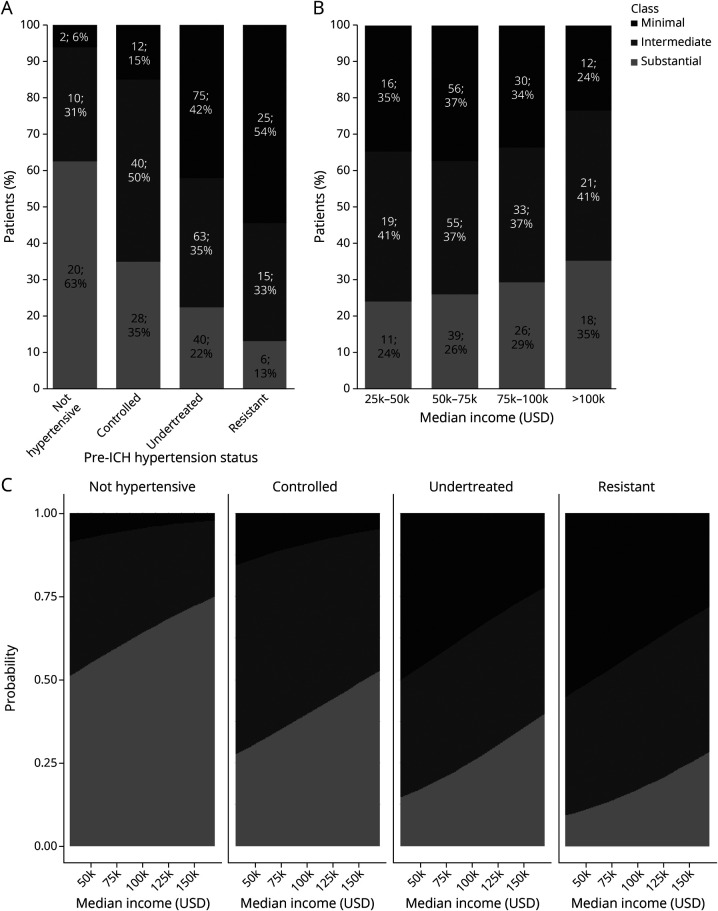
We conducted univariable analyses comparing individuals in classes reflecting improvement in hypertension severity before vs after ICH (Table 1). In univariable analyses, we identified the following potential factors associated with decreased hypertension severity after ICH (all p < 0.20): median neighborhood income, United States country of origin, prior history of ICH or dementia, larger hematoma volumes, and greater disability (higher modified Rankin Scale score at discharge). Potential factors associated with increased hypertension severity after ICH (all p < 0.20) included veterancy status, prehemorrhage history of undertreated or resistant hypertension, history of chronic kidney disease, and nonlobar (i.e., more likely hypertensive in etiology) hemorrhage. ICH survivors prescribed antiplatelets showed a potential association (p = 0.14) with intermediate hypertension severity improvement. In multivariable analysis (Table 2), we found that median neighborhood income, prior history of ICH, prior history of dementia, and larger hematoma volumes were independently associated with higher likelihood of intermediate or substantial improvement in hypertension severity after ICH (all p < 0.05). In contrast, veterancy status, prehemorrhage history of undertreated or resistant hypertension, and nonlobar ICH location were independently associated with lower likelihood of intermediate or substantial improvement in hypertension severity after ICH (all p < 0.05). We present visual representations of the relationship between hypertension severity improvement classes, pre-ICH hypertension treatment status, and neighborhood income in Figure 4. eTable 2 (links.lww.com/WNL/B806) presents results of multivariable comparisons across all 3 patient classes.
Table 2.
Multivariable Model for Patient Classes Reflecting Changes in Hypertension Severity After vs Before ICH
Figure 4. Hypertension Treatment Status before ICH, Neighborhood Income, and Improvement in Hypertension Severity After vs Before ICH.
