ABSTRACT
Background
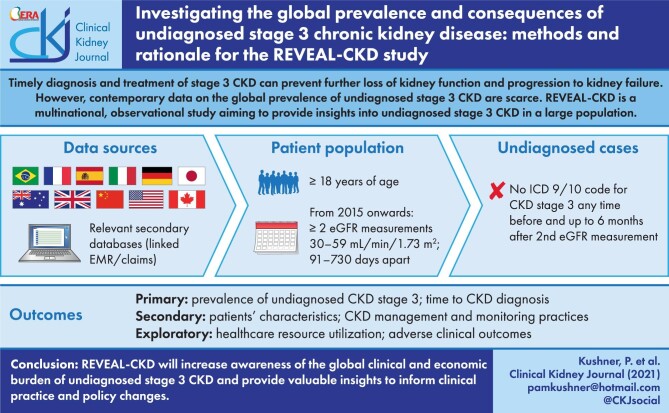
Timely diagnosis and treatment of stage 3 chronic kidney disease (CKD) can prevent further loss of kidney function and progression to kidney failure. However, contemporary data on the global prevalence of undiagnosed stage 3 CKD are scarce. REVEAL-CKD is a multinational, multifocal and observational study aiming to provide insights into undiagnosed stage 3 CKD in a large population.
Methods
Patients (aged ≥18 years) with data in selected secondary databases from 11 countries will be included if they have at least two estimated glomerular filtration rate (eGFR) measurements from 2015 onwards that are ≥30 and <60 mL/min/1.73 m2, recorded >90 and ≤730 days apart. Undiagnosed cases are those without an International Classification of Diseases 9/10 diagnosis code for CKD (any stage) any time before and up to 6 months after the second qualifying eGFR measurement. Time to diagnosis will be assessed using a Kaplan–Meier approach; patient characteristics associated with undiagnosed CKD will be assessed using adjusted logistical regression analyses.
Results
REVEAL-CKD will assess the point prevalence of undiagnosed stage 3 CKD and time to CKD diagnosis in initially undiagnosed cases overall and in individual countries. Trends in undiagnosed CKD prevalence by calendar year will be assessed. Patient characteristics, healthcare resource utilization, adverse clinical outcomes, and CKD management and monitoring practices in patients with versus without a CKD diagnosis will be compared.
Conclusions
REVEAL-CKD will increase awareness of the global clinical and economic burden of undiagnosed stage 3 CKD and provide valuable insights to inform clinical practice and policy changes.
Keywords: chronic kidney disease, epidemiology, methods and rationale, observational study, real world evidence
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) is a well-established global public health concern [1]. It can have a significant impact on patients, attributable to direct mortality and morbidity, as well as elevated risk of cardiovascular diseases [2]. The Global Burden of Disease study showed that, between 1990 and 2016, the global prevalence of CKD increased by approximately one-third, from 2804 per 100 000 persons to 3733 per 100 000 persons [3]. Country-specific microsimulations also project that the overall prevalence of CKD and renal replacement therapy will continue to increase from 2021 to 2026 [4]. This rising prevalence is related to aging populations, increased prevalence of CKD-associated risk factors including type 2 diabetes (T2D) and hypertension, and improved detection [5].
Early detection and management of CKD is crucial for delaying disease progression, because early stage CKD is primarily asymptomatic [6]. The initial diagnosis often occurs during advanced, symptomatic disease stages (stage G4/G5), when there are fewer opportunities to delay progression and prevent complications [7]. In the 2019 Kidney Disease: Improving Global Outcomes (KDIGO) conference entitled ‘Early Identification and Intervention in CKD’, patient representatives, patient advocates and healthcare professionals agreed that CKD screening and treatment should be implemented in a timely fashion, particularly in high-risk persons [8]. Recommended management to delay CKD progression includes lifestyle modifications and pharmacological treatment with renin–angiotensin–aldosterone system inhibitors [9]. More recently, renoprotective effects of sodium–glucose cotransporter-2 (SGLT-2) inhibitors have been demonstrated. The renal benefits of dapagliflozin were initially seen in patients with predominantly preserved renal function [10] and have since been shown in patients with CKD. The phase 3 Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial showed that in patients with CKD, with or without T2D, the addition of dapagliflozin to standard of care was associated with a significantly lower risk than placebo of a composite of a sustained decline in estimated glomerular filtration rate (eGFR) of ≥50%, end-stage kidney disease, or death from renal or cardiovascular causes, as well as a lower risk of death from cardiovascular causes or hospitalization for heart failure [11]. Renoprotective effects of other SGLT2 inhibitors such as canagliflozin have also been demonstrated, but only in those with CKD concomitant with T2D [12], and a trial to evaluate the renoprotective effects of empagliflozin in CKD is ongoing [13].
