Abstract
Administration of essential oils as natural plant products with antimicrobial activity might be an alternative to antibiotic treatment of bovine respiratory disease. The aim of this study was to analyse the in vitro antimicrobial activity of 11 essential oils against Pasteurella multocida isolated from the respiratory tract of calves using microdilution with determination of minimum inhibitory and bactericidal concentration as well as agar disc diffusion. Additionally, antimicrobial activity against Mannheimia haemolytica and bacteria in the Mannheimia clade was assessed by agar disc diffusion. Seven essential oil mixtures were also tested against all bacterial isolates. P. multocida was strongly inhibited by cinnamon cassia and lemongrass oil followed by coriander, winter savory, thyme, clove, and peppermint oil in the microdilution assays. Eucalyptus, wintergreen, spruce, and star anise oil showed lower activity. Comparison of both methods revealed an underestimation of cinnamon cassia oil activity by agar disc diffusion and conflicting results for wintergreen oil in microdilution, which precipitated in broth. Cinnamon cassia, thyme, wintergreen, lemongrass, and winter savory oil all showed strong antimicrobial activity against M. haemolytica . Bacteria in the Mannheimia clade were mostly inhibited by cinnamon cassia and thyme oil. Pasteurella isolates were more susceptible to inhibition by essential oils than Mannheimia isolates. Essential oil mixtures did not show stronger antibacterial activity than single essential oils. In conclusion, cinnamon cassia and lemongrass as well as coriander, winter savory, and thyme oil are promising candidates for treatment of P. multocida -associated bovine respiratory infections.
Key words: essential oils, Pasteurella multocida, Mannheimia haemolytica, bovine respiratory disease
Abbreviations
- ANOVA
analysis of variance
- BRD
bovine respiratory disease
- CAMHB
cation-adjusted Mueller-Hinton broth
- EO
essential oil
- IZR
inhibition zone radius
- MALDI-TOF MS
matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry
- MBC
minimum bactericidal concentration
- MIC
minimum inhibitory concentration
Introduction
Pasteurella multocida and Mannheimia haemolytica are commensals and also opportunistic pathogens in multifactorial BRD 1 , 2 . In cattle, BRD is a leading cause of morbidity and mortality 3 . Pathogenesis of BRD depends on multiple factors, such as the animal host, the environment, and involved pathogens 1 , 2 . Of these, P. multocida is an important causative agent in the development of BRD 4 . M. haemolytica is a major cause of severe fibrinous pleuropneumonia 5 . Clinical signs of infection involve respiratory distress, cough, fever, nasal discharge, and inappetence associated with weight loss 1 , 4 , 6 .
Often, antimicrobials are used for metaphylactic or therapeutic treatment of clinical BRD. A European study observed a still high susceptibility of P. multocida and M. haemolytica isolated from diseased, antimicrobial non-treated cattle against licensed antibiotics 7 . However, in North America, the prevalence of non-susceptible respiratory tract isolates in diseased animals is steadily increasing 8 . Several studies analysed the association of antimicrobial treatment and development of bacterial resistances. For example, multiple antibacterial treatments are associated with a higher probability of antimicrobial resistances in cattle 9 . Lower respiratory tract isolates of cattle receiving metaphylactic tulathromycin and chlortetracycline treatment showed a high macrolide resistance level 10 . Stanford et al. 11 found an increased antimicrobial resistance for macrolides, higher MICs across antimicrobial classes, and more resistance-related gene determinants in BRD isolates from cattle managed with antimicrobials in comparison to non-treated animals. This emphasises the importance of prudent antimicrobial use and the necessity of ongoing research on alternatives for prophylaxis, metaphylaxis, and treatment of BRD.
Medicinal plants containing EOs bear great potential for combined or alternative therapy of respiratory conditions in cattle 12 . EOs like thyme, eucalyptus, tea tree, and ravintsara are already used by some practicing veterinarians for the treatment of respiratory infections via oral, topic, or rectal application 13 . The inhalation of EOs is also recommended 14 . EOs are natural plant products and complex mixtures of volatile lipophilic compounds like monoterpenes, sesquiterpenes, and/or phenylpropanoids 15 . They are mainly produced by water or steam distillation or mechanical processing of plant material. As an in vitro screening method for antibacterial activity of EOs, agar disc diffusion can be used 16 , which provides an affordable method for susceptibility testing of EOs in veterinary routine diagnostics. Agar disc diffusion has disadvantages due to volatility and different solubility of EO components in the agar. The more elaborate broth dilution is the preferred method for analyses of EO activity, but hardly feasible in the daily laboratory routine.
Reports describing in vitro antibacterial activity of EOs against bacterial bovine respiratory pathogens are scarce 17 , 18 , 19 . Hence, further knowledge about the potential of EOs as an alternative treatment of BRD is needed. The aim of this study was (i) to analyse the in vitro antimicrobial activity of 11 EOs against P. multocida isolates from the respiratory tract of calves by microdilution, determining both the minimum inhibitory and bactericidal concentrations as well as by agar disc diffusion for comparison purposes. Additionally, (ii) to analyse antimicrobial activity of EOs against M. haemolytica and bacteria in the Mannheimia clade and (iii) antibacterial activity of seven EO mixtures against all bacterial isolates by agar disc diffusion. Thus, EOs for further research on phytotherapeutic prophylaxis and treatment of BRD should be identified.