(A) Stacked bar charts representing prevalence of minimal, intermediate, and substantial improvement in blood pressure (BP) control (as defined by latent class analysis) among study participants, subdivided by hypertension status before intracerebral hemorrhage (ICH). Data presented as absolute number of patients in each category and percentage. (B) Stacked bar charts representing prevalence of minimal, intermediate, and substantial improvement in BP control (as defined by latent class analysis) among study participants, subdivided by median residential income. Data presented as absolute number of patients in each category and percentage. (C) Predicted prevalence of minimal, intermediate, and substantial improvement in BP control (as defined by latent class analysis) as a function of participants’ hypertension status before ICH and median residential income. ICH survivors with prestroke history of undertreated or treatment-resistant hypertension did not experience the same degree of BP control improvement as their counterparts with no hypertension or controlled hypertension diagnoses, even with comparable median residential incomes. Values were computed for graphical illustration purposes from multinomial logistic regression results for a 75-year-old White man, not veteran status, without prior history of dementia or ICH before enrollment, and with an ICH volume of 6 mL (median value in cohort).
Racial and Ethnic Differences in Hypertension Severity After vs Before ICH
We first determined whether significant racial/ethnic differences existed for predictors of improvement in hypertension severity before vs after ICH, as determined from multivariable analyses (Table 2). ICH survivors differed by race/ethnicity in age (White: 75 ± 11 years, Asian: 76 ± 13 years, Black or African American: 65 ± 14 years, Hispanic or Latino: 69 ± 11 years, Other: 60 ± 23 years; p = 0.001), veteran status (White: 66/288 [23%], Asian: 1/14 [7%], Black or African American: 0/12, Hispanic or Latino: 0/19, Other: 0/3; p = 0.014), prior hemorrhage status (White: 35/288 [12%], Asian: 0/14, Black or African American: 5/12 [42%], Hispanic or Latino: 3/19 [16%], Other: 1/3 [33%]; p = 0.019), and median neighborhood income (White: $77,900 ± $2,800, Asian: $67,400 ± $2,900, Black or African American: $59,100 ± $1,400, Hispanic or Latino: $61,900 ± $2,700, Other: $84,800 ± $4,300; p = 0.015). Based on our prespecified analysis plan, we then conducted interaction analyses between factors associated with improvement in hypertension severity and self-reported race/ethnicity. We found that Black, Hispanic, and Asian participants with undertreated or treatment-resistant hypertension before ICH were all likely to experience minimal improvement in BP control after hemorrhagic stroke compared to their White counterparts (all 3 interactions, p < 0.001).
Discussion
We conducted a longitudinal analysis of hypertension severity in the 12 months preceding and following ICH in a single-center study. We leveraged LCA as an unbiased analytical tool to uncover underlying temporal trends in participants' BP measurements. We identified changes in hypertension severity after ICH (i.e., degree of improvement compared to before) as the most relevant temporal trend differentiating survivors. The patient classes we identified significantly differ in likelihood and magnitude of hypertension severity improvement after vs before ICH. Our analyses indicate that most ICH survivors experience very limited improvement in hypertension severity after ICH, with immediate implications for individuals as well as at a broader societal level. Furthermore, our findings support the hypothesis that challenges in controlling BP after ICH largely reflect the longstanding effects of social and biological determinants of health predating the acute hemorrhage.
We found that a history of undertreated and treatment-resistant hypertension before hemorrhagic stroke profoundly affected likelihood of hypertension severity decreasing after ICH. It is likely that the effect of biological and social factors acting as barriers to hypertension control before ICH persisted or even worsened after a major, destabilizing acute event such as hemorrhagic stroke.15 Conversely, a subset of individuals achieving BP control goals after ICH were also well-controlled before the acute hemorrhage event. While these patients may not be strictly considered to have experienced improvement in hypertension severity after ICH, preservation of optimal BP control in spite of the acute hemorrhage likely reflects underlying favorable genetic, environmental, and social effects.16 Overall, obtaining a detailed history of hypertension control before an acute intraparenchymal hemorrhage is critical to subsequent care of ICH survivors. Our results to do not imply an inevitable lack of progress in BP control for ICH survivors in general. Undertreated hypertension before ICH is a modifiable risk factor to be proactively addressed in the secondary prevention setting. Similarly, identifying ICH survivors with treatment-resistant hypertension before the acute hemorrhage would facilitate more rapid referral to clinics that specialize in its management.