Without appropriate and timely management, CKD can progress to end-stage kidney disease. The management of this advanced disease is very costly, in terms of both direct healthcare-related costs and the indirect losses in productivity for patients and their caregivers [14]. For example, in the USA, Medicare spending for those with CKD and/or end-stage kidney disease was estimated to be more than US $114 billion in 2016 [15]. The burden of disease for patients with end-stage kidney disease can be substantial, and many patients die before initiating renal replacement therapy [16]. This is due, in part, to the elevated risk of all-cause and cardiovascular mortality associated with CKD [17].
Previous studies have demonstrated potential gaps in guideline-recommended treatment and monitoring of CKD [18–20]. In addition, low diagnosis rates of early stage CKD have been reported in Italy [21], Sweden [20], the UK [22] and the USA [19, 23–26], and in patients with specific CKD aetiologies (e.g. diabetic kidney disease) [25, 27]. However, diagnosis rates in other countries and general populations are not available. Information on demographic and clinical factors associated with undiagnosed stage 3 CKD is also lacking.
REVEAL-CKD (NCT04847531) is a multinational, multifocal and observational study designed to fill this knowledge gap. The overall aim of the REVEAL-CKD study is to quantify the prevalence of, and factors associated with, undiagnosed stage 3 CKD in large populations across several countries.
MATERIALS AND METHODS
Study design
REVEAL-CKD is a multinational observational study using secondary data. Existing electronic medical record and healthcare claim databases will be used to extract data collected during routine clinical practice.
Study objectives
The primary objectives of REVEAL-CKD are to describe the point prevalence of undiagnosed stage 3 CKD and the time to CKD diagnosis in patients with undiagnosed CKD at the point of inclusion in the study.
Secondary objectives are to assess trends in the prevalence of undiagnosed stage 3 CKD by calendar year, to describe patient characteristics at the point of inclusion among those with diagnosed and undiagnosed stage 3 CKD, and to assess CKD management and monitoring practices in patients with undiagnosed versus diagnosed CKD.
Exploratory objectives include describing the risk of selected adverse clinical outcomes (all-cause mortality, end-stage kidney disease and major adverse cardiovascular events), healthcare resource utilization (HCRU) and associated healthcare costs among those with diagnosed versus undiagnosed CKD, as well as determining associations between the timing of CKD diagnosis and the risk of these adverse outcomes and HCRU. Additionally, the study will explore the prevalence of undiagnosed CKD, risk of adverse clinical outcomes, and HCRU and associated costs in patients with undiagnosed and diagnosed CKD in a cohort similar to those enrolled in the DAPA-CKD clinical trial [11].
Participating countries and data sources
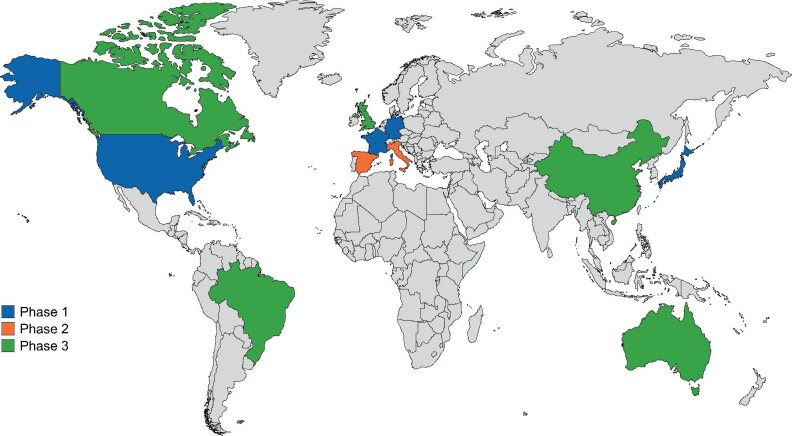
REVEAL-CKD is being conducted in 11 countries (Australia, Brazil, Canada, China (to be confirmed), France, Germany, Italy, Japan, Spain, the UK and the USA) in three phases (Figure 1), using data from contemporary country-specific databases (Supplementary data, Table S1), which meet the following criteria:
FIGURE 1:
Countries included in REVEAL-CKD by study phase.
total data availability spanning at least two calendar years;
recorded laboratory measurements of eGFR and availability of International Classification of Diseases (ICD) 9/10 diagnosis codes or other variables to determine the level of physician-diagnosed CKD;
claims data or records of primary care and/or outpatient consultations to capture CKD diagnosis using relevant coding.