Results
In microdilution, cinnamon cassia and lemongrass oil showed the strongest antibacterial activity against P. multocida with median MICs of 0.0078 and 0.0313%, respectively ( Fig. 1 a ). Coriander, winter savory, and thyme oil had an MIC of 0.125%, followed by clove (MIC of 0.1875%), peppermint (MIC of 0.25%), and eucalyptus and wintergreen oil (both: MIC of 0.5%). The weakest EOs were spruce (MIC of 1.0%) and star anise oil (MIC > 1.0%). It is important to mention that wintergreen oil immediately precipitated in the broth at the bottom of the well. Therefore, the visual determination of the MIC for wintergreen oil was not distinct. The MBC differed from the MIC, except for winter savory, thyme, and eucalyptus oil ( Fig. 1 a ). For several bacterial isolates, the MBC and MIC differed by one dilution step. This applies for spruce (10% of isolates), coriander, clove, peppermint oil (20 – 26% of isolates), lemongrass (43% of isolates), and cinnamon cassia oil (67% of isolates). For wintergreen and star anise oil, the MIC and MBC were not always possible to determine. MICs that did not have bactericidal effects led to a reduction of bacterial growth on plated agar plates, in contrast to growth controls. In agar disc diffusion, lemongrass oil (median IZR: 30.13 mm, interquartile range: 7.31) most effectively inhibited the growth of P. multocida , followed by cinnamon cassia (22.25 mm, 2.38), thyme (18.5 mm, 2.38), winter savory (16.75 mm, 0.94), coriander (15.00 mm, 2.88), wintergreen (14.5 mm, 1.25), peppermint (13.5 mm, 2.38), and clove oil (11.25 mm, 1.88) ( Fig. 1 b ). Comparison of the results of agar disc diffusion and microdilution of P. multocida ( Fig. 2 ) revealed two major differences in EO activity. Cinnamon cassia oil was much less effective than expected from the microdilution results. Wintergreen oil showed better activity in agar disc diffusion than expected from the microdilution results, where precipitation of the EO occurred. The other EOs showed concordant results of both methods. Thyme, winter savory, and coriander oil had similar activity in agar disc diffusion (IZR of 18.5 – 15.00 mm) and the same MIC (0.125%) in microdilution. Peppermint and clove oil also showed similar activity in agar disc diffusion (IZR of 13.5 – 11.25 mm) and microdilution (MIC values of 0.25 and 0.1875%). Star anise, spruce, and eucalyptus oil had the weakest activity in both agar disc diffusion (IZR of 7.50 – 5.00 mm) and microdilution (MIC of 0.5% – ≥ 1.0%).
Fig. 1.
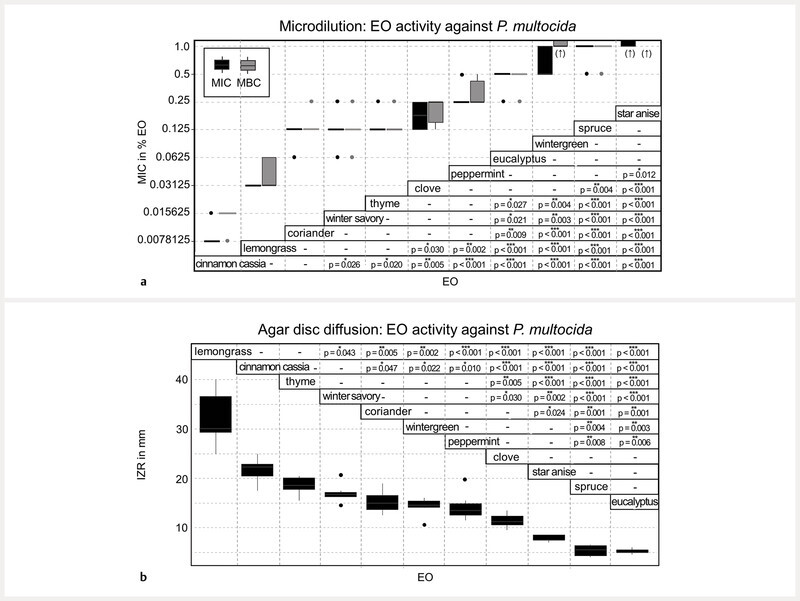
Depicted is ( a ) the minimum inhibitory and bactericidal concentration (MIC and MBC) in % of EOs and ( b ) the inhibition zone radius (IZR) in mm against P. multocida (n = 10). Statistically significant results of comparison of MICs or IZR of EOs are given. Conducted was a Kruskal-Wallis test (chi square = 100.94, df = 10, p < 0.001) and post hoc Dunn test with Benjamini-Hochberg adjustment for pairwise comparison. P values of statistically significant differences and indicating asterisks (*p value ≤ 0.05, **p value ≤ 0.01, ***p value ≤ 0.001, -not significant) are stated within the figure. (↑) MIC and MBC > 1%
Fig. 2.
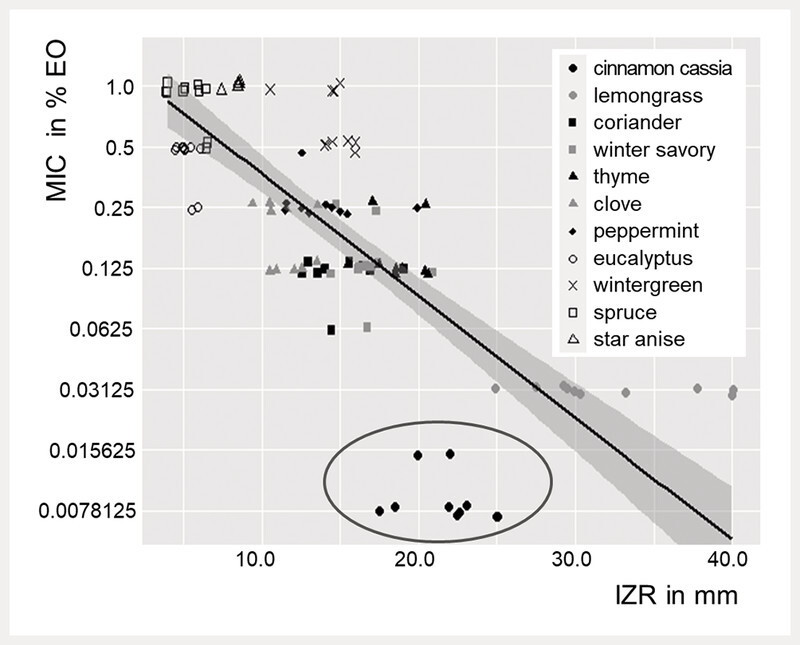
Plotted is the minimum inhibitory concentration (MIC) and the inhibition zone radius (IZR) of EOs against P. multocida and the regression line with 0.95 confidence interval of prediction. Cinnamon cassia oil, which shows largest divergence, is encircled.