We also identified associations between history of ICH and dementia and greater improvement in hypertension severity after ICH (vs before). From a care delivery standpoint, these findings may reflect greater attention to secondary prevention measures for individuals with more advanced cerebral disease. However, multiple hemorrhagic stroke events and higher incidence of cognitive impairment also identify survivors more likely to harbor cerebral amyloid angiopathy (CAA), rather than hypertensive arteriopathy, an underlying small vessel disease subtype.17-19 These individuals may therefore carry a lower underlying risk for severe hypertension, reflected in greater improvement in BP control after ICH. The association between lobar hematoma location and greater improvement in hypertension control is also likely to reflect underlying CAA, rather than anatomical considerations related to selective involvement of cortical structures.20-22 Ultimately, dedicated studies will be required to disentangle the effects of the acute hemorrhage anatomical characteristics, underlying small vessel disease subtype, and patient–physician behaviors on hypertension control after ICH.
We found that higher median neighborhood income served as a potent predictor of greater improvement in hypertension severity after ICH. However, neighborhood income likely represents a proxy for many more SDOH, both individual and structural.23-25 Some of these (e.g., housing stability, health literacy, environmental health, food security, healthful food availability) are modifiable by means of targeted interventions.26,27 Additional research focused on identifying modifiable SDOH associated with BP control after ICH are therefore warranted. Of note, we conducted our research in a high-resource setting; Massachusetts has one of the highest development indices in the world, as well as near-universal health care coverage (as also evident in our analyses).11 It is therefore not surprising that, in our analyses, some SDOH affecting hypertension control in other settings (e.g., health insurance coverage) did not play a crucial role. However, even in this setting, individuals' socioeconomic status exerted a significant effect on BP control after ICH. Our findings support expanding the scope of public health policy and future ICH research beyond focusing on health care availability and affordability alone.28
Existing evidence indicates that Black and Hispanic ICH survivors in the United States are at higher risk for recurrent ICH.8,9,29 We previously uncovered evidence of worse hypertension control after ICH among Black and Hispanic ICH survivors.8 In line with these prior findings, we found that Black, Hispanic, and Asian ICH survivors were more likely (compared to their White counterparts) to experience minimal improvement in BP measurements after ICH when displaying evidence of undertreated or treatment-resistant hypertension before the hemorrhage. Lower residential income among Black and Hispanic individuals further exacerbated this disparity, ultimately contributing to previously identified inequities in outcome after ICH. As previously mentioned, health care availability and insurance status may not be as relevant of a contributor to racial/ethnic disparities in BP control in our study setting. However, more complex disparities in care delivery, care performance, and barriers to a healthy lifestyle are still likely to be highly relevant. Future investigations of racial/ethnic disparities in ICH outcome will therefore benefit from incorporation of a broader array of structural, environmental, and individual-level SDOH.