Study population
Inclusion and exclusion criteria to define eligible patients are shown in Table 1 [28, 29].
Table 1.
REVEAL-CKD study inclusion and exclusion criteria
| Inclusion criteria |
| • ≥2 consecutive eGFR laboratory measurements recorded in 2015 or later, with values ≥30 and <60 mL/min/1.73 m2 (stage 3a/3b CKD using the CKD-EPI [28] (preferred) or MDRD [29] equation) that are >90 and ≤730 days apart |
| • ≥12 months of continuous presence in the database before the first qualifying eGFR measurement (look-back period) |
| • Aged ≥18 years at the index date (defined as the date of the second qualifying laboratory eGFR measurement indicative of stage 3a/3b CKD) |
| Exclusion criteria |
| • Solid organ transplant recorded before the index date |
| • Any evidence of advanced CKD (stages 4, 5 and end-stage renal disease) based on CKD diagnosis codes or renal replacement therapy before the index date |
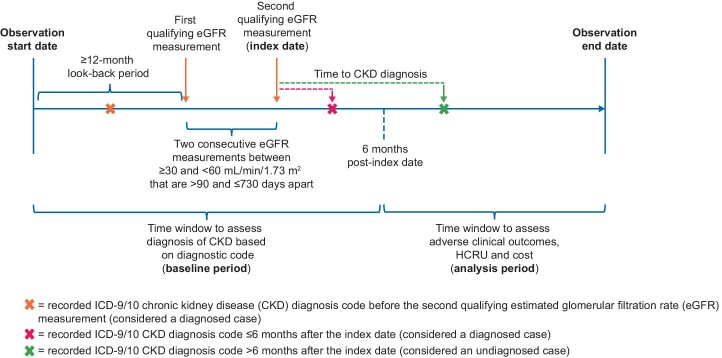
Figure 2 shows the study timeline for patients who are eligible for inclusion. The time window to assess undiagnosed cases of CKD (baseline period) spans from ≥12 months before the first qualifying eGFR measurement (look-back period) to 6 months after the second qualifying eGFR measurement (the index date). The analysis period for assessing HCRU, costs and adverse clinical outcomes associated with undiagnosed versus diagnosed CKD begins 6 months after the second qualifying eGFR measurement and continues until the end of available data coverage, which may be unique to each data source.
FIGURE 2:
Study timeline. HCRU, healthcare resource utilization.
Statistical analysis plan
General considerations
REVEAL-CKD is primarily a descriptive study. All analyses will be conducted separately for each included database; however, for countries with multiple databases, the per-database estimates of the prevalence of undiagnosed CKD may be pooled together in a weighted fashion to provide a single point estimate. The level of missing variables will be assessed. The potential impact of missing data on the outcomes will be investigated, and concerns for bias resulting from missing data will be addressed (e.g. via sensitivity analyses) as appropriate.
The prevalence of undiagnosed stage 3 CKD may be assessed in relevant subgroups that contain at least 20 patients, based on demographic and clinical characteristics. Subgroups examined as part of the study analysis will include age, sex, ethnicity and/or race, health insurance status and the healthcare setting in which eGFR was measured (inpatient versus outpatient). Additional subgroups based on comorbidities at the study index date include patients with T2D, heart failure, T2D with concomitant heart failure, anaemia, established cardiovascular disease and/or hypertension. Patients will also be divided into subgroups for analyses based on the aetiology of their CKD (glomerulonephritis, hypertensive kidney failure, diabetic nephropathy, acute kidney injury or unspecified kidney disease), CKD stage and urinary albumin-to-creatinine ratio (UACR). Depending on feasibility, a subgroup analysis of patients with undiagnosed CKD prescribed angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) will also be performed.
Analyses will also be conducted in a sub-population of patients similar to those enrolled in the phase 3 DAPA-CKD clinical trial [11]. Analysis of this patient population will provide insights into the number of patients with undiagnosed CKD who could benefit from dapagliflozin. Differential inclusion and exclusion criteria for this analysis are shown in Supplementary data, Table S2.