Isolates of M. haemolytica and bacteria in the Mannheimia clade were most efficiently inhibited by cinnamon cassia and thyme oil in agar disc diffusion ( Fig. 3 ). M. haemolytica was also inhibited by wintergreen, lemongrass, winter savory, clove, coriander, peppermint, and star anise oil in descending order. Bacteria in the Mannheimia clade were also inhibited by winter savory, coriander, wintergreen, lemongrass, peppermint, clove, and star anise oil. Eucalyptus and spruce oil only yielded small or no inhibition zones against all bacterial isolates. A two-way ANOVA was conducted to examine the effect of bacterial isolates and EOs on the size of the IZR. There was a statistically significant interaction between the EO and bacterial isolate (F = 12.37, df = 20, 279, p < 0.001). Predominantly, P. multocida isolates were more susceptible than Mannheimia isolates, except for star anise and eucalyptus oil ( Fig. 3 ).
Fig. 3.
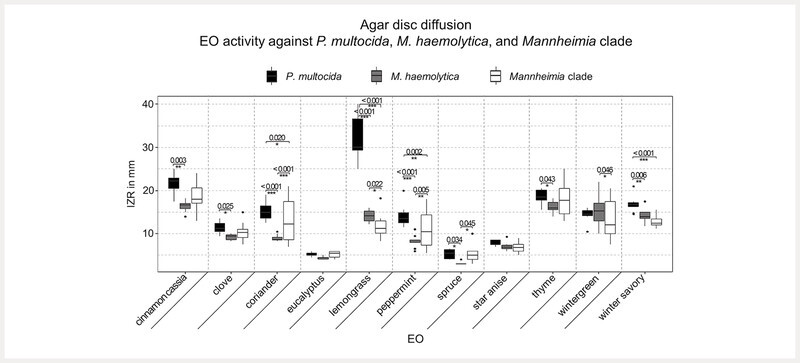
Depicted is the inhibition zone radius (IZR) in mm of EOs against P. multocida (n = 10), M. haemolytica (n = 10), and Mannheimia clade (n = 10). Only statistically significant results of comparison of differences in susceptibility of P. multocida vs. M. haemolytica vs. Mannheimia clade against the same EO are given. Conducted was a two-way ANOVA (interaction between EO and bacterial isolate: F = 12.37, df = 20, 279, p < 0.001) and a post hoc pairwise comparison with Benjamini-Hochberg adjustment. P values of statistically significant differences and indicating asterisks (*p value ≤ 0.05, **p value ≤ 0.01, ***p value ≤ 0.001) are stated with the significance bars.
Additionally, agar disc diffusion of EO mixtures, composed of the most potent single EOs, was performed. All EO mixtures showed antibacterial activity against all bacterial isolates ( Fig. 1S , Supporting Information). A two-way ANOVA was conducted that examined the effect of bacterial isolates and EO mixtures on the size of the IZR. There was no interaction between the EO and bacterial isolate (F = 1.14, df = 12, 189, p = 0.328), but the bacterial isolate (F = 266.44, df = 2, 189, p < 0.001) as well as the EO mixture (F = 8.01, df = 6, 189, p < 0.001) had an effect on the size of the IZR. Multiple comparison of the main effects revealed that P. multocida was more susceptible against the EO mixtures than M. haemolytica (p < 0.001) and bacteria in the Mannheimia clade (p < 0.001). M. haemolytica was also more susceptible than bacteria in the Mannheimia clade (p < 0.001) ( Fig. 1S , Supporting Information). The EO mixtures did not exceed antibacterial activity of the single EOs ( Fig. 2S , Supporting information).
Discussion
The analysis of antimicrobial activity of EOs is lacking standardised methods and is demanding due to their complexity, water insolubility, and volatility 20 . In agar disc diffusion, the size of the inhibition zone of bacterial growth depends on the diffusion of the EO components into the agar, which could be impaired by their hydrophobicity and volatility 16 . However, inhibition of bacterial growth proves antibacterial activity. Broth dilution enables direct contact between all EO components and the pathogen and allows for the determination of the MIC and subsequent determination of the MBC. Bacteriostatic activity has been defined as a ratio of MBC to MIC of > 4 21 , lower ratios indicate a bactericidal activity. For the EOs analysed in this study, a bactericidal mode of action can be assumed, because their MBC-MIC ratio is 1 – 2. Broth dilution is considered the more reliable method for analysis of antimicrobial activity of EOs. Nevertheless, agar disc diffusion is used in veterinary routine diagnostics to predict EO activity before therapeutic use (aromatogram). For the specific EOs analysed in this study, agar disc diffusion is generally valid, as shown by the comparison of the results of both methods. Two exceptions are the underestimation of cinnamon cassia oil activity and the conflicting results for wintergreen oil. Wintergreen oil immediately precipitates in the broth despite the presence of an emulsifier. In this case, the microdilution has to be interpreted with caution. The limitations of agar disc diffusion account for Pasteurella and Mannheimia isolates in the same manner and comparison of differences in their susceptibility are admissible. Predominantly, P. multocida isolates are more susceptible than Mannheimia isolates, which was also observed by others 17 , 19 . Moreover, agar disc diffusion of seven EO mixtures was performed. The good antimicrobial activity of all EO mixtures against P. multocida most likely depends on the high content of lemongrass oil (60 – 70%), which showed strong antimicrobial activity against P. multocida in agar disc diffusion. For determination of the synergistic effects of EO mixtures, determination of the fractional inhibitory concentration using checkerboard assays could be performed in future studies.