Our study has several limitations. We analyzed data from ICH survivors presenting to a single tertiary care center with expertise in ICH care, potentially leading to severity or referral bias and limiting the generalizability of our findings. However, joint analyses of data on hypertension control after ICH from MGH and the multicenter US Ethnic/Racial Variations in ICH study (enrolling at both academic centers and community hospitals) showed highly consistent findings in previous studies.8,30 Our findings are therefore likely to be generalizable to ICH survivors at large. By excluding those with no BP measurements in the year prior to ICH from the study, we may have excluded the more vulnerable patients who may not have regular access to care. However, this would likely result in an underestimation of the effect of prehemorrhage factors on BP measurements after ICH. Any bias introduced by this exclusion may also have been partially mitigated in our health care setting, due to the availability of near-universal health care coverage. Conducting our study in a high-resource setting with near-universal health care coverage limits generalizability of our findings, but allowed us to explore the effects of SDOH on hypertension severity after ICH in greater depth, beyond focusing on health care access and affordability alone. Our analyses were also limited in scope by the small sample size for participants self-reporting Black, Hispanic, and Asian race/ethnicity. Despite this significant limitation, we explored the effect of race and ethnicity on hypertension severity before and after ICH. Our approach identified interactions between Black, Hispanic, or Asian race/ethnicity and hypertension severity before ICH in determining BP control afterwards, implying substantial effect at both the individual and societal levels. Because of small sample size, these specific findings are to be considered preliminary, but support conducting dedicated studies of social determinants of hypertension control after ICH employing a balanced racial/ethnic recruitment scheme. Our analytical models, although helpful in providing additional insight into BP control after ICH, are not ready for implementation as bedside tools to guide individual patient care. Whereas this represents a highly relevant future direction, our findings have immediate implications in terms of clinical care, policy-making, and future research efforts. Finally, restriction of our analyses to individuals surviving at least 1 year after ICH is likely to limit generalizability to more severe hemorrhagic stroke cases.
Our study also displays several strengths. We gathered detailed information obtained from participants' interviews, semi-automated review of EHR, neuroimaging, and BP measurements before and after ICH in a highly consistent manner. As a result, our study is uniquely equipped to investigate the temporal trends in hypertension control before vs after ICH, as well as the complex network of biological and social factors contributing to them. We also leveraged LCA, an established and unbiased analytical tool, in an innovative way to explore the relevance of individual-level information in determining BP control temporal trends at a larger scale.
We leveraged BP measurements in the 12 months preceding and following ICH to categorize temporal trends in hypertension severity before vs after the acute hemorrhage. We found that most survivors did not display substantial improvement in hypertension severity after ICH, implicating persistence of longer-standing trends in its management. Socioeconomic status (reflected as residential median income) also affected likelihood of improvement in BP. Black, Hispanic, and Asian ICH survivors were disproportionately more likely to achieve minimal improvement when diagnosed with undertreated or treatment-resistant hypertension before ICH. Systematic differences in residential income further compounded this inequity. Additional studies are warranted to disentangle the complex network of biological and social factors influencing BP control before and after ICH.
Glossary
- ANOVA
analysis of variance
- BP
blood pressure
- CAA
cerebral amyloid angiopathy
- DBP
diastolic blood pressure
- EDW
enterprise data warehouse
- EHR
electronic health record
- ICH
intracerebral hemorrhage
- LCA
latent class analysis
- MGH
Massachusetts General Hospital
- SBP
systolic blood pressure
- SDOH
social determinants of health
- VIF
variance inflation factor
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
NIH K23NS100816, NIH R01NS093870, NIH R01NS103924, NIH R01AG26484.