Primary objectives
Undiagnosed cases are defined as those with two consecutive eGFR measurements indicating stage 3a/3b CKD with no corresponding ICD-9/10 diagnosis code for CKD (any stage, Supplementary data, Table S3) at any time during the ≥12-month period before the first qualifying eGFR measurement and ≤6 months after the index date (date of second qualifying eGFR measurement). The 6-month grace period after the second qualifying eGFR measurement will allow time for physicians to record a diagnosis of CKD after measurements have been taken. Included patients with at least one documented diagnosis code for CKD during this period are considered as having diagnosed stage 3 CKD. For the calculation of eGFR from serum creatinine measurements, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [28] will be preferred over the Modification of Diet in Renal Disease (MDRD) equation [29], as per current KDIGO guidelines [7].
In each database, the estimated prevalence of undiagnosed stage 3a/3b CKD will be calculated based on all patients who fulfill the criteria for undiagnosed stage 3a/3b CKD as described above, divided by all individuals meeting the inclusion criteria for the study. The binomial distribution will be used to calculate 95% confidence intervals (CIs) around prevalence.
To evaluate the robustness of the above primary definition for undiagnosed stage 3 CKD, two sensitivity analyses will be conducted. The first sensitivity analysis will include only ICD-9/10 diagnosis codes for stage 3a/3b CKD or higher. The second sensitivity analysis will include ICD-9/10 diagnosis codes for CKD based on a broader definition described by Winkelmayer et al. [30] (Supplementary data, Table S4). Additionally, to assess the suitability of defining CKD diagnosis state using ICD-9/10 diagnosis codes, the proportion of patients receiving treatment for CKD (ACE inhibitors or ARBs) within 12 months after the index date in the diagnosed and undiagnosed groups will be investigated.
To assess time to CKD diagnosis, patients will be followed from their index date to the earliest follow-up end date due to death or transfer out of the database, end of data coverage period or CKD diagnosis (Figure 2). The median time to CKD diagnosis for patients undiagnosed at their index date will be calculated overall and by patient characteristics and comorbidities using the Kaplan–Meier method. Patients who did not receive a CKD diagnosis will be censored at the last day of follow-up. Patient clinical and demographic characteristics associated with a delayed CKD diagnosis will be assessed using Cox regression analysis.
Secondary objectives
Trends in the prevalence of undiagnosed CKD over time will be examined by calculating the prevalence during each calendar year of available data. Patients with a second qualifying eGFR measurement for stage 3 CKD during a given calendar year will contribute data toward the calculation of prevalence for that year.
Patient characteristics at their index date, including demographic data, available physiological/laboratory values and medical history (Table 2), will be summarized overall and in patients with diagnosed and undiagnosed CKD, and compared using descriptive statistics and absolute standardized differences. Patient characteristics associated with undiagnosed CKD will be assessed using a logistic regression model, adjusted for covariates. Parameter estimates, odds ratios, 95% CIs and P-values will be reported.
Table 2.
Patient characteristics and comorbidities at their index date
| Demographic variables |
| •Age (in years) at the index date |
| • Sex |
| • Race/ethnicity (where possible) |
| • Insurance coverage |
| • Socioeconomic status (where possible) |
| • Family history of CKD |
| • Smoking status |
| Physiological/laboratory values |
| •Height |
| •Weight |
| • Body mass indexa |
| •Haemoglobin |
| •Haematocrit |
| • eGFRb |
| • Serum albumin |
| • Serum bicarbonate |
| • Serum calcium |
| • Serum potassium |
| • Serum uric acid |
| • UACR |
| • Albuminuria |
| • Total cholesterol |
| • LDL cholesterol |
| • HDL cholesterol |
| • Triglycerides |
| Medications |
| Cardiovascular |
| • ACE inhibitors |
| • ARBs |
| • Aldosterone receptor antagonists |
| • Angiotensin receptor-neprilysin inhibitors |
| • Loop diuretics |
| • Beta blockers |
| • Thiazide diuretics |
| • Calcium channel blockers |
| • Alpha blockers |
| • Lipid-lowering drugs |
| • Antithrombotic/antiplatelet agents |
| • Anticoagulants |
| Glucose-lowering |
| • Metformin |
| • Sulphonylureas |
| • Dipeptidyl-peptidase 4 inhibitors |
| • SGLT2 inhibitors |
| • Other oral glucose-lowering drugs |
| • Glucagon-like peptide-1 receptor agonists |
| • Insulin |
| Medical history/comorbidities |
| •Number of clinic visits ≤12 months prior to index date |
| • Atherosclerotic cardiovascular diseasec |
| • Atrial fibrillation |
| • Heart failure |
| • Peripheral arterial disease |
| • Type 1 and type 2 diabetes |
| •Hypertension |
| • Glomerulonephritis |
| •Hyperkalaemia |
| •Hypertensive kidney failure |
| • Diabetic nephropathy |
| • Acute kidney injury |
| • Gout |
| • Polycystic kidney disease |
| • Lupus |
| •COVID-19 infection |
Calculated as kg/m2, where kg is weight and m is height in meters.