Only a few studies investigated the antimicrobial activity of EOs against BRD pathogens. Two studies analysed the effect of winter savory and thyme oil against P. multocida 17 , 19 . Amat et al. 17 observed good activity of thyme oil ( Thymus zygis L.) against one bovine clinical respiratory tract isolate of P. multocida , with an MIC of 0.013%. They 17 also found the MIC equalled the MBC for this Pasteurella isolate, similar to the results obtained in our study. In a study from Salzmann 19 , winter savory and thyme oil ( Thymus vulgaris L.) showed strong antibacterial activity against bovine P. multocida and M. haemolytica isolates using agar disc diffusion and microdilution, with MICs of 0.39 and 0.63%, respectively. In the same study 19 , eucalyptus oil had a weak antibacterial effect against P. multocida in agar disc diffusion and inhibited bacterial growth in microdilution, with an MIC of 0.23%. Different MICs of thyme oil obtained in these studies could be explained by different plant species of the thyme used ( T. zygis L. and T. vulgaris L.), but all belonging to Thymi herba. EOs as natural plant products can vary in their composition of single chemical compounds due to the plant parts used, extraction methods, region of growth, or harvest season 22 . The antimicrobial activity of an EO can differ depending on its composition 23 . Thus, analysed antimicrobial activities apply for the specific batch of the tested EO within a study. A study by Kissels et al. 24 analysed the antimicrobial activity of the EO components thymol, carvacrol, and 1,8-cineole against bovine type strains of P. multocida and M. haemolytica using microdilution: thymol and carvacrol, which are major components of thyme and winter savory oil, exhibited strong antimicrobial activity. The monoterpenoid 1,8-cineole, which is a major component of eucalyptus oil, had no antibacterial effect 24 . This strong and weak activity of major EO components 24 equates the antimicrobial activity of their EOs found in our study.
Toxic side effects are not expected, as low MICs obtained for most EOs implicate a possible low dose application of EOs. EO preparations could be applied orally or by inhalation, direct nasal, or rectal application. In vitro and in vivo studies demonstrating EO safety and clinical efficiency on respiratory symptoms in animals and humans encourage their future use in veterinary medicine 17 , 25 , 26 , 27 , 28 . Fararh et al. 26 showed that an orally applied EO mixture of eucalyptus, lemon, cornment, rosemary, and Echinacea purpurea (0.015 – 0.03%) in combination with an injection of tulathromycin reduced respiratory manifestation and improved blood gas values in calves with respiratory disease caused by P. multocida and Haemophilus spp. Inhalation or direct nasal application of diluted EO preparations would enable specific targeting of the respiratory tract. In the literature, it has been reported 17 that in vitro , no cytotoxic effect against bovine turbinate cells could be detected by the use of diluted thyme or eucalyptus oil in concentrations of ≤ 0.4%. The MIC values for thyme and eucalyptus oil found in our study are below this value, thus safe application of those EOs should be possible while still exerting antibacterial activity. Further, only minimal or no cytotoxic effect could be observed in vitro by LeBel et al. 25 for diluted thyme and winter savory oil (≤ 0.078%) against porcine tracheal epithelial cells. Human studies showed a short-term beneficial effect of eucalyptus oil inhalation (final air concentration of 0.056%) 27 or nasal application of an EO spray mixture ( Eucalyptus citriodora and globulus, Mentha piperita, Origanum syriacim, Rosmarinus officinalis ) 28 .
Besides antimicrobial activity, other positive effects of EOs might contribute to the host defence against respiratory tract infections. Wu et al. 29 showed that linalool, a major compound of coriander and thyme oil (chemotype linalool), activated the Nrf-2 signalling pathway in a human lung cell line and impaired the expression of pro-inflammatory cytokines of P. multocida . Subcutaneous treatment with linalool also led to enhanced clearance of P. multocida , decreased lung neutrophil accumulation, and improved survival of mice 29 . Additionally, Amat et al. 30 observed only minimal in vitro effects of thyme and eucalyptus oil on commensal microbiota like Lactobacilli, Bacilli, and Staphylococci, which might be able to maintain respiratory health by inhibition of pathogen colonisation.
Overall, cinnamon cassia and lemongrass oil showed the best antimicrobial activity against bovine respiratory tract isolates of P. multocida , followed by coriander, winter savory, and thyme oil. Comparison of microdilution and agar disc diffusion of P. multocida isolates showed reliable results obtained in agar disc diffusion for the specific EOs analysed in this study, except for an underestimation of cinnamon cassia oil activity and inconsistent results of wintergreen oil. Mannheimia isolates were strongly inhibited by cinnamon cassia and thyme oil in agar disc diffusion. Further research is needed to validate the clinical potential, especially of cinnamon cassia, lemongrass, and thyme oil in BRD treatment.
Material and Methods
Bacterial isolates
Clinical bacterial isolates of P. multocida (n = 10), M. haemolytica (n = 10), and Mannheimia clade (n = 10) were analysed in this study. All P. multocida , three M. haemolytica , and all Mannheimia clade isolates were derived from deep nasopharyngeal swabs of veal calves, sampled within the “outdoor veal calf” project 31 . Nasopharyngeal swabs of the calves were streaked onto Pasteurella selective agar purchased from Thermo Scientific Oxoid. Plates were incubated at 37 °C for 24 h. One single colony per plate was selected for species identification with MALDI-TOF MS using Microflex LT from Bruker Daltonik GmbH. Colonies identified as P. multocida, M. haemolytica , and bacteria in the Mannheimia clade (identification on genus level) were purified on trypticase soy agar plates containing 5% sheep blood purchased from Becton Dickinson and incubated at 37 °C for 24 h. Identification was confirmed by MALDI-TOF MS and the isolates were frozen in 30% glycerol stocks at − 80 °C. Seven M. haemolytica isolates derived from bovine nose swabs which were sent in for veterinary routine diagnostics to Laboklin GmbH & Co.KG. Since these isolates originated from routine diagnostic samples, no further information on the sampled animals was available and isolates were chosen randomly from the diagnostic pool. Isolates were identified by colony morphology and MALDI-TOF MS with Microflex LT/SH by Bruker Daltonik GmbH. A confidence score was used to rate agreement between the created spectrum and reference spectra provided by the Bruker database. Isolates had confidence scores > 2 allowing for genus and species identification. Bacterial isolates were stored frozen with the CRYOBANK system from Mast Diagnostica at − 20 °C without additional additives.