Disclosure
S.M. Greenberg is supported by NIH R01AG26484. C.D. Anderson is supported by NIH R01NS103924, U01NS069763, and the AHA-Bugher Foundation, receives sponsored research support from Massachusetts General Hospital and Bayer AG, and reports consulting for ApoPharma and Invitae. L. Skolarus is supported by the AHA, NIH R01AG059733, U01MD010579, R01MD011516, and R21AG071796, and has consulted for Bracket Global. J. Rosand is supported by the AHA-Bugher Foundation, NIH R01NS036695, UM1HG008895, R01NS093870, and R24NS092983, and has consulted for Boehringer Ingelheim. A. Biffi is supported by Massachusetts General Hospital, the AHA-Bugher Foundation, and NIH K23NS100816. The remaining authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Biffi A, Bailey D, Anderson CD, et al. Risk factors associated with early vs delayed dementia after intracerebral hemorrhage. JAMA Neurol 2016;73(8):969-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biffi A, Anderson CD, Battey TW, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA. 2015;314(9):904-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casolla B, Moulin S, Kyheng M, et al. Five-year risk of major ischemic and hemorrhagic events after intracerebral hemorrhage. Stroke. 2019;50:1100-1107. [DOI] [PubMed] [Google Scholar]
- 4.Moulin S, Labreuche J, Bombois S, et al. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol. 2016;15(8):820-829. [DOI] [PubMed] [Google Scholar]
- 5.Biffi A, Murphy MP, Kubiszewski P, et al. APOE genotype, hypertension severity and outcomes after intracerebral haemorrhage. Brain Commun. 2019;1:fcz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arima H, Tzourio C, Anderson C, et al. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke. 2010;41:394-396. [DOI] [PubMed] [Google Scholar]
- 7.Zahuranec DB, Wing JJ, Edwards DF, et al. Poor long-term blood pressure control after intracerebral hemorrhage. Stroke. 2012;43:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Torres A, Murphy M, Kourkoulis C, et al. Hypertension and intracerebral hemorrhage recurrence among white, black, and Hispanic individuals. Neurology. 2018;91:e37–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh KB, Woo D, Sekar P, et al. Untreated hypertension: a powerful risk factor for lobar and nonlobar intracerebral hemorrhage in whites, blacks, and Hispanics. Circulation. 2016;134(19):1444-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herle M, Micali N, Abdulkadir M, et al. Identifying typical trajectories in longitudinal data: modelling strategies and interpretations. Eur J Epidemiol. 2020;35(3):205-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayanian JZ. The Massachusetts journey to expand health insurance coverage. J Gen Intern Med. 2012;27(2):139-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun H, Jain A, Leone MJ, et al. CoVA: an acuity score for outpatient screening that predicts coronavirus disease 2019 prognosis. J Infect Dis. 2021;223(1):38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Community Survey. Median Household Income by ZIP Code (2006-2010). Accessed November 23, 2020. https://www.psc.isr.umich.edu/dis/census/Features/tract2zip/.
- 14.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13–e115. [DOI] [PubMed] [Google Scholar]
- 15.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660-667. [DOI] [PubMed] [Google Scholar]
- 16.Cooper R. Hypertension, genes, and environment: challenges for prevention and risk prediction. Circulation. 2018;137(7):662-664. [DOI] [PubMed] [Google Scholar]
- 17.Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92(24):1146-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease: one peptide, two pathways. Nat Rev Neurol. 2020;16(1):30-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19:3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marini S, Crawford K, Morotti A, et al. Association of apolipoprotein E with intracerebral hemorrhage risk by race/ethnicity: a meta-analysis. JAMA Neurol. 2019;76(4):480-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68(6):934-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke. a J Cereb Circ. 2018;49:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnake-Mahl AS, Jahn JL, Subramanian SV, Waters MC, Arcaya M. Gentrification, neighborhood change, and population health: a systematic review. J Urban Health. 2020;97:1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858-867. [DOI] [PubMed] [Google Scholar]
- 25.Diez Roux AV, Mujahid MS, Hirsch JA, Moore K, Moore LV. The impact of neighborhoods on CV risk. Glob Heart.2016;11:353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor LA, Tan AX, Coyle CE, et al. Leveraging the social determinants of health: what works? PLoS One. 2016;11:e0160217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottlieb LM, Wing H, Adler NE. A systematic review of interventions on patients' social and economic needs. Am J Prev Med. 2017;53(5):719-729. [DOI] [PubMed] [Google Scholar]
- 28.Skolarus LE, Sharrief A, Gardener H, Jenkins C, Boden-Albala B. Considerations in addressing social determinants of health to reduce racial/ethnic disparities in stroke outcomes in the United States. Stroke.2020;51(11):3433-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo D, Haverbusch M, Sekar P, et al. Effect of untreated hypertension on hemorrhagic stroke. Stroke. 2004;35:1703-1708. [DOI] [PubMed] [Google Scholar]
- 30.Biffi A, Urday S, Kubiszewski P, et al. Combining imaging and genetics to predict recurrence of anticoagulation-associated intracerebral hemorrhage. Stroke. 2020;51:2153-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.