Calculated as mL/min/1.73 m2 using the CKD-EPI equation (preferred) or MDRD equation.
Includes myocardial infarction, stable and unstable angina, coronary revascularization and ischaemic or haemorrhagic stroke.
COVID-19, coronavirus disease 2019; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
To assess the management and monitoring of CKD in patients with undiagnosed versus diagnosed CKD, information on whether or not key quality indicators are met will be extracted from six domains (Table 3), adapted from previously published data and guidelines as defined by Bello et al. [18, 31, 32]. For these quality indicators, the start of the follow-up period will be defined as 6 months after the study index date, to allow for any potential delay in recording of ICD-9/10 codes. The proportion of patients with undiagnosed and diagnosed CKD achieving each of these quality indicators will be calculated. Each quality indicator will be calculated both for all patients and for the subgroup of patients with complete follow-up data during the specified interval over which the quality measure is to be assessed.
Table 3.
Quality indicators for CKD management and monitoring practices
| 1. Detection and recognition of CKD |
| • Patients receiving a UACR test within the first 6 months of the follow-up period, beginning 6 months after the study index date |
| 2. Monitoring of kidney function and complications |
| • Patients receiving the following tests within the first 18 months of follow-up |
| ○ Serum creatinine (as outpatients) |
| ○ UACR (as outpatients) and albuminuria |
| ○ Serum calcium |
| ○ Serum phosphate |
| ○ Serum albumin |
| ○ Serum bicarbonate |
| ○ Serum potassium |
| ○ Serum haemoglobin |
| 3. Use of recommended medication |
| •Statin prescription within the first 12 months of follow-up |
| •ACE inhibitor and/or ARB prescription within the first 12 months of follow-up |
| •SGLT2 inhibitor prescription at any time during follow-up in patients with T2D |
| •Influenza vaccination at any time during follow-up |
| 4. Kidney function monitoring after initiation of ACE inhibitors or ARBs |
| • Serum creatinine measurement 7–30 days after initial ACE inhibitor or ARB prescription |
| 5. Management of BP |
| • BP measurement(s) at any time during follow-upa |
| • At least one BP measurement within the first 6 months of follow-up |
| • BP measurement ≤140/90 mmHga within the first 12 and 24 months of follow-up |
| • BP measurement ≤130/80 mmHga in patients with evidence of albuminuria and/or diabetes within the first 12 and 24 months of follow-up |
| 6. Monitoring of glycaemic control in patients with T2D |
| • HbA1c test within the first 12 and 24 months of follow-up |
Taken using in-office sphygmomanometers as part of routine ambulatory clinical practice.
BP, blood pressure; HbA1c, glycated haemoglobin.
Exploratory objectives
The incidence of selected adverse clinical outcomes, outlined in Supplementary data, Table S5, will be assessed during the follow-up period starting 6 months post-index date in patients with diagnosed and undiagnosed CKD. If possible, the risk of adverse outcomes between the two groups will be compared in terms of hazard ratios using Cox regression analysis adjusted for baseline covariates.
To assess associations between the timing of CKD diagnosis and the incidence of the above adverse clinical outcomes, initially undiagnosed cases will be stratified based on time to CKD diagnosis, and the incidence of adverse clinical outcomes will be summarized by time stratum; those with no CKD diagnosis at any point during follow-up will be considered as a separate category. Cox regression models adjusted for potential confounders will be used to assess associations between time to CKD diagnosis and incidence of adverse clinical outcomes.
Where data are available, HCRU and costs will be examined by CKD diagnosis status (Supplementary data, Table S6). Descriptive analyses for annualized and cumulative HCRU and costs will be performed for the diagnosed and undiagnosed groups. Subgroup analyses will also be conducted for comorbidities and CKD-associated complications, including myocardial infarction, stroke, hospitalization for heart failure, hospitalization for unstable angina and acute kidney injury. HCRU and/or healthcare costs will be assessed in the first year, subsequent years and then cumulatively. Costs associated with HCRU will also be assessed per country for outcomes that are substantially more prevalent in undiagnosed versus diagnosed CKD populations.