Essential oils and essential oil mixtures
The tested EOs ( Table 1 ) and EO mixtures ( Table 2 ) were provided by SaluVet GmbH. Spruce oil was obtained from two different plant types. EO mixtures were composed of EOs that showed the strongest antibacterial activity tested individually. Lemongrass oil, according to results of previously carried out internal examinations, was expected to have a very strong antibacterial effect against all tested microorganisms. Therefore, it was chosen as the main component of all EO mixtures, with a content of at least 60%. Other oils were added in such a concentration that possible synergistic effects were expected to be detectable, whilst lemongrass oil still mainly determined the effect.
Table 1 The 11 analysed EOs with their common name, scientific name, plant species, used plant part, origin of plants, extraction method, and chemical composition according to manufacturer or literature.
| EO common name | Scientific name | Plant species | Plant part | Origin of used plants | Extraction method | Chemical composition given by the [A] manufacturer/[B] literature |
|---|---|---|---|---|---|---|
| Cinnamon cassia | Cinnamomi cassiae aetheroleum | Cinnamomum cassia (L.) J.Presl | leaves and young branches | China | steam distillation | [ A ] cinnamaldehyde 77.9%, trans -2-methoxycinnamaldehyde 9.1%, cinnamyl acetate 3.4%, coumarin 2.1%, eugenol 0.1% |
| Clove | Caryophylli aetheroleum | Syzygium aromaticum (L.) Merr. et L. M. Perry | leaves | Indonesia | steam distillation | [ B ] eugenol (60 – 95%) 39 ; (75 – 88%) 40 , α - and β -caryophyllene (5 – 10%) 39 ; (75 – 88%) 40 , acetyleugenol (2 – 27%) 39 , (4 – 15%) 40 , < 1%: 2-heptanone, ethyl hexanoate, humulenol; sesquiterpenes, α -humulene, α -humulene epoxide, β -humulene, β -caryophyllene oxide, α -cubebene, α -copaene, α -cadinene, δ -cadinene, α -ylangene, calacorene, calamenene 40 |
| Coriander | Coriandri aetheroleum | Coriandrum sativum L. | fruits | Russia | steam distillation | [ A ] linalool 71.04%, α -pinene 6.12%, γ -terpinene 4.91%, camphor 4.53%, geranyl acetate 3.74%, limonene 2.59%, geraniol 1.78%, p -cymene 0.65%, α -terpineole 0.28% |
| Eucalyptus | Eucalypti aetheroleum | Eucalyptus globulus Labill. | leaves and branches | China | steam distillation | [ B ] 1,8-cineole (54 – 95%), α -pinene (2.6%), p -cymene (2.7%) 39 |
| Lemongrass | Cymbopogoni aetheroleum | Cymbopogon flexuosus Nees ex Wats. | aerial parts | India | steam distillation | [ B ] citral (75%), geraniol (5.6%) 41 |
| Peppermint | Menthae piperitae aetheroleum | Mentha x piperita L. | aerial parts | India | steam distillation | [ A ] menthol 40.32%, menthone 25.22%, menthyl acetate 5.33%, cineole 4.6%, isomenthone 4.16%, menthofuran 2.79%, limonene 2.14%, pulegone 1.00%, isopulegol 0.19%, carvone 0.07% |
| Spruce | Piceae aetheroleum | Abies sibirica Ledeb.; Abies alba Mill. | needles and branches | Russia | steam distillation | [ B ] Abies sibirica : bornylacetate (30 – 40%), camphene (ca. 10%), santene, α - and β -pinene, α -phellandrene, limonene; Abies alba : bornylacetate (37 – 49%), camphene (10 – 17%), α - and β -pinene 38 |
| Star anise | Anisi stellati aetheroleum | Illicium verum Hook. fil. | fruits | China | steam distillation | [ B ] trans- anethole (80 – 90%), methylchavicol, foeniculin, cis -anethol, anisaldehyde, limonene, linalool, α -pinene 38 |
| Thyme | Thymus zygis aetheroleum | Thymus zygis L. | aerial parts | Spain | steam distillation | [ A ] thymol 46.69%, γ -terpinene 9.65%, p -cymol 19.56%, linalool 4.51%, carvacrol 3.04%, myrcene 1.87%, α -terpinene 1.72%, α -thujene 1.39%, terpinene-4-ol 1.01%, carvacrol methyl ether 0.34% |
| Wintergreen | Gaultheriae aetheroleum | Gaultheria procumbens L. | leaves | China | steam distillation | [ A ] methyl salicylate 98.90% |
| Winter savory | Saturejae montanae aetheroleum | Satureja montana L. | aerial parts | Southern Europe | steam distillation | [ B ] carvacrol (30 – 60%), p- cymene (10 – 20%), γ -terpinene (5 – 15%), borneole, carvone, β -caryophyllene 38 |
Table 2 The composition of the seven EO mixtures.