Sample size
If the prevalence of undiagnosed CKD is between 60 and 80%, a sample size of 1000 patients will be sufficient to estimate the prevalence with a margin of error of ±3% in the 95% CI per database/country. In total across all study phases 1 and 2 countries, data are expected to be collected from approximately one million patients.
DISCUSSION
Previous studies examining the prevalence of undiagnosed stage 3 CKD [19, 22–27, 30] have been limited to single countries or databases, were unable to explore and compare multiple patient subgroups (e.g. comorbidities), lacked a strict definition for undiagnosed CKD or were not designed to capture differences in outcomes between diagnosed and undiagnosed patient populations. Using a consistent, strict definition for undiagnosed CKD based on the internationally recognized KDIGO guidelines [7], REVEAL-CKD will be the first study to assess the prevalence of undiagnosed stage 3 CKD overall and in different patient subgroups by comorbidities such as T2D, heart failure and hypertension.
The study is designed to generate sufficient data to investigate a wide range of variables, such as highlighting factors associated with undiagnosed stage 3 CKD and quantifying the economic and health-related burden of delayed CKD diagnosis. A contemporary estimate of the prevalence of undiagnosed CKD will be useful to assess the impact of interventions aimed at improving early stage CKD diagnosis, such as the recently launched ‘Are You the 33%?’ initiative [33]. REVEAL-CKD will also provide valuable insights to inform the development of workable screening programmes and solutions for the management of patients with stage 3 CKD, particularly in primary care where such patients are most likely to be first seen and treated. For example, routine collection of UACR data in clinical practice would allow for UACR monitoring, which is important because evidence suggests that even very low levels of albuminuria correlate strongly with increased risk of cardiovascular disease [34–36].
Late-stage CKD has a large impact on patient health-related quality of life [2] and is associated with substantial healthcare costs [14]. As such, insights into the global prevalence of undiagnosed CKD will better characterize the need for monitoring and management of CKD at an early stage, at which progression and development of complications can be delayed. Data from REVEAL-CKD will also help to inform decisions for screening programmes by identifying important demographic and clinical predictors of undiagnosed stage 3 CKD. This can help to guide predictive models that inform patients or populations where screening programmes can be targeted. The study will also provide a solid evidence base to highlight potential health inequities in the countries examined and inform policy change to minimize such inequities. Additionally, REVEAL-CKD will generate evidence on how key quality indicators for the management of CKD are being met in the countries examined, potentially identifying opportunities for improvement.
Strengths and limitations
The primary strengths of REVEAL-CKD are the multinational nature of the study, the strict definition of undiagnosed CKD based on internationally recognized guidelines and the use of secondary databases with comprehensive patient medical records to capture a wide range of variables. The study will provide insights into the prevalence of undiagnosed CKD in multiple subgroups including stratifications by age and prior comorbidities such as T2D, heart failure and hypertension. In exploring these subgroups, REVEAL-CKD will generate information on the predictors of undiagnosed CKD, offering valuable insights to inform potential changes to current clinical practice. REVEAL-CKD will use large, retrospective databases to provide a comprehensive view of patients who are representative of real-world populations across several countries.
The identification of patients with undiagnosed stage 3 CKD relies on laboratory eGFR results, and the assumption that the absence of an associated CKD diagnosis code indicates that a patient is yet to be diagnosed by a physician. Because this information will be inferred from electronic medical records and claims data, this assumption may result in misclassification of patients due to incomplete reporting of diagnosis codes by physicians. Bias due to misclassification should be minimized, because the staging of CKD using eGFR results is based on an established clinical definition [7]. Inclusion in the study requires available recorded eGFR measurements, and therefore patients are likely to have been tested or screened for CKD, which may introduce a degree of selection bias. Because information on race is not collected in many of the databases selected for inclusion, race will not be included as a modifier for eGFR measurements. This is in line with current trends toward disregarding race as a modifier when calculating eGFR [37, 38] and will enable the pooling of data from all databases to provide a single estimate of the prevalence of undiagnosed stage 3 CKD. For patients with undiagnosed CKD, the use of therapies to mitigate CKD progression (e.g. ACE inhibitors or ARBs) will be investigated in the 12-month period following the index date to determine whether a patient is actually undiagnosed or is lacking a relevant diagnosis code but still receiving treatment for CKD. There is a risk of misclassification if a diagnosis of CKD has been made in clinical settings that do not contribute data to the selected database. Finally, the study will capture data from a range of countries, but results may not be generalizable to other countries, which may have significantly different healthcare systems.
CONCLUSIONS
REVEAL-CKD will provide valuable insights into the prevalence of undiagnosed stage 3 CKD in a large population and will increase disease awareness by providing contemporary, robust data from multiple countries. Insights from REVEAL-CKD will also help to inform interventions for efficient CKD screening in routine clinical practice, thereby increasing opportunities for early management of CKD now that appropriate treatments are available. In turn, these will reduce the risk of complications and progressions to later-stage disease, potentially reduce healthcare costs and improve patient outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
Medical writing support was provided by Bobby Thompson, MSc (Res), of Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca.
Contributor Information
Pamela Kushner, University of California, Irvine, CA, USA.
Emily Peach, Cardiovascular, Renal and Metabolism Epidemiology, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK.
Eric Wittbrodt, Cardiovascular, Renal and Metabolism Medical Affairs, BioPharmaceuticals Medical, AstraZeneca, Gaithersburg, MD, USA.
Salvatore Barone, Global Medical Affairs, BioPharmaceuticals Medical, AstraZeneca, Gaithersburg, MD, USA.
Hungta Chen, Medical/Payer Evidence Statistics, BioPharmaceuticals Medical, AstraZeneca, Gaithersburg, MD, USA.
Juan Jose Garcia Sanchez, Global Market Access and Pricing, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK.
Krister Järbrink, Cardiovascular, Renal and Metabolism Evidence, BioPharmaceuticals Medical, AstraZeneca, Gothenburg, Mölndal, Sweden.
Matthew Arnold, Real World Evidence Data & Analytics, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK.
Navdeep Tangri, Department of Internal Medicine, University of Manitoba, Winnipeg, MB, Canada.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
FUNDING
REVEAL-CKD is funded by AstraZeneca. It is a non-interventional observational study, and as such, no drugs are supplied or funded.
CONFLICT OF INTEREST STATEMENT
E.P., E.W., S.B., H.C., J.J.G.S. and K.J. are employees of AstraZeneca and hold stock options. M.A. is an employee of AstraZeneca. P.K. has received speaker's bureau and advisory board fees from AstraZeneca and Eli Lilly and Company, and honoraria from AstraZeneca and Eli Lilly and Company. N.T. has received grants from AstraZeneca, Boehringer Ingelheim/Eli Lilly and Company, Janssen Pharmaceuticals, Otsuka Pharmaceutical Co. Ltd and Tricida, Inc., has received honoraria from AstraZeneca, Boehringer Ingelheim/Eli Lilly and Company, Janssen Pharmaceuticals, Otsuka Pharmaceutical Co., Ltd and Tricida, Inc., and holds stock options from Mesentech, Renibus, Inc., pulseData and Tricida, Inc. The results presented in this paper have not been published previously in whole or part.
ETHICAL ASPECTS OF THE STUDY PROTOCOL
REVEAL-CKD uses de-identified data from existing databases and does not require data collection beyond that of routine clinical care. No identifiable information will be collected or examined as part of the study. All externally conducted analyses underwent local ethics review and approval. De-identified, internally licensed databases were shared with AstraZeneca by the licensee after ethics review and approval.