| EO mixtures | ||
|---|---|---|
| 2 EOs | 1 | 70% lemongrass + 30% cinnamon cassia |
| 2 | 70% lemongrass + 30% thyme | |
| 3 | 70% lemongrass + 30% wintergreen | |
| 4 | 70% lemongrass + 30% winter savory | |
| 3 EOs | 5 | 70% lemongrass + 30% cinnamon cassia + 10% thyme |
| 6 | 65% lemongrass + 20% winter savory + 15% cinnamon cassia | |
| 4 EOs | 7 | 60% lemongrass + 15% cinnamon cassia + 15% winter savory + 10% thyme |
Agar disc diffusion
All bacterial isolates [ P. multocida (n = 10), M. haemolytica (n = 10), and Mannheimia clade (n = 10)] were analysed by agar disc diffusion. Cryopreserved bacteria were recovered at 36 °C on Columbia agar with 5% sheep blood purchased from Becton Dickinson 24 h before performing agar disc diffusion. Agar disc diffusion of EOs or EO mixtures was performed on Mueller-Hinton agar with 5% sheep blood from Becton Dickinson as previously described by us 32 . Agar plates were inoculated with 100 µL of bacterial suspension adjusted to 0.5 McFarland in sterile 0.9% NaCl solution. Afterwards, a sterile 6-mm diameter blank filter paper disc purchased from Becton Dickinson was placed in the middle of the inoculated agar plate and loaded with 10 µL of the EO or EO mixture to be analysed. After overnight incubation at 36 °C, the inhibition zones were measured. Growth controls with only sterile blank filter paper discs and negative controls of EOs without bacterial inoculum were performed. Agar disc diffusion was run in duplicate. Since the blank filter paper discs had a radius of 3 mm, this was the smallest IZR to be determined.
Microdilution
Microdilution for determination of MIC and MBC was conducted for all 11 EOs against P. multocida isolates (n = 10) as described by Cermelli et al. 33 with broth and inoculum according to CLSI 34 . Cryopreserved bacteria were recovered at 36 °C on Columbia agar with 5% sheep blood 24 h before performing microdilution. A twofold serial dilution of EOs from 1.0 – 0.0039% was prepared in 96-well plates using CAMHB from Becton Dickinson with 0.5% Tween 20 from Merck Millipore as the emulsifier. Bacterial inoculum adjusted to 0.5 McFarland in sterile NaCl prediluted in CAMHB broth with 0.5% Tween 20 was seeded in prepared 96-well plates. Bacterial inoculum had a final dilution of 1 : 200 in 100 µL broth per well. A positive growth control with only CAMHB with 0.5% Tween 20 but no EO, and a negative control of the twofold serial dilution of EOs without bacterial inoculum was run alongside. The 96-well plates were sealed with a plastic sheet and incubated at 36 °C for 24 h. The MIC was determined visually as the lowest concentration of EO inhibiting visible bacterial growth in 96-well plates.
For determination of MBC, 100 µL of suitable wells were plated on Columbia agar with 5% sheep blood along with the positive and negative controls. The MBC was determined as the lowest concentration of EO inhibiting bacterial growth on inoculated agar plates.
Experimental design
In this study, activity of 11 EOs against P. multocida isolates (n = 10) was analysed using microdilution for the determination of MIC and MBC. Microdilution was performed in triplicate and the mean of each triplicate was used for further statistical analyses. Additionally, the 11 EOs and 7 EO mixtures were screened for their antimicrobial activity against bacterial isolates of P. multocida (n = 10), M. haemolytica (n = 10), and bacteria in the Mannheimia clade (n = 10). Agar disc diffusion was performed in duplicate, and the mean of each duplicate was used for further statistical analyses.
Statistical analyses
Analysis was conducted in “R” 35 and figures were produced using the package ggplot2 36 . The Shapiro-Wilk test was used to test for normality. Each group of bacterial isolate-EO combination was analysed individually for normality. The IZR of P. multocida and spruce oil [W(9) = 0.830, p = 0.033], star anise oil [W(9) = 0.771, p = 0.006], and wintergreen oil [W(9) = 0.792, p = 0.012], as well as M. haemolytica and coriander oil [W (9) = 0.843, p = 0.048], eucalyptus oil [W(9) = 0.833, p = 0.036], spruce oil [W(9) = 0.366, p < 0.001], and star anise oil [W(9) = 0.836, p = 0.040] as well as Mannheimia clade and spruce oil [W(9) = 0.837, p = 0.041] were not normally distributed.
Differences of EO activity for agar disc diffusion and microdilution of P. multocida were analysed with the Kruskal-Wallis test for global comparison of differences, followed by Dunnʼs post hoc test with Benjamini-Hochberg adjustment for multiple pairwise comparison. Differences of susceptibility of P. multocida vs. M. haemolytica vs. Mannheimia clade against the same EO in agar disc diffusion were analysed with the ARTool of “R” 37 as follows: after the alignment rank transformation to allow for analyses of nonparametric data, a two-way ANOVA was performed for global comparison of differences, followed by a post hoc pairwise comparison with ART-C and Benjamini-Hochberg adjustment.
Contributorsʼ Statement
Data collection: D. Bismarck, J. Becker, E. Müller, V. Becher, L. Nau, P. Mayer; design of the study: D. Bismarck, J. Becker, E. Müller, V. Becher, L. Nau, P. Mayer; statitical anlysis: D. Bismarck; interpretation of the data: D. Bismarck, J. Becker, E. Müller, V. Becher, L. Nau, P. Mayer; drafting the manuscript: D. Bismarck, J. Becker; revision of the manuscript: E. Müller, V. Becher, L. Nau, P. Mayer.
Acknowledgements
We thank Prof. Dr. Mireille Meylan from the Clinic for Ruminants, and Prof. Dr. Vincent Perreten from the Institute of Veterinary Bacteriology, Vetsuisse-Faculty, University Bern, for support and supplying bovine bacterial isolates. Bacterial isolates were sampled within the “outdoor veal calf” project [financed by the National Research Programme 72 (NRP72) of the Swiss National Science Foundation, project 407240_167083] and isolated at the Institute of Veterinary Bacteriology, Vetsuisse-Faculty, University Bern. This study was funded by SaluVet GmbH and Laboklin GmbH&Co.KG.
Footnotes
Conflict of Interest Philipp Mayer and Lisa Nau are employees of SaluVet GmbH, which provided the essential oils. Vera Becher was an employee of SaluVet GmbH when the study was conducted. The other authors declare that they have no conflict of interest.
Supporting Information
Supporting Information includes graphics on the EO mixture activity against P. multocida , M. haemolytica , and Mannheimia clade ( Fig. 1S ) and on the activity of EO mixtures and their single EO constituents against P. multocida , M. haemolytica , and Mannheimia clade ( Fig. 2S ).
References
- 1.Rice J A, Carrasco-Medina L, Hodgins D C, Shewen P E. Mannheimia haemolytica and bovine respiratory disease . Anim Health Res Rev. 2007;8:117–128. doi: 10.1017/S1466252307001375. [DOI] [PubMed] [Google Scholar]
- 2.Caswell J L. Failure of respiratory defenses in the pathogenesis of bacterial pneumonia of cattle. Vet Pathol. 2014;51:393–409. doi: 10.1177/0300985813502821. [DOI] [PubMed] [Google Scholar]
- 3.Murray G M, OʼNeill R G, More S J, McElroy M C, Earley B, Cassidy J P. Evolving views on bovine respiratory disease: An appraisal of selected key pathogens – Part 1. Vet J. 2016;217:95–102. doi: 10.1016/j.tvjl.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabo S M, Taylor J D, Confer A W. Pasteurella multocida and bovine respiratory disease . Anim Health Res Rev. 2007;8:129–150. doi: 10.1017/S1466252307001399. [DOI] [PubMed] [Google Scholar]
- 5.Confer A W, Ayalew S. Mannheimia haemolytica in bovine respiratory disease: Immunogens, potential immunogens, and vaccines . Anim Health Res Rev. 2018;19:79–99. doi: 10.1017/S1466252318000142. [DOI] [PubMed] [Google Scholar]
- 6.Friend S C, Wilkie B N, Thomson R G, Barnum D A. Bovine pneumonic pasteurellosis: Experimental induction in vaccinated and nonvaccinated calves. Can J Comp Med. 1977;41:77–83. [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong A, Thomas V, Simjee S, Moyaert H, El Garch F, Maher K, Morrissey I, Butty P, Klein U, Marion H, Rigaut D, Vallé M. Antimicrobial susceptibility monitoring of respiratory tract pathogens isolated from diseased cattle and pigs across Europe: The VetPath study. Vet Microbiol. 2014;172:202–215. doi: 10.1016/j.vetmic.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Portis E, Lindeman C, Johansen L, Stoltman G. A ten-year (2000–2009) study of antimicrobial susceptibility of bacteria that cause bovine respiratory disease complex– Mannheimia haemolytica, Pasteurella multocida , and Histophilus somni –in the United States and Canada . J Vet Diagn Invest. 2012;24:932–944. doi: 10.1177/1040638712457559. [DOI] [PubMed] [Google Scholar]
- 9.Coetzee J F, Magstadt D R, Sidhu P K, Follett L, Schuler A M, Krull A C, Cooper V L, Engelken T J, Kleinhenz M D, OʼConnor A M. Association between antimicrobial drug class for treatment and retreatment of bovine respiratory disease (BRD) and frequency of resistant BRD pathogen isolation from veterinary diagnostic laboratory samples. PLoS One. 2019;14:e0219104. doi: 10.1371/journal.pone.0219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timsit E, Hallewell J, Booker C, Tison N, Amat S, Alexander T W. Prevalence and antimicrobial susceptibility of Mannheimia haemolytica, Pasteurella multocida , and Histophilus somni isolated from the lower respiratory tract of healthy feedlot cattle and those diagnosed with bovine respiratory disease . Vet Microbiol. 2017;208:118–125. doi: 10.1016/j.vetmic.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Stanford K, Zaheer R, Klima C, McAllister T, Peters D, Niu Y D, Ralston B. Antimicrobial resistance in members of the bacterial bovine respiratory disease complex isolated from lung tissue of cattle mortalities managed with or without the use of antimicrobials. Microorganisms. 2020;8:288. doi: 10.3390/microorganisms8020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayrle H, Mevissen M, Kaske M, Nathues H, Gruetzner N, Melzig M, Walkenhorst M. Medicinal plants–prophylactic and therapeutic options for gastrointestinal and respiratory diseases in calves and piglets? A systematic review. BMC Vet Res. 2016;12:89. doi: 10.1186/s12917-016-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouy M. Baden, Switzerland; 2018. Ätherische Öle für Wiederkäuer – Bewährte Indikationen und Fallbeispiele aus der französischen Nutztierpraxis. 33. Schweizerische Jahrestagung für Phytotherapie 2018 – Ätherische Öle und ihr therapeutisches Potential. [Google Scholar]
- 14.Brendieck-Worm C, Klarer F, Stöger E.Heilende Kräuter für Tiere: Pflanzliche Hausmittel für Heim- und Nutztiere 2nd ed.ed.Bern: Haupt Verlag; 2018 [Google Scholar]
- 15.Reichling J, Schnitzler P, Suschke U, Saller R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties–an overview. Forsch Komplementmed. 2009;16:79–90. doi: 10.1159/000207196. [DOI] [PubMed] [Google Scholar]
- 16.Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 17.Amat S, Baines D, Timsit E, Hallewell J, Alexander T W. Essential oils inhibit the bovine respiratory pathogens Mannheimia haemolytica, Pasteurella multocida and Histophilus somni and have limited effects on commensal bacteria and turbinate cells in vitro. J Appl Microbiol. 2019;126:1668–1682. doi: 10.1111/jam.14238. [DOI] [PubMed] [Google Scholar]
- 18.Amat S, Baines D, Alexander T W. A vapour phase assay for evaluating the antimicrobial activities of essential oils against bovine respiratory bacterial pathogens. Lett Appl Microbiol. 2017;65:489–495. doi: 10.1111/lam.12804. [DOI] [PubMed] [Google Scholar]
- 19.Salzmann B A. Bern: Vetsuisse-Faculty; 2019. Antimikrobielle In-vitro -Wirkung von acht ätherischen Ölen auf Pasteurella multocida und Mannheimia haemolytica aus Nasentupferproben von Kälbern [Master Thesis] .