REFERENCES
- 1. Levey AS, Atkins R, Coresh J et al. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from kidney disease improving global outcomes. Kidney Int 2007; 72: 247–259 [DOI] [PubMed] [Google Scholar]
- 2. Bikbov B, Purcell CA, Levey AS et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie Y, Bowe B, Mokdad AH et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018; 94: 567–581 [DOI] [PubMed] [Google Scholar]
- 4. Garcia Sanchez JJ, Tangri N, Abdul Sultan A et al. POS-322 inside CKD: projecting the future burden of chronic kidney disease in the Americas and the Asia-Pacific region using microsimulation modelling. Kidney Int Rep 2021; 6: S138–S139 [Google Scholar]
- 5. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 6. Fraser SD, Blakeman T. Chronic kidney disease: identification and management in primary care. Pragmat Obs Res 2016; 7: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 8. Shlipak MG, Tummalapalli SL, Boulware LE et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2021; 99: 34–47 [DOI] [PubMed] [Google Scholar]
- 9. Xie X, Liu Y, Perkovic V et al. Renin–angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis 2016; 67: 728–741 [DOI] [PubMed] [Google Scholar]
- 10. Wiviott SD, Raz I, Bonaca MP et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 11. Heerspink HJL, Stefánsson BV, Correa-Rotter R et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446 [DOI] [PubMed] [Google Scholar]
- 12. Perkovic V, Jardine MJ, Neal B et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 13. Herrington WG, Preiss D, Haynes R et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang V, Vilme H, Maciejewski ML et al. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol 2016; 36: 319–330 [DOI] [PubMed] [Google Scholar]
- 15. Saran R, Robinson B, Abbott KC et al. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019; 73: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neovius M, Jacobson SH, Eriksson JK et al. Mortality in chronic kidney disease and renal replacement therapy: a population-based cohort study. BMJ Open 2014; 4: e004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci 2003; 325: 163–167 [DOI] [PubMed] [Google Scholar]
- 18. Bello AK, Ronksley PE, Tangri N et al. Quality of chronic kidney disease management in Canadian primary care. JAMA Network Open 2019; 2: e1910704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tuttle KR, Alicic RZ, Duru OK et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD registry. JAMA Network Open 2019; 2: e1918169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gasparini A, Evans M, Coresh J et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 2016; 31: 2086–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ravera M, Noberasco G, Weiss U et al. CKD awareness and blood pressure control in the primary care hypertensive population. Am J Kidney Dis 2011; 57: 71–77 [DOI] [PubMed] [Google Scholar]
- 22. de Lusignan S, Tomson C, Harris K et al. UK prevalence of chronic kidney disease for the adult population is 6.76% based on two creatinine readings. Nephron Clin Pract 2012; 120: c107 [Google Scholar]
- 23. Ryan TP, Sloand JA, Winters PC et al. Chronic kidney disease prevalence and rate of diagnosis. Am J Med 2007; 120: 981–986 [DOI] [PubMed] [Google Scholar]
- 24. Diamantidis CJ, Hale SL, Wang V et al. Lab-based and diagnosis-based chronic kidney disease recognition and staging concordance. BMC Nephrology 2019; 20: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bakris G. Prevalence and factors associated with undiagnosed chronic kidney disease in diabetes mellitus. In National Kidney Foundation 2019 Spring Clinical Meetings. Boston, MA. 2019 [Google Scholar]
- 26. Centers for Medicare and Medicaid Services . Chronic kidney disease often undiagnosed in medicare beneficiaries. https://www.cms.gov/files/document/ckd-data-highlight102020-2.pdf (12 March 2021, date last accessed) [Google Scholar]
- 27. Szczech LA, Stewart RC, Su H-L et al. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One 2014; 9: e110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levey AS, Bosch JP, Lewis JB et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 30. Winkelmayer WC, Schneeweiss S, Mogun H et al. Identification of individuals with CKD from medicare claims data: a validation study. Am J Kidney Dis 2005; 46: 225–232 [DOI] [PubMed] [Google Scholar]
- 31. Levin A, Hemmelgarn B, Culleton B et al. Guidelines for the management of chronic kidney disease. Can Med Assoc J 2008; 179: 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nash DM, Brimble S, Markle-Reid M et al. Quality of care for patients with chronic kidney disease in the primary care setting: a retrospective cohort study from Ontario, Canada. Can J Kidney Health Dis 2017; 4: 2054358117703059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Kidney Foundation . Nationwide kidney risk campaign relaunches by NKF, HHS, and ASN. https://www.kidney.org/news/nationwide-kidney-risk-campaign-relaunches-national-kidney-foundation-u-s-department-health-and (16 April 2020, date last accessed) [Google Scholar]
- 34. Volpe M. Microalbuminuria screening in patients with hypertension: recommendations for clinical practice. Int J Clin Pract 2008; 62: 97–108. [DOI] [PubMed] [Google Scholar]
- 35. Gerstein HC, Mann JF, Yi Q et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286: 421–426 [DOI] [PubMed] [Google Scholar]
- 36. Klausen K, Borch-Johnsen K, Feldt-Rasmussen B et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110: 32–35 [DOI] [PubMed] [Google Scholar]
- 37. Duggal V, Thomas I-C, Montez-Rath ME et al. National estimates of CKD prevalence and potential impact of estimating glomerular filtration rate without race. J Am Soc Nephrol 2021; 32: 1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diao JA, Wu GJ, Taylor HA et al. Clinical implications of removing race from estimates of kidney function. JAMA 2021; 325: 184–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.