- 20.Janssen A M, Scheffer J J, Baerheim Svendsen A. Antimicrobial activity of essential oils: A 1976–1986 literature review. Aspects of the test methods. Planta Med. 1987;53:395–398. doi: 10.1055/s-2006-962755. [DOI] [PubMed] [Google Scholar]
- 21.Pankey G A, Sabath L D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 22.Do T KT, Hadji-Minaglou F, Antoniotti S, Fernandez X. Authenticity of essential oils. TrAC Trends Anal Chem. 2015;66:146–157. [Google Scholar]
- 23.Lis-Balchin M, Deans S G, Eaglesham E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragr J. 1998;13:98–104. [Google Scholar]
- 24.Kissels W, Wu X, Santos R R. Short communication: Interaction of the isomers carvacrol and thymol with the antibiotics doxycycline and tilmicosin: In vitro effects against pathogenic bacteria commonly found in the respiratory tract of calves . J Dairy Sci. 2017;100:970–974. doi: 10.3168/jds.2016-11536. [DOI] [PubMed] [Google Scholar]
- 25.LeBel G, Vaillancourt K, Bercier P, Grenier D. Antibacterial activity against porcine respiratory bacterial pathogens and in vitro biocompatibility of essential oils . Arch Microbiol. 2019;201:833–840. doi: 10.1007/s00203-019-01655-7. [DOI] [PubMed] [Google Scholar]
- 26.Fararh K M, Farid S A, Abd EL-Hamied S S, El-Sharkawy R B. Clinicopathological changes in calves with respiratory diseases after treatment with essential volatile oil and other drugs. BVMJ. 2017;33:237–247. [Google Scholar]
- 27.Ben-Arye E, Dudai N, Eini A, Torem M, Schiff E, Rakover Y. Treatment of upper respiratory tract infections in primary care: a randomized study using aromatic herbs. Evid Based Complement Alternat Med. 2011;2011:690346. doi: 10.1155/2011/690346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen B M, Dressler W E. Acute aromatics inhalation modifies the airways. Effects of the common cold. Respiration. 1982;43:285–293. doi: 10.1159/000194496. [DOI] [PubMed] [Google Scholar]
- 29.Wu Q, Yu L, Qiu J, Shen B, Wang D, Soromou L W, Feng H. Linalool attenuates lung inflammation induced by Pasteurella multocida via activating Nrf-2 signaling pathway . Int Immunopharmacol. 2014;21:456–463. doi: 10.1016/j.intimp.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 30.Amat S, Timsit E, Baines D, Yanke J, Alexander T W. Development of bacterial therapeutics against the bovine respiratory pathogen Mannheimia haemolytica. Appl Environ Microbiol. 2019;85:e01359–1419. doi: 10.1128/AEM.01359-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker J, Schüpbach-Regula G, Steiner A, Perreten V, Wüthrich D, Hausherr A, Meylan M. Effects of the novel concept ‘outdoor veal calf’ on antimicrobial use, mortality and weight gain in Switzerland. Prev Vet Med. 2020;176:104907. doi: 10.1016/j.prevetmed.2020.104907. [DOI] [PubMed] [Google Scholar]
- 32.Bismarck D, Schneider M, Müller E. Antibakterielle In-vitro -Wirksamkeit ätherischer Öle gegen veterinärmedizinisch relevante Keime klinischer Isolate von Hunden, Katzen und Pferden . Complement Med Res. 2017;24:153–163. doi: 10.1159/000465519. [DOI] [PubMed] [Google Scholar]
- 33.Cermelli C, Fabio A, Fabio G, Quaglio P. Effect of eucalyptus essential oil on respiratory bacteria and viruses. Curr Microbiol. 2008;56:89–92. doi: 10.1007/s00284-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 34.CLSI . Wayne, PA: Clinical and Laboratory Standards Institute; 2018. Performance Standards for antimicrobial Disk and Dilution Susceptibility Tests for Bacteria isolated from Animals. 4th ed. CLSI supplement VET08. [Google Scholar]
- 35.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria 2021. Accessed November 2, 2021 at:https://www.R-project.org/
- 36.Wickham H. New York: Springer; 2009. ggplot2: Elegant graphics for data analysis. [Google Scholar]
- 37.Elkin L A, Kay M, Higgins J J, Wobbrock J O. New York: ACM; 2021. An Aligned Rank Transform Procedure for Multifactor Contrast Tests; pp. 754–768. [Google Scholar]
- 38.Hiller K, Melzig M F.Lexikon der Arzneipflanzen und Drogen 2nd ed.ed.Heidelberg: Spektrum Akademischer Verlag; 2010 [Google Scholar]
- 39.World Health Organization . Geneva: World Health Organization; 2002. WHO monographs on selected medicinal plants, Volume 2. [Google Scholar]
- 40.European Scientific Cooperative on Phytotherapy . Exeter: European Scientific Cooperative on Phytotherapy; 2014. ESCOP Monographs Online Series, Caryophylli Aetheroleum – clove oil. [Google Scholar]
- 41.Nath S C, Saha B N, Bordoloi D N, Mathur R K, Leclercq P A. The chemical composition of the essential oil of Cymbopogon flexuosus (Steud) Wats. Growing in Northeast India . J Essent Oil Res. 1994;6:85–87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information includes graphics on the EO mixture activity against P. multocida , M. haemolytica , and Mannheimia clade ( Fig. 1S ) and on the activity of EO mixtures and their single EO constituents against P. multocida , M. haemolytica , and Mannheimia clade ( Fig. 2S ).


